Abstract
Editing reactions catalyzed by aminoacyl-tRNA synthetases are critical for accurate translation of the genetic code. To date, this activity, whereby misactivated amino acids are hydrolyzed either before or after transfer to noncognate tRNAs, has been characterized extensively only in the case of class I synthetases. Class II synthetases have an active-site architecture that is completely distinct from that of class I. Thus, findings on editing by class I synthetases may not be applicable generally to class II enzymes. Class II Escherichia coli proline-tRNA synthetase is shown here to misactivate alanine and to hydrolyze the noncognate amino acid before transfer to tRNAPro. This enzyme also is capable of rapidly deacylating a mischarged Ala-tRNAPro variant. A single cysteine residue (C443) that is located within the class II-specific motif 3 consensus sequence was shown previously to be dispensable for proline-tRNA synthetase aminoacylation activity. We show here that C443 is critical for the hydrolytic editing of Ala-tRNAPro by this class II synthetase.
The faithful translation of the genetic code is crucial for the survival of all organisms. The aminoacyl-tRNA synthetases play a central role in ensuring the fidelity of this process through selection and activation of the correct amino acid and by specific tRNA recognition (1–3). Aminoacylation of tRNAs is carried out in a two-step process. In the first step, the amino acid is activated with ATP to form the aminoacyl adenylate; in the second step, the activated amino acid is transferred to the tRNA. Misactivation of amino acids can be corrected through a number of different pathways (1). In the so-called “pretransfer” editing pathway, the noncognate aminoacyl adenylate is hydrolyzed by the synthetase in either a tRNA-dependent or tRNA-independent manner. In the “posttransfer” pathway, a tRNA aminoacylated with a noncognate amino acid is deacylated rapidly by the synthetase. A third pathway that may be used to expel a noncognate amino acid from the synthetase active site uses the chemical characteristics of the amino acid. For example, Escherichia coli methionine-tRNA synthetase misactivates homocysteine, which has been shown to cyclize via nucleophilic attack of the sulfur atom to form homocysteine thiolactone, with loss of AMP (4).
Amino acid editing was discovered first in class I isoleucine-tRNA synthetase (5, 6). Isoleucine-tRNA synthetase misactivates valine (7), hydrolyzes Val-AMP in a tRNAIle-dependent manner (5), and deacylates Val-tRNAIle (6). It was determined that the D arm sequence of tRNAIle is a critical determinant for triggering pretransfer editing (8) and appears to be required for efficient translocation of misactivated Val-AMP or Val-tRNAIle from the synthetic to the editing active site (9). A large insertion in the active site of IleRS, designated connective polypeptide 1, has been shown to be responsible for the editing activity (10–13).
The editing reactions of class II synthetases have been much less studied than those of class I. Class II phenylalanine-tRNA synthetase specifically deacylates Ile-tRNAPhe (14), and alanine-tRNA synthetase has been shown to hydrolyze misactivated serine and glycine (15). E. coli lysine-tRNA synthetase hydrolyzes misactivated homocysteine, homoserine, cysteine, threonine, and alanine, whereas aspartic acid-tRNA synthetase and serine-tRNA synthetase do not (16). Additionally, lysine-tRNA synthetase apparently does not possess posttransfer editing (17). Although E. coli threonine-tRNA synthetase does not appear to edit via the pretransfer route, it misactivates serine, and recent experiments indicate that this class II enzyme can deacylate Ser-tRNAThr (18, 19). An N-terminal domain has been proposed to function as the editing domain in this system (18, 19). E. coli proline-tRNA synthetase (ProRS) contains a large insertion domain of unknown function within its amino acid activation site (20). We hypothesized that ProRS might misactivate noncognate amino acids smaller than proline, such as alanine, and, therefore, would require editing activity. With this in mind, in this study, we examine amino acid misactivation and both pre- and posttransfer editing activities of this class II synthetase.
Materials and Methods
RNA Preparation.
Wild-type E. coli and human tRNAPro and the E. coli tRNAPro G1:C72/U70 triple mutant were prepared as described (20, 21). RNA was prepared by in vitro transcription with T7 RNA polymerase (22). To determine the required time to reach plateau levels of aminoacylation, assays were performed at room temperature according to published conditions (23). Reactions to isolate mischarged tRNA contained 1 μM E. coli alanine-tRNA synthetase, 2 units/ml inorganic pyrophosphatase, and 10 μM E. coli G1:C72/U70-tRNAPro. All of the amino acids (at least 100 pmol) present in the reaction were tritiated. At the desired time, acetic acid was added to 1% final concentration to quench the reaction. The [3H]Ala-tRNA was purified by repeated phenol extractions, followed by ethanol precipitation. Phenol was equilibrated against diethyl pyrocarbonate-treated water. Charged tRNA was quantified by scintillation counting.
Enzyme Preparation.
Wild-type and C443G E. coli ProRS were prepared as described (24). E. coli alanine-tRNA synthetase was prepared according to the published protocol (25). Enzyme concentrations were determined by active-site titration (26).
Enzyme Assays.
ATP-PPi exchange assays were performed according to published conditions (27). Amino acid concentrations were as follows: 0.05–2 mM proline and 25–500 mM alanine. The E. coli ProRS concentration was 1 nM in experiments with proline and 20 nM in experiments with alanine. Kinetic parameters were determined from Lineweaver–Burk plots and represent the average of at least three determinations.
ATP hydrolysis assays were carried out essentially as described (7), with the following modifications: reactions contained 4 units/ml inorganic pyrophosphatase and were quenched with 25 vol of 7% HClO4, 10 mM NaPPi, and 3% charcoal. ProRS concentrations ranged from 1 to 4 μM. The charcoal-bound ATP/AMP was separated from the [32P]Pi in solution by centrifugation. A 50-μl aliquot of the supernatant injected into 5 ml of scintillation fluor was quantified by liquid scintillation counting. For determination of kinetic parameters, the alanine concentration range used was 10–580 mM.
Aminoacylation assays to detect mischarged tRNA were carried out under standard assay conditions (23) with 2–5 μM ProRS, 8–10 μM tRNAPro, and either 214 μM [14C]alanine or 22.5 μM [3H]alanine. Proline aminoacylation assays were carried out under standard conditions of 0.1 μM ProRS, 2 μM tRNAPro, and 22.5 μM [3H]proline (28).
Deacylation assays were performed as described (7), except that each reaction contained 150 mM KPO4 (pH 7.0), 10 mM MgCl2, 2 units/ml inorganic pyrophosphatase, and 0.1 mg/ml BSA.
Results
Noncognate Amino Acid Activation by E. coli ProRS.
To test the hypothesis that E. coli ProRS has editing activity, we first measured kinetic parameters for alanine activation by ProRS. Indeed, we found that this enzyme misactivates alanine, albeit with a significantly reduced kcat and elevated KM relative to cognate proline (Table 1). Based on these data, the in vitro “discrimination factor” [1/(relative kcat/KM)] for activation of alanine compared with proline is 23,000. However, in E. coli, alanine is present at a much higher concentration than proline (148 μM vs. 9 μM, respectively) (29). Thus, the “effective discrimination factor” (30–32), which takes into account the higher frequency with which ProRS will encounter alanine in vivo, is only ≈1,200. Amino acid editing is predicted to be necessary when the discrimination factor is less than ≈3,300, the observed overall error rate for protein synthesis (33). Therefore, E. coli ProRS is likely to require editing to maintain fidelity in translation.
Table 1.
Kinetic parameters for activation of proline and alanine by E. coli ProRS
| Amino acid | kcat, sec−1 | KM, mM | kcat/KM, sec−1⋅mM−1 | kcat/KM (relative) |
|---|---|---|---|---|
| Proline | 70 ± 25 | 0.25 ± 0.035 | 280 | 1 |
| Alanine | 1.7 ± 0.56 | 140 ± 65 | 0.012 | 4.3 × 10−5 |
Pretransfer Editing by E. coli ProRS.
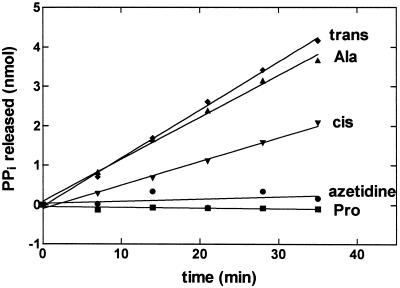
We next wanted to establish whether E. coli ProRS carries out pretransfer editing in vitro. ATP hydrolysis activity is considered diagnostic of hydrolytic editing (1). The ATP hydrolysis activity of E. coli ProRS is stimulated in the presence of alanine but, as expected, not in the presence of 2–500 mM cognate proline (Fig. 1 and data not shown). Based on ATPase assays performed over a broad range of alanine concentrations, we determined a KMAla of 216 mM and a pretransfer editing rate of 0.035 sec−1. Using this assay, we determined that E. coli ProRS also edits the proline analogs cis- and trans-4-hydroxy-proline (Fig. 1). However, ProRS is unable to hydrolyze activated azetidine-4-carboxylic acid, a four-membered ring analog of proline that is toxic to E. coli cells (34, 35). Notably, the presence of an unmodified tRNAPro transcript, or a mixture of native E. coli tRNAs, does not further stimulate ATP hydrolysis activity (data not shown).
Figure 1.
Noncognate amino acids stimulate ATP hydrolysis by E. coli ProRS. Graph showing the ATP hydrolysis activity of ProRS (2 μM) in the presence of 250 mM trans-4-hydroxyproline (trans) (⧫), 500 mM alanine (▴), 250 mM cis-4-hydroxyproline (cis) (▾), 250 mM azetidine-4-carboxylic acid (azetidine) (●), and 2 mM proline (■).
Posttransfer Editing by E. coli ProRS.
Posttransfer editing assays typically require the measurement of deacylation rates of preformed mischarged tRNAs (7). E. coli ProRS misaminoacylates tRNAPro with alanine at an approximately 50,000-fold reduced efficiency relative to aminoacylation with proline. We also attempted to misaminoacylate tRNAPro with alanine by using Caenorhabditis elegans mitochondrial and E. coli alanine-tRNA synthetases. These misaminoacylation assays were performed under conditions reported to increase mischarging, including the addition of up to 20% DMSO or methanol and inorganic pyrophosphatase (36). Using these methods, we were unable to produce sufficient amounts of mischarged tRNAPro for use in deacylation assays.
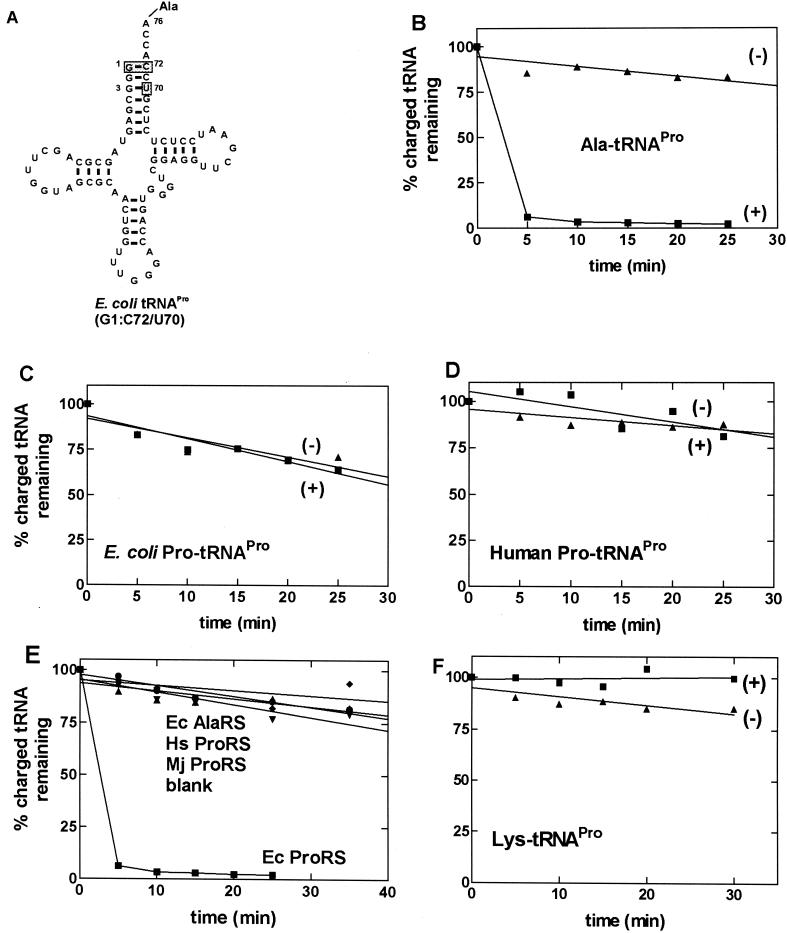
Because misacylated wild-type tRNAPro was difficult to obtain in good yield, we decided to use an E. coli tRNAPro variant containing three acceptor stem mutations, C1:G72 → G1:C72 and C70 → U70 (Fig. 2A) (21). This mutant is charged by E. coli ProRS, albeit at a reduced efficiency because of the absence of a major ProRS recognition element, G72 (28, 37). However, this variant is an excellent substrate for E. coli alanine-tRNA synthetase because it contains the G3:U70 base pair, a major determinant for aminoacylation with alanine (38, 39), and lacks G72, which is a known negative or blocking element (21). Using this triple mutant, it was possible to isolate mischarged G1:C72/U70-[3H]Ala-tRNAPro in high yield. We found that E. coli ProRS rapidly deacylates this Ala-tRNAPro variant (Fig. 2B) but does not deacylate E. coli or human [3H]Pro-tRNAPro (Fig. 2 C and D). The rapid deacylation activity was specific to E. coli ProRS, because other enzymes, including human and Methanococcus jannaschii ProRSs and E. coli alanine-tRNA synthetase, did not exhibit this activity (Fig. 2E). To ensure that deacylation of G1:C72/U70-Ala-tRNAPro does not result from the introduction of the three acceptor stem mutations, we also prepared G1:C72/U70-Lys-tRNAPro. This was accomplished by using human lysine-tRNA synthetase, which has nonspecific charging capabilities (T. Stello and K.M.-F., unpublished data). This mischarged variant was not deacylated by E. coli ProRS (Fig. 2F).
Figure 2.
Deacylation of wild-type tRNAPro and mischarged tRNAPro variants. (A) The sequence of alanine-accepting E. coli tRNAPro. The shaded boxes indicate nucleotides that are mutated relative to wild-type E. coli tRNAPro. (B–D) Graphs showing the deacylation of G1:C72/U70-[3H]Ala-tRNAPro (B), E. coli [3H]Pro-tRNAPro (C), and human [3H]Pro-tRNAPro (D) in the presence (+) and absence (−) of E. coli ProRS. (E) Graph showing that the efficient deacylation of the Ala-tRNAPro variant is specific for E. coli (Ec) ProRS (0.5 μM). E. coli AlaRS (1 μM) (⧫), human (Hs) ProRS (0.6 μM) (●), and M. jannaschii (Mj) ProRS (0.4 μM) (▾) were unable to deacylate E. coli G1:C72/U70-Ala-tRNAPro. (F) G1:C72/U70-[3H]Lys-tRNAPro is not deacylated by E. coli ProRS. In B-–F, the data obtained in the presence of E. coli ProRS are represented by ■, and the control reaction carried out in the absence of enzyme is represented by ▴.
Role of Single Cysteine in E. coli ProRS Editing Activities.
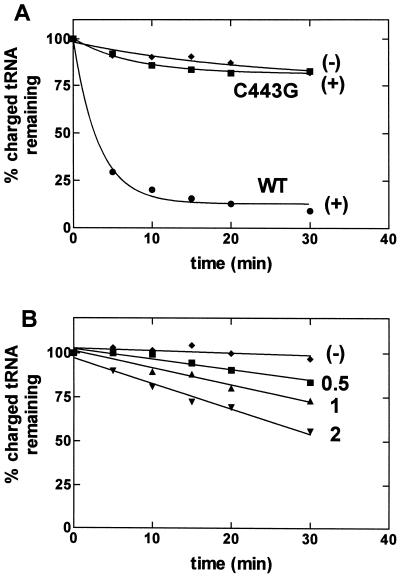
E. coli ProRS contains a single cysteine residue that is located in the class II-specific motif 3 sequence and aligns with residues that have been implicated in amino acid binding specificity in other class II systems (ref. 24 and references therein). Mutagenesis of C443 to alanine and glycine has a relatively small effect (4- and 7-fold, respectively) on the overall rate of the aminoacylation reaction (24). Our previous results suggested that the thiol located at position 443 is not essential for aminoacylation activity but is likely to form the prolyl-adenylate substrate-binding pocket (24). To establish the role of this single cysteine residue in amino acid editing activity, we tested the C443G mutant, which maintained the highest aminoacylation activity, in both pre- and posttransfer editing assays. Fig. 3A shows that the C443G mutation abolishes the rapid deacylation of the Ala-tRNAPro variant that is observed with wild-type ProRS. In this assay, the mutant enzyme concentration was the same as that of the wild-type enzyme (0.1 μM). When the concentration of C443G-ProRS is increased, weak deacylation activity is observed (Fig. 3B). The mutant enzyme does not deacylate cognate E. coli Pro-tRNAPro under these high enzyme conditions (data not shown). Thus, the activity is specific for the mischarged tRNA and is not a result of an increase in the nonspecific background rate of deacylation that some synthetases have been shown to exhibit (6, 40). Based on the weak deacylation activity observed at high mutant ProRS concentrations, we determined that the C443G mutation results in a 150-fold (±44) decrease in the efficiency of posttransfer editing. In contrast, C443G-ProRS exhibited wild-type levels of pretransfer editing of alanine, as measured by the ATP hydrolysis assay (data not shown).
Figure 3.
Deacylation of G1:C72/U70-Ala-tRNAPro by wild-type (WT) and C443G ProRS. (A) Deacylation by 0.1 μM C443G-ProRS (■) and wild-type ProRS (●). (B) Deacylation by 0.5 μM (■), 1 μM (▴), and 2 μM (▾) C443G-ProRS. In both graphs, an assay performed in the absence of ProRS (⧫) is shown.
Discussion
We show here that class II E. coli ProRS misactivates the noncognate amino acid alanine and possesses both pre- and posttransfer editing activity. Additionally, we find that some nonproteinaceous amino acids appear to be misactivated and subsequently hydrolyzed by E. coli ProRS. 4-Hydroxy-proline is incorporated into collagen via a posttranslational modification (41). To avoid its random incorporation into proteins, its adenylate, when formed, must be prevented from being transferred to tRNAPro. Indeed, cis-4-hydroxy-proline and its isomer, trans-4-hydroxy-proline, both stimulate ATP hydrolysis, suggesting a pretransfer editing pathway. In contrast to misactivated 4hydroxy-proline, the adenylate of the four-membered ring analog of proline, azetidine-4-carboxylic acid, is not hydrolyzed by E. coli ProRS. This is consistent with the finding that this compound is toxic to E. coli and can be incorporated into proteins in place of proline (34, 35, 42).
We also show that the rapid, alanine-specific posttransfer deacylation activity observed in the presence of E. coli ProRS depends on a single cysteine residue located within the amino acid activation active site. This residue is located at position 443 within motif 3, and sequence alignments indicate that there is a conserved cysteine, serine, or threonine at this position in all ProRSs sequenced to date (20). The C443 residue previously was shown to be dispensable for aminoacylation (24) and, as shown here, does not appear to be important for pretransfer editing. In contrast, the active-site cysteine plays a critical role in the observed posttransfer deacylation activity. Although C443G-ProRS is severely defective in posttransfer hydrolysis, it is not completely inactive. However, a much higher concentration of enzyme is required to observe deacylation in the absence of C443. From the data presented here, we cannot distinguish between a direct or an indirect involvement of C443 in the catalytic reaction. Based on the “double-sieve” mechanism of amino acid editing, a second active site that is distinct from the activation active site contains key catalytic residues responsible for editing (32). This mechanism indeed has been observed in the case of several class I enzymes (10–13, 43). A recent report on class II threonine-tRNA synthetase suggests that a separate editing active site also may be present in this enzyme (18, 19). By analogy, the large insertion domain located between motifs 2 and 3 of prokaryotic-like ProRSs is a good candidate for the second sieve (20, 44). Therefore, an indirect role for C443 in posttransfer editing is more likely than a direct catalytic role. Although C443 is dispensable for aminoacylation, it is involved in the formation of the proline-binding pocket (24). Thus, this residue may be required for optimal binding of the end of a mischarged tRNA before its transfer to the editing active site. The efficiency of transfer of alanine to tRNAPro by C443G-ProRS is not increased by the mutation. However, this is not too surprising given the observation that the mutant enzyme maintains full pretransfer editing capabilities.
Taken together, our results strongly suggest that in addition to C443G, other residues are likely to be involved in both editing reactions. By analogy to class I synthetases, these catalytic residues may be located in a domain that is distinct from the activation active site.
Acknowledgments
We thank Profs. Paul Schimmel and Hung-wen Liu and Drs. Tamara Hendrickson and Tim Stello for helpful discussions. We also thank Paul Schimmel for comments on the manuscript and Mr. Brian Burke and Dr. Tim Stello for providing materials. We thank Prof. Joe Chihade for the gift of C. elegans AlaRS, Ms. Fei Chen for purifying the C443G-ProRS, and Prof. Jack Horowitz and Mr. Keith Tardif (Iowa State University) for providing us with an unpublished protocol for the ATP hydrolysis assay. We thank Prof. Susan Martinis (University of Houston) for the protocol for isolating mischarged tRNA. This work was funded by Grant GM49928 from the National Institutes of Health.
Abbreviation
- ProRS
proline-tRNA synthetase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Jakubowski H, Goldman E. Microbiol Rev. 1992;56:412–429. doi: 10.1128/mr.56.3.412-429.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carter C W., Jr Annu Rev Biochem. 1993;62:715–748. doi: 10.1146/annurev.bi.62.070193.003435. [DOI] [PubMed] [Google Scholar]
- 3.Giegé R, Sissler M, Florentz C. Nucleic Acids Res. 1998;26:5017–5035. doi: 10.1093/nar/26.22.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jakubowski H, Fersht A R. Nucleic Acids Res. 1981;9:3105–3117. doi: 10.1093/nar/9.13.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldwin A N, Berg P. J Biol Chem. 1966;241:839–845. [PubMed] [Google Scholar]
- 6.Eldred E W, Schimmel P R. J Biol Chem. 1972;247:2961–2964. [PubMed] [Google Scholar]
- 7.Schmidt E, Schimmel P. Science. 1994;264:265–267. doi: 10.1126/science.8146659. [DOI] [PubMed] [Google Scholar]
- 8.Hale S P, Auld D S, Schmidt E, Schimmel P. Science. 1997;276:1250–1252. doi: 10.1126/science.276.5316.1250. [DOI] [PubMed] [Google Scholar]
- 9.Farrow M A, Nordin B E, Schimmel P. Biochemistry. 1999;38:16898–16903. doi: 10.1021/bi9920782. [DOI] [PubMed] [Google Scholar]
- 10.Lin L, Hale S P, Schimmel P. Nature (London) 1996;384:33–34. doi: 10.1038/384033b0. [DOI] [PubMed] [Google Scholar]
- 11.Nureki O, Vassylyev D G, Tateno M, Shimada A, Nakama T, Fukai S, Konno M, Hendrickson T L, Schimmel P, Yokoyama S. Science. 1998;280:578–582. doi: 10.1126/science.280.5363.578. [DOI] [PubMed] [Google Scholar]
- 12.Nomanbhoy T K, Hendrickson T L, Schimmel P. Mol Cell. 1999;4:519–528. doi: 10.1016/s1097-2765(00)80203-8. [DOI] [PubMed] [Google Scholar]
- 13.Silvian L F, Wang J, Steitz T A. Science. 1999;285:1074–1077. [PubMed] [Google Scholar]
- 14.Yarus M. Proc Natl Acad Sci USA. 1972;69:1915–1919. doi: 10.1073/pnas.69.7.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsui W-C, Fersht A R. Nucleic Acids Res. 1981;9:4627–4637. doi: 10.1093/nar/9.18.4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jakubowski H. Biochemistry. 1997;36:11077–11085. doi: 10.1021/bi970589n. [DOI] [PubMed] [Google Scholar]
- 17.Jakubowski H. Biochemistry. 1999;38:8088–8093. doi: 10.1021/bi990629i. [DOI] [PubMed] [Google Scholar]
- 18.Sankaranarayanan R, Dock-Bregeon A-C, Rees B, Bovee M, Caillet J, Romby P, Francklyn C S, Moras D. Nat Struct Biol. 2000;7:461–465. doi: 10.1038/75856. [DOI] [PubMed] [Google Scholar]
- 19.Musier-Forsyth K, Beuning P J. Nat Struct Biol. 2000;7:435–436. doi: 10.1038/75816. [DOI] [PubMed] [Google Scholar]
- 20.Stehlin C, Burke B, Yang F, Liu H, Shiba K, Musier-Forsyth K. Biochemistry. 1998;37:8605–8613. doi: 10.1021/bi980364s. [DOI] [PubMed] [Google Scholar]
- 21.Liu H, Yap L-P, Musier-Forsyth K. J Am Chem Soc. 1996;118:2523–2524. [Google Scholar]
- 22.Liu H, Musier-Forsyth K. Biochemistry. 1994;33:12708–12714. doi: 10.1021/bi00208a023. [DOI] [PubMed] [Google Scholar]
- 23.Musier-Forsyth K, Scaringe S, Usman N, Schimmel P. Proc Natl Acad Sci USA. 1991;88:209–213. doi: 10.1073/pnas.88.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stehlin C, Heacock D H, Liu H, Musier-Forsyth K. Biochemistry. 1997;36:2932–2938. doi: 10.1021/bi962295s. [DOI] [PubMed] [Google Scholar]
- 25.Beuning P J, Gulotta M, Musier-Forsyth K. J Am Chem Soc. 1997;119:8397–8402. [Google Scholar]
- 26.Fersht A R, Ashford J S, Bruton C J, Jakes R, Koch G L E, Hartley B S. Biochemistry. 1975;14:1–4. doi: 10.1021/bi00672a001. [DOI] [PubMed] [Google Scholar]
- 27.Heacock D, Forsyth C J, Shiba K, Musier-Forsyth K. Bioorg Chem. 1996;24:273–289. [Google Scholar]
- 28.Liu H, Peterson R, Kessler J, Musier-Forsyth K. Nucleic Acids Res. 1995;23:165–169. doi: 10.1093/nar/23.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raunio R, Rosenqvist H. Acta Chem Scand. 1970;24:2737–2744. doi: 10.3891/acta.chem.scand.24-2737. [DOI] [PubMed] [Google Scholar]
- 30.Freist W, Sternbach H, Pardowitz I, Cramer F. J Theor Biol. 1998;193:19–38. doi: 10.1006/jtbi.1998.0672. [DOI] [PubMed] [Google Scholar]
- 31.Ninio J. Biochimie. 1975;57:587–595. doi: 10.1016/s0300-9084(75)80139-8. [DOI] [PubMed] [Google Scholar]
- 32.Fersht A R. Enzyme Structure and Mechanism. San Francisco: Freeman; 1985. pp. 347–368. [Google Scholar]
- 33.Fersht A R. Proc R Soc London Ser B. 1981;212:351–379. doi: 10.1098/rspb.1981.0044. [DOI] [PubMed] [Google Scholar]
- 34.Fowden L, Richmond M H. Biochim Biophys Acta. 1963;71:459–461. [Google Scholar]
- 35.Rabinovitz M, Finkleman A, Reagan R L, Breitman T R. J Bacteriol. 1969;99:336–338. doi: 10.1128/jb.99.1.336-338.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giegé R, Kern D, Ebel J P, Taglang R. FEBS Lett. 1971;15:281–285. doi: 10.1016/0014-5793(71)80638-5. [DOI] [PubMed] [Google Scholar]
- 37.McClain W H, Schneider J, Gabriel K. Nucleic Acids Res. 1994;22:522–529. doi: 10.1093/nar/22.3.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hou Y-M, Schimmel P. Nature (London) 1988;333:140–145. doi: 10.1038/333140a0. [DOI] [PubMed] [Google Scholar]
- 39.McClain W H, Chen Y-M, Foss K, Schneider J. Science. 1988;242:1681–1684. doi: 10.1126/science.2462282. [DOI] [PubMed] [Google Scholar]
- 40.Schreier A A, Schimmel P R. Biochemistry. 1972;11:1582–1589. doi: 10.1021/bi00759a006. [DOI] [PubMed] [Google Scholar]
- 41.Bornstein P. Annu Rev Biochem. 1974;43:567–603. doi: 10.1146/annurev.bi.43.070174.003031. [DOI] [PubMed] [Google Scholar]
- 42.Deming T J, Fournier M J, Mason T L, Tirrell D A. Macromolecules. 1996;29:1442–1444. [Google Scholar]
- 43.Chen J-F, Guo N-N, Li T, Wang E-D, Wang Y-L. Biochemistry. 2000;39:6726–6731. doi: 10.1021/bi000108r. [DOI] [PubMed] [Google Scholar]
- 44.Cusack S, Yaremchuk A, Krikliviy I, Tukalo M. Structure (London) 1998;6:101–108. doi: 10.1016/s0969-2126(98)00011-2. [DOI] [PubMed] [Google Scholar]