Abstract
Phosphoprotein enriched in astrocytes-15 kDa (PEA-15), a phosphoprotein enriched in astrocytes, inhibits both apoptosis and proliferation in normal and cancerous cells. Here, analysis of PEA-15 expression in glioblastoma organotypic cultures revealed low levels of PEA-15 in tumor cells migrating away from the explants, regardless of the expression levels in the originating explants. Because glioblastomas are highly invasive primary brain tumors that can originate from astrocytes, we explored the involvement of PEA-15 in the control of astrocyte migration. PEA-15−/− astrocytes presented an enhanced motility in vitro compared with their wild-type counterparts. Accordingly, NIH-3T3 cells transfected by green fluorescent protein-PEA-15 displayed a reduced migration. Reexpression of PEA-15 restored PEA-15−/− astrocyte motility to wild-type levels. Pharmacological manipulations excluded a participation of extracellular signal-regulated kinase/mitogen-activated protein kinase, phosphatidylinositol 3-kinase/Akt, and calcium/calmodulin-dependent protein kinase II in this effect of PEA-15. In contrast, treatment by bisindolylmaleimide, Gö6976, and rottlerin, and chronic application of phorbol 12-myristate 13-acetate and/or bryostatin-1 indicated that PKCδ mediated PEA-15 inhibition of astrocyte migration. PEA-15−/− astrocytes constitutively expressed a 40-kDa form of PKCδ that was down-regulated upon PEA-15 reexpression. Together, these data reveal a new function for PEA-15 in the inhibitory control of astrocyte motility through a PKCδ-dependent pathway involving the constitutive expression of a catalytic fragment of PKCδ.
INTRODUCTION
Astrocytes are one of the cell types from which gliomas, the main primitive brain tumors of adulthood originate (Kleihues et al., 2002). In contrast to astrocytes, gliomal cells exhibit high motility. Their ability to infiltrate rapidly the normal brain parenchyma limits the efficacy of surgical resection and targeted radiotherapy. Thus, understanding the intracellular mechanisms underlying cell migration within the CNS is of great importance for prognosis and development of therapeutics.
Control of cell motility has been shown to depend on protein kinase C (PKC) activity. PKCs form a family of at least 11 isoforms. Based on their structural and biochemical properties, these PKC isoforms can be divided into three major groups: 1) classical PKCs (α, β1, β2, and γ), which are activated by diacylglycerol (DAG) and are Ca2+ dependent; 2) novel PKCs (nPKC: δ, ε, η, θ, and μ), which are activated by DAG but are Ca2+ independent; and 3) the atypical PKCs (ζ, λ, and ι), which do not respond to either DAG or calcium. PKC isozymes have been implicated in the three independent but highly coordinated cellular processes involved during tumor cell migration: 1) cell attachment to extracellular matrix or basement membrane (Fagerholm et al., 2002); 2) cell motility, which involves the reorganization of the actin cytoskeleton (Iwabu et al., 2004); and 3) cell invasion, through extracellular matrix degradation by proteolytic enzymes (Woo et al., 2004). Accordingly, inhibition of PKC has been shown to inhibit cell motility and invasiveness (Kermorgant et al., 2001). Interestingly, phosphoprotein enriched in astrocytes-15 kDa (PEA-15), a major small cytoplasmic astrocytic phosphoprotein, is a PKC substrate. Furthermore, PEA-15–induced insulin resistance in type 2 diabetes results from a dysregulation of the balance between the activities of PKCα and ζ (Condorelli et al., 2001).
PEA-15 was first identified as an abundant phosphoprotein in brain astrocytes (Araujo et al., 1993); subsequently, it was shown to be widely expressed in different tissues and highly conserved among vertebrates (Danziger et al., 1995; Estelles et al., 1996). It is composed of an N-terminal death effector domain and a C-terminal tail of irregular structure that contains the serines phosphorylated by PKC (S104) or by Akt/calcium/calmodulin-dependent protein kinase II (CaMKII) (S116) (Renault et al., 2003). PEA-15 inhibits apoptosis (Estelles et al., 1999; Condorelli et al., 1999; Kitsberg et al., 1999; Hao et al., 2001). In human malignant glioma cell lines, PEA-15 expression and phosphorylation on its PKC site are required for resistance to tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis (Hao et al., 2001). PEA-15 also modifies extracellular signal-regulated kinase (ERK) signaling by controlling ERK subcellular localization (Formstecher et al., 2001; Whitehurst et al., 2004) and thereby restricts cell proliferation. Recently, the phosphorylation of PEA-15 was reported to determine whether PEA-15 binds ERK or FADD (Krueger et al., 2005; Renganathan et al., 2005). Therefore, PEA-15 occurs as a regulator protein that controls cell apoptosis as well as proliferation, two cell functions dysregulated in cancer. PEA-15 involvement in cancer is complex. In transformed and metastatic murine squamous carcinoma cells, the pea-15 gene is up-regulated (Dong et al., 2001) and PEA-15 overexpression favors skin tumors (Formisano et al., 2005). In contrast, a tumor suppressor function was recently reported for PEA-15 in cellular models of breast and ovary tumors (Gaumont-Leclerc et al., 2004; Bartholomeusz et al., 2006). This led us to investigate human tumors arising from astrocytes.
Here, examining human glioblastomas, we observed ex vivo that cells migrating away from the tumor core express low levels of PEA-15, regardless of the expression level in the original tumor. This prompted us to explore the role of PEA-15 in the motility of astrocytes. We report that loss of PEA-15 expression results in an increased astrocyte motility by a PKCδ-dependent mechanism related to the constitutive expression of a novel 40-kDa form of PKCδ.
MATERIALS AND METHODS
Reagents
Phorbol 12-myristate 13-acetate (PMA), KN-62, U0126, LY294002, Gö6976, ZVAD, and DEVD were from Calbiochem (San Diego, CA). Fibronectin from human plasma, leptomycin B, aphidicolin from Nigrospora sphaerica, rottlerin, and bryostatin-1 were from Sigma (Lyon, France). Recombinant human transforming growth factor (TGF)α was obtained from AbCys (Paris, France). PEA-15 (Ab-7) rabbit polyclonal antibody has been characterized previously (Sharif et al., 2004). PKCδ (C20), PKCε (C15), PKCη (C15), PKCμ (C20), and PKCθ (C18) polyclonal antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). PKCε mouse monoclonal antibody (clone 21, ref 610085) was from BD Biosciences Transduction Laboratories (Lille, France). PKCθ mouse antibody (2059), phospho-PKCδ (Thr505), myosin light chain (MLC) and phospho-MLC (serine 19) antibodies were from Cell Signaling Technology (Ozyme, St. Quentin-en-Yvelines, France). Mouse green fluorescent protein (GFP) antibody was from Roche Diagnostics (Mannheim, Germany). MIB (Ki-67) and glial fibrillary acidic protein antibodies were from Dako Denmark (Glostrup, Denmark).
Plasmids
GFP-PKCδ Construct. Rat PKCδ cDNA was provided by Prof. Peter Parker (CRUK Institute for Cancer Studies, London Research Institute, London, United Kingdom). Rat PKCδ open reading frame was amplified by polymerase chain reaction (PCR) by using 5′-CGGAATTCATGGCACCGTTCCTGCGCA-3′ as forward primer and 5′-GGGGGTACCCTATTCCAGGAATTGCTC-3′ as reverse primer. The product of PCR was then digested with EcoRI and KpnI restriction enzymes and was cloned into pEGFP C2 vector (Clonetech, Mountain View, CA). GFP-PEA-15 (Kitsberg et al., 1999) and GFP-CF-PKCδ (DeVries et al., 2002) have been described previously.
Cell Culture
Glial cultures were prepared as described previously (Araujo et al., 1993). Briefly, culture dishes were coated with 1.5 μg/ml poly-l-ornithine (mol. wt. 40,000; Sigma). Striatal and cortical cells from 1-d-postnatal wild-type or PEA-15−/− mice were dissociated and plated in culture defined minimal essential medium (MEM)-F-12 medium consisting of a 1:1 mixture of MEM and F-12 nutrient (Invitrogen, Cergy Pontoise, France) supplemented with 33 mM glucose, pH 7.4, 2 mM glutamine, 3 mM sodium bicarbonate, 5 mM HEPES, 50 IU/ml penicillin, 5 μg/ml streptomycin (all from Invitrogen), and 10% fetal bovine serum (Perbio Science France, Brebières, France). The culture medium was changed every 3 d. When cells were almost confluent, around day 7, cells were treated by 5 μM cytosine arabinoside for 2 d.
Tumor Explants Collection and Culture
Glioblastomas (n = 11) were collected by an anatomopathologist in the surgical room and examined immediately by smears technique (Beuvon et al., 2000) to make sure that sample contained a majority of tumor cells. These samples were either directly fixed by formolzinc solution (formaldehyde 2% with 8 g/l NaCl, 3 g/l ZnSO4) for an histopathological control or cut by 1 cm3 in a transport medium and then minced in 1-mm3 fragments and maintained on surgical sponge of gelatin (Gelfoam; Pharmacia and Upjohn, Guyancourt, France; also available upon request) in RPMI 1640 medium (Sigma) containing 2 g/l sodium bicarbonate, 100 IU/ml penicillin, 100 IU/ml streptomycin supplemented with 6% fetal calf serum at 37°C, and 5% CO2 from 10 to 30 d. The blocks of gelatin containing tumor explant were then fixed and paraffin embedded, before being cut into 4-μm-thick sections by using a microtome (Microm HM340E; Electron Microscopy Sciences, Fort Washington, PA).
Incubation of the Ab-7 Antibody with Glutathione S-Transferase (GST) or GST-PEA-15 Proteins
PEA-15 Ab-7 antibody diluted 1/1000 in phosphate-buffered saline (PBS) buffer (without Ca2+ or Mg2+, pH 7.4; Sigma) was incubated 24 h at 4°C, with rotation, with 1.5 μg of GST-PEA-15 or GST adsorbed on glutathione-Sepharose beads.
Immunohistochemistry
Tissue sections were first deparaffinized before being incubated in 0.3% H2O2 in methanol for 5 min. Tissue sections were then washed twice in PBS before being immunostained for 1 h at room temperature with the indicated antibodies diluted in PBS containing 0.3% Triton X-100. Immunohistochemical detection was achieved using the avidin–biotin complex immunoperoxidase technique and the diaminobenzidine chromogen (Vector Laboratories, Burlingame, CA). Hematoxylin and eosin (Mayer hematoxylin; Merck, Darmstadt, Germany) staining was then performed to visualize cells nuclei and bodies, respectively.
Immunocytochemistry and Confocal Laser Scanning Microscopy
PEA-15 immunocytochemistry was performed as described previously (Sharif et al., 2004). Actin staining was performed using phalloidin coupled to Alexa 488 (Invitrogen). Nuclei were stained with TO-PRO-3 iodide (Invitrogen) according to manufacturer's specifications. Cells were examined using a Leica TS2 (Leica, Wetzlar, Germany) confocal microscope with appropriate filters.
Wound Scratch Assay
Astrocytes were replated between day 11 and 13 in vitro on polyornithine-coated 12-well culture dishes (Falcon; BD Biosciences Discovery Labware, Bedford, MA). The next day, a scratch was done in the confluent monolayer by using a sterile pipette tip. Then, cells were washed three times with PBS buffer (without Ca2+ or Mg2+, pH 7.4; Sigma) and replaced in MEM-F-12 medium supplemented with 10% fetal calf serum or 50 ng/ml TGFα for the indicated times. Pictures of marked fields were taken using an inverted phase contrast microscope (Nikon Diaphot) at different time intervals. By using Lucia software (Laboratory Imaging, Hostiva, Czek Republic), areas of fields that were not yet colonized by cells were measured.
Astrocyte Adhesion Assay
Immulon-2 96-well plates were coated overnight (4°C) with 10 μg/ml human fibronectin. Uncoated control wells were blocked with 2% heat-inactivated bovine serum albumin (BSA) (Sigma) for 4 h (4°C). Wild-type and knockout astrocytes were subsequently added (2 × 105 cells/well) in serum-free media and incubated for 60 min (37°C). Wells were washed twice with PBS before be fixed for 20 min with 2% glutaraldehyde (Electron Microscopy Service Laboratories, Westmont, NJ). The fixed cells were washed once with PBS and then stained with 0.1% crystal violet (Serva Biochemicals, Hauppauge, NY) for 45 min. Adherent astrocytes were washed three times with PBS, solubilized in 0.1 N sodium citrate (Sigma) for 30 min, and absorbance at 595 nm was determined using an ELISA plate reader (Molecular Devices, Sunnyvale, CA). To determine 100% of cells for quantitation, a set of wells was fixed and stained without being washed. Data represent experiments done in triplicate.
Transwell Assay
The underside of the polycarbonate membranes of transwells (6.5 mm in diameter, 10 μm in thickness, 8-μm pores; Corning Life Sciences, Acton, MA) were coated with 10 μg/ml human fibronectin for 3 h at 37°C. Primary astrocytes were trypsinized at day 13 in vitro, stained with trypan blue, and counted using a hemocytometer. Cells (2 × 104) were plated onto the uncoated topside of the filter in MEM-F-12 medium in presence of drugs for 45 min (for 2 h with leptomycin B), before adding 50 ng/ml TGFα. The inferior well contained same concentrations of drugs and TGFα. After 12 h of migration at the incubator (37°C, 5% CO2), cells were fixed 20 min at room temperature with paraformaldehyde (4% in PBS, pH 7.5). The topside of the transwell was washed, and each filter was swabbed with a cotton-tipped applicator to remove cells that did not migrate through the filter. Cells were stained using Hoechst dye (sanofi-aventis, Bridgewater, NJ) according to manufacturer's specifications. Filters were cut out and mounted on slides in Fluoromount-G medium (Southern Biotech, United Kingdom, obtained through Cliniscience, Maubeuge, France). By using Lucia software (Laboratory Imaging) connected to an epifluorescence microscope, 15 fields per membrane were counted. For PMA-induced PKC down-regulation experiments, astrocytes were treated 48 h by 1 μM PMA before migration assay.
Purification of Protein Transducer Domain (PTD)-Fusion Proteins
PTD-PEA-15 and PTD-GFP constructs have been described previously (Caron et al., 2001; Embury et al., 2001). The isolation and purification were done as described previously (Embury et al., 2001). Titration of proteins obtained was performed comparing increasing volumes of PTD-fusion protein with standards of BSA loaded on a 15% polyacrylamide gel and then stained with Coomassie blue. Astrocytes were treated with 4 μM PTD-fusion proteins for 2 h before trypsinization and during the duration of migration.
Immunoblotting
After treatment, cells were rinsed twice with PBS and then scraped on ice in lysis buffer containing 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.5% NP-40, 2 mM EDTA, 2 mM EGTA, and 2 mM sodium orthovanadate, supplemented with phosphatase inhibitor cocktails 1 and 2 (Sigma) and Mini Complete protease inhibitor cocktail without EDTA (Roche Diagnostics) following manufacturer's specifications. Cell lysates were passed thrice through a 26-gauge needle and then centrifuged at 13,000 × g during 20 min at 4°C. After protein titration by microBCA protein assay (Pierce Chemical, Rockford, IL), supernatants were mixed with sample buffer (Laemli), boiled, and then separated on 10% polyacrylamide gels (8 and 13% for blotting PKCμ and PEA-15, respectively). After transfer to a polyvinylidene difluoride membrane (Immobilon-P; Millipore, Billerica, MA) blots were probed with indicated primary antibodies before visualizing with horseradish peroxidase-conjugated secondary antibodies followed by development with an enhanced ECL kit (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom).
RESULTS
Cells Migrating Away from Tumor Explants Maintained in Organotypic Cultures Express Low Levels of PEA-15
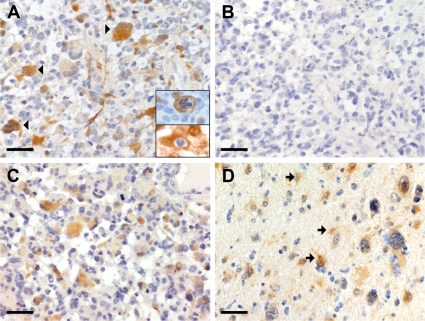
Histochemical staining of human glioblastomas (World Health Organization [WHO] grade IV) was performed using a specific antibody against PEA-15 that we recently characterized (Sharif et al., 2004). In the seven glioblastoma biopsies examined, a strong PEA-15-immunoreactive signal was observed in tumor cells of the tumor solid core (Figure 1A). However, in agreement with the well-known heterogeneity of glioblastomas, we also observed some areas of the tumor core that were not PEA-15 positive. The tumor cells were identified by a histopathologist with regard to their morphological characteristics and their positive MIB immunolabeling, the latter identifying proliferating cells (Figure 1A, inset). Specificity of this labeling was confirmed through the preabsorption of the PEA-15 antibody with a GST-PEA-15 fusion protein that suppressed any immunohistochemical staining (Figure 1B), whereas preabsorption with GST alone did not extinguish the signal (Figure 1C). In the parenchyma surrounding the tumor solid core, which corresponds to the infiltrative component of the tumor, both tumor cells and reactive astrocytes were positive for PEA-15 (Figure 1D). Whereas in reactive astrocytes the immunohistochemical staining was always localized in the cytoplasm, some tumoral cells exhibited in addition a nuclear localization of the signal. Because in these tumors only a few tumor cells exhibited a positive MIB immunolabeling, it remained difficult to identify every malignant cell inside the tumor infiltrative component, thus preventing the in vivo study of PEA-15 expression in tumor cells.
Figure 1.
PEA-15-immunohistochemical staining in human glioblastomas (WHO grade IV). PEA-15-immunoreactivity is indicated in brown. Hematoxylin and eosin counterstaining was used to label the cell cytoplasm (pink) and nucleus (blue). (A) PEA-15-immunoreactive tumor cells are observed within the tumor mass (arrowheads). Top inset, PEA-15-immunopositive cell (brown) that exhibits the typical appearance of malignancy. Note the nuclear anomalies. Bottom inset, PEA-15-immunopositive cell (brown) that exhibits a positive nuclear immunolabeling for MIB (blue), without any counterstaining. (B) Immunohistochemical staining of a section adjacent to A with the PEA-15 antibody preincubated with GST-PEA-15. Note the total lack of immunoreactive signal demonstrating the specificity of the Ab-7 antibody in human. (C) Immunohistochemical staining of a section adjacent to A and C with the PEA-15 antibody preincubated with GST alone. Note the maintenance of the immunoreactive signal. (D) Reactive astrocytes (arrows) exhibiting PEA-15 immunolabeling of their cytoplasm in the peritumoral infiltration zone. Bar, 100 μm.
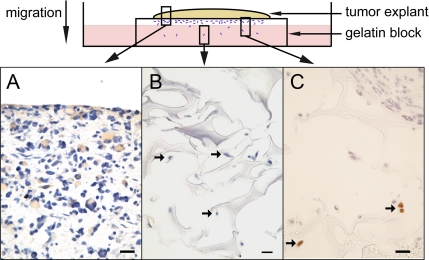
Further immunohistochemical analysis was thus performed on human glioma explants grown at the top of surgical sponges from 10 to 30 d. This model confirmed that initial explants were largely composed of tumor cells. Moreover, such organotypic cultures have the advantage that they preserve the histological integrity of the tumor for several weeks (Rubinstein et al., 1973), whereas they allow monitoring of the robust invasive capacities of the tumor cells that characterize high-grade gliomas (Sorour et al., 1975). Accordingly, tumor cells, identified by their MIB-positive immunolabeling (Figure 2C), migrated away from the explant and infiltrated the depths of the sponges in 11 of the 12 samples studied. Macrophages, distinguished from tumor cells by their very different morphology (round and voluminous cytoplasm) as well as their CD68-immunohistochemical staining also infiltrated the depths of the sponges, as observed in tumors in vivo (our unpublished data). The level of the PEA-15–immunoreactive signal in the glioma explants varied from high to low, but it was always detectable (Figure 2A). Regardless of the intensity of the PEA-15–immunoreactive signal in the explant, all cells migrating away exhibited weak or no PEA-15 immunoreactivity (Figure 2B). These observations led us to test whether PEA-15 expression can alter cell motility.
Figure 2.
Migrating tumor cells express low levels of PEA-15, regardless of the expression levels in the originating tumors. Localization of A, B, and C is indicated by boxes in the schematic description of the experimental model. (A) Example of PEA15-immunoreactive tumoral cells (brown) in explants of glioblastomas maintained in organotypic culture for 30 d. (B) Lack of PEA-15 immunoreactivity in cells migrating away (arrows) from the explant depicted in A. (C) Tumoral nature of cells (arrows) migrating from glioblastoma explant, demonstrated according to their MIB immunolabeling (brown). Cell bodies are colored by eosin in pink, and nuclei by hematoxylin in blue. Bar, 100 μm.
The Lack of PEA-15 Expression Enhances Migration of Normal Astrocytes
Astrocytes constitute one of the cell types from which gliomas arise and are known to express high levels of PEA-15 protein (Araujo et al., 1993). We thus compared motility of astrocytes grown in primary culture from either PEA-15 knockout or wild-type mice.
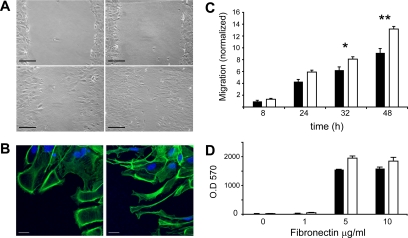
Cellular motility was first analyzed at the edge of a scratch wound made across an astrocyte monolayer in the presence of fetal calf serum (FCS). As expected, wounding induced migration of the remaining cell sheet into the gap (Figure 3A). Wild-type astrocytes extended lamellipodial protrusions in the direction of migration (Figure 3B, left). Wounding induced much more drastic morphological changes in PEA-15−/− astrocytes, which emitted long oriented protrusions (Figure 3B, right). PEA-15−/− astrocytes recolonized the wound faster than their wild-type counterparts (Figures 3A, right, and C) (p < 0.05 at 32 h and p < 0.001 at 48 h).
Figure 3.
Accelerated wound healing in astrocyte monolayers lacking PEA-15 expression. (A) Confluent monolayers of wild-type (left column) or PEA-15−/− astrocytes (right column) were scratched. The recolonization of wounded areas was studied using an inverted phase-contrast microscope from t = 0 (top) to 48 h (bottom). Images shown are representative of three independent experiments. Bars, 100 μm. (B) Actin staining (green) using phalloidin reveals that wounding induces lamellipodia extension in wild-type astrocytes (left), whereas the morphology of PEA-15−/− astrocytes is distinct, with cells emitting long protrusions (right picture). Nuclei were stained (blue) with TO-PRO3 iodide. Bars, 20 μm. (C) Quantitation of mean distances moved by migrating cell leading edge at different intervals of time (black bars, wild-type astrocytes; white bars, PEA-15−/− astrocytes). Normalization is based on the value of wild-type astrocyte migration at t = 8 h. Data shown are mean + SD of one experiment representative of three independent experiments (n = 10, *p < 0.05, **p < 0.01. Two-way analysis of variance (ANOVA) followed by Bonferroni posttest). (D) Wild-type (black bars) and PEA-15−/− astrocytes (white bars) adhesion on various concentrations of fibronectin is similar.
Because the difference between the migratory capabilities of wild-type and PEA-15−/− astrocytes may result from changes in adhesion properties, adhesion of wild-type and PEA-15−/− astrocytes on various concentrations of fibronectin was analyzed revealing no difference (Figure 3D).
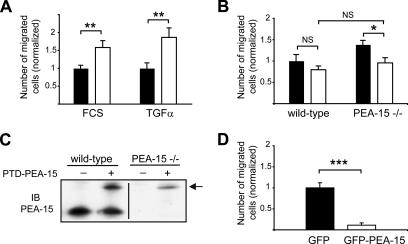
Because some discrepancies concerning the effect of other proteins on migration, e.g., p27 (Assoian, 2004), were likely related to the experimental procedures used, we further confirmed the PEA-15 effect on astrocyte migration, by using another migration assay, the transwell assay. In the transwell assay, in agreement with the results obtained using the wound scratch assay, in the presence of 10% FCS PEA-15−/− astrocytes presented a significant 1.6 ± 0.58-fold enhanced migration compared with wild-type astrocytes (Figure 4A). On a second type of stimulation, in the presence of 50 ng/ml TGFα, which has been previously described as a strong stimulator of astrocyte motility (Faber-Elman et al., 1996), PEA-15−/− astrocytes migrated 1.9 ± 0.65-fold more than their wild-type counterparts (Figure 4A). Considering the more robust effect of PEA-15 in the presence of TGFα, further studies were done using TGFα as a migration inducer. Whereas the wound assay mimics a directed migration of cells still contacting their neighboring cells, the transwell assay, which requires trypsinization of cells, measures haptokinesis toward fibronectin of independent cells. The influence of PEA-15 on cell migration thus seems to be relatively independent of the extracellular context. The transwell assay was used for further studies because this migration assay reveals a difference of migratory capability between both types of astrocytes in less time (12 h) compared with the wound scratch assay.
Figure 4.
PEA-15 expression controls cell migration in a transwell assay. (A) PEA-15−/− astrocytes (white bars) show an enhanced migration in comparison with wild-type astrocytes (black bars) in the transwell assay in the presence of either 10% FCS or 50 ng/ml human recombinant TGFα. (B) Wild-type or PEA-15−/− astrocytes were treated with 4 μM PTD-PEA-15 (white bars) or PTD-GFP (black bars) assay. Note that PTD-PEA-15 treatment reduces significantly the migration of PEA-15−/− astrocytes, whereas having a minor effect on wild-type astrocytes. (C) Transduction by PTD-PEA-15 led to PEA-15 reexpression in wild-type and PEA-15−/− astrocytes. Note that the PTD-PEA-15 fusion protein (arrow) has an enhanced apparent molecular weight in comparison with endogenous PEA-15. The line on the image indicates where an irrelevant section has been deleted. (D) Transient transfection by GFP-PEA-15 drastically decreases migration of NIH-3T3 cells demonstrating that the control exerted by PEA-15 on cell migration is not restricted to astrocytes. Cell migration assays are done in the presence of 50 ng/ml TGFα, in triplicate and data shown are mean ± SEM of three independent experiments (n = 9; NS, not statistically significant; *p < 0.05, **p < 0.01, ***p < 0.001, two-way ANOVA analysis and Bonferroni posttest).
Migration is a highly integrated cellular process composed of multiple coordinated events involving a great number of molecules (Lauffenburger and Horwitz, 1996). It was therefore necessary to rule out the possibility that the enhanced migration of PEA-15−/− astrocytes was related to an epiphenomenon, rather than to a direct effect of PEA-15. We thus analyzed PEA-15−/− astrocyte motility by using the transwell assay after reintroducing PEA-15. The poor efficiency of cDNA transfection for astrocytes in primary cultures led us to restore PEA-15 expression by using a fusion protein composed of the PTD and PEA-15 (PTD-PEA-15) (Embury et al., 2001). The 11 amino acids of the PTD sequence allow the transduction into living cells of fused proteins dissolved in aqueous solution (Schwarze et al., 1999). Neither the fusion protein control PTD-GFP, at any concentration used, nor a low dose of 0.4 μM PTD-PEA-15 had a significant effect on astrocyte motility. Treatment by 4 μM PTD-PEA-15, although having no significant effect on wild-type astrocyte migration, led to a major reduction of the extent of the migration of PEA-15−/− astrocytes (Figure 4B). Western blotting experiments confirmed the efficiency of the transduction by PTD-PEA-15 (Figure 4C). It is noteworthy that although the theoretical weight of PTD-PEA-15 is 16 kDa, the apparent molecular weight on a polyacrylamide gel of the protein is 26 kDa, probably due to the presence of numerous charged amino acids in the PTD sequence. Together these data demonstrate that expression of PEA-15 inhibits astrocyte motility. In addition, transient transfection of NIH-3T3 cells by GFP-PEA-15 cDNA drastically reduced migration on transwell assay, whereas transfection by GFP alone had no effect (Figure 4D), thus indicating that the control exerted by PEA-15 on motility is not restricted to astrocytes.
The Antiproliferative and Antimigratory Functions of PEA-15 Are Distinct
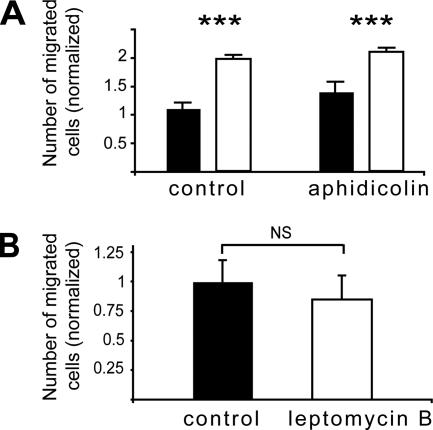
PEA-15 inhibits astrocyte proliferation (Formstecher et al., 2001). The increased number of migrating PEA-15−/− astrocytes could thus be a consequence of increased cell division. To investigate this hypothesis, migration assays were done in the presence of 10 μg/ml aphidicholin, a mitotic inhibitor reported to have no effect on the motility of astrocytes stimulated by basic fibroblast growth factor (Milner et al., 1999). 5-Bromo-2-deoxyuridine incorporation experiments confirmed that aphidicholin completely inhibited both wild-type and PEA-15−/− astrocyte proliferation for at least 48 h (our unpublished data), a period of time longer than the duration of the transwell experiments (12 h). Inhibition of proliferation by aphidicholin did not alter the migration of either wild-type or PEA-15−/− astrocytes (Figure 5A). This result demonstrates that the enhanced migration of PEA-15−/− astrocytes is not a consequence of their increased proliferation.
Figure 5.
The control of astrocyte migration by PEA-15 is independent of its control of proliferation. (A) Migration of wild-type (black bars) and PEA-15−/− astrocytes (white bars) in the presence of an antimitotic, 10 μg/ml aphidicolin, does not alter the enhanced migration of PEA-15−/− astrocytes. (B) The effect of PEA-15 on migration is independent of the nuclear export machinery. The inhibition of the nuclear export protein CRM1, by 10 ng/ml leptomycin B, does not modify the migration of wild-type astrocytes. Cell migration assays are done in the presence of 50 ng/ml TGFα, in triplicates and data shown are mean + SEM of three independent experiments (n = 9; NS, not statistically significant; ***p < 0.001, two-way ANOVA analysis and Bonferroni posttest).
The Effect of PEA-15 on Migration Is Independent of the Nuclear Export Machinery
PEA-15 blocks ERK-dependent proliferation by binding ERK1/2 and preventing their prolonged localization in the nucleus (Formstecher et al., 2001). PEA-15 contains a nuclear export sequence required for its capacity to dynamically localize ERK in the cytoplasm (Formstecher et al., 2001). As reported previously, treatment of wild-type astrocytes with 10 ng/ml leptomycin B, a potent inhibitor of the nuclear export machinery protein CRM1 (Nishi et al., 1994; Ossareh-Nazari et al., 1997) led to the relocalization of PEA-15 in the nucleus, as observed by confocal microscopy (our unpublished data). In the presence of leptomycin B, the motility of wild-type astrocytes was not modified (Figure 5B). It thus seems that the mechanisms whereby PEA-15 controls cell migration differ from those involved in its control of cell proliferation.
ERK/Mitogen-activated Protein Kinase (MAPK), Phosphatidylinositol 3-Kinase/Akt, and CaMKII Are Not Required for the PEA-15 Effect on Migration
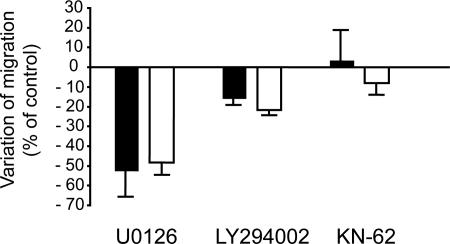
Our cell motility assays use FCS or TGFα as inducers of motility. Both FCS and TGFα are known to activate MAPK/ERK and PI3K/Akt pathways. We therefore tested whether these pathways are involved in the effect of PEA-15 on cell migration. The presence of 10 μM U0126, a specific inhibitor of mitogen-activated protein kinase kinase (Favata et al., 1998) during migration experiments reduced similarly by ∼50% the migration extent of both cell types (Figure 6) (52 ± 13.5 and 48 ± 6.0% for wild-type and PEA-15−/− astrocytes, respectively). Therefore, the migration of astrocytes in presence of TGFα depends on ERK/MAPK activity, but this signaling pathway is not involved in the specific effect of PEA-15. PI3K inhibition by 30 μM LY294002 decreased the migration extent of wild-type astrocytes by 15.4 ± 3.6% and did the same for PEA-15−/− astrocytes (−21.7% ± 2.5%) (Figure 6), demonstrating that Akt activity is also required for full astrocyte motility but that it does not interfere with the specific effect of PEA-15 on cell migration. Next, we tested the CaMKII, which can regulate cell migration (Pauly et al., 1995). The inhibition of CaMKII by 10 μM KN-62 did not significantly modify the migration of either wild-type or PEA-15−/− astrocytes. (Figure 6), suggesting that CaMKII is not involved in astrocyte motility.
Figure 6.
ERK, PI3K, and CaMKII activities are not required for PEA-15 inhibition of astrocyte migration. Inhibition of ERK/MAPK pathway (10 μM U0126), or PI3K pathway (30 μM LY294002) or CaMKII (10 μM KN-62) have similar effects on wild-type (black bars) and PEA-15−/− astrocytes (white bars), indicating that these pathways are not implicated in the effect of PEA-15 on astrocyte migration. Histograms represent variations relatives to respective controls. Cell migration assays are done in the presence of 50 ng/ml TGFα, in triplicates and data shown are mean + SEM of three independent experiments.
A Member of the Novel PKC Family Mediates PEA-15 Inhibition of Astrocyte Motility
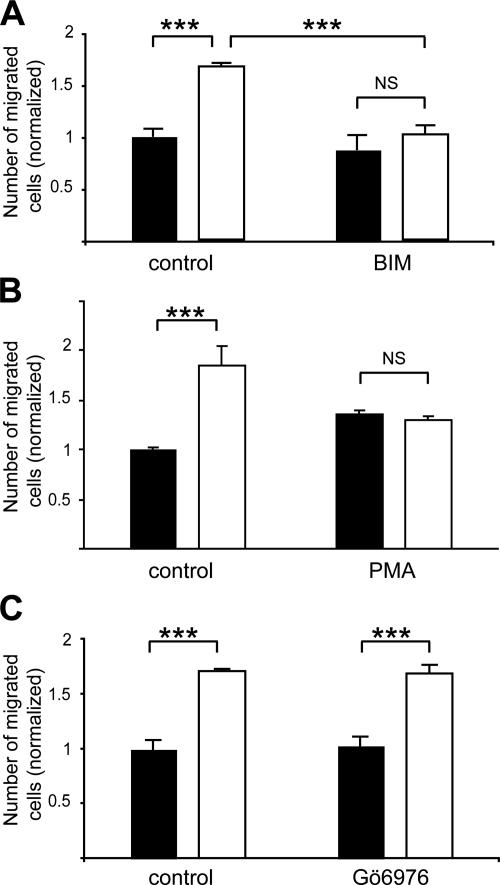
Members of the PKC family are known to modulate cell migration (Gomez et al., 1999). We thus investigated their role in the effect of PEA-15 on astrocyte migration. In the presence of 5 μM bisindoylmaleimide (BIM), a broad-spectrum inhibitor of PKC, the migration of PEA-15−/− astrocytes was reduced by 38.3 ± 11.9%, matching the extent of migration of wild-type astrocytes, whereas the migration of these latter was not significantly affected (Figure 7A). This result implicates a PKC in the enhanced migration observed for PEA-15−/− astrocytes. The isoforms of PKC are classified in three families based on their activation requirements. Long-term treatment (48 h) with 1 μM PMA is known to desensitize PKCs belonging to the classical and novel PKC families, through down-regulation of PKC protein levels (Blackshear, 1988). PMA treatment inhibited dramatically the migration of PEA-15−/− astrocytes by 45 ± 4.9% (Figure 7B), allowing PEA-15−/− astrocytes to return to the same migration rate as their wild-type counterparts. This result indicates that the PKC isoform implicated belongs to either the classical or the novel PKC family. Treatment of astrocytes with 100 nM Gö6976, previously shown to inhibit selectively the family of classical PKCs (Martiny-Baron et al., 1993), did not alter the migration of either PEA-15−/− or wild-type cells (Figure 7C), therefore excluding the participation of these classical PKCs and thereby demonstrating that PEA-15 inhibition of astrocyte motility is mediated by a PKC belonging to the novel PKC family.
Figure 7.
The control exerted by PEA-15 on astrocyte migration is mediated by an isoform of PKC belonging to the novel PKC family (black bars, wild-type; white bars, PEA-15−/−). (A) Broad-spectrum inhibition of PKCs by 5 μM BIM inhibits the enhanced migration of PEA-15−/− astrocytes, whereas not affecting the migration of wild-type astrocytes. (B) Down-regulation of classical and novel PKCs by long-term exposure to 1 μM PMA equalizes the migration of wild-type and PEA-15−/− astrocytes. (C) Isoforms of the classical PKC family are not implicated in the effect of PEA-15 on migration because inhibition of these isoforms by 100 nM Gö6976 does not affect the migration of either PEA-15−/− or wild-type astrocytes. Cell migration assays are done in the presence of 50 ng/ml TGFα, in triplicates, and data shown are mean + SEM of three independent experiments (n = 9; NS, not statistically significant; **p < 0.01, ***p < 0.001, two-way ANOVA analysis and Bonferroni posttest).
A 40-kDa Form of PKCδ Mediates PEA-15 Inhibition of Astrocyte Migration
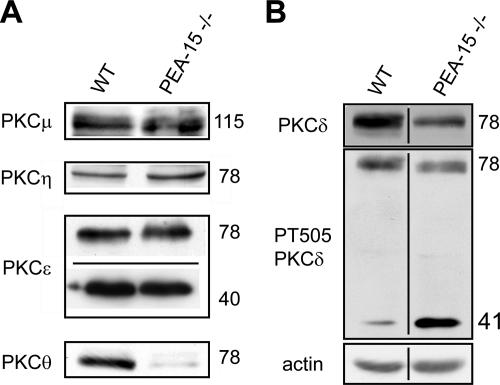
The expression of nPKC isoforms was therefore investigated in wild-type and PEA-15−/− astrocytes (Figure 8). Because cleavage of nPKC by various proteases can generate catalytic fragments of PKC around 40 kDa located in their C-terminal half (Schaap et al., 1990; Emoto et al., 1995; Datta et al., 1997; Endo et al., 2000), we therefore explored nPKC expression by using antibodies recognizing epitopes located in the C-terminal part of the enzymes. All nPKC isoforms were expressed in wild-type astrocytes. PKCε, PKCμ, and PKCη were similarly expressed in wild-type and PEA-15−/− astrocytes (Figure 8A). Western blot analysis revealed a weak down-regulation of endogenous 78-kDa PKCδ in PEA-15−/− astrocytes in comparison with their wild-type counterparts (ratio knockout/wild type = 67 ± 8%, n = 3; Figure 8B). In addition, we observed a strong down-regulation of PKCθ in PEA-15−/− astrocytes that was confirmed using a second antibody recognizing an epitope located in the N-terminal domain of the enzyme (our unpublished data).
Figure 8.
Expression patterns of novel PKCs display two major differences between wild-type and PEA-15−/− astrocytes. (A) Contrary to PKCμ, η, and ε that display similar expression, PKCθ is strongly down-regulated in PEA-15−/− astrocytes. The line on the image of PKCε Western blot indicates where an irrelevant section has been deleted. (B) Whereas the holoenzyme PKCδ is weakly down-regulated in PEA-15−/− astrocytes, please note the presence in PEA-15−/− astrocytes of a 40-kDa PKCδ exhibiting a strong phosphorylation on its threonine 505. The line on the image indicates where an irrelevant section has been deleted. Eighty micrograms of astrocyte lysates was loaded onto polyacrylamide gels and immunoblotted with indicated antibodies. Shown are representative blots of at least three repeats.
In both types of astrocytes, we noted the presence of two forms of PKCε, one isoform corresponding to the holoenzyme, and the other isoform corresponding to the low-molecular-weight C-terminal fragment. Regarding PKCδ, use of the antibody directed against the PKCδ phospho-threonine 505, located in the catalytic moiety, revealed the presence only in PEA-15−/− astrocytes of a 40-kDa form of PKCδ.
PKCδ and PKCθ have both been reported to control cell migration (Tang et al., 1997, 1999; Kruger and Reddy, 2003; Li et al., 2003; Iwabu et al., 2004); therefore, we determined their implication in the control exerted by PEA-15 on astrocyte migration.
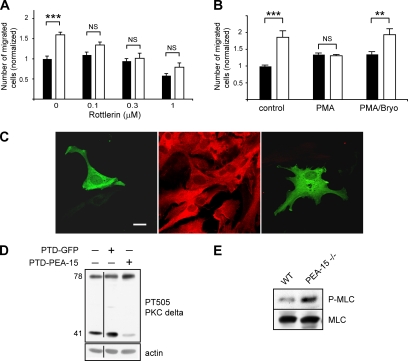
We first performed dose-response experiments in presence of rottlerin, initially described as a specific inhibitor of PKCδ (Gschwendt et al., 1994) that was further shown to inhibit PKCθ (Villalba et al., 1999). Very low doses of rottlerin, 0.1 and 0.3 μM, had a significant inhibitory effect only on the migration of PEA-15−/− astrocytes, and they abolished the difference between wild-type and PEA-15−/− astrocyte motilities (Figure 9A). The down-regulation of PKCθ in PEA-15−/− astrocytes implies that the enhanced motility of PEA-15−/− astrocytes relies on an increased enzymatic activity associated to PKCδ. This was further confirmed by coapplication of 300 nM bryostatin-1, known to specifically protect PKCδ during PMA-induced down-regulation, contrary to all other PMA-sensitive PKC isoforms that are down-regulated (Szallasi et al., 1994a, b; Lu et al., 1997). In this condition, PEA-15−/− astrocytes conserved their enhanced migration (Figure 9B). Together, these results indicate that PEA-15 inhibits astrocyte migration by inhibiting a PKCδ-dependent stimulatory pathway.
Figure 9.
Evidence for an involvement of PKCδ in the inhibitory control exerted by PEA-15 on astrocyte motility. (A) Low doses of rottlerin decrease significantly the enhanced migration of PEA-15−/− astrocytes (white bars), whereas not affecting significantly the motility of wild-type astrocytes (black bars), thus abrogating the difference in migration between PEA-15−/− astrocytes and wild-type astrocytes. (B) Although PMA-induced down-regulation of novel and classical PKC isozymes equalizes the migration of wild-type and PEA-15−/− astrocytes, note that specific protection of PKCδ down-regulation by coapplication of PMA and bryostatin-1 restores the enhanced migration of PEA-15−/− astrocytes but did not affect their wild-type counterparts. Cell migration assays are done in the presence of 50 ng/ml TGFα, in triplicates, and data shown are mean + SEM of three independent experiments (n = 9; NS, not statistically significant; **p < 0.01, ***p < 0.001, two-way ANOVA and Bonferroni posttest). (C) The localization of GFP-PKCδ in astrocytes is not affected by the expression of PEA-15. GFP-PKCδ is localized mainly in the cytoplasm of wild-type (left) and PEA-15−/− astrocytes (right), similarly to PEA-15 in wild-type astrocytes (middle). Bar, 15 μm. (D) Reexpression of PEA-15 in PEA-15−/− astrocytes by transduction of PTD-PEA-15 strongly down-regulates the 40-kDa form of PKCδ. The line on the image indicates where an irrelevant section has been deleted. (E) Phosphorylation of myosin light chain is strongly enhanced in PEA-15−/− astrocytes. Eighty micrograms of astrocyte lysates was loaded onto polyacrylamide gels and immunoblotted with indicated antibodies. Shown are representative blots of at least three repeats.
Because biological activity of a kinase results also from its intracellular localization, we verified the localization of PKCδ in wild-type and PEA-15−/− astrocytes. Probably because of low expression levels of PKCδ in astrocytes, we were unable to detect endogenous PKCδ by immunocytochemistry. We thus transfected GFP-PKCδ into wild-type and PEA-15−/− astrocytes and confocal analysis revealed a mainly cytoplasmic localization in both cases (Figure 9C). In addition, GFP-PKCδ did not colocalize with the cytoskeletal proteins actin and tubulin or with the focal adhesion protein vinculin (our unpublished data).
To more precisely test the involvement of the 40-kDa form of PKCδ observed in PEA-15−/− astrocytes in their enhanced migration, we examined the effect of PTD-PEA-15 transduction of PEA-15−/− astrocytes. Whereas treatment with PTD-GFP had no effect, reexpression of PEA-15 led to the total disappearance of the 40-kDa form of PKCδ in PEA-15−/− astrocytes (Figure 9D).
Phosphorylation of the threonine 505 located in the activation loop is considered as a reliable marker of PKCδ activation (Stempka et al., 1999; Rybin et al., 2003). Lack of a specific immunoprecipitating antibody against the 40-kDa isoform prevented a direct kinase assay to precisely quantify the enzymatic activity of the 40-kDa-PKCδ in PEA-15−/− astrocytes lysates. We next tried to quantify the total PKCδ enzymatic activity in astrocyte lysates to demonstrate a global increase of the enzymatic activity of PKCδ in PEA-15−/− astrocytes. Unfortunately, we were unable to immunoprecipitate the protein from astrocyte lysates, thus preventing the determination of this enzymatic activity. This was probably due to the low endogenous expression level of PKCδ in astrocytes, since we were able to immunoprecipitate PKCδ from lysates of Chinese hamster ovary cells overexpressing PKCδ (our unpublished data), wherein the catalytic fragment of PKCδ is not present.
We thus searched for indirect evidence of modified PKCδ activity in PEA-15−/− cells. PKCδ has been shown to mediate a signaling cascade during epidermal growth factor (EGF)-induced cell migration, leading to the phosphorylation of MLC on its serine 19 (Iwabu et al., 2004). Accordingly, we observed by Western blotting an enhanced phosphorylation of the MLC on its serine 19 in PEA-15−/− astrocytes in comparison with their wild-type counterparts (Figure 9E).
Together, these results strongly suggest that the presence of the 40-kDa form of PKCδ in PEA-15−/− astrocytes is responsible for the enhanced migration of these cells.
A 41-kDa fragment of PKCδ (catalytic fragment [CF]-PKCδ) exhibiting a constitutive enzymatic activity has been reported after induction of apoptosis (Emoto et al., 1995; Ghayur et al., 1996; Steinberg, 2004). CF-PKCδ results from caspase 3-dependent cleavage of the holoenzyme and is proapoptotic. We transfected wild-type and PEA-15−/− astrocytes with a cDNA encoding this CF-PKCδ fused to GFP. Both types of astrocytes died after apoptosis (our unpublished data). Contrary to CF-PKCδ, the 40-kDa PKCδ observed is constitutively expressed in PEA-15−/− astrocytes, and no sign of apoptosis was observed, suggesting they represent two different forms of the kinase.
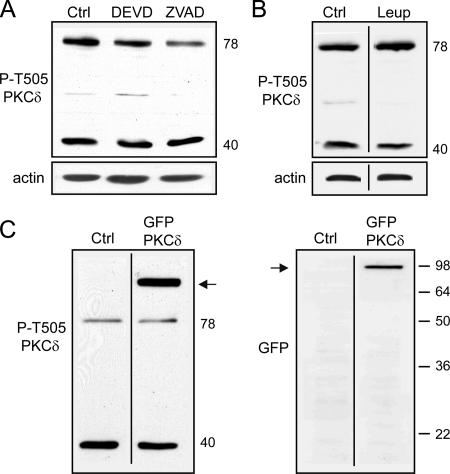
We envisaged that the 40-kDa PKCδ expressed in PEA-15−/− astrocytes resulted from a cleavage by a caspase. PEA-15−/− cells were treated by a caspase 3 inhibitor, DEVD (50 μM), or a pan-caspase inhibitor, ZVAD (50 μM), during 12 h, a time sufficient for the disappearance of this fragment upon treatment by PTD-PEA-15 (Figure 9D). Both specific caspase 3 and broad-spectrum caspase inhibition did not modify the expression level of the 40-kDa PKCδ in PEA-15−/− astrocytes (Figure 10A), whereas the strong decrease of the 40-kDa fragment of PKCε observed in both types of astrocytes was used as an internal control of inhibitors efficiency (our unpublished data). Furthermore, broad-spectrum inhibition of serine and thiol proteases by 100 μM leupeptin had no effect on the expression level of the 40-kDa PKCδ in PEA-15−/− astrocytes (Figure 10B). These results strongly suggested an absence of cleavage of PKCδ in PEA-15−/− astrocytes. To further test this hypothesis, we transfected a cDNA encoding GFP-PKCδ, wherein the GFP is fused to the N-terminal extremity of PKCδ. No cleaved form of GFP-PKCδ could be detected up to 72 h posttransfection in PEA-15−/− astrocytes (Figure 10C).
Figure 10.
The 40-kDa form of PKCδ expressed in PEA-15−/− astrocytes does not result from a proteolytic cleavage. (A) Specific inhibition of caspase 3 by 50 μM DEVD, or pan-caspase inhibition by 50 μM ZVAD, does not change the expression level of the 40-kDa form of PKCδ in PEA-15−/− astrocytes. (B) Broad-spectrum inhibition of serine and thiol proteases by 100 μM leupeptin had no effect on the expression level of the 40-kDa form of PKCδ in PEA-15−/− astrocytes. (C) Forty-eight hours after transfection by cDNA encoding GFP-PKCδ, wherein GFP is fused to the amino-terminal part of the PKCδ, PEA-15−/− astrocytes were lysed, and evidence for cleavage of the fusion protein was determined. Blotting using either an antibody recognizing an epitope located in the carboxy-terminal part of PKCδ (left blot), or an anti-GFP (right blot), did not evidence any short fragments of GFP-PKCδ (arrows). Eighty micrograms of astrocyte lysates was loaded onto polyacrylamide gels and immunoblotted with indicated antibodies. Shown are representative blots of at least three repeats. The lines in B and C indicate where irrelevant sections have been deleted.
Altogether, these results strongly suggest that the 40-kDa fragment of PKCδ observed in PEA-15−/− astrocytes does not result from a proteolytic cleavage.
DISCUSSION
Here, we describe a new role for PEA-15 as an inhibitor of astrocyte migration, one cell type thought to originate gliomas, highly invasive tumors. Reexpression of PEA-15 by using PTD–PEA-15 fusion protein restores the migration of PEA-15−/− astrocytes to wild-type levels, demonstrating that the loss of PEA-15 is indeed responsible for the increased motility of PEA-15−/− astrocytes. Moreover, transfecting NIH-3T3 fibroblasts with GFP-PEA-15, we demonstrated that the control exerted by PEA-15 on cell migration is not restricted to astrocytes.
Cell migration involves two sets of factors. Intracellular factors determine intrinsic cell properties, adhesion, and cell migration, whereas external factors, including extracellular matrix (ECM) and cell–cell interaction, control tissue permissivity to cell migration. A rich field of research revealed the complexity of brain ECM components as well as some of their remodeling during development or pathologies. For example, fibronectin deposited in the ECM of brain tumors strongly promotes the migration of glioma cells (Ohnishi et al., 1998). Interestingly, PEA-15 has been shown to block H-Ras–initiated inhibition of integrin activation (Ramos et al., 1998). PEA-15 binding to ERK is required for this modulation (Chou et al., 2003). Increased integrin activity is known to inhibit migration and would be consistent with PEA-15 inhibition of cell migration. However, comparing wild-type and PEA-15−/− astrocytes we have not seen any difference in integrin activation (Ramos, unpublished data), a result in agreement with the observation that both types of astrocytes have similar adhesion on fibronectin. Further studies should reveal if PEA-15 expression modulates cell migration differently according to the matrix composition.
Most of PKC isozymes have been implicated in cell migration. Western blotting experiments as well as pharmacological manipulations on PKC isozymes allowed us to demonstrate that PEA-15 inhibits astrocyte migration by a PKCδ-dependent mechanism. PKCδ has been particularly implicated in cell migration (Kruger and Reddy, 2003; Li et al., 2003; Iwabu et al., 2004). PKCδ is indeed responsible for a major part of the EGF-induced fibroblast contractile force generation, because RNA interference-mediated PKCδ depletion prevents MLC phosphorylation, which in turn promotes motility (Iwabu et al., 2004). Increased PKCδ has also been correlated with enhanced metastatic potential in mammary tumor cells (Kiley et al., 1999).
The expression of a 40-kDa form of PKCδ (40-kDa-PKCδ) in PEA-15−/− astrocytes seems to account for their enhanced migration. Indeed, reexpression of PEA-15 in PEA-15−/− astrocytes upon transduction with PTD-PEA-15 simultaneously decreased astrocyte migration and expression of this 40-kDa-PKCδ. The strong phosphorylation of threonine 505 of 40-kDa-PKCδ in PEA-15−/− astrocytes suggests a constitutively active form, because this phosphorylation has been reported as a reliable marker of PKCδ activation (Stempka et al., 1999; Rybin et al., 2003). The enhanced phosphorylation of myosin light chain in PEA-15−/− astrocytes is in agreement with an increased PKCδ activity. However, the biological effect of this 40-kDa-PKCδ may also result from phosphorylation of specific substrates that are not affected by the holoenzyme.
Contrary to the proapoptotic CF-PKCδ previously observed upon apoptosis induction (Steinberg, 2004), the 40-kDa-PKCδ is constitutively expressed in PEA-15−/− astrocytes. Although PEA-15−/− astrocytes exhibit an increased sensitivity to various inducers of apoptosis (Kitsberg et al., 1999; Renault et al., 2003), no sign of apoptosis was observed in the cells in any of the culture conditions used in this study. Furthermore, apoptosis was induced after transfection of PEA-15−/− astrocytes with GFP-CF-PKCδ, indicating that they did not develop a resistance to apoptosis and further supporting that the 40-kDa-PKCδ expressed in these cells differs from CF-PKCδ. Accordingly, whereas the CF-PKCδ results from the proteolytic cleavage of the holoenzyme by caspase 3 (Emoto et al., 1995; Ghayur et al., 1996), the lack of effect of the inhibition of various proteases as well as the absence of cleavage of transfected GFP-PKCδ in PEA-15−/− astrocytes suggest that the 40-kDa-PKCδ does not result from proteolytic cleavage. The size and the enzymatic activity of the 40-kDa-PKCδ indicate that it is not encoded by the two alternative splicing variants of PKCδ that have already been described in mammals (Ueyama et al., 2000; Sakurai et al., 2001). The molecular mechanisms linking PEA-15 expression and 40-kDa-PKCδ generation remain to be elucidated.
In L6 skeletal muscle cells, PEA-15 action on glucose transport is mediated by an enhanced activation of the classical PKCα that in turn inhibits PKCζ (Condorelli et al., 2001). In astrocytes migration, the inefficiency of Gö6976, the inhibitor of classical PKC, to reverse the difference between wild-type and PEA-15−/− astrocyte motilities, rules out the involvement of PKCα. It is noteworthy that, contrary to the desensitization of PKC induced by PMA, which is actually a down-regulation, the inhibition of PKC activity by BIM had no significant effect on wild-type astrocyte migration. This suggests that down-regulation of PKCα by PMA abrogates its inhibition of PKCζ, this latter isoform being known to stimulate astrocyte polarization (Etienne-Manneville and Hall, 2001, 2003) and cell migration (Crean et al., 2004; Petit et al., 2005). Contrary to PMA, BIM should inhibits both PKCα and PKCζ, although less efficiently for this latter, thus possibly explaining the different effects exerted by PMA and BIM on wild-type astrocyte migration. Whether PEA-15 expression modulates PKC signaling, i.e., PKCδ or PKCα, through a common molecular mechanism or whether it is specific of each isoform needs further investigation.
It remains also to be determined whether phosphorylation of PEA-15 can modulate its control of cell migration. The observation that CaMKII or PI3K/Akt inhibition does not interfere with PEA-15 effect on astrocyte migration suggests that phosphorylation of PEA-15 on its serine 116 is not involved. Because PEA-15 contains a serine substrate of PKC (serine 104) (Kubes et al., 1998), a cross-regulation between PKC and PEA-15 may tune astrocytes motility.
In consideration of the different known cellular functions of PEA-15 in different chronic disorders, including cancer (Eramo et al., 2005; Stassi et al., 2005) and diabetes (Condorelli et al., 1998; Condorelli et al., 2001; Vigliotta et al., 2004), the novel observation that PEA-15 inhibits cellular motility may have a very significant impact on future investigation of key areas such as metastasis and diabetes complications.
ACKNOWLEDGMENTS
We are grateful to Prof. Jacques Glowinski for constant support. We thank Amelia Dias-Morais and Joelle Lacombe for expert technical help in genotyping and immunohistochemistry experiments, respectively. We thank Dr. Mary Reyland for kind gift of GFP-CF-PKCδ construct, Dr. Ricardo Pastori for gift of PTD-PEA-15 construct, Dr. Jacques Bertoglio for gift of PTD-GFP construct, Dr. Denis Hervé for gift of BIM, and Eric Etienne for confocal assistance. We are grateful to Drs. Charles-Felix Calvo, Etienne Formstecher, Laurent Muller, and Catherine Monnot for fruitful discussions. This research was supported by the Association pour la Recherche contre la Cancer (ARC, Grants 3500 and 4621 to H.C.). F.R.M. is a recipient from study fellowships from French Ministry of Research and from the Académie Nationale de Médecine. J.W.R. is supported by National Cancer Institute Grant CA-93849 from the National Institutes of Health.
Abbreviations used:
- CaMKII
calcium/calmodulin-dependent protein kinase II
- CF-PKCδ
catalytic fragment of protein kinase Cδ
- ERK
extracellular signal-regulated kinase
- GST
glutathione S-transferase
- MAPK
mitogen-activated protein kinase
- MLC
myosin light chain
- PEA-15
phosphoprotein enriched in astrocytes-15 kDa
- PI3K
phosphatidylinositol 3-kinase
- PKC
protein kinase C
- PMA
phorbol 12-myristate 13-acetate
- PTD
protein transducer domain
- TGF
transforming growth factor
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-11-1072) on September 20, 2006.
REFERENCES
- Araujo H., Danziger N., Cordier J., Glowinski J., Chneiweiss H. Characterization of PEA-15, a major substrate for protein kinase C in astrocytes. J. Biol. Chem. 1993;268:5911–5920. [PubMed] [Google Scholar]
- Assoian R. K. Stopping and going with p27kip1. Dev. Cell. 2004;6:458–459. doi: 10.1016/s1534-5807(04)00103-0. [DOI] [PubMed] [Google Scholar]
- Bartholomeusz C., Itamochi H., Nitta M., Saya H., Ginsberg M. H., Ueno N.T. Antitumor effect of E1A in ovarian cancer by cytoplasmic sequestration of activated ERK by PEA15. Oncogene. 2006;25:79–90. doi: 10.1038/sj.onc.1209014. [DOI] [PubMed] [Google Scholar]
- Beuvon F., Varlet P., Fallet-Bianco C., Daumas-Duport C. The “smears” technique for the extemporaneous examination: diagnostic contribution to neurosurgical pathology. Ann. Pathol. 2000;20:499–506. [PubMed] [Google Scholar]
- Blackshear P. J. Approaches to the study of protein kinase C involvement in signal transduction. Am. J. Med. Sci. 1988;296:231–240. doi: 10.1016/s0002-9629(15)40866-3. [DOI] [PubMed] [Google Scholar]
- Caron N. J., Torrente Y., Camirand G., Bujold M., Chapdelaine P., Leriche K., Bresolin N., Tremblay J. P. Intracellular delivery of a Tat-eGFP fusion protein into muscle cells. Mol. Ther. 2001;3:310–318. doi: 10.1006/mthe.2001.0279. [DOI] [PubMed] [Google Scholar]
- Chou F. L., Hill J. M., Hsieh J. C., Pouyssegur J., Brunet A., Glading A., Uberall F., Ramos J. W., Werner M. H., Ginsberg M. H. PEA-15 binding to ERK1/2 MAPKs is required for its modulation of integrin activation. J. Biol. Chem. 2003;278:52587–52597. doi: 10.1074/jbc.M309322200. [DOI] [PubMed] [Google Scholar]
- Condorelli G., Vigliotta G., Cafieri A., Trencia A., Andalo P., Oriente F., Miele C., Caruso M., Formisano P., Beguinot F. PED/PEA-15, an anti-apoptotic molecule that regulates FAS/TNFR1-induced apoptosis. Oncogene. 1999;18:4409–4415. doi: 10.1038/sj.onc.1202831. [DOI] [PubMed] [Google Scholar]
- Condorelli G., et al. PED/PEA-15 gene controls glucose transport and is overexpressed in type 2 diabetes mellitus. EMBO J. 1998;17:3858–3866. doi: 10.1093/emboj/17.14.3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condorelli G., Vigliotta G., Trencia A., Maitan M. A., Caruso M., Miele C., Oriente F., Santopietro S., Formisano P., Beguinot F. Protein kinase C (PKC)-alpha activation inhibits PKC-zeta and mediates the action of PED/PEA-15 on glucose transport in the L6 skeletal muscle cells. Diabetes. 2001;50:1244–1252. doi: 10.2337/diabetes.50.6.1244. [DOI] [PubMed] [Google Scholar]
- Crean J. K., Furlong F., Finlay D., Mitchell D., Murphy M., Conway B., Brady H. R., Godson C., Martin F. Connective tissue growth factor [CTGF]/CCN2 stimulates mesangial cell migration through integrated dissolution of focal adhesion complexes and activation of cell polarization. FASEB J. 2004;18:1541–1543. doi: 10.1096/fj.04-1546fje. [DOI] [PubMed] [Google Scholar]
- Danziger N., Yokoyama M., Jay T., Cordier J., Glowinski J., Chneiweiss H. Cellular expression, developmental regulation, and phylogenic conservation of PEA-15, the astrocytic major phosphoprotein and protein kinase C substrate. J. Neurochem. 1995;64:1016–1025. doi: 10.1046/j.1471-4159.1995.64031016.x. [DOI] [PubMed] [Google Scholar]
- Datta R., Kojima H., Yoshida K., Kufe D. Caspase-3-mediated cleavage of protein kinase C theta in induction of apoptosis. J. Biol. Chem. 1997;272:20317–20320. doi: 10.1074/jbc.272.33.20317. [DOI] [PubMed] [Google Scholar]
- DeVries T. A., Neville M. C., Reyland M. E. Nuclear import of PKCdelta is required for apoptosis: identification of a novel nuclear import sequence. EMBO J. 2002;21:6050–6060. doi: 10.1093/emboj/cdf606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G., Loukinova E., Chen Z., Gangi L., Chanturita T. I., Liu E. T., Van Waes C. Molecular profiling of transformed and metastatic murine squamous carcinoma cells by differential display and cDNA microarray reveals altered expression of multiple genes related to growth, apoptosis, angiogenesis, and the NF-kappaB signal pathway. Cancer Res. 2001;61:4797–4808. [PubMed] [Google Scholar]
- Embury J., et al. Proteins linked to a protein transduction domain efficiently transduce pancreatic islets. Diabetes. 2001;50:1706–1713. doi: 10.2337/diabetes.50.8.1706. [DOI] [PubMed] [Google Scholar]
- Emoto Y., et al. Proteolytic activation of protein kinase C delta by an ICE-like protease in apoptotic cells. EMBO J. 1995;14:6148–6156. doi: 10.1002/j.1460-2075.1995.tb00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo K., Oki E., Biedermann V., Kojima H., Yoshida K., Johannes F. J., Kufe D., Datta R. Proteolytic cleavage and activation of protein kinase C [micro] by caspase-3 in the apoptotic response of cells to 1-β-d-arabinofuranosylcytosine and other genotoxic agents. J. Biol. Chem. 2000;275:18476–18481. doi: 10.1074/jbc.M002266200. [DOI] [PubMed] [Google Scholar]
- Eramo A., et al. Inhibition of DNA methylation sensitizes glioblastoma for tumor necrosis factor-related apoptosis-inducing ligand-mediated destruction. Cancer Res. 2005;65:11469–11477. doi: 10.1158/0008-5472.CAN-05-1724. [DOI] [PubMed] [Google Scholar]
- Estelles A., Charlton C. A., Blau H. M. The phosphoprotein protein PEA-15 inhibits Fas- but increases TNF-R1-mediated caspase-8 activity and apoptosis. Dev. Biol. 1999;216:16–28. doi: 10.1006/dbio.1999.9510. [DOI] [PubMed] [Google Scholar]
- Estelles A., Yokoyama M., Nothias F., Vincent J. D., Glowinski J., Vernier P., Chneiweiss H. The major astrocytic phosphoprotein PEA-15 is encoded by two mRNAs conserved on their full length in mouse and human. J. Biol. Chem. 1996;271:14800–14806. doi: 10.1074/jbc.271.25.14800. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S., Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106:489–498. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S., Hall A. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature. 2003;421:753–756. doi: 10.1038/nature01423. [DOI] [PubMed] [Google Scholar]
- Faber-Elman A., Solomon A., Abraham J. A., Marikovsky M., Schwartz M. Involvement of wound-associated factors in rat brain astrocyte migratory response to axonal injury: in vitro simulation. J. Clin. Investig. 1996;97:162–171. doi: 10.1172/JCI118385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerholm S., Morrice N., Gahmberg C.G., Cohen P. Phosphorylation of the cytoplasmic domain of the integrin CD18 chain by protein kinase C isoforms in leukocytes. J. Biol. Chem. 2002;277:1728–1738. doi: 10.1074/jbc.M106856200. [DOI] [PubMed] [Google Scholar]
- Favata M. F., et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Formisano P., et al. Raised expression of the antiapoptotic protein ped/pea-15 increases susceptibility to chemically induced skin tumor development. Oncogene. 2005;24:7012–7021. doi: 10.1038/sj.onc.1208871. [DOI] [PubMed] [Google Scholar]
- Formstecher E., et al. PEA-15 mediates cytoplasmic sequestration of ERK MAP kinase. Dev. Cell. 2001;1:239–250. doi: 10.1016/s1534-5807(01)00035-1. [DOI] [PubMed] [Google Scholar]
- Gaumont-Leclerc M. F., Mukhopadhyay U. K., Goumard S., Ferbeyre G. PEA-15 is inhibited by adenovirus E1A and plays a role in ERK nuclear export and Ras-induced senescence. J. Biol. Chem. 2004;279:46802–46809. doi: 10.1074/jbc.M403893200. [DOI] [PubMed] [Google Scholar]
- Ghayur T., et al. Proteolytic activation of protein kinase C delta by an ICE/CED 3-like protease induces characteristics of apoptosis. J. Exp. Med. 1996;184:2399–2404. doi: 10.1084/jem.184.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez D. E., Skilton G., Alonso D. F., Kazanietz M. G. The role of protein kinase C and novel phorbol ester receptors in tumor cell invasion and metastasis. Oncol. Rep. 1999;6:1363–1370. doi: 10.3892/or.6.6.1363. [DOI] [PubMed] [Google Scholar]
- Gschwendt M., Muller H. J., Kielbassa K., Zang R., Kittstein W., Rincke G., Marks F. Rottlerin, a novel protein kinase inhibitor. Biochem. Biophys. Res. Commun. 1994;199:93–98. doi: 10.1006/bbrc.1994.1199. [DOI] [PubMed] [Google Scholar]
- Hao C., Beguinot F., Condorelli G., Trencia A., Van Meir E. G., Yong V. W., Parney I. F., Roa W. H., Petruk K. C. Induction and intracellular regulation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) mediated apoptosis in human malignant glioma cells. Cancer Res. 2001;61:1162–1170. [PubMed] [Google Scholar]
- Iwabu A., Smith K., Allen F. D., Lauffenburger D. A., Wells A. Epidermal growth factor induces fibroblast contractility and motility via a protein kinase C delta-dependent pathway. J. Biol. Chem. 2004;279:14551–14560. doi: 10.1074/jbc.M311981200. [DOI] [PubMed] [Google Scholar]
- Kermorgant S., Aparicio T., Dessirier V., Lewin M. J., Lehy T. Hepatocyte growth factor induces colonic cancer cell invasiveness via enhanced motility and protease overproduction. Evidence for PI3 kinase and PKC involvement. Carcinogenesis. 2001;22:1035–1042. doi: 10.1093/carcin/22.7.1035. [DOI] [PubMed] [Google Scholar]
- Kiley S. C., Clark K. J., Goodnough M., Welch D. R., Jaken S. Protein kinase C delta involvement in mammary tumor cell metastasis. Cancer Res. 1999;59:3230–3238. [PubMed] [Google Scholar]
- Kitsberg D., et al. Knock-out of the neural death effector domain protein PEA-15 demonstrates that its expression protects astrocytes from TNFalpha-induced apoptosis. J. Neurosci. 1999;19:8244–8251. doi: 10.1523/JNEUROSCI.19-19-08244.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleihues P., Louis D. N., Scheithauer B. W., Rorke L. B., Reifenberger G., Burger P. C., Cavenee W. K. The WHO classification of tumors of the nervous system. J. Neuropathol. Exp. Neurol. 2002;61:215–225. doi: 10.1093/jnen/61.3.215. discussion 226–219. [DOI] [PubMed]
- Krueger J., Chou F. L., Glading A., Schaefer E., Ginsberg M. H. Phosphorylation of phosphoprotein enriched in astrocytes (PEA-15) regulates extracellular signal-regulated kinase-dependent transcription and cell proliferation. Mol. Biol. Cell. 2005;16:3552–3561. doi: 10.1091/mbc.E04-11-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger J. S., Reddy K. B. Distinct mechanisms mediate the initial and sustained phases of cell migration in epidermal growth factor receptor-overexpressing cells. Mol. Cancer Res. 2003;1:801–809. [PubMed] [Google Scholar]
- Kubes M., Cordier J., Glowinski J., Girault J. A., Chneiweiss H. Endothelin induces a calcium-dependent phosphorylation of PEA-15 in intact astrocytes: identification of Ser104 and Ser116 phosphorylated, respectively, by protein kinase C and calcium/calmodulin kinase II in vitro. J. Neurochem. 1998;71:1307–1314. doi: 10.1046/j.1471-4159.1998.71031307.x. [DOI] [PubMed] [Google Scholar]
- Lauffenburger D. A., Horwitz A. F. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Li C., Wernig F., Leitges M., Hu Y., Xu Q. Mechanical stress-activated PKCdelta regulates smooth muscle cell migration. FASEB J. 2003;17:2106–2108. doi: 10.1096/fj.03-0150fje. [DOI] [PubMed] [Google Scholar]
- Lu Z., Hornia A., Jiang Y. W., Zang Q., Ohno S., Foster D. A. Tumor promotion by depleting cells of protein kinase C delta. Mol. Cell. Biol. 1997;17:3418–3428. doi: 10.1128/mcb.17.6.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martiny-Baron G., Kazanietz M. G., Mischak H., Blumberg P. M., Kochs G., Hug H., Marme D., Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J. Biol. Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- Milner R., Huang X., Wu J., Nishimura S., Pytela R., Sheppard D., ffrench-Constant C. Distinct roles for astrocyte alphavbeta5 and alphavbeta8 integrins in adhesion and migration. J. Cell Sci. 1999;112:4271–4279. doi: 10.1242/jcs.112.23.4271. [DOI] [PubMed] [Google Scholar]
- Nishi K., Yoshida M., Fujiwara D., Nishikawa M., Horinouchi S., Beppu T. Leptomycin B targets a regulatory cascade of crm1, a fission yeast nuclear protein, involved in control of higher order chromosome structure and gene expression. J. Biol. Chem. 1994;269:6320–6324. [PubMed] [Google Scholar]
- Ohnishi T., Hiraga S., Izumoto S., Matsumura H., Kanemura Y., Arita N., Hayakawa T. Role of fibronectin-stimulated tumor cell migration in glioma invasion in vivo: clinical significance of fibronectin and fibronectin receptor expressed in human glioma tissues. Clin. Exp. Metastasis. 1998;16:729–741. doi: 10.1023/a:1006532812408. [DOI] [PubMed] [Google Scholar]
- Ossareh-Nazari B., Bachelerie F., Dargemont C. Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science. 1997;278:141–144. doi: 10.1126/science.278.5335.141. [DOI] [PubMed] [Google Scholar]
- Pauly R. R., Bilato C., Sollott S. J., Monticone R., Kelly P. T., Lakatta E. G., Crow M. T. Role of calcium/calmodulin-dependent protein kinase II in the regulation of vascular smooth muscle cell migration. Circulation. 1995;91:1107–1115. doi: 10.1161/01.cir.91.4.1107. [DOI] [PubMed] [Google Scholar]
- Petit I., Goichberg P., Spiegel A., Peled A., Brodie C., Seger R., Nagler A., Alon R., Lapidot T. Atypical PKC-zeta regulates SDF-1-mediated migration and development of human CD34+ progenitor cells. J. Clin. Investig. 2005;115:168–176. doi: 10.1172/JCI21773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos J. W., Kojima T. K., Hughes P. E., Fenczik C. A., Ginsberg M. H. The death effector domain of PEA-15 is involved in its regulation of integrin activation. J. Biol. Chem. 1998;273:33897–33900. doi: 10.1074/jbc.273.51.33897. [DOI] [PubMed] [Google Scholar]
- Renault F., Formstecher E., Callebaut I., Junier M.P., Chneiweiss H. The multifunctional protein PEA-15 is involved in the control of apoptosis and cell cycle in astrocytes. Biochem. Pharmacol. 2003;66:1581–1588. doi: 10.1016/s0006-2952(03)00514-8. [DOI] [PubMed] [Google Scholar]
- Renganathan H., Vaidyanathan H., Knapinska A., Ramos J.W. Phosphorylation of PEA-15 switches its binding specificity from ERK/MAPK to FADD. Biochem. J. 2005;390:729–735. doi: 10.1042/BJ20050378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein L. J., Herman M. M., Foley V. L. In vitro characteristics of human glioblastomas maintained in organ culture systems. Light microscopy observations. Am. J. Pathol. 1973;71:61–80. [PMC free article] [PubMed] [Google Scholar]
- Rybin V. O., Sabri A., Short J., Braz J. C., Molkentin J. D., Steinberg S. F. Cross-regulation of novel protein kinase C (PKC) isoform function in cardiomyocytes. Role of PKC epsilon in activation loop phosphorylations and PKC delta in hydrophobic motif phosphorylations. J. Biol. Chem. 2003;278:14555–14564. doi: 10.1074/jbc.M212644200. [DOI] [PubMed] [Google Scholar]
- Sakurai Y., Onishi Y., Tanimoto Y., Kizaki H. Novel protein kinase C delta isoform insensitive to caspase-3. Biol. Pharm. Bull. 2001;24:973–977. doi: 10.1248/bpb.24.973. [DOI] [PubMed] [Google Scholar]
- Schaap D., Hsuan J., Totty N., Parker P.J. Proteolytic activation of protein kinase C-epsilon. Eur. J. Biochem. 1990;191:431–435. doi: 10.1111/j.1432-1033.1990.tb19139.x. [DOI] [PubMed] [Google Scholar]
- Schwarze S. R., Ho A., Vocero-Akbani A., Dowdy S. F. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- Sharif A., Renault F., Beuvon F., Castellanos R., Canton B., Barbeito L., Junier M. P., Chneiweiss H. The expression of PEA-15 (phosphoprotein enriched in astrocytes of 15 kDa) defines subpopulations of astrocytes and neurons throughout the adult mouse brain. Neuroscience. 2004;126:263–275. doi: 10.1016/j.neuroscience.2004.02.039. [DOI] [PubMed] [Google Scholar]
- Sorour O., Raafat M., El-Bolkainy N., Rifaat M. Infiltrative potentiality of brain tumors in organ culture. J. Neurosurg. 1975;43:742–749. doi: 10.3171/jns.1975.43.6.0742. [DOI] [PubMed] [Google Scholar]
- Stassi G., Garofalo M., Zerilli M., Ricci-Vitiani L., Zanca C., Todaro M., Aragona F., Limite G., Petrella G., Condorelli G. PED mediates AKT-dependent chemoresistance in human breast cancer cells. Cancer Res. 2005;65:6668–6675. doi: 10.1158/0008-5472.CAN-04-4009. [DOI] [PubMed] [Google Scholar]
- Steinberg S. F. Distinctive activation mechanisms and functions for protein kinase Cdelta. Biochem. J. 2004;384:449–459. doi: 10.1042/BJ20040704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stempka L., Schnolzer M., Radke S., Rincke G., Marks F., Gschwendt M. Requirements of protein kinase cdelta for catalytic function. Role of glutamic acid 500 and autophosphorylation on serine 643. J. Biol. Chem. 1999;274:8886–8892. doi: 10.1074/jbc.274.13.8886. [DOI] [PubMed] [Google Scholar]
- Szallasi Z., Denning M. F., Smith C. B., Dlugosz A. A., Yuspa S. H., Pettit G. R., Blumberg P. M. Bryostatin 1 protects protein kinase C-δ from down-regulation in mouse keratinocytes in parallel with its inhibition of phorbol ester-induced differentiation. Mol. Pharmacol. 1994a;46:840–850. [PubMed] [Google Scholar]
- Szallasi Z., Smith C. B., Pettit G. R., Blumberg P. M. Differential regulation of protein kinase C isozymes by bryostatin 1 and phorbol 12-myristate 13-acetate in NIH 3T3 fibroblasts. J. Biol. Chem. 1994b;269:2118–2124. [PubMed] [Google Scholar]
- Tang S., Gao Y., Ware J. A. Enhancement of endothelial cell migration and in vitro tube formation by TAP20, a novel beta 5 integrin-modulating, PKC theta-dependent protein. J. Cell Biol. 1999;147:1073–1084. doi: 10.1083/jcb.147.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S., Morgan K. G., Parker C., Ware J. A. Requirement for protein kinase C theta for cell cycle progression and formation of actin stress fibers and filopodia in vascular endothelial cells. J. Biol. Chem. 1997;272:28704–28711. doi: 10.1074/jbc.272.45.28704. [DOI] [PubMed] [Google Scholar]
- Ueyama T., Ren Y., Ohmori S., Sakai K., Tamaki N., Saito N. cDNA cloning of an alternative splicing variant of protein kinase C delta (PKC deltaIII), a new truncated form of PKCdelta, in rats. Biochem. Biophys. Res. Commun. 2000;269:557–563. doi: 10.1006/bbrc.2000.2331. [DOI] [PubMed] [Google Scholar]
- Vigliotta G., et al. Overexpression of the ped/pea-15 gene causes diabetes by impairing glucose-stimulated insulin secretion in addition to insulin action. Mol. Cell. Biol. 2004;24:5005–5015. doi: 10.1128/MCB.24.11.5005-5015.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba M., Kasibhatla S., Genestier L., Mahboubi A., Green D. R., Altman A. Protein kinase ctheta cooperates with calcineurin to induce Fas ligand expression during activation-induced T cell death. J. Immunol. 1999;163:5813–5819. [PubMed] [Google Scholar]
- Whitehurst A. W., Robinson F. L., Moore M. S., Cobb M. H. The death effector domain protein PEA-15 prevents nuclear entry of ERK2 by inhibiting required interactions. J. Biol. Chem. 2004;279:12840–12847. doi: 10.1074/jbc.M310031200. [DOI] [PubMed] [Google Scholar]
- Woo J. H., Lim J. H., Kim Y. H., Suh S. I., Min do S., Chang J. S., Lee Y. H., Park J. W., Kwon T. K. Resveratrol inhibits phorbol myristate acetate-induced matrix metalloproteinase-9 expression by inhibiting JNK and PKC delta signal transduction. Oncogene. 2004;23:1845–1853. doi: 10.1038/sj.onc.1207307. [DOI] [PubMed] [Google Scholar]