Abstract
We have previously demonstrated that knockout of the calcineurin gene or inhibition of calcineurin activity by immunosuppressants resulted in hypersensitivity to Cl− in fission yeast. We also demonstrated that knockout of the components of the Pmk1 mitogen-activated protein kinase (MAPK) pathway, such as Pmk1 or Pek1 complemented the hypersensitivity to Cl−. Using this interaction between calcineurin and Pmk1 MAPK, here we developed a genetic screen that aims to identify new regulators of the Pmk1 signaling and isolated vic (viable in the presence of immunosuppressant and chloride ion) mutants. One of the mutants, vic1-1, carried a missense mutation in the cpp1+ gene encoding a β subunit of the protein farnesyltransferase, which caused an amino acid substitution of aspartate 155 of Cpp1 to asparagine (Cpp1D155N). Analysis of the mutant strain revealed that Rho2 is a novel target of Cpp1. Moreover, Cpp1 and Rho2 act upstream of Pck2–Pmk1 MAPK signaling pathway, thereby resulting in the vic phenotype upon their mutations. Interestingly, compared with other substrates of Cpp1, defects of Rho2 function were more phenotypically manifested by the Cpp1D155N mutation. Together, our results demonstrate that Cpp1 is a key component of the Pck2–Pmk1 signaling through the spatial control of the small GTPase Rho2.
INTRODUCTION
The mitogen-activated protein kinase (MAPK) pathway is one of the most important intracellular signaling that plays a crucial role in cell proliferation, cell differentiation, and cell cycle regulation (Nishida and Gotoh, 1993; Marshall, 1994; Herskowitz, 1995; Levin and Errede, 1995). The Pmk1 MAPK, a homologue of the mammalian extracellular signal-regulated kinase (ERK)/MAPK, regulates cell morphology and cell integrity in fission yeast Schizosaccharomyces pombe (Toda et al., 1996; Zaitsevskaya-Carter and Cooper, 1997). A functional connection between the Pmk1 pathway and the protein kinase C homologues Pck1 and Pck2 has been suggested, and differential roles of Pck1 and Pck2 in the regulation of cell integrity also have been suggested; however, it is unclear whether these protein kinase C homologues act upstream of the Pmk1 pathway or synergistically regulate independent aspects of cell integrity (Toda et al., 1996;Sengar et al., 1997; Arellano et al., 1999; Calonge et al., 2000).
We have previously demonstrated that knockout of the fission yeast calcineurin gene ppb1+ or inhibition of calcineurin activity by immunosuppressants results in hypersensitivity to Cl−, and that calcineurin and Pmk1 MAPK play antagonistic roles in Cl− homeostasis (Sugiura et al., 1998). Based on this genetic interaction between calcineurin and Pmk1 MAPK, we screened for multicopy suppressors of the Cl−-hypersensitive phenotype of the calcineurin knockout and identified genes encoding an MAPK phosphatase Pmp1 (Sugiura et al., 1998); MAPK kinase (MAPKK) Pek1 (Sugiura et al., 1999), and a novel KH-type RNA-binding protein Rnc1 that binds and stabilizes Pmp1 mRNA (Sugiura et al., 2003).
We also demonstrated that knockout of the components of the Pmk1 MAPK pathway, such as Pmk1 or Pek1 complemented the Cl−-hypersensitive phenotype of calcineurin knockout. Notably, these knockout strains are viable in the presence of immunosuppressant FK506 and high concentrations of MgCl2, whereas the wild-type cells are inviable in the same condition (Sugiura et al., 1998, 1999). These results prompted us to perform a novel genetic screen to isolate vic (viable in the presence of immunosuppressant and chloride ion) mutants, aiming to identify novel components of the Pmk1 MAPK pathway.
We have identified a vic1-1/cpp1-v1 mutant, in addition to the mutation allele in the known components of the Pmk1 MAPK pathway, including pmk1+ (MAPK), pek1+ (MAPKK), mkh1+ (MAPKK kinase, MAPKKK) as well as pck2+ (protein kinase C). The cpp1+ gene encodes a β subunit of the farnesyltransferase (FTase) that is highly conserved through evolution.
In mammals, many proteins, including Ras family small GTPases, nuclear lamins A and B, transducin, rhodopsin kinase, and a peroxisomal protein termed PxF, have been reported as substrates for FTase (Glomset and Farnsworth, 1994; Zhang and Casey, 1996; Casey and Seabra, 1996; Gelb, 1997; Mumby, 1997). There is widespread interest in FTase because Ras proteins are modified by FTase and such a modification is critical for the oncogenic transformation (Hancock et al., 1989; Jackson et al., 1990; Kato et al., 1992). Several FTase inhibitors (FTIs) have been in clinical trials for the treatment of cancer (Johnston, 2001; Ayral-Kaloustian and Salaski, 2002). Also, recent studies have reported that abnormal persistence of the farnesyl modifications on the nuclear prelamin A has been implicated in the pathogenesis of Hutchinson–Gilford progeria syndrome, a devastating premature aging disease (Pollex and Hegele, 2004). Thus, FTIs represent a possible therapeutic option for cancer as well as for individuals with Hutchinson–Gilford progeria syndrome.
In budding yeast Saccharomyces cerevisiae, α-factor mating pheromone and Ras2 involved in the cAMP pathway have been identified as substrates of FTase (Goodman et al., 1990; He et al., 1991). In fission yeast Schizosaccharomyces pombe, FTase Cpp1 has been reported to play a critical role in sexual differentiation and morphogenesis through Ras1 (Yang et al., 2000) and also has been reported to play a role in cell cycle progression through Rheb (Yang et al., 2001).
Here, we show that the small GTPase Rho2 is a novel target of Cpp1 and that Cpp1 and Rho2 act upstream of Pck2-Pmk1 MAPK cell integrity signaling pathway.
MATERIALS AND METHODS
Strains, Media, and Genetic and Molecular Biology Methods
The S. pombe strains used in this study are listed in Table 1. The complete medium yeast extract-peptone-dextrose (YPD) and the minimal medium Edinburgh minimal medium (EMM) have been described previously (Toda et al., 1996). Standard genetic and recombinant DNA methods (Moreno et al., 1991) were used except where noted. FK506 was provided by Fujisawa Pharmaceutical Co. (Osaka, Japan).
Table 1.
S. pombe strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| HM123 | h−leu1-32 | Our stock |
| HM528 | h+his2 | Our stock |
| KP928 | h+his2 leu1 ura4-D18 | Our stock |
| KP119 | h+leu1-32 ura4-D18 ppb1::ura4+ | Our stock |
| KP208 | h−leu1-32 ura4-D18 pmk1::ura4+ | Our stock |
| KP330 | h−leu1-32 pck2::LEU2 | Our stock |
| KP452 | h−leu1-32 ura4-D18 mkh1::ura4+ | Our stock |
| KP454 | h−leu1-32 ura4-D18 pek1::ura4+ | Our stock |
| KP251 | h−leu1-32 ura4-D18 ppb1::ura4+pmk1::ura4+ | Our stock |
| KP327 | h−leu1-32 ura4-D18 ppb1::ura4+pck2::LEU2 | Our stock |
| KP468 | h−leu1-32 ura4-D18 ppb1::ura4+mkh1::ura4+ | Our stock |
| KP550 | h−leu1-32 ura4-D18 ppb1::ura4+pek1::LEU2 | Our stock |
| KP754 | h−leu1-32 cpp1-v1 | This study |
| KP1855 | h−leu1-32 ura4-D18 cpp1::ura4+ | This study |
| KP2436 | h−leu1-32 rho2::KanMX6 | This study |
| KP2451 | h+his2 leu1-32 ura4-D18 rho2::KanMX6 | This study |
| KP2174 | h−leu1-32 ura4-D18 ras1::ura4+ | This study |
| KP1375 | h−leu1-32 ura4-D18 rho3::ura4+ | This study |
| KP1825 | h−leu1-32 ura4-D18 pck1::ura4+ | Our stock |
| KP2462 | h−leu1-32 ura4-D18 mkh1::ura4+rho2::KanMX6 | This study |
| KP2465 | h−leu1-32 ura4-D18 pck1::ura4+rho2::KanMX6 | This study |
| KP2466 | h−leu1-32 ura4-D18 pmk1::ura4+rho2::KanMX6 | This study |
| KP2468 | h−leu1-32 ura4-D18 pck2::LEU2 rho2::KanMX6 | This study |
Isolation of vic1-1/cpp1-v1 Mutant
The vic1-1/cpp1-v1 mutant was isolated in a screen of cells that had been mutagenized with nitrosoguanidine as described previously (Zhang et al., 2000). Mutants were spread on YPD plates to give ∼1000 cells/plate, and the plates were incubated at 27°C for 4 d. The plates were then replica-plated at 27°C onto plates containing 0.5 μg/ml FK506 and 0.2 M MgCl2. Mutants that grew in the plates were selected and designated as vic mutants. The original mutants isolated were backcrossed three times to wild-type strains HM123 and HM528.
Cloning and Knockout of the cpp1+ Gene
To clone the vic1+ gene, the temperature sensitivity of vic1-1 mutants (KP754) was used. The vic1-1 mutants were grown at 27°C and transformed with an S. pombe genomic DNA library constructed in the vector pDB248 (Beach et al., 1982). Leu+ transformants were replica-plated onto YPD plates at 36°C, and the plasmid DNA was recovered from transformants that showed plasmid-dependent rescue. These plasmids complemented both the temperature sensitivity and vic phenotype of the vic1-1 mutant. By DNA sequencing, the suppressing plasmids were identified to contain the cpp1+ gene (SPAC17G6.04c). To investigate the relationship between the cloned cpp1+ gene and vic1-1 mutant, linkage analysis was performed as follows. The entire cpp1+ gene was subcloned into the pUC-derived plasmid containing S. cerevisiae LEU2 gene and integrated by homologous recombination into the genome of the wild-type strain HM123. The integrant was mated with the vic1-1 mutant. The resulting diploid was sporulated, and tetrads were dissected. In total, 30 tetrads were dissected. In all cases, only parental ditype tetrads were found, indicating allelism between the cpp1+ gene and the vic1-1 mutation (our unpublished data).
To knockout cpp1+ gene, a one-step gene disruption by homologous recombination was performed as described previously (Rothstein, 1983). The cpp1::ura4+ disruption was constructed as follows. The BamHI fragment containing the cpp1+ gene was subcloned into the BamHI site of BlueScriptSK(+) (Stratagene, La Jolla, CA). Then, a PstI/SmaI fragment containing the ura4+ gene was inserted into the NsiI/EcoRV site of the previous construct. The fragment containing the disrupted cpp1+ gene was transformed into haploid cells. Stable integrants were selected on medium lacking uracil, and knockout of the gene was checked by genomic Southern hybridization and tetrad analysis (our unpublished data).
Cloning and Tagging of the rho1+, rho2+, rho3+, and ras1+ Genes
The rho1+, rho2+, rho3+, and ras1+ genes were amplified by PCR with the genomic DNA of wild-type cells as a template. The primers used were summarized in Table 2. The amplified products containing theses genes were digested with BamHI, and the resulting fragments were subcloned into BlueScriptSK(+).
Table 2.
S. pombe primers used in this study
| Gene | Primer |
|---|---|
| Rho1 sense | 5′-CGG GAT CCC ATA TGG CGA CAG AAC TTC GC-3′ |
| Rho1 antisense | 5′-CGG GAT CCT TAC AAC AAG ATA CAA CGC-3′ |
| Rho2 sense | 5′-CGC GGA TCC CAT ATG TTG CAA TCT CAA CC-3′ |
| Rho2 antisense | 5′-CGC GGA TCC TTA TGA AAT GAT GCA GC-3′ |
| Rho2CIIL antisense | 5′-CGC GGA TCC TTA TAA AAT GAT GCA GC-3′ |
| Rho2SIIS antisense | 5′-CGC GGA TCC TTA TGA AAT GAT GGA GCA TTT TGT AG-3′ |
| Rho3 sense | 5′-CGC GGA TCC CAT ATG TCA AGC TGT TTC GG-3′ |
| Rho3 antisense | 5′-CGG GAT CCT CAA GCA ATG ATA CAT CCG GTA-3′ |
| Ras1 sense | 5′-CGG GAT CCC ATA TGA GGG TAA GTC TAA GCA ATG-3′ |
| Ras1 antisense | 5′-CGG GAT CCC TAA CAT ATA ACA CAA CAT TTA G-3′ |
For ectopic expression of proteins, we used the thiamine-repressible nmt1 promoter (Maundrell, 1993). Expression was repressed by the addition of 4 μM thiamine to EMM and was induced by washing and then incubating the cells in EMM lacking thiamine. To express green fluorescent protein (GFP)-Rho1, Rho2, Rho3, or Ras1, these genes were tagged at its N terminus with GFP carrying the S65T mutation. Rho2CIIL and Rho2SIIS were made in the same way except that the antisense primers were specifically designed for amplifying Rho2CIIL and Rho2SIIS. Similarly, Rho2, Ras1, or Rho3 was tagged at its N terminus with FLAG. These constructs were confirmed to be fully functional as their expression complemented the phenotypes associated with Δras1, Δrho2, and Δrho3, respectively (our unpublished data).
Microscopy and Miscellaneous Methods
Methods in light microscopy, such as fluorescence microscopy and differential interference contrast microscopy, were performed as described previously (Kita et al., 2004). Cell extract preparation and immunoblot analysis were performed as described previously (Sio et al., 2005).
RESULTS
Isolation of vic Mutants
To identify new components of the protein kinase C-Pmk1 MAPK signaling pathway, we performed a genetic screen in S. pombe based on the functional interaction that calcineurin and Pmk1 MAPK play antagonistic roles in Cl− homeostasis (Sugiura et al., 1998). On inhibition of the Pmk1 MAPK signaling, the Cl−-hypersensitive phenotype of calcineurin knockout was complemented, i.e., the knockout of pmk1+, pek1+, mkh1+, or pck2+ all complemented the Cl−-hypersensitive phenotype of calcineurin knockout (Figure 1A, +0.2 M MgCl2). The inhibition of calcineurin signaling is also achieved by the immunosuppressant FK506, a specific inhibitor of calcineurin, because wild-type cells failed to grow in the presence of FK506 and 0.2 M MgCl2 (Figure 1A, wt, +FK506 + 0.2 M MgCl2). Consistently, knockout of the components of protein kinase C-MAPK signaling makes cells grow in the presence of FK506 and MgCl2 (Figure 1A, +FK506 + 0.2 M MgCl2). We therefore hypothesized that if we isolate mutants that can grow in the presence of FK506 and 0.2 M MgCl2, the genes responsible for the mutation are expected to function in the protein kinase C–Pmk1 MAPK signaling. These may include mutations in the unknown factor downstream of Pmk1 or in novel factors required for the activation or function of Pmk1 MAPK. We then performed the isolation of the mutants that are viable in the presence of immunosuppressant and chloride ion; hence, we named these as vic mutants. By this genetic approach, we isolated several novel mutants, in addition to mkh1, pek1, pmk1, and pck2 mutants (our unpublished data). Here, we focus on the characterization of the vic1-1 mutant.
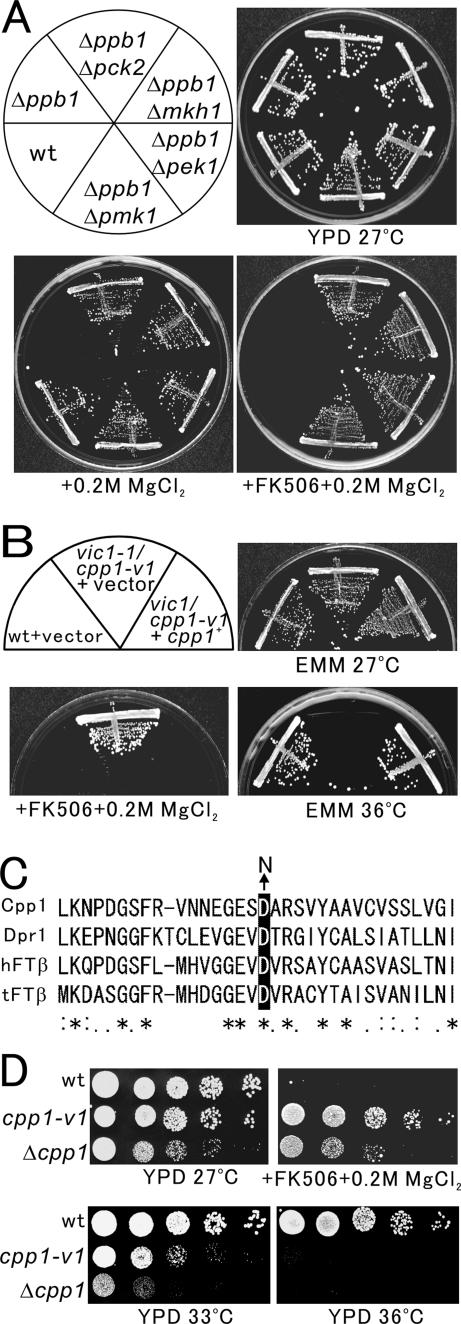
Figure 1.
A mutation in the vic1+/cpp1+ gene causes vic and temperature-sensitive phenotypes. (A) Knockout of the components of the Pmk1 MAPK pathway (Δmkh1, Δpek1, or Δpmk1) or the protein kinase C (Δpck2) suppressed the Cl− hypersensitivity caused by calcineurin knockout (Δppb1). The cells as shown were streaked onto the plates as indicated, then incubated for 4 d at 27°C. (B) The vic and temperature-sensitive phenotypes of vic1-1/cpp1-v1 mutant cells. Cells transformed with the multicopy vector pDB248 or the vector containing the cpp1+ gene were streaked onto the plates as indicated and then incubated for 4 d at 27°C or 3 d at 36°C, respectively. (C) Partial alignment of protein sequences of S. pombe Cpp1 with related proteins from S. cerevisiae (Dpr1p), human (hFTβ), and tomato (tFTβ). Sequence alignment was performed using the ClustalW program. Asterisks indicate identical amino acids, and colons indicate similar amino acids. Arrow indicates the mutation site in the aspartic acid 155 of Cpp1, which when mutated to asparagine resulted in vic and temperature-sensitive phenotypes in Cpp1. (D) The phenotypes of cpp1-v1 and Δcpp1. Wild-type cells, cpp1-v1 mutant, and Δcpp1 cells were dropped onto the plates as indicated and then incubated for 4 d at 27°C or 3 d at 33 and 36°C, respectively.
As shown in Figure 1B, the vic1-1 mutants grew well in the presence of FK506 and 0.2 M MgCl2 at 27°C wherein wild-type cells failed to grow (Figure 1B, + FK506 + 0.2 M MgCl2). However, vic1-1 mutant cells could not grow at 36°C whereas wild-type cells grew normally (Figure 1B, EMM 36°C). As predicted, the vic1-1Δppb1 double mutant cells were able to grow in the presence of 0.2 M MgCl2 (our unpublished data), indicating that the vic1-1 mutation suppresses the Cl− sensitivity of calcineurin knockout and suggesting that the gene product is involved in the regulation of the Pmk1 MAPK pathway.
The vic1-1/cpp1-v1 Is an Allele of the cpp1+ Gene That Encodes a β Subunit of Protein FTase
The vic1+ gene was cloned by complementation of the temperature-sensitive growth defect of vic1-1 mutant cells (Figure 1B, EMM 36°C, +cpp1+). The vic1+ gene also complemented the vic phenotype of vic1-1 mutant cells, because these cells failed to grow in the presence of FK506 and 0.2 M MgCl2 at 27°C when the vic1+ gene was introduced (Figure 1B, + FK506 + 0.2 M MgCl2, + cpp1+). Nucleotide sequencing of the cloned DNA fragment revealed that the vic1+ gene is identical to the cpp1+ gene (SPAC17G6.04c), which encodes the β subunit of the protein FTase of 382 amino acids that is highly similar to the human hFTβ and S. cerevisiae Dpr1p. Linkage analysis was performed (see Materials and Methods), and results indicated the allelism between the cpp1+ gene and the vic1-1 mutation. We therefore renamed vic1-1 as cpp1-v1.
To identify the mutation site in the cpp1-v1 allele, the genomic DNA from cpp1-v1 mutant cells was isolated, and the full-length coding region of the cpp1-v1 gene was sequenced. The G-to-A nucleotide substitution caused a highly conserved aspartic acid to be altered to an asparagine residue at the amino acid position 155 (Figure 1C, black arrow). We therefore refer to the protein product of the cpp1-v1 gene as Cpp1D155N.
We constructed Cpp1 knockout (see Materials and Methods), and Δcpp1 cells displayed a slow growth rate even at the permissive temperature of 27°C as reported by Yang et al. (2000). In contrast, cpp1-v1 mutant cells grew normally at the permissive temperature of 27°C (Figure 1D, YPD 27°C). The Δcpp1 cells also grew in the presence of FK506 and 0.2 M MgCl2; however, the growth rate was significantly slower compared with that of the cpp1-v1 mutant cells (Figure 1D, + FK506 + 0.2 M MgCl2). Thus, in contrast to the cpp1-v1 mutant cells, the Δcpp1 cells did not display a markedly clear vic phenotype. Notably, the degree of the temperature-sensitive growth defect was more severe in Δcpp1 cells, because Δcpp1 cells failed to grow even at 33°C, whereas cpp1-v1 mutant cells grew well (Figure 1D, YPD 33°C). Thus, the cpp1-v1 mutant and Δcpp1 cells displayed similar but distinct phenotypes, suggesting that FTase activity is partially deficient in cpp1-v1 mutant cells. Consistently, overexpression of Cpp1D155N suppressed the temperature-sensitive phenotype of Δcpp1 cells (our unpublished data).
Rho2 Is the Target of Cpp1 Responsible for vic Phenotype
FTase catalyzes the posttranslational modification of Ras and other Ras family proteins. The target proteins have a consensus CAAX motif (where C represents cysteine, A represents aliphatic amino acid, and X preferentially represents methionine, cysteine, serine, alanine, or glutamine) at the C terminus (Maltese, 1990; Cox and Der, 1992; Magee et al., 1992).
In fission yeast, Ras1 (a known target of Cpp1), Rho2, and Rho3 small GTPases contain CAAX motif modified by FTase (Figure 2A, FTase), and Rho1 small GTPase contains CAAL motif modified by geranylgeranyltransferase I (Figure 2A, GGTase I). We examined the intracellular localization of Ras1, Rho2, Rho3, and Rho1 in wild-type, cpp1-v1 mutant, or Δcpp1 cells, based on the assumption that a mutation in the cpp1+ gene should cause a defect in the farnesylation of the target small GTPases, thereby leading to a defect in their intracellular localization.
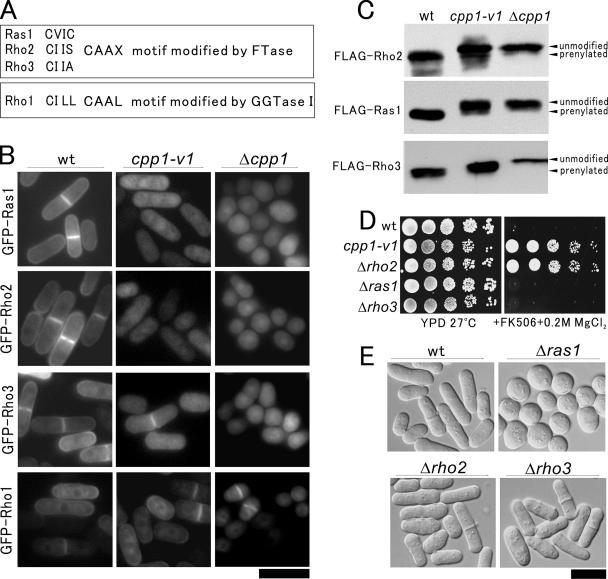
Figure 2.
Rho2 is the target of Cpp1 responsible for vic phenotype. (A) The C-terminal sequence of Ras1, Rho2, Rho3, and Rho1 in fission yeast. Ras1, Rho2, and Rho3 contain CAAX motif modified by FTase, and Rho1 contains CAAL motif modified by GGTase I. (B) Intracellular localization of various small GTPases in wild-type, cpp1-v1 mutant, and Δcpp1 cells. The GFP-fused Ras1, Rho2, Rho3, or Rho1 was transformed into wild-type, cpp1-v1 mutant, or Δcpp1 cells. The transformants were grown to early log phase in EMM containing 4 μM thiamine and were examined under the fluorescence microscope. Bar, 10 μm. (C) Defective modification of Rho2, Ras1, and Rho3 in cpp1-v1 mutant and Δcpp1 cells. The FLAG-fused Rho2, Ras1, or Rho3 was transformed into wild-type or cpp1-v1 mutant cells. The transformants were grown in EMM in the absence of thiamine at 27°C for 20 h, and then the cells were collected and lysed. Cell extracts were subjected to 15% polyacrylamide gel and immunoblotted using anti-FLAG antibody. Prenylated and unmodified forms of Rho2, Ras1, or Rho3 are indicated. (D) Δrho2 cells showed vic phenotype. The cells as shown were dropped onto the plates as indicated and then incubated for 4 d at 27°C. (E) Morphology of Δrho2, Δras1, and Δrho3 cells. Cells were grown to mid-log phase in YPD at 27°C and were observed under the differential interference contrast microscopy. Bar, 10 μm.
As shown in Figure 2B, in wild-type cells all these small GTPases predominantly localized to the plasma membrane. However, in cpp1-v1 mutant cells and Δcpp1 cells, the plasma membrane localization of Ras1 or Rho2 was not clearly observed. In contrast, in cpp1-v1 mutant cells the plasma membrane localization of Rho3 was clearly observed, and in Δcpp1 cells it was abolished. Note that the localization of CAAL-ending GTPase Rho1 was not affected in either cpp1-v1 mutant or Δcpp1 cells. Δcpp1 cells displayed the round cell shape as reported previously owing to the defective farnesylation of Ras1 (Yang et al., 2000, 2001) (Figure 2B, Δcpp1). In clear contrast, the cpp1-v1 mutant cells displayed cylindrical cell shape (Figure 2B, cpp1-v1), suggesting that farnesylation of Ras1 in cpp1-v1 mutant cells is not considerably defective so as to affect the cell shape. These results suggest that CAAX-ending Ras1, Rho2, and Rho3 are targets of Cpp1 in fission yeast. More importantly, the partial loss of the farnesyltransferease activity caused by the cpp1-v1 mutation differentially impacts the function of these small GTPases.
To investigate whether there is a defect in the farnesylation of these three substrates in cpp1-v1 mutant cells, we constructed FLAG-tagged Rho2, Ras1, and Rho3, and we expressed these in wild-type, cpp1-v1 mutant, and Δcpp1 cells. As shown in Figure 2C, Rho2, Ras1, and Rho3 extracted from Δcpp1 cells migrated slower (unmodified), compared with those from wild-type cells (prenylated). Rho2, Ras1, and Rho3 extracted from the cpp1-v1 mutant cells exhibited significantly different patterns of migration from the wild-type or Δcpp1 cells (Figure 2C). Notably, in cpp1-v1 mutant cells, prenylation of Ras1 and Rho2 was severely impaired, whereas that of Rho3 was modestly impaired (Figure 2C).
Because our intention is to identify components of the protein kinase C–Pmk1 MAPK signaling by isolating vic mutants, we next investigated whether the knockout cells of the three targets exhibit vic phenotype similar to that of cpp1-v1/vic1-1 cells. Results clearly showed that Δrho2 cells, but not Δras1 or Δrho3 cells, were able to grow in the presence of FK506 and 0.2M MgCl2 (Figure 2D). These results raise the possibility that Cpp1-Rho2 is involved in the protein kinase C-Pmk1 MAPK signaling. We also examined the morphology of Δrho2, Δras1, and Δrho3 cells to understand the differential roles of different small GTPases in cellular morphogenesis. Δras1 cells displayed the round cell shape similar to that of Δcpp1 cells, whereas neither Δrho2 nor Δrho3 cells showed obvious defect in morphology (Figure 2E).
Farnesylation by Cpp1 Is Essential for the Membrane Localization and Function of Rho2
To examine whether the membrane localization of Rho2 is critical for its function, we created various mutant versions of Rho2 at its C terminus. As shown in Figure 2B and here, wild-type Rho2 (Rho2CIIS) localized to the plasma membrane in wild-type cells, but not in cpp1-v1 mutant or Δcpp1 cells (Figure 3A, GFP-Rho2CIIS). In contrast, the geranylgeranylated mutant form of Rho2 (Rho2CIIL), which bypasses the farnesylation requirement, localized to the plasma membrane both in wild-type cells and in cpp1 mutant cells, indicating that geranylgeranylation allows Rho2 to associate with the membrane even in the absence of farnesylation (Figure 3A, GFP-Rho2CIIL). However, the prenylation-defective Rho2 (Rho2SIIS), wherein the cysteine in the CAAX-motif was replaced with serine, failed to localize to the plasma membrane even in wild-type cells (Figure 3A, GFP-Rho2SIIS).
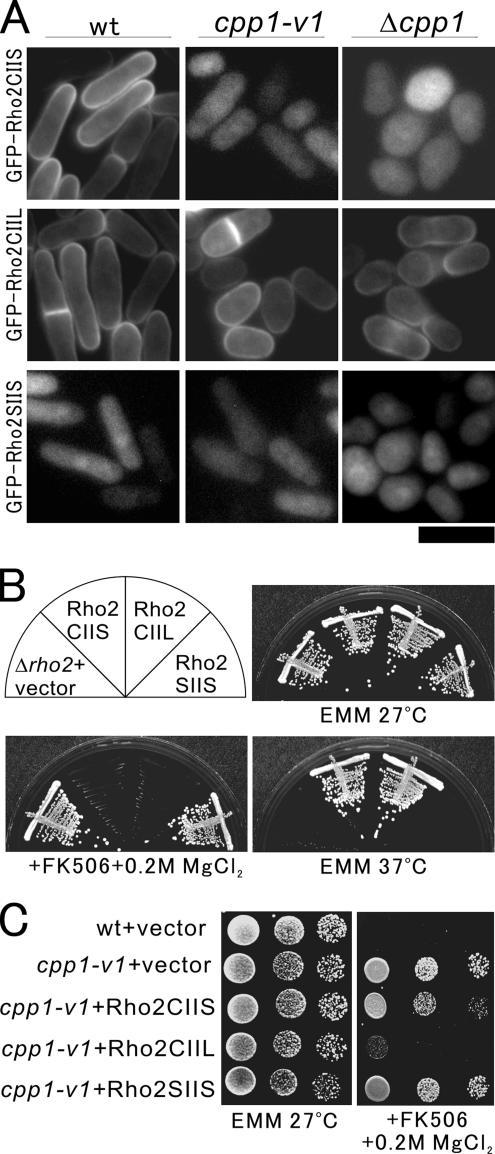
Figure 3.
Farnesylation by Cpp1 is essential for the proper membrane localization and function of Rho2. (A) Intracellular localization of GFP-fused wild-type Rho2 (Rho2CIIS), the geranylgeranylated mutant version of Rho2 (Rho2CIIL), or the prenylation-defective mutant version of Rho2 (Rho2SIIS) in wild-type, cpp1-v1 mutant or Δcpp1 cells. The GFP-Rho2CIIS, GFP-Rho2CIIL, or GFP-Rho2SIIS was transformed into wild-type, cpp1-v1 mutant, or Δcpp1 cells. The transformants were cultured and examined as in Figure 2B. (B) The plasma membrane localization is essential for Rho2 function. Wild-type Rho2 (Rho2CIIS) and the geranylgeranylated mutant version of Rho2 (Rho2CIIL), but not the prenylation-defective mutant version of Rho2 (Rho2SIIS), suppressed the phenotypes. The Δrho2 cells harboring the control vector, Rho2CIIS, Rho2CIIL, or Rho2SIIS were streaked onto the plates as indicated and then incubated for 4 d at 27°C or 3 d at 37°C, respectively. (C) Geranylgeranylated mutant version of Rho2 (Rho2CIIL) suppressed the vic phenotype of cpp1-v1 mutant cells. The cpp1-v1 mutant cells harboring the control vector, Rho2CIIS, Rho2CIIL, or Rho2SIIS were dropped onto the plates as indicated and then incubated for 4 d at 27°C.
Notably, Rho2CIIL, which localized to the plasma membrane, fully suppressed the temperature-sensitive and vic phenotypes of Δrho2 cells, suggesting that geranylgeranylated Rho2, which bypasses farnesylation, can function as well as farnesylated Rho2 (Figure 3B, +Rho2CIIL). On the other hand, Rho2SIIS, which failed to localize to the plasma membrane, could not suppress the phenotypes (Figure 3B, +Rho2CIIS), suggesting that the plasma membrane localization of Rho2 is critical for its function.
To address whether the vic phenotype of cpp1-v1 mutant cells is because of the defective farnesylation of Rho2, we overexpressed these mutant forms of Rho2 in cpp1-v1 mutant cells. As expected, only Rho2CIIL, which can localize to the plasma membrane even in cpp1-v1 mutant cells, reversed the vic phenotype of cpp1-v1 mutant cells, whereas Rho2CIIS and Rho2SIIS could not (Figure 3C). Thus, Rho2 is the target of Cpp1, that is responsible for the vic phenotype of cpp1-v1 mutant cells, strongly suggesting that Cpp1 functions in Pmk1 MAPK signaling through Rho2 regulation.
Cpp1 and Rho2 Act Upstream of the Pck2–Pmk1 MAPK Pathway
As a first step to examine the functional relationship between Cpp1-Rho2 and Pmk1 signaling, we examined whether the vic phenotype is a specific indication for Pmk1 MAPK signaling, or whether it is shared by other MAPK pathways in fission yeast. For this, null cells of sty1+ encoding a stress-activated MAPK or spk1+ encoding a MAPK involved in meiosis was compared with rho2 null cells, cpp1 mutant cells, together with null cells of the components of the Pmk1 MAPK pathway. Results clearly showed that Δrho2, cpp1 mutant cells, as well as Δpmk1, Δpek1, Δmkh1, or Δpck2 grew in the presence of FK506 and 0.15M MgCl2, but Δsty1 or Δspk1 could not grow (Figure 4A, +FK506 + 0.15M MgCl2), indicating that the vic phenotype is specifically shared by the mutations in the components of the protein kinase C–Pmk1 MAPK signaling pathway.
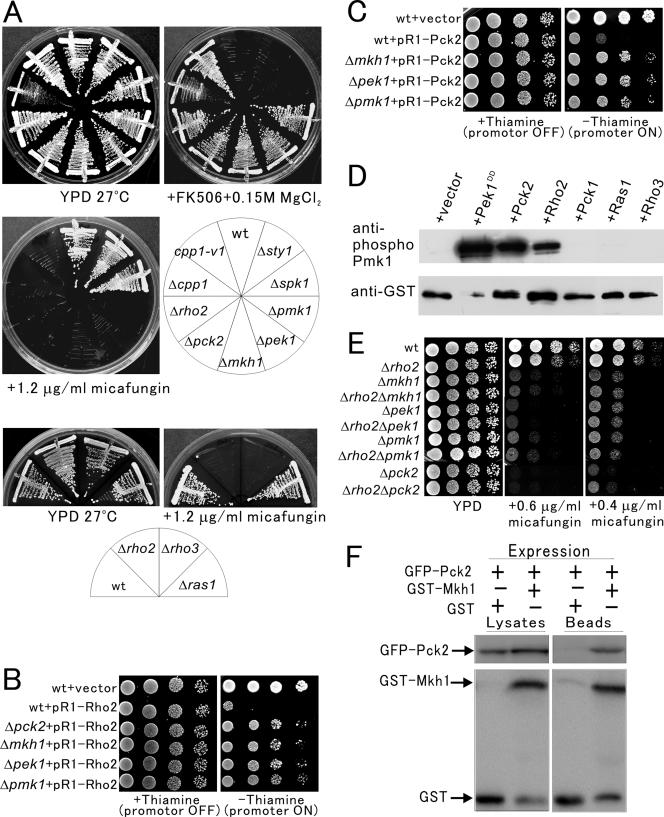
Figure 4.
Rho2 functions upstream of the Pck2–Pmk1 MAPK pathway. (A) Knockout of the rho2+, pck2+, and the component of the Pmk1 MAPK pathway exhibited vic phenotype and micafungin sensitivity. The cells as indicated were streaked onto the indicated plates and then incubated for 4 d at 27°C. (B) Overexpression of Rho2 showed toxicity to wild-type cells, but not to Δpck2, Δmkh1, Δpek1, or Δpmk1 cells. The cells as indicated transformed with pREP1-GFP-Rho2 were dropped onto EMM plate with or without 4 μM thiamine and then incubated for 4 d at 27°C. (C) Overexpression of Pck2 showed toxicity to wild-type cells but not to Δmkh1, Δpek1, or Δpmk1 cells. The cells as shown harboring pREP1-GFP-Pck2 were dropped onto the plate as indicated and then incubated as in Figure 4B. (D) Rho2 stimulates the phosphorylation of Pmk1 in vivo. Wild-type cells containing pREP42-GST-Pmk1 and either pREP1-vector (+vector), pREP1-pek1DD (+Pek1DD), pREP1-Pck2 (+Pck2), pREP1-Rho2 (+Rho2), pREP1-Pck1 (+Pck1), pREP1-Ras1 (+Ras1), or pREP1-Rho3 (+Rho3) were grown in EMM. Immunoblotting using anti-phospho Pmk1 (top) and anti-GST (bottom) antibodies showed that overproduction of Pek1DD, Pck2 as well as Rho2 but not that of Pck1 increased the levels of phosphorylation of Pmk1. (E) Δrho2Δmkh1, Δrho2Δpek1, Δrho2Δpmk1, or Δrho2Δpck2 double mutants did not show synergism in the sensitivity to micafungin. The cells as indicated were dropped onto the plate as indicated and then incubated as in Figure 4B. (F) Pck2 associates with Mkh1. Cells integrated with GFP-Pck2 were transformed with the control GST vector or pREP1—GST–Mkh1 and then grown in EMM medium in the absence of thiamine for 20 h. GST-Mkh1 and GST were precipitated by glutathione beads, washed extensively, and subjected to SDS-PAGE and immunoblotted using anti-GFP or anti-GST antibodies.
Because mutations that perturb the signaling through the protein kinase C–Pmk1 MAPK pathway are known to result in defective cell integrity (Toda et al., 1996), we examined whether knockout of the components of the protein kinase C–Pmk1 MAPK signaling pathway display hypersensitivity to the cell wall-damaging agent micafungin, an inhibitor of (1,3)-β-d-glucan synthase (Carver, 2004). The Δrho2, Δcpp1 as well as Δpck2 or knockout of the components of Pmk1 MAPK pathway were hypersensitive to micafungin, whereas the growth of Δsty1 or Δspk1 cells was not inhibited in the presence of 1.2 μg/ml micafungin (Figure 4A, top, + 1.2 μg/ml micafungin). Thus, the vic phenotype and the sensitivity to micafungin are specific indications of defective protein kinase C–Pmk1 MAPK signaling. Interestingly, Δrho3 cells, but not Δras1 cells, displayed hypersensitivity to micafungin (Figure 4A, bottom), whereas neither of them showed the vic phenotype (Figure 2D).
We next examined whether Rho2 acts upstream of the protein kinase C–Pmk1 MAPK signaling. As reported by Calonge et al. (2000) and as shown here (Figure 4B), overexpression of Rho2 is toxic to wild-type cells, but not to Δpck2 cells. If the toxicity of Rho2 overexpression is caused by the hyperactivation of Pmk1 MAPK pathway, it would be expected that this toxicity could be complemented by knockout of the components of Pmk1 signaling pathway. As expected, the overexpression of Rho2 was not toxic to Δmkh1, Δpek1, and Δpmk1 cells (Figure 4B), indicating that the toxicity of Rho2 overexpression is mediated by Pck2 and Mkh1–Pek1–Pmk1 signaling pathway. Similarly, overexpression of Pck2 caused the lethality in wild-type cells, but not in Δmkh1, Δpek1 and Δpmk1 cells (Figure 4C), indicating that the lethality caused by Pck2 overexpression is mediated by Mkh1–Pek1–Pmk1 signaling. Together, these genetic analyses strongly suggest that Rho2 functions upstream of Pck2–Mkh1–Pek1–Pmk1 signaling.
To further confirm that Rho2–Pck2 activates and transmits signaling to Pmk1, we examined the effects of Rho2, Pck2, or Pck1 overexpression on the phosphorylation levels of Pmk1 MAPK by using anti-phospho Pmk1 antibodies (Sugiura et al., 1999). As shown in Figure 4D, overexpression of Rho2 and Pck2 dramatically increased the phosphorylation levels of Pmk1 similar to that obtained from the overexpression of Pek1DD, a constitutively active MAPKK for Pmk1 (Figure 4D). In clear contrast, overexpression of Pck1 did not increase the phosphorylation level of Pmk1 (Figure 4D). Thus, Rho2–Pck2 acts upstream of the Pmk1 MAPK pathway and stimulates the Pmk1 signaling in vivo. In addition, overexpression of Ras1 or Rho3 did not increase the phosphorylation level of Pmk1 (Figure 4D). Together with the results of Figures 2D and 4A, this suggests that Rho3 is involved in the regulation of cell wall integrity but independently of the Pmk1 signaling pathway.
Next, we constructed a series of double mutants between Δrho2 and knockout of the components of the protein kinase C–Pmk1 MAPK signaling and compared the sensitivity to micafungin with each single mutant. The Δrho2 cells showed a relatively weak sensitivity to micafungin, because Δrho2 cells grew in the presence of 0.6 μg/ml micafungin, wherein Δmkh1, Δpek1, Δpmk1, or Δpck2 mutants failed to grow. However, Δrho2Δmkh1, Δrho2Δpek1, Δrho2Δpmk1 or Δrho2Δpck2 double knockout mutant cells failed to grow in the presence of 0.4 μg/ml micafungin and do not show synergism in the sensitivity to micafungin compared with the parental single knockout (Figure 4E).
The above-mentioned results strongly suggest that Pck2 functions upstream of Pmk1. We thus examined whether Pck2 associates with the MAPKKK Mkh1. For this, we expressed glutathione S-transferase (GST)-fused Mkh1 in strains where GFP-Pck2 is chromosomally integrated. In the GST pull-down assay, GFP–Pck2 associates with GST–Mkh1 but not with control GST vector alone (Figure 4F), indicating a protein–protein interaction between Pck2 and Mkh1.
Pck1 Is Involved in Cell Integrity Signaling and Acts Independently of the Pmk1 MAPK Pathway
In the study by Toda et al. (1993), Pck1 and Pck2 have been reported to share an overlapping function for cell viability and to partially complement each other. We then examined whether Pck2 and Pck1 share an overlapping function in cell integrity and chloride ion homeostasis.
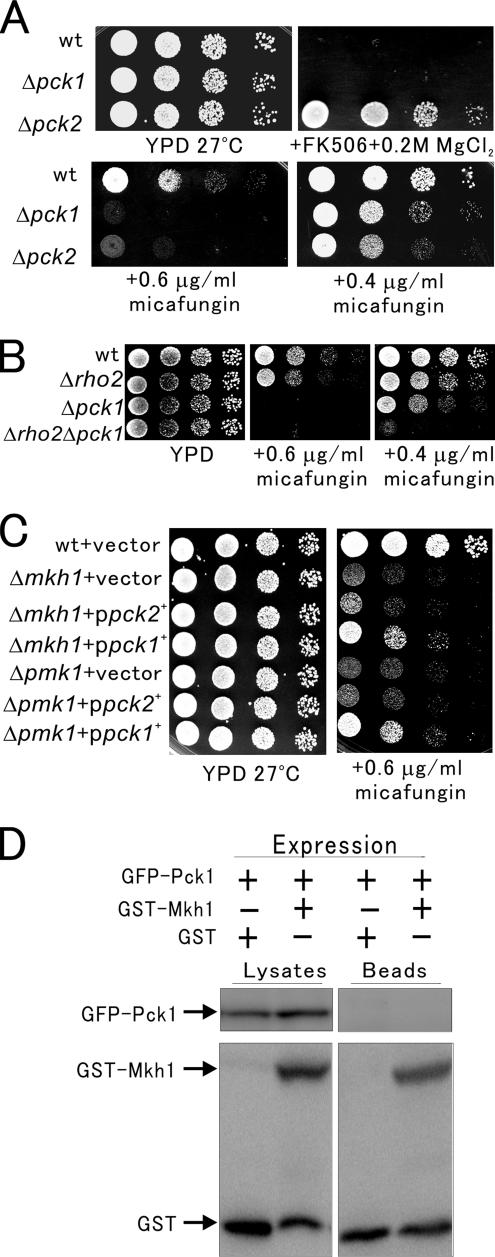
For this, we first compared Δpck1 and Δpck2 cells regarding vic phenotype. Results clearly showed that Δpck2, but not Δpck1, grew in the presence of FK506 and 0.2 M MgCl2 (Figure 5A), suggesting that Pck1 is not involved in Cl− homeostasis. We also compared Δpck1 and Δpck2 cells regarding the cell integrity defect. As shown in Figure 5B, at a higher concentration of micafungin (0.6 μg/ml), the growth of Δpck1 and Δpck2 cells were significantly inhibited, but at a lower concentration of micafungin (0.4 μg/ml), the growth of Δpck2 cells was markedly inhibited compared with that of Δpck1 cells. These results indicate that although Δpck1 and Δpck2 cells are both sensitive to micafungin, Δpck1 cells displayed a weaker sensitivity to micafungin compared with Δpck2 cells.
Figure 5.
Pck1 is involved in cell integrity signaling and acts independently of the Pmk1 MAPK pathway. (A) The phenotypes of Δpck2 and Δpck1. Wild-type, Δpck2, or Δpck1 cells were dropped onto the plates as indicated and then incubated for 4 d at 27°C. (B) Δrho2Δpck1 double mutants showed synergism in the sensitivity to micafungin. Wild-type, Δpck1, Δrho2, or Δrho2Δpck1 cells were dropped onto the plates as indicated and then incubated for 4 d at 27°C. (C) pck1+, but not pck2+, partially suppressed the micafungin sensitivity of Δmkh1 and Δpmk1 cells. The Δmkh1 or Δpmk1 cells were transformed with the control vector, pck1+ or pck2+ gene. The transformants were dropped onto the plates as indicated and then incubated for 4 d at 27°C. (D) Pck1 does not associate with Mkh1. GFP-Pck1 integrated cells were transformed with the control GST vector or pREP1-GST-Mkh1 and then grown in EMM medium in the absence of thiamine for 20 h. Pull-down assay was performed as described in Figure 4E.
As shown in Figure 4D, Δrho2Δpck2 double mutants showed micafungin sensitivity to the same extent as that of Δpck2 mutants. Here, we constructed Δrho2Δpck1 double mutants and investigated the micafungin sensitivity. Although both Δpck1 cells and Δrho2 cells can grow in the presence of 0.4 μg/ml micafungin, Δrho2Δpck1 double mutants failed to grow in the presence of 0.4 μg/ml micafungin, indicating a synergism in the sensitivity to micafungin compared with that of the parental single knockout (Figure 5B). Thus, Pck1 seems to play a role in cell integrity in parallel to the Rho2–Pck2–Mkh1–Pek1–Pmk1 signaling pathway. Consistently, our recent study showed that Pmk1 is required for the stimulation of calcineurin via Yam8/Cch1-mediated Ca2+ influx and that knockout of pck2+, but not pck1+ gene, markedly diminished the Yam8/Cch1-dependent stimulation of calcineurin activity, suggesting that Pck2 acts upstream of the Pmk1 in this signaling pathway (Deng et al., 2006).
Furthermore, overexpression of pck1+, but not pck2+, partially suppressed the micafungin sensitivity of Δmkh1 and Δpmk1 cells (Figure 5C). Overexpression of pck1+ also partially suppressed the micafungin sensitivity of Δpek1 cells (our unpublished data). These results indicate that Pck1 stimulates the cell integrity signaling independently of the Pmk1 MAPK pathway, whereas Pck2 exerts its function on cell integrity through the MAPK pathway. In addition, a pull-down experiment demonstrated that Pck1 does not associate with Mkh1 (Figure 5D), in clear contrast to the association observed between Pck2 and Mkh1 (Figure 4F). These results are in good agreement with the findings obtained with phosphorylation experiments.
DISCUSSION
In the present study, we performed a novel genetic screen that aims to identify new regulators of the Pmk1 signaling, and we identified a missense mutation of the cpp1+ gene encoding FTase. Substitution of a highly conserved aspartic acid to an asparagine residue at the amino acid position 155 resulted in the partial but severe impairment of the FTase activity in cpp1-v1 mutant cells.
Interestingly, the cpp1-v1 mutation caused the differential effects on the function of its substrates. That is, the membrane distribution of Ras1 and Rho2 was apparently abolished but that of Rho3 was still maintained in cpp1-v1 mutant cells, indicating that Rho3 farnesylation is not completely abolished in cpp1-v1 mutant cells. This is in good correlation with the moderate defect of prenylation as shown in Figure 2C. Furthermore, although Ras1 prenylation was severely impaired in cpp1-v1 mutant cells (Figure 2C), the function of Ras1 seemed to be maintained in the cpp1-v1 mutant cells because their cell shape was not so much affected compared with that of Δcpp1 cells. In clear contrast, the function of Rho2 seemed to be severely impaired in the cpp1-v1 mutant cells, because the mutant cells showed a clear vic phenotype similar to that of the Δrho2 cells. These results suggest that Rho2 function is preferentially sensitive to defect of FTase activity in the cpp1-v1 mutant cells.
Although a numbers of studies showed that functions of Ras proteins depend on its farnesylation, the identities of the relevant farnesylated proteins in human oncogenesis are not fully resolved. FTIs clearly have the potential to inhibit oncogenic Ras signaling, but FTIs are also effective on tumor cell lines that do not contain mutant Ras (Sepp-Lorenzino et al., 1995; End et al., 2001), suggesting that the pharmacological effects extend outside of the Ras protein (Tamanoi et al., 2001). RhoB, a small GTPase of the mammalian Rho family has been suggested as a potential relevant FTI target (Prendergast, 2001; Cox and Der, 2002). RhoB exists as both farnesylated and geranylgeranylated forms, whereas the highly homologous RhoA and RhoC isoforms are solely geranylgeranylated (Adamson et al., 1992). Treatment of cells with FTIs causes a loss of farnesylated RhoB and a consequent increase in geranylgeranylated RhoB (Lebowitz et al., 1997), and these changes in prenylation have been implicated in the antineoplastic responses to FTIs (Liu et al., 2000).
Given the high degree of conservation of FTase, Ras and Rho proteins as well as its involvement in human oncogenesis, the cpp1-v1 mutant may be a useful model for studying the conserved molecular mechanism of protein farnesylation and the differential effects of FTase inhibition on the various substrates.
In conclusion, the identification and analyses of cpp1-v1 mutant cells have demonstrated for the first time the functional importance of the posttranslational modification of Rho GTPase protein in the activation of cell integrity signaling through protein kinase C–MAPK pathway. Because of the high similarity between the fission yeast and mammalian MAPK pathway, the screen of vic mutants would further provide an excellent opportunity to identify novel components of MAPK cascade and analyze regulatory mechanisms of MAPK signaling in higher eucaryotes.
ACKNOWLEDGMENTS
We thank Drs. Takashi Toda, Mitsuhiro Yanagida, and Masayuki Yamamoto for providing strains and plasmids; Susie O. Sio for critical reading of the manuscript; and Fujisawa Pharmaceutical Co. for the gift of FK506. This work was supported by the 21st Century Centers of Excellence Program, the Asahi Glass Foundation, the Uehara Memorial Foundation, and research grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-08-0688) on September 27, 2006.
REFERENCES
- Adamson P., Marshall C. J., Hall A., Tilbrook P. A. Post-translational modifications of p21rho proteins. J. Biol. Chem. 1992;267:20033–20038. [PubMed] [Google Scholar]
- Arellano M., Valdivieso M. H., Calonge T. M., Coll P. M., Duran A., Perez P. Schizosaccharomyces pombe protein kinase C homologues, pck1p and pck2p, are targets of rho1p and rho2p and differentially regulate cell integrity. J. Cell Sci. 1999;112:3569–3578. doi: 10.1242/jcs.112.20.3569. [DOI] [PubMed] [Google Scholar]
- Ayral-Kaloustian S., Salaski E. J. Protein farnesyltransferase inhibitors. Curr. Med. Chem. 2002;9:1003–1032. doi: 10.2174/0929867024606687. [DOI] [PubMed] [Google Scholar]
- Beach D., Piper M., Nurse P. Construction of a Schizosaccharomyces pombe gene bank in a yeast bacterial shuttle vector and its use to isolate genes by complementation. Mol. Gen. Genet. 1982;187:326–329. doi: 10.1007/BF00331138. [DOI] [PubMed] [Google Scholar]
- Calonge T. M., Nakano K., Arellano M., Arai R., Katayama S., Toda T., Mabuchi I., Perez P. Schizosaccharomyces pombe rho2p GTPase regulates cell wall α-glucan biosynthesis through the protein kinase pck2p. Mol. Biol. Cell. 2000;11:4393–4401. doi: 10.1091/mbc.11.12.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver P. L. Micafungin. Ann. Pharmacother. 2004;38:1707–1721. doi: 10.1345/aph.1D301. [DOI] [PubMed] [Google Scholar]
- Casey P. J., Seabra M. C. Protein prenyltransferases. J. Biol. Chem. 1996;271:5289–5292. doi: 10.1074/jbc.271.10.5289. [DOI] [PubMed] [Google Scholar]
- Cox A. D., Der C. J. Protein prenylation: more than just glue? Curr. Opin. Cell Biol. 1992;4:1008–1016. doi: 10.1016/0955-0674(92)90133-w. [DOI] [PubMed] [Google Scholar]
- Cox A. D., Der C. J. Farnesyltransferase inhibitors: promises and realities. Curr. Opin. Pharmacol. 2002;2:388–393. doi: 10.1016/s1471-4892(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Deng L., Sugiura R., Takeuchi M., Suzuki M., Ebina H., Takami T., Koike A., Iba S., Kuno T. Real-time monitoring of calcineurin activity in living cells: evidence for two distinct Ca2+-dependent pathways in fission yeast. Mol. Biol. Cell. 2007 doi: 10.1091/mbc.E06-06-0526. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- End D. W., et al. Characterization of the antitumor effects of the selective farnesyl protein transferase inhibitor R115777 in vivo and in vitro. Cancer Res. 2001;61:131–137. [PubMed] [Google Scholar]
- Gelb M. H. Protein prenylation, et cetera: signal transduction in two dimensions. Science. 1997;275:1750–1751. doi: 10.1126/science.275.5307.1750. [DOI] [PubMed] [Google Scholar]
- Glomset J. A., Farnsworth C. C. Role of protein modification reactions in programming interactions between ras-related GTPases and cell membranes. Annu. Rev. Cell Biol. 1994;10:181–205. doi: 10.1146/annurev.cb.10.110194.001145. [DOI] [PubMed] [Google Scholar]
- Goodman L. E., Judd S. R., Farnsworth C. C., Powers S., Gelb M. H., Glomset J. A., Tamanoi F. Mutants of Saccharomyces cerevisiae defective in the farnesylation of Ras proteins. Proc. Natl. Acad. Sci. USA. 1990;87:9665–9669. doi: 10.1073/pnas.87.24.9665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock J. F., Magee A. I., Childs J. E., Marshall C. J. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989;57:1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- He B., Chen P., Chen S. Y., Vancura K. L., Michaelis S., Powers S. RAM2, an essential gene of yeast, and RAM1 encode the two polypeptide components of the farnesyltransferase that prenylates a-factor and Ras proteins. Proc. Natl. Acad. Sci. USA. 1991;88:11373–11377. doi: 10.1073/pnas.88.24.11373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz I. MAP kinase pathways in yeast: for mating and more. Cell. 1995;80:187–197. doi: 10.1016/0092-8674(95)90402-6. [DOI] [PubMed] [Google Scholar]
- Jackson J. H., Cochrane C. G., Bourne J. R., Solski P. A., Buss J. E., Der C. J. Farnesol modification of Kirsten-ras exon 4B protein is essential for transformation. Proc. Natl. Acad. Sci. USA. 1990;87:3042–3046. doi: 10.1073/pnas.87.8.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston S. R. Farnesyl transferase inhibitors: a novel targeted therapy for cancer. Lancet Oncol. 2001;2:18–26. doi: 10.1016/s1470-2045(00)00191-1. [DOI] [PubMed] [Google Scholar]
- Kato K., Cox A. D., Hisaka M. M., Graham S. M., Buss J. E., Der C. J. Isoprenoid addition to Ras protein is the critical modification for its membrane association and transforming activity. Proc. Natl. Acad. Sci. USA. 1992;89:6403–6407. doi: 10.1073/pnas.89.14.6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita A., et al. Loss of Apm1, the μ1 subunit of the clathrin-associated adaptor-protein-1 complex, causes distinct phenotypes and synthetic lethality with calcineurin deletion in fission yeast. Mol. Biol. Cell. 2004;15:2920–2931. doi: 10.1091/mbc.E03-09-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebowitz P. F., Casey P. J., Prendergast G. C., Thissen J. A. Farnesyltransferase inhibitors alter the prenylation and growth-stimulating function of RhoB. J. Biol. Chem. 1997;272:15591–15594. doi: 10.1074/jbc.272.25.15591. [DOI] [PubMed] [Google Scholar]
- Levin D. E., Errede B. The proliferation of MAP kinase signaling pathways in yeast. Curr. Opin. Cell Biol. 1995;7:197–202. doi: 10.1016/0955-0674(95)80028-x. [DOI] [PubMed] [Google Scholar]
- Liu A., Du W., Liu J. P., Jessell T. M., Prendergast G. C. RhoB alteration is necessary for apoptotic and antineoplastic responses to farnesyltransferase inhibitors. Mol. Cell. Biol. 2000;20:6105–6113. doi: 10.1128/mcb.20.16.6105-6113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee A. I., Newman C. M., Giannakouros T., Hancock J. F., Fawell E., Armstrong J. Lipid modifications and function of the ras superfamily of proteins. Biochem. Soc. Trans. 1992;20:497–499. doi: 10.1042/bst0200497. [DOI] [PubMed] [Google Scholar]
- Maltese W. A. Posttranslational modification of proteins by isoprenoids in mammalian cells. FASEB J. 1990;4:3319–3328. doi: 10.1096/fasebj.4.15.2123808. [DOI] [PubMed] [Google Scholar]
- Marshall C. J. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr. Opin. Genet. Dev. 1994;4:82–89. doi: 10.1016/0959-437x(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Maundrell K. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene. 1993;123:127–130. doi: 10.1016/0378-1119(93)90551-d. [DOI] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Mumby S. M. Reversible palmitoylation of signaling proteins. Curr. Opin. Cell Biol. 1997;9:148–154. doi: 10.1016/s0955-0674(97)80056-7. [DOI] [PubMed] [Google Scholar]
- Nishida E., Gotoh Y. The MAP kinase cascade is essential for diverse signal transduction pathways. Trends Biochem. Sci. 1993;18:128–131. doi: 10.1016/0968-0004(93)90019-j. [DOI] [PubMed] [Google Scholar]
- Pollex R. L., Hegele R. A. Hutchinson-Gilford progeria syndrome. Clin. Genet. 2004;66:375–381. doi: 10.1111/j.1399-0004.2004.00315.x. [DOI] [PubMed] [Google Scholar]
- Prendergast G. C. Farnesyltransferase inhibitors define a role for RhoB in controlling neoplastic pathophysiology. Histol. Histopathol. 2001;16:269–275. doi: 10.14670/HH-16.269. [DOI] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Sengar A. S., Markley N. A., Marini N. J., Young D. Mkh1, a MEK kinase required for cell wall integrity and proper response to osmotic and temperature stress in Schizosaccharomyces pombe. Mol. Cell. Biol. 1997;17:3508–3519. doi: 10.1128/mcb.17.7.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepp-Lorenzino L., Ma Z., Rands E., Kohl N. E., Gibbs J. B., Oliff A., Rosen N. A peptidomimetic inhibitor of farnesyl:protein transferase blocks the anchorage-dependent and -independent growth of human tumor cell lines. Cancer Res. 1995;55:5302–5309. [PubMed] [Google Scholar]
- Sio S. O., Suehiro T., Sugiura R., Takeuchi M., Mukai H., Kuno T. The role of the regulatory subunit of fission yeast calcineurin for in vivo activity and its relevance to FK506 sensitivity. J. Biol. Chem. 2005;280:12231–12238. doi: 10.1074/jbc.M414234200. [DOI] [PubMed] [Google Scholar]
- Sugiura R., Kita A., Shimizu Y., Shuntoh H., Sio S. O., Kuno T. Feedback regulation of MAPK signalling by an RNA-binding protein. Nature. 2003;424:961–965. doi: 10.1038/nature01907. [DOI] [PubMed] [Google Scholar]
- Sugiura R., Toda T., Dhut S., Shuntoh H., Kuno T. The MAPK kinase Pek1 acts as a phosphorylation-dependent molecular switch. Nature. 1999;399:479–483. doi: 10.1038/20951. [DOI] [PubMed] [Google Scholar]
- Sugiura R., Toda T., Shuntoh H., Yanagida M., Kuno T. pmp1+, a suppressor of calcineurin deficiency, encodes a novel MAP kinase phosphatase in fission yeast. EMBO J. 1998;17:140–148. doi: 10.1093/emboj/17.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamanoi F., Gau C. L., Jiang C., Edamatsu H., Kato-Stankiewicz J. Protein farnesylation in mammalian cells: effects of farnesyltransferase inhibitors on cancer cells. Cell. Mol. Life Sci. 2001;58:1636–1649. doi: 10.1007/PL00000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Dhut S., Superti-Furga G., Gotoh Y., Nishida E., Sugiura R., Kuno T. The fission yeast pmk1+ gene encodes a novel mitogen-activated protein kinase homolog which regulates cell integrity and functions coordinately with the protein kinase C pathway. Mol. Cell. Biol. 1996;16:6752–6764. doi: 10.1128/mcb.16.12.6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Shimanuki M., Yanagida M. Two novel protein kinase C-related genes of fission yeast are essential for cell viability and implicated in cell shape control. EMBO J. 1993;12:1987–1995. doi: 10.1002/j.1460-2075.1993.tb05848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Tabancay A. P., Jr, Urano J., Tamanoi F. Failure to farnesylate Rheb protein contributes to the enrichment of G0/G1 phase cells in the Schizosaccharomyces pombe farnesyltransferase mutant. Mol. Microbiol. 2001;41:1339–1347. doi: 10.1046/j.1365-2958.2001.02599.x. [DOI] [PubMed] [Google Scholar]
- Yang W., Urano J., Tamanoi F. Protein farnesylation is critical for maintaining normal cell morphology and canavanine resistance in Schizosaccharomyces pombe. J. Biol. Chem. 2000;275:429–438. doi: 10.1074/jbc.275.1.429. [DOI] [PubMed] [Google Scholar]
- Zaitsevskaya-Carter T., Cooper J. A. Spm1, a stress-activated MAP kinase that regulates morphogenesis in S. pombe. EMBO J. 1997;16:1318–1331. doi: 10.1093/emboj/16.6.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F. L., Casey P. J. Protein prenylation: molecular mechanisms and functional consequences. Annu. Rev. Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Sugiura R., Lu Y., Asami M., Maeda T., Itoh T., Takenawa T., Shuntoh H., Kuno T. Phosphatidylinositol 4-phosphate 5-kinase Its3 and calcineurin Ppb1 coordinately regulate cytokinesis in fission yeast. J. Biol. Chem. 2000;275:35600–35606. doi: 10.1074/jbc.M005575200. [DOI] [PubMed] [Google Scholar]