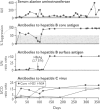
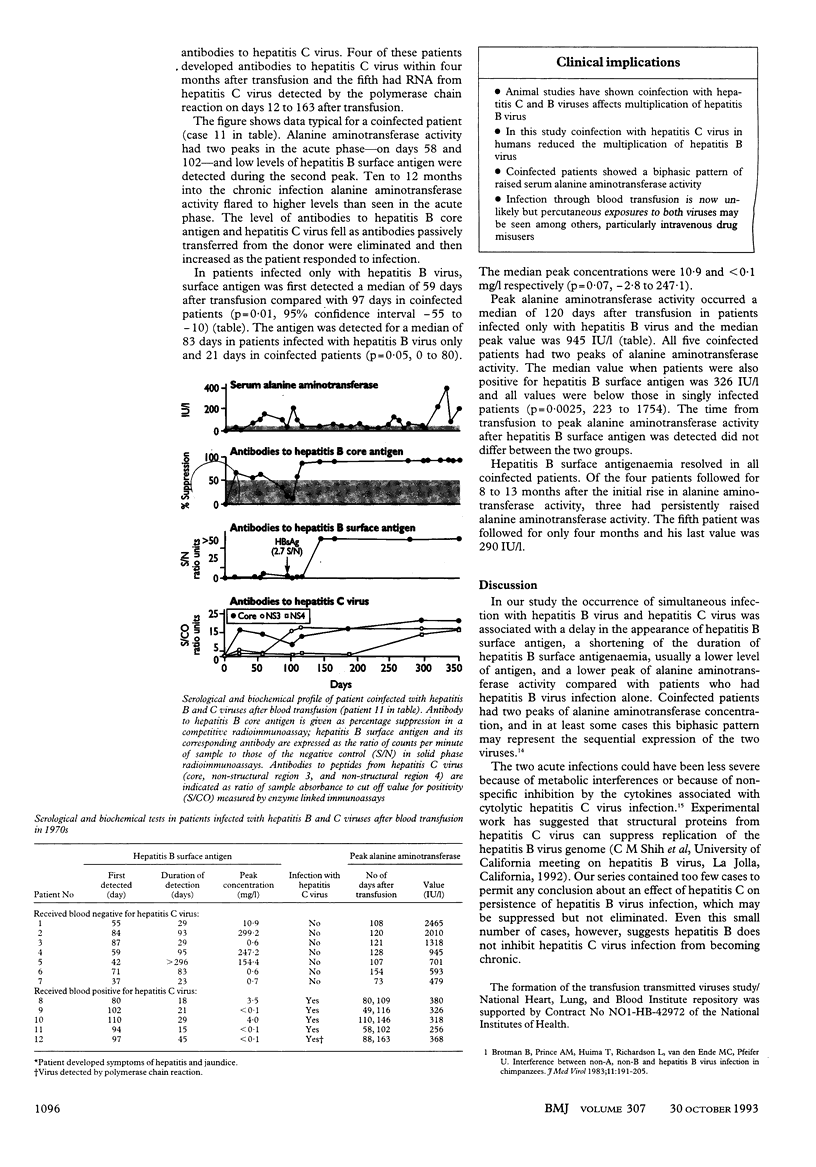
Abstract
OBJECTIVE--To investigate the possible interference with acute hepatitis B virus infection by co-infection with hepatitis C virus. DESIGN--Analysis of stored sera collected for transfusion transmitted viruses study in 1970s. SETTING--Four major medical centres in the United States. PATIENTS--12 recipients of blood infected with hepatitis B virus. MAIN OUTCOME MEASURES--In 1970s, presence of antibodies in hepatitis B virus and raised serum alanine aminotransferase concentration; detection of antibodies to hepatitis C virus with new enzyme linked immunoassays. RESULTS--Five of the 12 patients were coinfected with hepatitis C virus. Hepatitis B surface antigen was first detected at day 59 in patients infected with hepatitis B virus alone and at day 97 in those coinfected with hepatitis C virus (p = 0.01); median durations of antigenaemia were 83 and 21 days respectively (p = 0.05), and the antigen concentration was lower in the coinfected patients. Alanine aminotransferase patterns were uniphasic when hepatitis B virus infection occurred alone (range 479-2465 IU/l) and biphasic in patients with combined acute infection (no value > 380 IU/l; p = 0.0025). Four coinfected recipients developed chronic hepatitis C virus infection. The fifth patient was followed for only four months. CONCLUSIONS--Acute coinfection with hepatitis C virus and hepatitis B virus inhibits hepatitis B virus infection in humans, and onset of hepatitis B may reduce the severity of hepatitis C virus infection but not frequency of chronicity. Alanine aminotransferase concentration showed a biphasic pattern in dual infection.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aach R. D., Stevens C. E., Hollinger F. B., Mosley J. W., Peterson D. A., Taylor P. E., Johnson R. G., Barbosa L. H., Nemo G. J. Hepatitis C virus infection in post-transfusion hepatitis. An analysis with first- and second-generation assays. N Engl J Med. 1991 Nov 7;325(19):1325–1329. doi: 10.1056/NEJM199111073251901. [DOI] [PubMed] [Google Scholar]
- Aach R. D., Szmuness W., Mosley J. W., Hollinger F. B., Kahn R. A., Stevens C. E., Edwards V. M., Werch J. Serum alanine aminotransferase of donors in relation to the risk of non-A,non-B hepatitis in recipients: the transfusion-transmitted viruses study. N Engl J Med. 1981 Apr 23;304(17):989–994. doi: 10.1056/NEJM198104233041701. [DOI] [PubMed] [Google Scholar]
- Brotman B., Prince A. M., Huima T., Richardson L., van den Ende M. C., Pfeifer U. Interference between non-A, non-B and hepatitis B virus infection in chimpanzees. J Med Virol. 1983;11(3):191–205. doi: 10.1002/jmv.1890110303. [DOI] [PubMed] [Google Scholar]
- Bukh J., Purcell R. H., Miller R. H. Importance of primer selection for the detection of hepatitis C virus RNA with the polymerase chain reaction assay. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):187–191. doi: 10.1073/pnas.89.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eble K., Clemens J., Krenc C., Rynning M., Stojak J., Stuckmann J., Hutten P., Nelson L., DuCharme L., Hojvat S. Differential diagnosis of acute viral hepatitis using rapid, fully automated immunoassays. J Med Virol. 1991 Mar;33(3):139–150. doi: 10.1002/jmv.1890330302. [DOI] [PubMed] [Google Scholar]
- Gilles P. N., Fey G., Chisari F. V. Tumor necrosis factor alpha negatively regulates hepatitis B virus gene expression in transgenic mice. J Virol. 1992 Jun;66(6):3955–3960. doi: 10.1128/jvi.66.6.3955-3960.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimms L., Vallari D., Ducharme L., Holland P., Kuramoto I. K., Zeldis J. Specificity of anti-HCV ELISA assessed by reactivity to three immunodominant HCV regions. Lancet. 1990 Dec 22;336(8730):1590–1591. doi: 10.1016/0140-6736(90)93377-2. [DOI] [PubMed] [Google Scholar]
- Mosley J. W., Aach R. D., Hollinger F. B., Stevens C. E., Barbosa L. H., Nemo G. J., Holland P. V., Bancroft W. H., Zimmerman H. J., Kuo G. Non-A, non-B hepatitis and antibody to hepatitis C virus. JAMA. 1990 Jan 5;263(1):77–78. [PubMed] [Google Scholar]
- Stevens C. E., Aach R. D., Hollinger F. B., Mosley J. W., Szmuness W., Kahn R., Werch J., Edwards V. Hepatitis B virus antibody in blood donors and the occurrence of non-A, non-B hepatitis in transfusion recipients. An analysis of the Transfusion-Transmitted Viruses Study. Ann Intern Med. 1984 Dec;101(6):733–738. doi: 10.7326/0003-4819-101-6-733. [DOI] [PubMed] [Google Scholar]
- Tateda A., Kikuchi K., Numazaki Y., Shirachi R., Ishida N. Non-B hepatitis in Japanese recipients of blood transfusions: clinical and serologic studies after the introduction of laboratory screening of donor blood for hepatitis B surface antigen. J Infect Dis. 1979 May;139(5):511–518. doi: 10.1093/infdis/139.5.511. [DOI] [PubMed] [Google Scholar]
- Tsiquaye K. N., Portmann B., Tovey G., Kessler H., Hu S., Lu X. Z., Zuckerman A. J., Craske J., Williams R. Non-A, non-B hepatitis in persistent carriers of hepatitis B virus. J Med Virol. 1983;11(3):179–189. doi: 10.1002/jmv.1890110302. [DOI] [PubMed] [Google Scholar]
- Vallari D. S., Jett B. W., Alter H. J., Mimms L. T., Holzman R., Shih J. W. Serological markers of posttransfusion hepatitis C viral infection. J Clin Microbiol. 1992 Mar;30(3):552–556. doi: 10.1128/jcm.30.3.552-556.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]