Abstract
Dictyostelium amoebae grow as single cells but upon starvation they initiate multicellular development. Phg2 was characterized previously as a kinase controlling cellular adhesion and the organization of the actin cytoskeleton. Here we report that Phg2 also plays a role during the transition between growth and multicellular development, as evidenced by the fact that phg2 mutant cells can initiate development even in the presence of nutrients. Even at low cell density and in rich medium, phg2 mutant cells express discoidin, one of the earliest predevelopmental markers. Complementation studies indicate that, in addition to the kinase domain, the core region of Phg2 is involved in the initiation of development. In this region, a small domain contiguous with a previously described ras-binding domain was found to interact with the Dictyostelium ortholog of the mammalian adhesion-regulating molecule (ADRM1). In addition, adrm1 knockout cells also exhibit abnormal initiation of development. These results suggest that a Phg2-Adrm1 signaling pathway is involved in the control of the transition from growth to differentiation in Dictyostelium. Phg2 thus plays a dual role in the control of cellular adhesion and initiation of development.
INTRODUCTION
Cellular adhesion is an essential element in many physiological events such as cell migration, multicellular development, metastasis, and phagocytosis. It is a complex process that involves receptors at the cell surface, intracellular signaling, and the actin cytoskeleton. Local signaling pathways that control cellular adhesion also influence global cell physiology. For example, the focal adhesion kinase (FAK) can be activated by integrins or by growth factor receptors, and it not only participates in cellular adhesion and migration, but also regulates cell proliferation and survival (Gabarra-Niecko et al., 2003). An intricate intracellular signaling network thus links local cellular adhesion events and the more general regulation of cellular physiology. Elucidating the organization and function of such a signaling network is key to understanding many crucial events such as tissue morphogenesis and tumor progression (Christofori, 2006).
Dictyostelium amoebae grow as single cells in the soil, where they feed on microorganisms. They harbor a small haploid genome and are easily amenable to genetic analysis; therefore they have been used extensively as a model organism to study cellular adhesion, motility, and phagocytosis (Cardelli, 2001). When starved, Dictyostelium cells cease to proliferate and aggregate to form a multicellular structure. The control of the transition from growth to differentiation has been the subject of many studies (reviewed in Maeda, 2005). It would seem logical that these two cellular processes be regulated in a coordinated manner, because changes in cellular adhesion are crucial for switching from single phagocytic cells to multicellular aggregates. To date, however, no molecular link has been established to our knowledge between cellular adhesion and the control of cell proliferation in Dictyostelium.
The study of cellular adhesion in Dictyostelium has been achieved in part by isolating a number of mutants defective in cellular adhesion (Cornillon et al., 2000; Fey et al., 2002; Gebbie et al., 2004). Among these, phg2 knockout cells present defects in adhesion, the organization of the actin cytoskeleton and cell motility (Gebbie et al., 2004). Phg2 is a serine/threonine kinase with several potential functional domains, notably a putative ras-binding domain (RBD), which can bind Rap1, a member of the ras family of GTP-binding proteins (Gebbie et al., 2004; Kortholt et al., 2006). In addition, a phosphatidylinositol 4,5-bisphosphate-binding domain in its N-terminal region targets Phg2 to the plasma membrane (Blanc et al., 2005). The structure of Phg2 suggests that like other kinases involved in cellular adhesion, Phg2 functions as a platform for the binding of many cellular proteins, and might regulate many aspects of cellular physiology.
Here we show that in addition to its role in cell adhesion, Phg2 is involved in the initiation of multicellular development in Dictyostelium. Furthermore, we report that Phg2 interacts with Adrm1, previously identified in mammalian cells as an adhesion-related molecule (Simins et al., 1999; Lamerant and Kieda, 2005) and that Adrm1 also participates in the control of the initiation of development in Dictyostelium. Phg2 appears to be a common element between the cell adhesion machinery and a signal transduction pathway controlling the initiation of multicellular development.
MATERIALS AND METHODS
Cell Culture and Mutagenesis
Unless otherwise specified, all cells used in this study were derived from the DH1-10 subclone (Cornillon et al., 2000) of the Dictyostelium wild-type DH1 strain (Caterina et al., 1994). Cells were grown at 21°C in HL5 medium (Cornillon et al., 1998) and subcultured twice a week to maintain the cell density below 2 × 106 cells/ml. The phg2 and myoVII mutants were described previously (Gebbie et al., 2004). Phagocytosis of latex beads was assessed as described previously (Cornillon et al., 2000).
To generate adrm1 knockout cells, AX2 cells were transfected with the pBluescript plasmid containing the sequence of ADRM1, where the exon 2 and intron 2 were replaced with a blasticidin-resistance cassette. Transfected cells were grown in HL5 containing blasticidin (10 μg/ml), and adrm1 knockout cells were identified by PCR (Charette and Cosson, 2004; Charette et al., 2006).
The cDNA sequence coding for wild-type Phg2 or Phg2 lacking the kinase domain or the core region were cloned into the pDXA-GFP2 expression vector (Levi et al., 2000). Phg2 mutant cells were transfected with these vectors and selected for their capacity to grow in the presence of G418 (15 μg/ml).
Multicellular Development
To induce multicellular development in submerged cultures, cells were harvested at a density of 1–2 × 106 cells/ml, washed in HL5 medium, and plated at 106 cells/ml in Petri dishes (94-mm diameter) containing HL5 diluted in phosphate buffer (2 mM Na2HPO4, 14.7 mM KH2PO4, pH 6.5) at the indicated dilution. Cells were allowed to develop and the presence of multicellular aggregates and the expression of contact site A (csA) were assessed after 24 h.
To induce the formation of fruiting bodies, wild-type or phg2 mutant cells were washed in phosphate buffer containing 1% HL5, resuspended at 50 × 106 cells/ml, and plated on 0.45-μm membrane filters laid on two layers of grade 1 Whatman paper soaked with phosphate buffer (Sussman, 1987). In our hands, the presence of minute amounts of nutrients preserved cell viability and did not interfere with multicellular development. Filters were placed in Petri dishes with adequate humidity and incubated at 21°C.
To test whether the developmental anomaly seen in phg2 mutant cells was cell-autonomous, wild-type cells stably expressing the green fluorescent protein (WT-GFP) were mixed with phg2 mutant cells, respectively, at concentrations of 0.4 × 105 cells/ml and 0.52 × 105 cells/ml and cocultured in HL5 medium for 48 h. Cells were then harvested, washed in HL5 medium, and plated at 2 × 106 cells/ml in Petri dishes (94-mm diameter) containing phosphate buffer with 9% HL5 medium. Formation of multicellular aggregates was observed at the onset of development, at a time when the presence of fluorescent cells in aggregates could be assessed with precision. Experiments where phg2 mutant cells stably expressing the green fluorescent protein (phg2-GFP) were mixed with wild-type cells were performed identically.
Immunodetection
The csA and discoidin proteins were detected with monoclonal antibodies 33-294-17 (Bertholdt et al., 1985), and 80-52-13 (Wetterauer et al., 1993), respectively. To assess csA expression after 24 h of development, 1.5 × 106 cells were harvested, washed in H2O, and lysed in 40 μl of sample buffer (0.103 g/ml sucrose, 5 × 10−2 M Tris, pH 6.8, 5 × 10−3 M EDTA, 0.5 mg/ml bromophenol blue, 2% SDS). Fifteen microliters of each sample was separated on a 10% acrylamide gel in reducing conditions. Proteins were transferred onto a nitrocellulose BA 85 membrane (Schleicher & Schuell, Dassel, Germany). Membranes were incubated with the anti-CsA or anti-discoidin I antibody and then with a horseradish peroxidase–coupled donkey anti-mouse Ig (Amersham Biosciences, Little Chalfont, Buckinghamshire, United Kingdom), washed, and revealed by enhanced chemiluminescence.
Testing Protein–Protein Interactions by Two-Hybrid Assay
Two-hybrid assays were carried out using the Matchmaker LexA two-hybrid system (Clontech Laboratories, Palo Alto, CA). The DNA sequence encoding the Phg2 core domain (residues 573-755) was fused to the DNA-binding protein LexA in the expression vector pEG202. This bait construct was used to screen a Dictyostelium cDNA library kindly provided by Dr. R. Firtel (University of California, San Diego). Among the positive clones, one sequence containing the Nt domain of Dictyostelium Adrm1 (DDB0167941; residues 1-117) specifically allowed EGY48 yeast cells expressing the plasmid p80-LacZ to grow on selective plates (synthetic complete medium without leucine and containing galactose) and to give a blue color on X-gal supplemented plates.
To determine more precisely the regions of the Phg2 core domain required for Adrm1 and Rap1 interaction, the DNA sequences encoding the Nt domain of Adrm1 and the constitutively active forms of Rap1 (Rap1(G12T) were fused to the B42 activation domain in the vector pJG4-5 containing the inducible GAL1 promoter. Sequences corresponding to different regions of the Phg2 core domain were cloned into the expression vector pEG202 and cotransfected in the reporter yeast with Adrm1- and Rap1-containing constructs. Transformed yeast cells were tested for their ability to grow on galactose plates without leucine and give a blue color on X-gal supplemented plates. For greater precision, the β-galactosidase activity was determined in suspension for a fixed number of yeast cells using O-nitrophenyl-β-d-galactopyranoside as a substrate. The background activity measured in cells expressing only the B42 activation domain fusion protein was subtracted.
RESULTS
Initiation of Development Is Abnormal in phg2 Mutant Cells
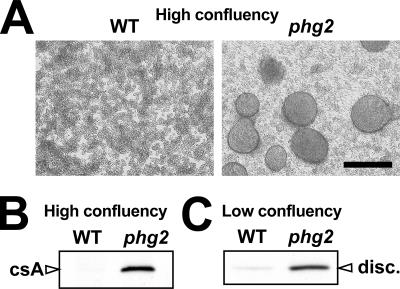
This study was initiated by the observation that phg2 mutant cells grown in HL5 medium were able to form tight aggregates when they reached high density (Figure 1A). These well-delimitated aggregates differed from the loose cellular aggregates frequently observed in highly confluent Dictyostelium cultures, but were similar to those formed by starved wild-type cells in submerged conditions (Marin, 1976 and unpublished data). Indeed, the formation of aggregates by phg2 mutant cells in these conditions coincided with an induction of contact site A (csA), a well-characterized marker of multicellular development in Dictyostelium (Noegel et al., 1986), which was not expressed by wild-type cells in the same conditions (Figure 1B). This result suggested that phg2 mutant cells initiated multicellular development in an old culture medium, from which nutrients were partially depleted and in which secreted extracellular factors had accumulated.
Figure 1.
Phg2 mutant cells present signs of abnormal development initiation. (A) Cultures of wild-type or phg2 mutant cells grown to their maximal density in HL5 medium (4 × 106 cells/ml) were incubated for an additional 3 d. Phg2 mutants formed well-delimited cellular aggregates, whereas wild-type cells did not. Bar, 200 μm. (B) The formation of phg2 aggregates in these conditions coincided with an induction of contact site A (csA) expression as shown by immunodetection with an anti-csA antibody. (C) The expression of discoidin I (disc.) was assessed in cells grown at low cellular densities (below 106 cells/ml). In these conditions, discoidin was overexpressed in phg2 mutant cells compared with wild-type cells.
The prestarvation factor (PSF) is an extracellular factor continuously secreted by growing cells, and at high concentrations it can induce the expression of several early developmental genes (Rathi and Clarke, 1992). Discoidin genes are among the first to be induced by PSF when the cellular density increases while the concentration of nutrients decreases in the culture medium, a stage often referred to as the prestarvation stage (Maeda, 2005). To test whether this initial step of cellular differentiation was also altered in phg2 mutant cells, we assessed the expression of discoidin in cells cultivated at low confluence. In these conditions, the discoidin protein was present at low levels in wild-type cells as described previously (Clarke et al., 1987), but highly expressed in phg2 mutant cells (Figure 1C), indicating that prestarvation was also induced abnormally in phg2 mutant cells.
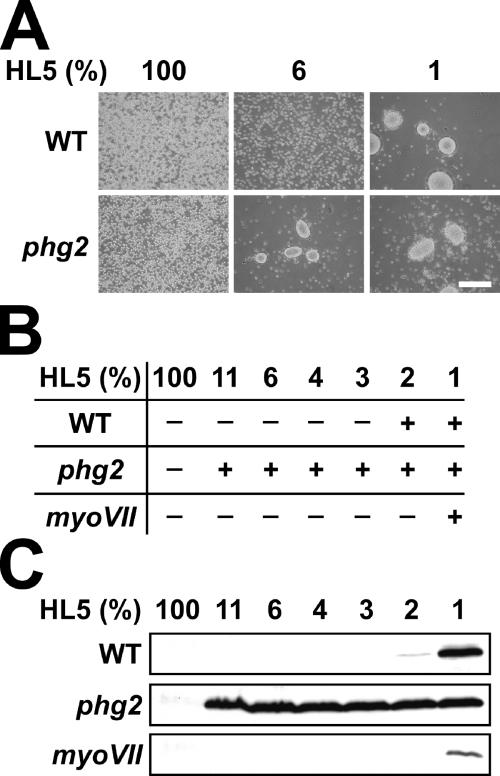
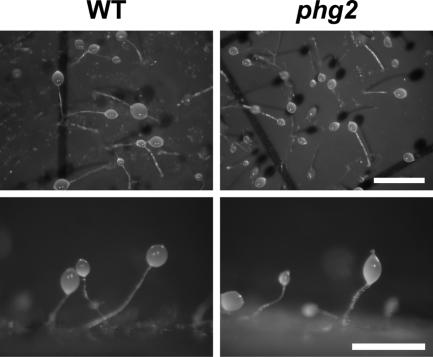
Together these results suggested that phg2 mutant cells did not respond normally to the presence of nutrients and generally behaved as cells partially deprived of nutrients. To further test this possibility, cells were incubated in medium containing defined concentrations of nutrients, obtained by diluting HL5 culture medium with phosphate buffer. After 24 h, multicellular development was monitored by assessing the presence of tight cellular aggregates as well as the expression of csA. Low amounts of nutrients (3% HL5) were sufficient to inhibit development of wild-type cells, whereas phg2 mutant cells initiated development at much higher concentrations of nutrients (up to 11% HL5; Figure 2). MyoVII mutant cells, which exhibit an adhesion defect similar to that seen in phg2 mutant cells (Gebbie et al., 2004), did not show anomalies in the initiation of development in this assay (Figure 2). This result indicated that the abnormal initiation of multicellular development in phg2 mutant cells was not a secondary consequence of a defect in cellular adhesion but resulted rather from a defect in the cellular response to nutrients. Interestingly, development of starved phg2 mutant cells was morphologically identical to that of wild-type cells (Figure 3), indicating that Phg2 is involved in the control of the initiation of development, but not essential at later stages.
Figure 2.
Phg2 mutant cells initiate multicellular development in the presence of abnormally high concentrations of nutrients. (A and B) Wild-type or mutant cells were placed for 24 h in a medium containing a defined amount of nutrients, obtained by diluting HL5 medium with phosphate buffer. After 24 h, multicellular development was monitored by assessing the presence of tight cellular aggregates. Bar, 200 μm. +, presence of tight cellular aggregates; −, absence of aggregates. Phg2 mutant cells initiated multicellular development at concentrations of nutrients that inhibited development of wild-type or myoVII cells. (C) Cells treated as in A were harvested and the expression of csA was determined by Western blot analysis.
Figure 3.
Multicellular development is unaffected in starved phg2 mutant cells. Wild-type and phg2 mutant cells were harvested, resuspended in phosphate buffer, and plated on nitrocellulose filters. Multicellular development was observed after 24 h. Bar, 1 mm.
Abnormal Initiation of Development in phg2 Mutant Is Cell Autonomous
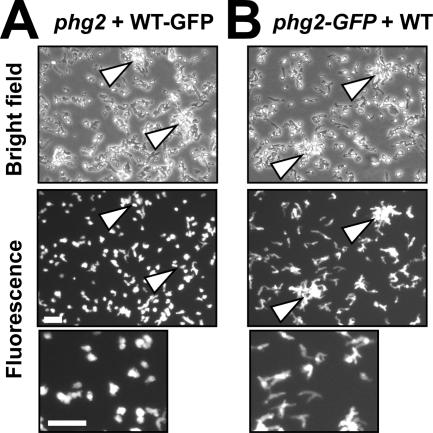
The abnormal initiation of multicellular development in phg2 mutant cells might conceivably result from an anomaly in intracellular signaling, or from an abnormal secretion of signaling molecules in the extracellular medium. To distinguish between these two possibilities, wild-type cells expressing the green fluorescent protein (WT-GFP) were mixed with phg2 mutant cells, cocultured in HL5 medium for 48 h, and then transferred to phosphate buffer containing 9% HL5, a condition where phg2 mutant cells initiated development, whereas wild-type cells did not (see Figure 2). The cells were then observed at the onset of development, when cellular aggregates were still small and easily visualized. At this time, fluorescent wild-type cells clearly did not accumulate in aggregates (Figure 4A). In addition, wild-type cells retained the mostly spherical morphology typical of unstarved cells (Figure 4A). Conversely, when phg2 mutant cells expressing GFP (phg2-GFP) were mixed with wild-type cells, they were clearly present in cellular aggregates at the onset of development, and they adopted the elongated morphology typical of starved cells (Figure 4B). These observations demonstrated that the abnormal development of phg2 mutant cells was cell autonomous.
Figure 4.
Abnormal initiation of development in phg2 mutant cells is cell-autonomous. (A) Wild-type cells expressing the green fluorescent protein (WT-GFP) were mixed with phg2 mutants, cocultured for 48 h, and then incubated in a medium containing 9% HL5. At the onset of multicellular development, fluorescent wild-type cells did not enter multicellular aggregates (indicated by arrows). Examination of the cells at high magnification in the same experiment (lower panel) revealed that wild-type cells retained a mostly spherical shape typical of unstarved cells. (B) Phg2-GFP cells were mixed with wild-type cells and treated as described in A. Phg2 mutant cells accumulated in cellular aggregates and adopted the elongated shape typical of developing cells (lower panel). Bar, 50 μm.
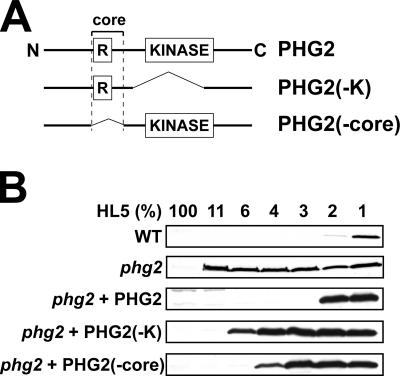
The Core Region of Phg2 Is Involved in the Control of Development Initiation
To determine the role of various domains of Phg2 in its function, we first analyzed by complementation the putative role of the kinase and ras-binding domains in controlling the initiation of multicellular development. For this, phg2 mutant cells were transfected with plasmids expressing either the full-length or truncated version of the Phg2 protein (Figure 5A), and the effect of nutrients on the initiation of development was determined by assessing the expression of csA (Figure 5B). Expression of full-length Phg2 in phg2 mutant cells restored normal sensitivity to nutrients, but expression of a kinase-deleted Phg2 did not (Figure 5B). Deletion of the core region encompassing the RBD also produced a Phg2 protein that only partially restored sensitivity to nutrients (Figure 5B), despite the fact that it was expressed at levels much higher than those of Phg2 in wild-type cells (unpublished data). These results suggested that both the kinase domain and the core region of Phg2 are implicated in intracellular signaling, implying that the core region interacts with cellular proteins involved in intracellular signaling.
Figure 5.
The core region of Phg2 is implicated in the control of development initiation. (A) Schematic structure of the Phg2 protein and of two deletion mutants. (B) The ability of phg2 mutant cells expressing wild-type or mutated versions of Phg2 to initiate multicellular development was assessed as described in Figure 2. Although high levels of Phg2 were detected in cells complemented with Phg2-K and Phg2-core (unpublished data), the initiation of development remained abnormal.
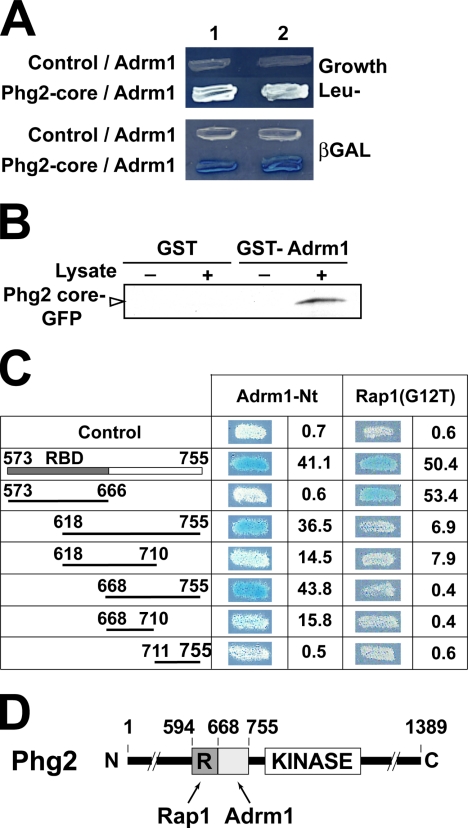
Interaction of Phg2 with Adhesion-regulating Molecule
We used the yeast two-hybrid system to search for proteins interacting with the Phg2 core region in a cDNA library. Growth in Leu- medium as well as production of β-galactosidase indicated that the Phg2 core region interacted with the Dictyostelium adhesion-regulating molecule (Adrm1) protein (Figure 6A). To confirm this observation, a GST-Adrm1 fusion protein was produced in bacteria and immobilized on glutathione-Sepharose beads. The beads were then incubated with a lysate of wild-type cells expressing the Phg2 core region fused with the GFP. As shown in Figure 6B, the Phg2 core region bound to beads coated with GST-Adrm1, but not to beads coated with GST alone. This suggested further that the Phg2 core region interacted with Adrm1.
Figure 6.
A specific domain of Phg2 interacts with the Adrm1 protein. (A) Two distinct clones (1 and 2) expressing the indicated proteins are shown. Growth in Leu- medium (top panel) as well as production of β-galactosidase (blue color, lower panel) indicated that the core region of Phg2 interacted with the Adrm1 protein in a yeast two-hybrid assay. (B) A GST-Adrm1 fusion protein was immobilized on glutathione beads and mixed with a lysate of Dictyostelium cells overexpressing the Phg2 core region tagged with GFP. Binding of the GFP fusion protein to GST-Adrm1 beads was detected with an anti-GFP antibody. (C) A collection of mutants of the Phg2 core region was constructed, and their interaction with Adrm1 and Rap1 was tested in a two-hybrid assay. The production of β-galactosidase was visualized on plates. It was also evaluated more precisely in a liquid assay and is indicated for each construct (arbitrary units). (D) Schematic representation of Phg2. The core region contains two distinct regions: the RBD (R) interacts with Rap1, whereas the adjacent region interacts with Adrm1.
We also used the two-hybrid assay described above to delineate the region of Phg2 interacting with Adrm1. Interestingly, the Phg2 RBD interacted with Rap1 as previously reported (Gebbie et al., 2004), but it was not necessary for the interaction between Phg2 and Adrm1 (Figure 6C). A region of 87 amino acid residues adjacent to the Phg2 RBD interacted with Adrm1 (Figure 6C). Thus the Phg2 core region comprised two separate binding sites, one for Rap1 (residues 594-668), and one for Adrm1 (residues 668-755; Figure 6D).
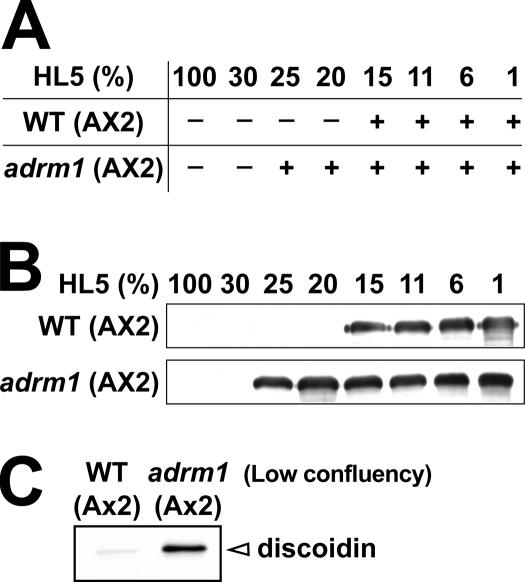
To define more precisely the role of Adrm1, the ADRM1 gene was disrupted in Dictyostelium. Although all Dictyostelium strains used in this study were derived from the wild-type DH1–10 strain, this strain harbors a large duplication in the chromosome 2, which includes ADRM1 (Eichinger et al., 2005). Consequently we were obliged to derive adrm1 knockout cells from an AX2 wild-type strain, where the ADRM1 gene is not duplicated (Eichinger et al., 2005). Adrm1 mutant cells grew normally and were not impaired in their ability to internalize latex beads (96% of wild-type cells), suggesting that cellular adhesion, the first step of the phagocytic process, was not affected (Gebbie et al., 2004). Adrm1 knockout cells were also allowed to develop in defined media in order to assess the role of Adrm1 in intracellular signaling. Wild-type AX2 cells developed more readily than DH1-10 cells, and multicellular development was initiated even at relatively high concentrations of HL5 (15%; Figure 7). The development of starved adrm1 mutant cells was morphologically identical to that of wild-type cells (unpublished data). However, adrm1 mutant cells were able to develop at even higher concentrations of HL5 (25%; Figure 7) and the formation of multicellular aggregates coincided with an induction of csA (Figure 7B). This suggests that, like Phg2, Adrm1 is involved in the control of multicellular development initiation. We also assessed the expression of discoidin in adrm1 mutant cells cultivated at low confluency. In these conditions, the discoidin protein was detected at very low levels in wild-type cells but was highly expressed in adrm1 mutant cells (Figure 7C), indicating that prestarvation was abnormally induced in adrm1 mutant cells, like in phg2 mutant cells.
Figure 7.
Initiation of multicellular development is altered in adrm1 mutant cells. (A and B). Adrm1 mutant cells were generated in an AX2 Dictyostelium strain. Wild-type or mutant cells were incubated in medium containing defined amounts of nutrients, and multicellular development was determined by assessing the formation of multicellular aggregates (A) and the expression of csA (B), as described in Figure 2. (C) The expression of discoidin I was assessed in cells grown at low cellular densities (below 106 cells/ml). In these conditions, discoidin was overexpressed in adrm1 mutant cells compared with wild-type cells.
DISCUSSION
Our results indicate that in addition to its role in cellular adhesion, Phg2 is involved in nutrient-controlled initiation of multicellular development in Dictyostelium. Because phg2 mutant cells develop more readily than wild-type cells (i.e., in the presence of higher concentrations of nutrients), Phg2 must act as a negative regulator of development initiation. Remarkably, phg2 mutant cells also aberrantly express discoidin, one of the first markers of transition from growth to development in Dictyostelium, suggesting that the regulatory role of Phg2 is exerted at the very first stages of the initiation of development.
The early steps of multicellular development in Dictyostelium have been extensively studied. Particular attention has been paid to the crucial role of cAMP signaling once the developmental program is initiated (Loomis, 1998). By contrast, relatively few studies have investigated the molecular mechanisms controlling the transition of Dictyostelium cells from unicellular growth to multicellular development (Maeda, 2005). This switch is controlled notably by the nutritional state of the cells, as well as by the extracellular accumulation of secreted signaling molecules. One of the first steps occurs in cells that are still growing, when PSF (prestarvation factor), a secreted factor, accumulates in the medium above a certain threshold, while the concentration of nutrients decreases. This induces the expression of some very early developmental genes including discoidin. The expression of discoidin shown in the phg2 mutant cells at low confluency could not be explained by higher amounts of extracellular factors because our results indicate that the anomaly in development initiation was cell autonomous. This suggests that Phg2 is involved in a signal transduction pathway that controls the first steps in the initiation of development. Two previously isolated mutants, gdt1 and gdt2, show premature development and aberrantly induce discoidin at low cell densities like phg2 mutants (Chibalina et al., 2004). A few other gene products might also control the transition to multicellular development (PKA, YakA, Dia1, Dia2, AmiA, AmiB, PufA), but their exact role in the induction of the prestarvation stage is less clear, and several of them may rather be involved at later stages of multicellular development initiation (Maeda, 2005). Further studies are needed to determine how Phg2 interacts with other signaling components implicated in the control of development initiation.
We have also identified by two-hybrid screening a potential element of the signaling pathway allowing Phg2 to control development: the Adrm1 protein interacts with a region adjacent to the previously identified RBD. Interestingly, Adrm1 was previously identified as a protein up-regulated in metastatic tumor cells, and its overexpression increased the propensity of cells to engage in cell–cell interactions (Simins et al., 1999; Lamerant and Kieda, 2005). Adrm1 is conserved in many species, including plants, Dictyostelium amoebae, Caenorhabditis elegans, Drosophila flies, mouse, or human. Its mechanism of action is unknown, as are its putative functional partners. Our observation that Phg2 interacts with Adrm1 indicates a potential link between Adrm1 and the adhesion machinery. Analysis of adrm1 knockout cells further suggested that Adrm1 participates, like Phg2, in the control of development initiation in Dictyostelium. We did not, however, observe a defect in cellular adhesion in adrm1 mutant cells, indicating that either Adrm1 does not play a direct role in cellular adhesion or its function is redundant with that of other cellular proteins in Dictyostelium. Although this study further emphasizes the role of Phg2 in controlling cell adhesion and physiology, it seems likely that several other proteins will be found to interact with Phg2. Further analysis will be necessary to extensively identify the functional partners of Phg2 and to define their precise role in various cellular functions.
ACKNOWLEDGMENTS
We thank Dr. Prashant Nair for critical reading of the manuscript. This work was supported by the Fonds National Suisse de la Recherche Scientifique (P.C.) and the Association pour la Recherche sur le Cancer (ARC, F.L.). P.C. participates in the NEMO network supported by the 3R Foundation. S.J.C. received a fellowship from the Human Frontier Science Program.
Abbreviations used:
- RBD
ras-binding domain
- Adrm1
adhesion-regulating molecule
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-07-0619) on September 20, 2006.
REFERENCES
- Bertholdt G., Stadler J., Bozzaro S., Fichtner B., Gerisch G. Carbohydrate and other epitopes of the contact site A glycoprotein of Dictyostelium discoideum as characterized by monoclonal antibodies. Cell Differ. 1985;16:187–202. doi: 10.1016/0045-6039(85)90516-0. [DOI] [PubMed] [Google Scholar]
- Blanc C., Charette S., Cherix N., Lefkir Y., Cosson P., Letourneur F. A novel phosphatidylinositol 4,5-bisphosphate-binding domain targeting the Phg2 kinase to the membrane in Dictyostelium cells. Eur. J. Cell Biol. 2005;84:951–960. doi: 10.1016/j.ejcb.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Cardelli J. Phagocytosis and macropinocytosis in Dictyostelium: phosphoinositide-based processes, biochemically distinct. Traffic. 2001;2:311–320. doi: 10.1034/j.1600-0854.2001.002005311.x. [DOI] [PubMed] [Google Scholar]
- Caterina M. J., Milne J. L., Devreotes P. N. Mutation of the third intracellular loop of the cAMP receptor, cAR1, of Dictyostelium yields mutants impaired in multiple signaling pathways. J. Biol. Chem. 1994;269:1523–1532. [PubMed] [Google Scholar]
- Charette S. J., Cornillon S., Cosson P. Identification of low frequency knockout mutants in Dictyostelium discoideum created by single or double homologous recombination. J. Biotechnol. 2006;122:1–4. doi: 10.1016/j.jbiotec.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Charette S. J., Cosson P. Preparation of genomic DNA from Dictyostelium discoideum for PCR analysis. Biotechniques. 2004;36:574–575. doi: 10.2144/04364BM01. [DOI] [PubMed] [Google Scholar]
- Chibalina M. V., Anjard C., Insall R. H. Gdt2 regulates the transition of Dictyostelium cells from growth to differentiation. BMC Dev. Biol. 2004;4:8. doi: 10.1186/1471-213X-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofori G. New signals from the invasive front. Nature. 2006;441:444–450. doi: 10.1038/nature04872. [DOI] [PubMed] [Google Scholar]
- Clarke M., Kayman S. C., Riley K. Density-dependent induction of discoidin-I synthesis in exponentially growing cells of Dictyostelium discoideum. Differentiation. 1987;34:79–87. doi: 10.1111/j.1432-0436.1987.tb00053.x. [DOI] [PubMed] [Google Scholar]
- Cornillon S., Olie R. A., Golstein P. An insertional mutagenesis approach to Dictyostelium cell death. Cell Death Differ. 1998;5:416–425. doi: 10.1038/sj.cdd.4400361. [DOI] [PubMed] [Google Scholar]
- Cornillon S., Pech E., Benghezal M., Ravanel K., Gaynor E., Letourneur F., Bruckert F., Cosson P. Phg1p is a nine-transmembrane protein superfamily member involved in dictyostelium adhesion and phagocytosis. J. Biol. Chem. 2000;275:34287–34292. doi: 10.1074/jbc.M006725200. [DOI] [PubMed] [Google Scholar]
- Eichinger L., et al. The genome of the social amoeba Dictyostelium discoideum. Nature. 2005;435:43–57. doi: 10.1038/nature03481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey P., Stephens S., Titus M. A., Chisholm R. L. SadA, a novel adhesion receptor in Dictyostelium. J. Cell Biol. 2002;159:1109–1119. doi: 10.1083/jcb.200206067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabarra-Niecko V., Schaller M. D., Dunty J. M. FAK regulates biological processes important for the pathogenesis of cancer. Cancer Metastasis Rev. 2003;22:359–374. doi: 10.1023/a:1023725029589. [DOI] [PubMed] [Google Scholar]
- Gebbie L., et al. Phg2, a kinase involved in adhesion and focal site modeling in Dictyostelium. Mol. Biol. Cell. 2004;15:3915–3925. doi: 10.1091/mbc.E03-12-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortholt A., Rehmann H., Kae H., Bosgraaf L., Keizer-Gunnink I., Weeks G., Wittinghofer A., Van Haastert P. J. Characterization of the GbpD activated Rap1 pathway regulating adhesion and cell polarity in Dictyostelium discoideum. J. Biol. Chem. 2006;281:23367–23376. doi: 10.1074/jbc.M600804200. [DOI] [PubMed] [Google Scholar]
- Lamerant N., Kieda C. Adhesion properties of adhesion-regulating molecule 1 protein on endothelial cells. FEBS J. 2005;272:1833–1844. doi: 10.1111/j.1742-4658.2005.04613.x. [DOI] [PubMed] [Google Scholar]
- Levi S., Polyakov M., Egelhoff T. T. Green fluorescent protein and epitope tag fusion vectors for Dictyostelium discoideum. Plasmid. 2000;44:231–238. doi: 10.1006/plas.2000.1487. [DOI] [PubMed] [Google Scholar]
- Loomis W. F. Role of PKA in the timing of developmental events in Dictyostelium cells. Microbiol. Mol. Biol. Rev. 1998;62:684–694. doi: 10.1128/mmbr.62.3.684-694.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y. Regulation of growth and differentiation in Dictyostelium. Int. Rev. Cytol. 2005;244:287–332. doi: 10.1016/S0074-7696(05)44007-3. [DOI] [PubMed] [Google Scholar]
- Marin F. T. Regulation of development in Dictyostelium discoideum: I. Initiation of the growth to development transition by amino acid starvation. Dev. Biol. 1976;48:110–117. doi: 10.1016/0012-1606(76)90050-6. [DOI] [PubMed] [Google Scholar]
- Noegel A., Gerisch G., Stadler J., Westphal M. Complete sequence and transcript regulation of a cell adhesion protein from aggregating Dictyostelium cells. EMBO J. 1986;5:1473–1476. doi: 10.1002/j.1460-2075.1986.tb04384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathi A., Clarke M. Expression of early developmental genes in Dictyostelium discoideum is initiated during exponential growth by an autocrine-dependent mechanism. Mech. Dev. 1992;36:173–182. doi: 10.1016/0925-4773(92)90068-u. [DOI] [PubMed] [Google Scholar]
- Simins A. B., Weighardt H., Weidner K. M., Weidle U. H., Holzmann B. Functional cloning of ARM-1, an adhesion-regulating molecule upregulated in metastatic tumor cells. Clin. Exp. Metastasis. 1999;17:641–648. doi: 10.1023/a:1006790912877. [DOI] [PubMed] [Google Scholar]
- Sussman M. Cultivation and synchronous morphogenesis of Dictyostelium under controlled experimental conditions. Methods Cell Biol. 1987;28:9–29. doi: 10.1016/s0091-679x(08)61635-0. [DOI] [PubMed] [Google Scholar]
- Wetterauer B., Jacobsen G., Morandini P., MacWilliams H. Mutants of Dictyostelium discoideum with defects in the regulation of discoidin I expression. Dev. Biol. 1993;159:184–195. doi: 10.1006/dbio.1993.1232. [DOI] [PubMed] [Google Scholar]