Abstract
The Golgi apparatus (GA) is the organelle where complex glycan formation takes place. In addition, it is a major sorting site for proteins destined for various subcellular compartments or for secretion. Here we investigate β1,4-galactosyltransferase 1 (galT) and α2,6-sialyltransferase 1 (siaT), two trans-Golgi glycosyltransferases, with respect to their different pathways in monensin-treated cells. Upon addition of monensin galT dissociates from siaT and the GA and accumulates in swollen vesicles derived from the trans-Golgi network (TGN), as shown by colocalization with TGN46, a specific TGN marker. We analyzed various chimeric constructs of galT and siaT by confocal fluorescence microscopy and time-lapse videomicroscopy as well as Optiprep density gradient fractionation. We show that the first 13 amino acids of the cytoplasmic tail of galT are necessary for its localization to swollen vesicles induced by monensin. We also show that the monensin sensitivity resulting from the cytoplasmic tail can be conferred to siaT, which leads to the rapid accumulation of the galT–siaT chimera in swollen vesicles upon monensin treatment. On the basis of these data, we suggest that cycling between the trans-Golgi cisterna and the trans-Golgi network of galT is signal mediated.
INTRODUCTION
Since the discovery of the Golgi apparatus (GA) as the site of complex glycosylation (Neutra and Leblond, 1966), interest focused on the molecular organization of the glycosylation machinery. The key mediators of glycosylation are the glycosyltransferases, which are type 2 membrane proteins (Paulson and Colley, 1989). Those involved in N-glycosylation of proteins (Rabouille et al., 1995) appear to be arranged from the cis to the trans face of the GA like an assembly line, as proposed two decades ago (Berger, 1985). One of the best studied Golgi-associated glycosyltransferases is β1,4-galactosyltransferase 1 (galT), which traditionally has been used as Golgi marker enzyme in fractionation studies (Bretz et al., 1980 and references cited therein). Early work on immunolocalization of galT determined its steady state localization to the trans side of the GA and its exclusion from the trans-most cisterna, which can be considered as part of the TGN (Roth and Berger, 1982; Griffiths and Simons, 1986); this confinement to the trans side (Rabouille et al., 1995; Kweon et al., 2004) has to our knowledge not been called into question. However, intracellular trafficking of galT and its retention specifically to trans located cisternae is insufficiently understood. The enzyme follows the secretory pathway as determined by pulse-chase experiments (Strous and Berger, 1982) and is somehow retained in the trans cisternae, whereas a small fraction of galT moves, in defined cell types, to the plasma membrane (Hathaway et al., 2003) and possibly leaves the cell by shedding (for review see Strous, 1986). The site and mechanisms of cleavage of galT are unknown. More recently, an additional pathway of trafficking has been proposed: GFP-tagged forms of galT appear to recycle to the endoplasmic reticulum (ER; Sciaky et al., 1997; Storrie, 2005). This retrograde transport was postulated to be mediated by tubules carrying galT-GFP back to the ER, a process much enhanced by brefeldin A (BFA; Lippincott-Schwartz et al., 1990). In no instances galT-GFP was found in structures near or at the plasma membrane. Therefore, post-Golgi trafficking of this enzyme remains mysterious because at least two different routes (transport to the cell surface and secretion by shedding as well as recycling to the ER) have been described. Post-Golgi trafficking intermediates containing galT have indeed not been characterized.
Golgi disturbing agents such as BFA and monensin have been useful to dissect some of the mechanisms involved in transport through the GA (for review see Dinter and Berger, 1998). Monensin, a cationophore exchanging luminal H+ for Na+, leads to the formation of swollen vesicles mainly in post-Golgi/endosomal compartments by Na+-driven water influx (Zhang et al., 1993; for review see Mollenhauer et al., 1990). Surprisingly, galT was found by immunoelectron microscopy to decorate the membranes of these swollen vesicles (Strous et al., 1985), an effect interpreted as swelling of trans-Golgi cisternae along the lines of the then widely accepted view of monensin being a Golgi transport disruptor (Tartakoff, 1983). However, α2,6-sialyltransferase 1 (siaT), a trans-Golgi enzyme that colocalizes with galT (Taatjes et al., 1987; Kweon et al., 2004), dissociates from galT in monensin-treated cells (Berger et al., 1993), leading to the notion of displacement of galT to TGN-derived swollen vesicles, where galT colocalizes with the cation insensitive mannose-6-phosphate receptor (Berger et al., 2001).
The present work was carried out to demonstrate monensin-induced dissociation of galT from siaT by live microscopy and its colocalization with the authentic TGN marker TGN46 by double immunofluorescence to swollen vesicles and to quantify the morphological data by subcellular fractionation. Moreover, we show that this displacement is mediated by 13 N-terminal amino acids of galT and is necessary and in the context of the entire cytosolic tail sufficient to mediate anterograde transport of glycosyltransferases from the trans-Golgi cisterna to the TGN.
MATERIALS AND METHODS
Materials
Enzymes used in molecular cloning were obtained from New England Biolabs (Ipswich, MA), Promega (Madison, WI), Roche Diagnostics (Basel, Switzerland), or Sigma-Aldrich (St. Louis, MO). Polyethylenimine (PEI; linear, Mr 25,000) was from Polysciences (Warrington, PA), Lipofectamine, DMEM, and bovine fetal calf serum were from Invitrogen (Carlsbad, CA). Disposables and cell culture dishes were from BD Biosciences (Franklin Lakes, NJ) or Nunc (Rochester, NY). Oligonucleotides were synthesized by Microsynth GmBH (Galbach, Switzerland). Optiprep was from Sigma-Aldrich; nitrocellulose was from Whatman (Brentford. United Kingdom); enhanced chemiluminescence (ECL) Western blotting reagents from PerkinElmer Life Sciences (Wellesley, MA); goat anti-rabbit, goat anti-mouse, and donkey anti-sheep antibodies conjugated with Alexa488 or Alexa568 were obtained from Invitrogen. Monoclonal antibodies against giantin (G1/133), GPP130 (A1/118), and p63 (G1/296) were obtained from Axxora Life Sciences (San Diego, CA); antibodies against GFP were from Roche Diagnostics (Basel, Switzerland). The goat anti-mouse antibody conjugated with horseradish peroxidase was from GE Healthcare (Chalfont St. Giles, United Kingdom). The polyclonal antibody sheep anti-human TGN46 was from Serotec (Kidlington, United Kingdom). The mAb for Na+-K+-ATPase (N1/123) was a gift from H. P. Hauri (Marxer et al., 1989). The mAb to galT was galT#2/36/118 (Berger et al., 1986), the polyclonal antibodies to galT were described in Watzele et al. (1991) and to siaT in Berger et al. (1993).
Recombinant DNA
All basic DNA procedures were as described (Sambrook et al., 1998). The PCR procedure of Ho et al. (1989) was used to generate the mutants and the galT–siaT fusion chimeras as described (Rohrer and Kornfeld, 2001). The final PCR products were subcloned into pcDNA3.1(−) (Invitrogen) as described (Rohrer et al., 1995). In brief, galT-GFP and siaT-GFP were produced by PCR using pUC18-galT (Watzele and Berger, 1990) and pIC20H-siaT (Berger et al., 1993) as templates with respective oligos (list of oligos used can be found in Supplementary Material) containing restriction sites NotI and BglII. The PCR products were subsequently digested for 2 h at 37°C using NotI and BglII. BglII and HindIII restriction sites were introduced into GFP using the same method, and the resulting PCR product was digested. The vector pcDNA 3.1(−) was cut using NotI/HindIII. Subsequently the fragments were analyzed and purified by agarose gel electrophoresis. The three fragments (galT or siaT, GFP, and vector) were extracted from the agarose gel using the QIAEX II kit form Qiagen (Valencia, CA) and were assembled in an overnight ligation at 15°C.
To create chimeras of galT and siaT, two separate PCR fragments of the two parts to be fused together were generated using galT-GFP or siaT-GFP as templates. The fragments were analyzed and purified by agarose gel electrophoresis. In a second PCR these purified fragments were merged together using outer oligos (Ho et al., 1989). The resulting fragment was subcloned into pcDNA 3.1(−), as described for galT-GFP and siaT-GFP above. The tail mutants were created similarly, using galT-GFP as template and overlapping oligos containing the mutations (Ho et al., 1989).
Cell Culture and Transfection
HepG2 cells were grown in DMEM supplemented with 10% FCS to 20–30% confluency in six-well plates before transfection with 1.4 μg of linearized plasmid DNA combined with 2.2 μg PEI according to the protocol from Polyplus-Transfection (Illkirch, France). Selection for resistance to neomycin (G418) was carried out using 1 mg/ml G418 as the final concentration. Resistant colonies were picked individually and screened for the expression of the various GFP-chimeras by fluorescence analysis, or several colonies were pooled to give a mixture of cells expressing the construct at various levels.
Confocal Immunofluorescence Microscopy
Confocal immunofluorescence microscopy was carried out as described (Rohrer and Kornfeld, 2001). HepG2 cells were grown to 30–50% confluency on coverslips in DMEM + 10% FCS. The cells were then incubated for 30 min in DMEM + 10% FCS containing 2 μM monensin before fixation with 3% paraformaldehyde. After fixation, the cells were permeabilized using 0.1% saponin in PBS for 20 min, incubated with suitably diluted primary antibody in 0.1% saponin in PBS for 30 min, then washed in 0.1% saponin in PBS three times and incubated with secondary antibodies in 0.1% saponin in PBS, and finally washed three times in PBS. The coverslips were mounted on glass slides with ProLong Antifade (Invitrogen) for viewing with a Leica TCS SP2 AOBS (Leica, Wetzlar, Germany) confocal laser-scanning microscope. The resulting stacks of images were exported as Tiff files and analyzed with the Imaris program (Bitplane).
Live Confocal Immunofluorescence Microscopy and Deconvolution
Live confocal immunofluorescence microscopy was carried out on a Leica SP2 microscope equipped with an incubation chamber (Life Imaging Services, Reinach, Switzerland). They were recorded at an xy-resolution of 512 pixels and 4–6 z-slices. The 4D stacks were processed using AutoDeblur (Media Cybernetics, Silver Spring, MD) and compiled using the Imaris Software (Bitplane).
Optiprep Density Gradient
Optiprep density gradients were performed as described in the manufacturer's protocol (Axis Shield, Roskilde, Denmark; protocol S36). Briefly, HepG2 cells were grown to 70–80% confluency on 10-cm dishes and incubated for 30 min in DMEM + 10% FCS containing 2 μM monensin before harvest by a rubber policeman and collection in 2 ml homogenization buffer (0.25 M sucrose, 1 mM EDTA, 10 mM HEPES-NaOH, pH 7.4). Cells were then broken using 15 strokes in a ball bearing homogenizer (Balch and Rothman, 1985) with a clearance of 16 μm, followed by removal of nuclei by low-speed centrifugation (800 × g). The homogenate (0.8 ml) was then loaded on top of a step gradient containing 2.5–30% Optiprep (final volume of 9.2 ml: 2.5%, 0.8 ml; 5%, 1.3 ml; 7.5%, 1.3 ml; 10%, 1.3 ml; 12.5%, 0.8 ml; 15%, 1.3 ml; 17.5%, 0.8 ml; 20%, 0.8 ml; and 30%, 0.8 ml) and centrifuged for 2.5 h at 200,000 × gav in the Sorvall TH641 swing-out rotor. Ten 1-ml fractions were collected from the bottom by pinching a hole in the tube and letting the gradient slowly drop into the collecting tubes. The resulting fractions were analyzed by Western blotting techniques using mAb against GFP and secondary antibody anti-mouse conjugated with horse-radish peroxidase.
SDS-PAGE and Immunoblotting
Proteins were separated on 10% SDS-polyacrylamide minigels (Bio-Rad, Hercules, CA) by using the Laemmli system (Laemmli, 1970). After electrophoresis the proteins were transferred from the gels onto nitrocellulose membranes according to the method of Towbin et al. (1979). The immunoblotting was performed as previously described (Rohrer et al., 1995). The chemoluminescence was recorded by using a quantitative densitometer from Raytest (Straubenhardt, Germany), and signals were quantified by calculating the total fluorescence of each band and correcting it by subtracting the background fluorescence using the Aida Software (Straubenhardt, Germany).
RESULTS
GalT-GFP Separates from siaT-GFP and Giantin in Cells Treated with Monensin
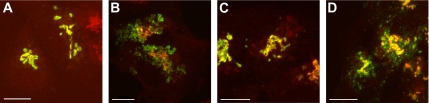
Previously, we have shown that in HepG2 cells treated with monensin, the endogenously expressed trans-Golgi enzyme galT moves to swollen vesicles, whereas the endogenous siaT, which also localizes to the trans cisterna of the Golgi remains associated with the GA, which was little disturbed by monensin (Berger et al., 1993). Subsequently, this monensin-induced dissociation was reproduced in CHO cells transfected with both galT and siaT (Berger et al., 2001), indicating a general rather than a cell-specific phenomenon for HepG2 cells. To further investigate the dynamics of this dissociation, time-lapse videomicroscopy was carried out. Both enzymes exhibit a similar domain structure consisting of a short cytoplasmic tail, a transmembrane domain, a stem region, and a catalytic domain (Paulson and Colley, 1989). This latter domain was replaced in both enzymes with the fluorescent protein GFP or RFP. The resulting constructs were stably transfected into HepG2 cells, and to avoid potential problems by overexpressing the constructs, we isolated individual colonies with modest expression levels. Using a mAb specific for galT (Berger et al., 1986) or a polyclonal antibody specific for siaT (Berger et al., 1993), we could show by indirect confocal immunofluorescence that the two chimeras almost perfectly colocalized with their respective endogenous counterparts in the GA, as indicated by the predominant yellow staining in the perinuclear region (Figure 1, A and C). Furthermore, we could demonstrate that galT-GFP is dispersed to the same swollen vesicles upon treatment with 2 μM monensin for 30 min as endogenous galT (Figure 1B), whereas siaT-GFP is not affected by monensin treatment and remains in the GA together with endogenous siaT (Figure 1D). To directly compare the dynamics of the two chimeras in presence of monensin, we transiently expressed siaT-RFP in HepG2 cells stably transfected with galT-GFP. The cells were cultured in glass-bottom dishes where they can readily be examined by live videomicroscopy on an inverted microscope equipped with an incubation chamber. For live experiments the medium was replaced by PBS supplemented by 10% FCS. Both chimeras were colocalized before monensin treatment and also immediately after the addition of 2 μM monensin (Figure 2A, 0 min) in the perinuclear region, representing the GA. However, upon treatment of double-labeled cells with monensin, a rapid separation of the two chimeras was observed. Although the localization siaT-GFP within the GA remained almost unchanged over the entire time course of 30 min, galT-GFP was found in swollen vesicles, which appeared early in the Golgi region, and then separated from the Golgi-localized siaT-RFP (Figure 2A, Supplementary Movie S1). There are two remarkable features of these swollen vesicles: first they appear within minutes after treatment (Figure 2A, 3 min) and second, their size reached a diameter of up to 2 μm, which is rather gigantic compared with other vesicles such as COPII-coated vesicles that are 50–60 nm in size (Figure 2A; Barlowe, 1995). In addition, to identify the Golgi scaffold we used the marker giantin, a vesicle-tethering protein localized to the cytoplasmic side of the GA (Linstedt and Hauri, 1993). Under control conditions, where the cells are grown in regular growth media supplemented with FCS before fixation, galT-GFP (Figure 2B) and siaT-GFP (Figure 2D) both showed substantial costaining with giantin. However, upon treatment with 2 μM monensin for 30 min, siaT-GFP remained colocalized with giantin (Figure 2E), whereas galT-GFP dissociated from giantin (Figure 2C) and is found in swollen vesicles. These results show that monensin treatment did not affect the scaffold of the Golgi in general but rather the localization of individual components, i.e., galT-GFP.
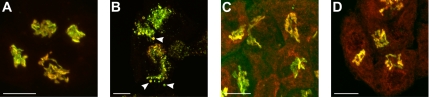
Figure 1.
Double-staining of galT-GFP and siaT-GFP with endogenous galT and siaT in HepG2 cells treated with monensin. GalT-GFP and siaT-GFP colocalize with their endogenous counterpart in HepG2 cells. (A) In the GA of control cells, stably transfected galT-GFP (green) colocalizes with endogenous galT (red) labeled with mAb against galT in the GA. (B) On treatment with 2 μM monensin for 30 min both galT-GFP (green) and endogenous galT (red) are redistributed to swollen vesicles (arrowheads). (C) In control cells, stably transfected siaT-GFP (green) colocalizes with endogenous siaT (red) labeled with polyclonal antibody against siaT. (D) On treatment with 2 μM monensin for 30 min both siaT-GFP (green) and endogenous siaT (red) remain together and show little change. Bar, 10 μm.
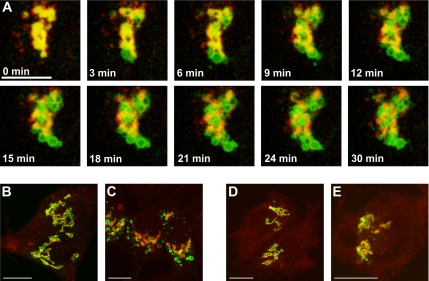
Figure 2.
Double-staining time-lapse videomicroscopy of galT-GFP and siaT-RFP in HepG2 cells treated with monensin. GalT-GFP rapidly separates from siaT-RFP and endogenous giantin upon monensin treatment. (A) HepG2 cells stably transfected with galT-GFP (green) were transiently transfected with siaT-RFP (red). Seventy-two hours after transfection of siaT-RFP, monensin was added to a final concentration of 2 μM, and the movie (3 frames/min) was recorded on a confocal microscope, starting ∼1 min after addition of monensin. Bar, 10 μm. See also Supplementary Movie S1. (B and C) Double immunofluorescence of galT-GFP (green) and giantin (red) in control cells (B) and cells treated with 2 μM monensin for 30 min (C). (D and E) Double immunofluorescence of siaT-GFP (green) and giantin (red) in control cells (D) and cells treated with 2 μM monensin for 30 min (E). Bar, 10 μm.
GalT-GFP Trapped in Monensin-induced Swollen Vesicles Is Insensitive to BFA
To further substantiate the dissociation of galT from siaT, we tested their respective dynamics to the fungal metabolite BFA with and without pretreatment with monensin. By inhibiting the Arf1 GTP exchange factor (Donaldson et al., 1992; Cherfils and Melancon, 2005) BFA forces genuine Golgi membranes to fuse with the ER, thereby transporting their content to the ER (Lippincott-Schwartz et al., 1989; Strous et al., 1990). In contrast, the TGN proteins do not redistribute to the ER but rather form a tubular network in an early step and later are in close proximity to the microtubule organization center (Lippincott-Schwartz et al., 1991). In control experiments without monensin pretreatment, the cells were monitored by time-lapse videomicroscopy after the addition of BFA at a concentration of 10 μM. Both galT-GFP (Figure 3A and Supplementary Movie S2) and siaT-GFP (Figure 3C and Supplementary Movie S4) were relocated from the GA to the ER. Pretreatment of the cells expressing galT-GFP with 2 μM monensin for 15 min before addition of BFA led to the initial formation of swollen vesicles containing galT-GFP (Figure 3B, 0 min). The subsequent addition of 10 μM BFA had no effect on these swollen vesicles as they could be observed over the entire time course (Figure 3B, Supplementary Movie S3). In contrast, cells expressing siaT-GFP were insensitive to the pretreatment with 2 μM monensin for 15 min (Figure 3D, 0 min), but were redistributed to the ER upon addition of BFA (Figure 3D, Supplementary Movie S5). These data support our contention that monensin treatment forms swollen vesicles in which galT is trapped and remained incompetent for its return to the GA and BFA-induced backflow to the ER.
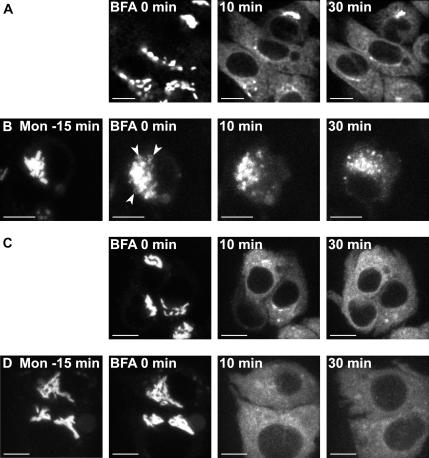
Figure 3.
Dynamics of galT-GFP and siaT-GFP in monensin-treated cells followed by addition of BFA in HepG2 cells. The time-lapse video starts immediately after addition of 10 μM BFA (0 min). (A and C) Control experiments in absence of monensin; both galT-GFP (A, Supplementary Movie S2) and siaT-GFP (C, Supplementary Movie S4) relocated to the ER after treatment with 10 μM BFA. (B and D) Fifteen-minute monensin (2 μM) pretreatment: galT-GFP (B, Supplementary Movie S3) is present in swollen vesicles (arrowheads) at the time of addition of BFA and remains in swollen vesicles. (D) Supplementary Movie S5: SiaT-GFP is relocated to the ER also in presence of 2 μM monensin. Bar, 10 μm.
GalT-GFP Colocalizes in Swollen Vesicles with TGN46
To determine whether galT-containing swollen vesicles are derived from the TGN as previously suggested, by showing colocalization of galT with the cation-independent mannose 6-phosphate receptor (Berger et al., 2001) we investigated the presence of TGN46, a marker of the TGN excluded from the GA (Banting and Ponnambalam, 1997; Prescott et al., 1997), in the swollen vesicles by confocal immunofluorescence microscopy. As expected for proteins localized to compartments that are in such close proximity as the trans-Golgi cisterna and the TGN, we could show that both galT-GFP and siaT-GFP staining overlap partially with TGN46 staining in the Golgi area of untreated cells, with TGN46 being also present in peripheral structures (Figure 4, A and C), which are likely endosomes. The formation of TGN46-positive swollen vesicles is clearly visible upon treatment with monensin (Figure 4, B and D), which also contain galT-GFP (Figure 4B) but are devoid of siaT-GFP (Figure 4D).
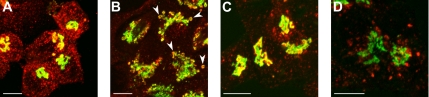
Figure 4.
Double-staining of galT-GFP or siaT-GFP with TGN6 in monensin-treated HepG2 cells. GalT-GFP but not siaT-GFP can be found in TGN46-positive swollen vesicles. HepG2 cells stably transfected with galT-GFP or siaT-GFP were immunolabeled using anti TGN46 antibody. (A and C) Significant overlap between TGN46 (red) and galT-GFP (A, green) or siaT-GFP (C, green) in the Golgi region, with additional peripheral staining of TGN46, possibly in endosomes. (B) On treatment with 2 μM monensin for 30 min both galT-GFP and TGN46 are found in swollen vesicles (arrowheads). (D) Although treatment of the cells with 2 μM monensin for 30 min does not affect siaT-GFP localization, TGN46 is redistributed to swollen vesicles, and the Golgi region stained with siaT-GFP is devoid of TGN46. Bar, 10 μm.
Quantification of galT Segregation to Swollen Vesicles
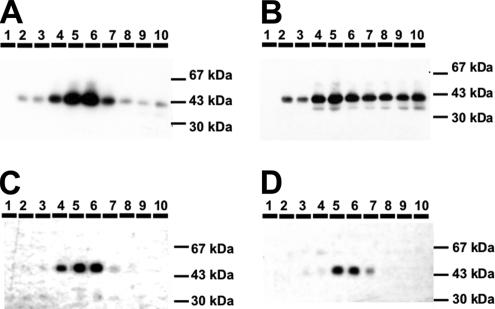
To quantify the redistribution of the GFP-chimeras, we established an iso-osmotic density gradient using Optiprep, which allows separating monensin-induced swollen vesicles from the GA. The membranes were recovered by additional high-speed centrifugation steps and finally resuspended in sample buffer and loaded on a SDS-PAGE. The chimeras were visualized by a mAb specific for GFP followed by ECL and quantified. Figure 5 shows that both galT-GFP and siaT-GFP are enriched in fractions 5 and 6, with ∼20% in the lighter fractions (7–10) in untreated cells (Table 1). In cells treated with monensin before fractionation, galT-GFP accumulated in fractions 7–10 to 59%, which is an increase by 39% (Table 1), whereas no significant shift was observed in siaT-GFP–transfected cells (Table 1). The results for TGN46 were comparable to those of galT-GFP: 26% of TGN46 were found in fractions 7–10 of untreated cells and in cells treated with monensin TGN46 accumulated to 52% in the light fractions (7–10; Table 1).
Figure 5.
Optiprep density gradient of HepG2 cells treated with monensin. Fraction 1 contains densest membranes; fraction 10 contains lightest membranes. GalT-GFP (A) as well as siaT-GFP (C) from control are enriched in fractions 5 and 6. (B) GalT-GFP from cells treated with 2 μM monensin for 30 min are distributed in fractions 4–10. (D) SiaT-GFP from cells treated with 2 μM monensin for 30 min remains unchanged and remain enriched in fractions 5 and 6. For densitometric analysis, see Table 1.
Table 1.
Quantitative evaluation of protein accumulation in Optiprep density gradient
| % in Fractions 7–10 | ||
|---|---|---|
| Control | 2 μM monensin | |
| GalT-GFP (n = 5) | 20.2 ± 8.5 | 58.6 ± 11.4 |
| SiaT-GFP (n = 6) | 21.3 ± 6.2 | 22.3 ± 7.5 |
| TGN46 (n = 7) | 26.2 ± 7.7 | 52.2 ± 11.6 |
| GSS-GFP (n = 5) | 26.0 ± 4.0 | 44.7 ± 3.8 |
| SGG-GFP (n = 5) | 18.5 ± 8.1 | 22.6 ± 7.8 |
Table shows percentage of total protein in the light fractions 7–10 in untreated (control) cells and cells treated with monensin ± SD. Densitometric analysis is as described in Materials and Methods.
The Cytoplasmic Tail of galT Is Sufficient for Monensin Sensitivity
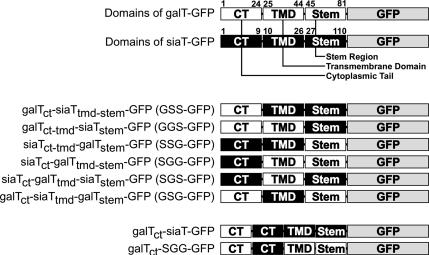
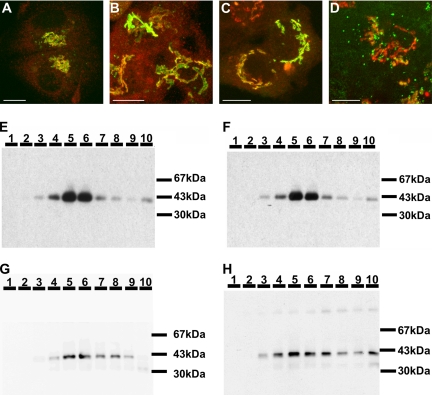
To determine which domain of galT is involved in its dramatic displacement to monensin-induced swollen vesicles (for simplicity referred to as “response to monensin” or “monensin sensitivity”), we generated a series of chimeras in which domains between galT and siaT were swapped (Figure 6). We stably expressed these constructs in HepG2 cells to investigate the effect of monensin on these chimeras, by fluorescence microscopy and by Optiprep density gradients. The SGG-GFP chimera only containing the cytoplasmic tail of siaT was insensitive to monensin as shown initially by immunofluorescence, where no separation of this chimeric protein from giantin was observed after the addition of monensin (Figure 7B), and by Optiprep density gradient, where it shows no increased accumulation in lighter fractions and remains accumulated in fractions 5–6 in treated cells (Figure 7, E and F, and Table 1). This distribution on the density gradients is representative for the entire group of chimeras containing the cytoplasmic tail of siaT (SGG-GFP, SSG-GFP, and SGS-GFP), as they all were insensitive to monensin (Figure 7, A and B, and Supplementary Figure S1). Conversely, the GSS-GFP chimera was included in swollen vesicles and separated from the GA stained with antibodies to giantin upon addition of monensin (Figure 7D). The monensin sensitivity of GSS-GFP was confirmed by Optiprep density gradient, where its distribution is clearly shifted toward the light fractions (7–10) containing 45% of GSS-GFP in treated cells versus 26% in control cells (Figure 7, G and H, and Table 1). The other chimeras containing the cytoplasmic tail of galT (GGS-GFP and GSG-GFP) also remained sensitive to monensin, as determined by immunofluorescence (Supplementary Figure S2). Although in most cells a large fraction of the GGS-GFP chimera was found in mitochondria as confirmed by double-staining using Mitotracker (Invitrogen; Supplementary Figure S3), the Golgi staining was predominant in some cells (Supplementary Figure S2). The fusion of the cytoplasmic tail and TMD of galT to the stem region of siaT possibly created a new cryptic mitochondrial targeting sequence, which is partially active. To avoid any confusion with the additional staining pattern, we selected cells with predominant Golgi localization of the GGS-GFP chimera and could show that the fraction of GGS-GFP localized in the GA showed extensive formation of swollen vesicles upon treatment with monensin in live videomicroscopy (Supplementary Figure S2 and Supplementary Movie S6).
Figure 6.
Chimeras of galT and siaT fused to GFP. For easy reference the constructs were designated with a three-letter code for the three domains possibly involved (cytoplasmic tail, transmembrane domain, and stem region), indicating the origin of the domain: G for galT derived and S for siaT derived. The numbers refer to the amino acid number of the respective full-length protein (galT, GenBank accession no. NP_001497.2; siaT, GenBank accession no. BC040009).
Figure 7.
Response of various galT/siaT–GFP chimeras to monensin. (A and C) control; (B and D) monensin-treated HepG2 cells (2 μM monensin, 30 min). Double-immunofluorescent pictures of SGG-GFP (A and B, green) and GSS-GFP (C and D, green), and giantin (red). (A and C) The GFP-chimeras and giantin colocalize under control conditions. (B) SGG-GFP remains colocalized with giantin in monensin-treated cells. (D) In monensin-treated cells, GSS-GFP separates from giantin and is found in swollen vesicles. (E–H) Optiprep density gradients. SGG-GFP (E and F) and GSS-GFP (G and H). (E and G) Both SGG-GFP (E) and GSS-GFP (G) are enriched in fractions 5 and 6 in untreated cells. (F) SGG-GFP remains enriched in fractions 5 and 6 after treatment with 2 μM monensin for 30 min. (H) GSS-GFP shifts toward less dense fractions upon treatment of the cells with 2 μM monensin for 30 min.
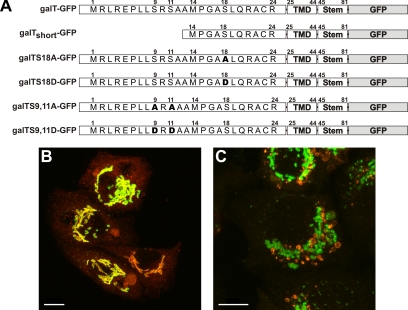
The Short Form of galT Is Insensitive to Monensin
Translation initiation of galT can occur alternatively at either one of the two in frame methionines, resulting in a long form (24 amino acids) and a short form (11 amino acids) of the cytosolic tail (Figure 8; Strous et al., 1988). Both iso-forms have been localized to the trans Golgi cisterna (Russo et al., 1992), with a small fraction of the long form present at the plasma membrane (Shur et al., 1998). To further investigate the involvement of the cytoplasmic tail in monensin sensitivity of galT, we compared the two start variants of galT. Although the amount of the short form expressed in cells stably transfected with galT-GFP has not been determined, all the green fluorescence in cells stably transfected with galTctshort-GFP can be attributed to the short form. The short form entirely colocalized with endogenous galT in the GA (Figure 8B). GalTctshort-GFP remains in the GA in monensin-treated cells (Figure 8C), showing that the sensitivity to monensin is highly reduced in galTctshort-GFP.
Figure 8.
Scheme of mutants of galT and response to monensin of the long iso-form. (A) Schematic drawing of the various chimeras. (B) Stably transfected galTshort-GFP (green) colocalizes with immunostained endogenous galT (red) in control cells. (C) On monensin treatment endogenous galT is found in swollen vesicles, whereas galTshort-GFP is mostly found in the Golgi.
The Potential Phosphorylation Sites Are Not Involved in Monensin Sensitivity of galT
GalT has been shown to be phosphorylated on serine residues within the cytosolic tail (Strous et al., 1987), which might affect its trafficking behavior (Hathaway et al., 2003) and/or monensin sensitivity. We analyzed all three potential phosphorylation sites (Ser9 and Ser11 as pair or Ser18 alone) by mutating them either to alanine to prevent any phosphorylation or to aspartic acid to mimic the negative charge conferred by phosphorylation (Casanova et al., 1990). All mutant constructs were stably transfected into HepG2 cells and analyzed by double immunofluorescence with giantin as a Golgi marker. Preventing phosphorylation (galT-S9,11A-GFP, and galT-S18A-GFP) did not have any effect on the localization of the mutants, and monensin sensitivity was preserved in all of the mutants (Supplementary Figure S4). These results demonstrated that phosphorylation is not required for monensin sensitivity. Changing the serine residues to aspartic acid residues (galT-S9,11D-GFP, and galT-S18D-GFP) did not affect the localization in the GA or the monensin sensitivity of the mutants (Supplementary Figure S5). This indicates that monensin sensitivity of galT is not affected by phosphorylation or alternatively, that aspartic acid is not capable of mimicking phosphorylation of the cytoplasmic tail of galT.
Transfer of the Cytoplasmic Tail of galT Renders siaT Sensitive to Monensin
To demonstrate that the cytoplasmic tail alone is not only necessary but also sufficient to confer monensin sensitivity, we added the complete cytoplasmic tail of galT to the 5′ end of siaT-GFP and SGG-GFP, respectively, to obtain the constructs GSSS-GFP and GSGG-GFP. In these constructs we also removed the starting methionine of the cytoplasmic tail of siaT to prevent alternative translation initiation. Both chimeras were found in the GA in stably transfected HepG2 cells, as indicated by a colabeling with giantin by confocal immunofluorescence (Figure 9, A and C). On treatment with 2 μM monensin both chimeras were separated from giantin into swollen vesicles (Figure 9, B and D). This clearly demonstrated that the cytoplasmic domain of galT is necessary and sufficient to target a protein into swollen vesicles induced by treatment with monensin.
Figure 9.
Fusion of the cytoplasmic tail of galT to siaT-GFP confers monensin sensitivity. (A and C) In the GA of control cells, stably transfected GSSS-GFP (A, green) and GSGG-GFP (C, green) colocalize with endogenous giantin (red). (B and D) On treatment with 2 μM monensin for 30 min both GSSS-GFP (B, green) and GSGG-GFP (D, green) dissociate from giantin (red) and are redistributed to swollen vesicles. Bar, 10 μm.
DISCUSSION
In this study we demonstrate by time-lapse videomicroscopy that there are two classes of Golgi glycosyltransferases with respect to their trafficking behavior in the presence of pH-disturbing agents such as monensin. This has been documented by comparing galT and siaT, two glycosyltransferases colocalized in the trans cisternae of the GA (Rabouille et al., 1995; Kweon et al., 2004): a dramatic shift of galT to swollen vesicles could be observed within minutes upon addition of monensin, whereas siaT remained Golgi-associated (Figure 2). The separation of these two enzymes in the presence of monensin was confirmed by quantitative subcellular fractionation. Furthermore, the origin of the swollen vesicles was shown to be derived from the TGN by colocalization of galT with the established marker protein TGN46 (Prescott et al., 1997) upon treatment with monensin. The 13 N-terminal amino acids of galT were identified as necessary and the entire cytoplasmic tail as sufficient to confer monensin sensitivity, suggesting the presence of sorting signals that mediate a continuous cycling of galT from its major localization in the trans-Golgi stack to the TGN and back.
Mechanism of Monensin Effect on the Secretory Pathway
In contrast to our findings, monensin was previously suggested to impose a trafficking block between the medial and the trans cisternae of the Golgi (Griffiths et al., 1983; Quinn et al., 1983). In fact, a medial Golgi block was always difficult to reconcile with the presence of galT, an established trans-Golgi marker, in monensin-induced swollen vesicles, as first described by Strous (1986). Additional reports made clear that Golgi proteins can be separated into two groups: one group that is affected by treatment with monensin such as galT (Berger et al., 1993), α1,3-fucosyltransferase 6 (Borsig et al., 1999), Golgi phosphoprotein of 130 kDa (GPP130), and Golgi protein-73 (GP73; Puri et al., 2002) and redistributes to swollen vesicles, and another group that includes proteins such as glucose-6-phosphatase (Griffiths et al., 1983), siaT (Berger et al., 1993), mannosidase II, N-acetylgalactosaminyltransferase 2, and giantin (Berger et al., 2001), which show little disturbance in presence of monensin. Such a spatial separation of the enzymes upon monensin treatment into different compartments obviously leads to a heterogeneous phenotype of impaired glycosylation. For review see Dinter and Berger (1998).
The Nature of galT-containing Swollen Vesicles
The strongest evidence of the present study that the swollen vesicles induced by monensin originate from the TGN is, on one hand, their fast appearance after the addition of monensin from Golgi (-like) structures (Figure 2), indicating that they are not derived from endosomes or ER and, on the other hand, the colocalization of galT containing swollen vesicles with TGN46 (Figure 4), a marker protein of the TGN (Prescott et al., 1997). Additional evidence that the swollen vesicles are TGN derived is provided by showing that in monensin-treated cells, galT-GFP was insensitive to BFA and remained in swollen vesicles (Figure 3). As shown by Berger et al. (2001), galT trapped in these vesicles does not return to the GA when monensin is replaced by chloroquine, an agent which blocks exit from an acidic post-Golgi compartment (as shown for TGN38; Chapman and Munro, 1994). The swollen vesicles may still release a small fraction of galT to an anterograde pathway leading to shedding, because monensin only slows but does not block post-Golgi trafficking of galT (Strous et al., 1985). That swollen vesicles originate from the TGN was first observed in a very elegant study of Zhang et al. (1993) conducted in sycamore maple suspension cells, showing that swollen vesicles were formed specifically from the TGN upon treatment with monensin and that the organization of the remaining Golgi stacks stayed intact as analyzed by electron microscopy.
Distinction between TGN and trans-Golgi Cisternae
Because morphological assignments based on single labeling experiments are not precise enough to differentiate trans-Golgi and TGN, siaT was often referred to as a TGN-localized transferase (see for example, Gleeson et al., 2004). If the TGN is operationally defined as the part of the secretory pathway that is not subject to BFA-induced retrograde flow, our data clearly associate siaT to the trans-Golgi cisternae codistributed with galT (Figure 3), which was also demonstrated by previous fractionation studies (Strous et al., 1993). Indeed, qualitatively we observed no difference in reactivity to BFA for both galT and siaT (Figure 3). Importantly, the TGN can also be delineated by the presence of specific markers such as TGN38/46, which revealed a different reaction to BFA, i.e., a collapse of TGN membranes around the microtubule organizing center (MTOC; Reaves and Banting, 1992). Transition of membrane or cargo proteins from the proximal to the distal part of the BFA divide is poorly understood and may involve vesicular transfer, fusion of trans-Golgi subdomains with the TGN, or maturation of (parts of) trans-Golgi cisternae to become the TGN.
Evidence for Golgi-sorting Signals Mediating Golgi-to-TGN Transition
In many instances, molecular determinants were localized within proteins mediating either their vesicular transfer or transition via a microdomain (for recent reviews see Aridor and Traub, 2002; Bonifacino and Traub, 2003; Robinson, 2004). Therefore, we investigated whether exchanging the domains (cytoplasmic tail, transmembrane domain, and stalk region) between galT-GFP and siaT-GFP would provide evidence for such determinants. Indeed, the cytoplasmic tail of galT was found necessary for monensin sensitivity: replacing the cytoplasmic tail of galT by the cytoplasmic tail of siaT (SGG-GFP) made galT insensitive to monensin; conversely replacing the cytosolic tail of siaT by the cytosolic tail of galT (GSS) conferred monensin sensitivity to siaT (Figure 7). Furthermore, by transferring the cytoplasmic tail of galT onto full-length siaT-GFP (GSSS-GFP), we could show that the monensin-induced redistribution to swollen vesicles is a dominant effect (Figure 9), suggesting a positive sorting signal that is sufficient to mediate the transport from the trans-Golgi to the TGN. A more detailed analysis of the short and the long iso-form of galT, which are the result of an alternative start methionine within the cytosolic tail, demonstrated that only the first 13 amino acids are required for monensin sensitivity.
Current Concepts of Trafficking of galT
GalT's primary localization is the GA where, in steady state, more than 90% of total cell-associated enzyme is accumulated (Rhee et al., 2005). Over time a small fraction is shed from the cell in a soluble and enzymatically active form (Strous and Berger, 1982). Of the internal pool, the fraction of galT that is not localized to the GA appears to be cycling through proximal compartments of the secretory pathway (Zaal et al., 1999; Rhee et al., 2005). In addition, the two iso-forms of galT, resulting from alternative translation initiation, display a difference in post-Golgi trafficking. Although the long isoform of galT has been shown to move to the plasma membrane, the short isoform has not been found at the plasma membrane (Hathaway et al., 2003). Our data support the finding that the two isoforms exhibit different intracellular trafficking properties and that the short isoform may be a true Golgi resident. Shur and associates (Hathaway et al., 2003) showed that phosphorylation of the long isoform of galT promotes its retention in the Golgi. However, in our hands phosphorylation mutants of the long isoform do not influence monensin sensitivity. This also indicates that phosphorylation is not critical for the transition from trans-Golgi to the TGN because the phosphorylation mutants are also found in monensin-induced swollen vesicles derived from the TGN.
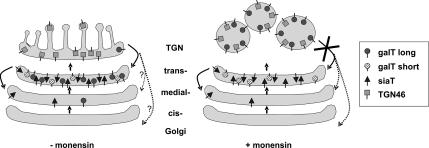
Taking all these findings together, we propose a model (Figure 10), in which galT is localized to the Golgi by its transmembrane domain as shown previously by several groups (reviewed by Colley, 1997). Although the short isoform of galT is resident to the trans cisterna, the long isoform contains a forward signal mediating its transport from the trans-Golgi cisterna to the TGN and an additional signal for its return to the trans-Golgi, permitting this form to constantly cycle between the two compartments. On monensin treatment, retrograde transport from the trans-Golgi network back to the trans-Golgi cisterna, but not anterograde transport (Strous et al., 1985), is blocked and galT is found in TGN-derived swollen vesicles together with other TGN proteins such as TGN46 or cation-independent mannose 6-phosphate receptor. Although phosphorylation appears to have no influence on the cycling of galT between trans-Golgi cisterna and TGN, it might well be that it is involved in a regulated exit from the TGN to the plasma membrane, as suggested by the group of Shur (Youakim et al., 1994). The difference in behavior of galT and siaT upon monensin treatment could also explain the different findings regarding Golgi inheritance during mitosis. Although galT-GFP was found within the ER during mitosis (Altan-Bonnet et al., 2006), siaT-FKBP could not be trapped in the ER during mitosis (Pecot and Malhotra, 2004). Whether this difference goes along with the different sensitivity to monensin may be clarified by further work by using both proteins under both experimental conditions.
Figure 10.
Model for GA-to-TGN transition. In the steady state, long and short iso-forms of galT as well as siaT are accumulated in the trans-Golgi. GalT is assumed to constantly cycle from the trans-Golgi to the TGN and back to nondefined proximal sites of the secretory pathway. In presence of monensin, galT is arrested in TGN-derived swollen vesicles where it colocalizes with TGN46. Anterograde transport from those sites is retarded only (Strous et al., 1985), whereas retrograde transport is blocked, permitting accumulation in swollen vesicles.
Taken together, our data show different dynamics of siaT and galT and suggest the existence of molecular determinants that mediate trans-Golgi to TGN transition of galT as well the recycling from TGN back to the trans-Golgi.
Supplementary Material
ACKNOWLEDGMENTS
We thank H. P. Hauri for kindly providing antibody against Na+K+-ATPase. This work was supported by Swiss National Science Foundation Grant 3100A0-109782 to E.B. and Grant 3100A0-108231 to J.R.
Abbreviations used:
- galT
β1,4-galactosyltransferase 1
- siaT
α2,6-sialyltransferase 1
- GA
Golgi apparatus
- TGN
trans-Golgi network
- ER
endoplasmic reticulum
- BFA
brefeldin A
- ECL
enhanced chemiluminescence.
Footnotes

 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-08-0665) on October 4, 2006.
REFERENCES
- Altan-Bonnet N., Sougrat R., Liu W., Snapp E. L., Ward T., Lippincott-Schwartz J. Golgi inheritance in mammalian cells is mediated through endoplasmic reticulum export activities. Mol. Biol. Cell. 2006;17:990–1005. doi: 10.1091/mbc.E05-02-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aridor M., Traub L. M. Cargo selection in vesicular transport: the making and breaking of a coat. Traffic. 2002;3:537–546. doi: 10.1034/j.1600-0854.2002.30804.x. [DOI] [PubMed] [Google Scholar]
- Balch W. E., Rothman J. E. Characterization of protein transport between successive compartments of the Golgi apparatus: asymmetric properties of donor and acceptor activities in a cell-free system. Arch. Biochem. Biophys. 1985;240:413–425. doi: 10.1016/0003-9861(85)90046-3. [DOI] [PubMed] [Google Scholar]
- Banting G., Ponnambalam S. TGN38 and its orthologues: roles in post-TGN vesicle formation and maintenance of TGN morphology. Biochim. Biophys. Acta. 1997;1355:209–217. doi: 10.1016/s0167-4889(96)00146-2. [DOI] [PubMed] [Google Scholar]
- Barlowe C. COPII: a membrane coat that forms endoplasmic reticulum-derived vesicles. FEBS Lett. 1995;369:93–96. doi: 10.1016/0014-5793(95)00618-j. [DOI] [PubMed] [Google Scholar]
- Berger E. G. How Golgi-associated glycosylation works. Cell Biol. Int. Rep. 1985;9:407–417. doi: 10.1016/0309-1651(85)90149-3. [DOI] [PubMed] [Google Scholar]
- Berger E. G., Aegerter E., Mandel T., Hauri H. P. Monoclonal antibodies to soluble, human milk galactosyltransferase (lactose synthase A protein) Carbohydr. Res. 1986;149:23–33. doi: 10.1016/s0008-6215(00)90366-5. [DOI] [PubMed] [Google Scholar]
- Berger E. G., Berger B., Höchli M., Dinter A. Colocalization of beta 1,4 galactosyltransferase with mannose 6-phosphate receptor in monensin-induced TGN-derived structures. Histochem. Cell Biol. 2001;115:157–168. doi: 10.1007/s004180000231. [DOI] [PubMed] [Google Scholar]
- Berger E. G., Grimm K., Bächi T., Bosshart H., Kleene R., Watzele M. Double immunofluorescent staining of alpha 2,6 sialyltransferase and beta 1,4 galactosyltransferase in monensin-treated cells: evidence for different Golgi compartments? J. Cell. Biochem. 1993;52:275–288. doi: 10.1002/jcb.240520304. [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S., Traub L. M. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- Borsig L., Imbach T., Höchli M., Berger E. G. alpha 1,3 Fucosyltransferase VI is expressed in HepG2 cells and codistributed with beta1,4galactosyltransferase I in the Golgi apparatus and monensin-induced swollen vesicles. Glycobiology. 1999;9:1273–1280. doi: 10.1093/glycob/9.11.1273. [DOI] [PubMed] [Google Scholar]
- Bretz R., Bretz H., Palade G. E. Distribution of terminal glycosyltransferases in hepatic Golgi fractions. J. Cell Biol. 1980;84:87–101. doi: 10.1083/jcb.84.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J. E., Breitfeld P. P., Ross S. A., Mostov K. E. Phosphorylation of the polymeric immunoglobulin receptor required for its efficient transcytosis. Science. 1990;248:742–745. doi: 10.1126/science.2110383. [DOI] [PubMed] [Google Scholar]
- Chapman R. E., Munro S. Retrieval of TGN proteins from the cell surface requires endosomal acidification. EMBO J. 1994;13:2305–2312. doi: 10.1002/j.1460-2075.1994.tb06514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherfils J., Melancon P. On the action of brefeldin A on Sec7-stimulated membrane-recruitment and GDP/GTP exchange of Arf proteins. Biochem. Soc. Trans. 2005;33:635–638. doi: 10.1042/BST0330635. [DOI] [PubMed] [Google Scholar]
- Colley K. J. Golgi localization of glycosyltransferases: more questions than answers. Glycobiology. 1997;7:1–13. doi: 10.1093/glycob/7.1.1-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinter A., Berger E. G. Golgi-disturbing agents. Histochem. Cell Biol. 1998;109:571–590. doi: 10.1007/s004180050256. [DOI] [PubMed] [Google Scholar]
- Donaldson J. G., Finazzi D., Klausner R. D. Brefeldin A inhibits Golgi membrane-catalysed exchange of guanine nucleotide onto ARF protein. Nature. 1992;360:350–352. doi: 10.1038/360350a0. [DOI] [PubMed] [Google Scholar]
- Gleeson P. A., Lock J. G., Luke M. R., Stow J. L. Domains of the TGN: coats, tethers and G proteins. Traffic. 2004;5:315–326. doi: 10.1111/j.1398-9219.2004.00182.x. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Quinn P., Warren G. Dissection of the Golgi complex. I. Monensin inhibits the transport of viral membrane proteins from medial to trans Golgi cisternae in baby hamster kidney cells infected with Semliki Forest virus. J. Cell Biol. 1983;96:835–850. doi: 10.1083/jcb.96.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Simons K. The trans Golgi network: sorting at the exit site of the Golgi complex. Science. 1986;234:438–443. doi: 10.1126/science.2945253. [DOI] [PubMed] [Google Scholar]
- Hathaway H. J., Evans S. C., Dubois D. H., Foote C. I., Elder B. H., Shur B. D. Mutational analysis of the cytoplasmic domain of beta 1,4-galactosyltransferase I: influence of phosphorylation on cell surface expression. J. Cell Sci. 2003;116:4319–4330. doi: 10.1242/jcs.00720. [DOI] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Kweon H.-S., et al. Golgi enzymes are enriched in perforated zones of Golgi cisternae but are depleted in COPI vesicles. Mol. Biol. Cell. 2004;15:4710–4724. doi: 10.1091/mbc.E03-12-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linstedt A. D., Hauri H. P. Giantin, a novel conserved Golgi membrane protein containing a cytoplasmic domain of at least 350 kDa. Mol. Biol. Cell. 1993;4:679–693. doi: 10.1091/mbc.4.7.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Donaldson J. G., Schweizer A., Berger E. G., Hauri H. P., Yuan L. C., Klausner R. D. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990;60:821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L., Tipper C., Amherdt M., Orci L., Klausner R. D. Brefeldin A's effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell. 1991;67:601–616. doi: 10.1016/0092-8674(91)90534-6. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L. C., Bonifacino J. S., Klausner R. D. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989;56:801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marxer A., Stieger B., Quaroni A., Kashgarian M., Hauri H. P. (Na++ K+)-ATPase and plasma membrane polarity of intestinal epithelial cells: presence of a brush border antigen in the distal large intestine that is immunologically related to beta subunit. J. Cell Biol. 1989;109:1057–1069. doi: 10.1083/jcb.109.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollenhauer H. H., Morre D. J., Rowe L. D. Alteration of intracellular traffic by monensin; mechanism, specificity and relationship to toxicity. Biochim. Biophys. Acta. 1990;1031:225–246. doi: 10.1016/0304-4157(90)90008-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neutra M., Leblond C. P. Synthesis of the carbohydrate of mucus in the Golgi complex as shown by electron microscope radioautography of goblet cells from rats injected with glucose-H3. J. Cell Biol. 1966;30:119–136. doi: 10.1083/jcb.30.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson J., Colley K. Glycosyltransferases. Structure, localization, and control of cell type- specific glycosylation. J. Biol. Chem. 1989;264:17615–17618. [PubMed] [Google Scholar]
- Pecot M. Y., Malhotra V. Golgi membranes remain segregated from the endoplasmic reticulum during mitosis in mammalian cells. Cell. 2004;116:99–107. doi: 10.1016/s0092-8674(03)01068-7. [DOI] [PubMed] [Google Scholar]
- Prescott A. R., Lucocq J. M., James J., Lister J. M., Ponnambalam S. Distinct compartmentalization of TGN46 and beta 1,4-galactosyltransferase in HeLa cells. Eur. J. Cell Biol. 1997;72:238–246. [PubMed] [Google Scholar]
- Puri S., Bachert C., Fimmel C. J., Linstedt A. D. Cycling of early Golgi proteins via the cell surface and endosomes upon lumenal pH disruption. Traffic. 2002;3:641–653. doi: 10.1034/j.1600-0854.2002.30906.x. [DOI] [PubMed] [Google Scholar]
- Quinn P., Griffiths G., Warren G. Dissection of the Golgi complex. II. Density separation of specific Golgi functions in virally infected cells treated with monensin. J. Cell Biol. 1983;96:851–856. doi: 10.1083/jcb.96.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabouille C., Hui N., Hunte F., Kieckbusch R., Berger E. G., Warren G., Nilsson T. Mapping the distribution of Golgi enzymes involved in the construction of complex oligosaccharides. J. Cell Sci. 1995;108:1617–1627. doi: 10.1242/jcs.108.4.1617. [DOI] [PubMed] [Google Scholar]
- Reaves B., Banting G. Perturbation of the morphology of the trans-Golgi network following Brefeldin A treatment: redistribution of a TGN-specific integral membrane protein, TGN38. J. Cell Biol. 1992;116:85–94. doi: 10.1083/jcb.116.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. W., Starr T., Forsten-Williams K., Storrie B. The steady-state distribution of glycosyltransferases between the Golgi apparatus and the endoplasmic reticulum is approximately 90:10. Traffic. 2005;6:978–990. doi: 10.1111/j.1600-0854.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- Robinson M. S. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004;14:167–174. doi: 10.1016/j.tcb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Rohrer J., Kornfeld R. Lysosomal hydrolase mannose 6-phosphate uncovering enzyme resides in the trans-Golgi network. Mol. Biol. Cell. 2001;12:1623–1631. doi: 10.1091/mbc.12.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer J., Schweizer A., Johnson K. F., Kornfeld S. A determinant in the cytoplasmic tail of the cation-dependent mannose 6-phosphate receptor prevents trafficking to lysosomes. J. Cell Biol. 1995;130:1297–1306. doi: 10.1083/jcb.130.6.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Berger E. G. Immunocytochemical localization of galactosyltransferase in HeLa cells: codistribution with thiamine pyrophosphatase in trans-Golgi cisternae. J. Cell Biol. 1982;93:223–229. doi: 10.1083/jcb.93.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo R. N., Shaper N. L., Taatjes D. J., Shaper J. H. Beta 1,4-galactosyltransferase: a short NH2-terminal fragment that includes the cytoplasmic and transmembrane domain is sufficient for Golgi retention. J. Biol. Chem. 1992;267:9241–9247. [PubMed] [Google Scholar]
- Sambrook J., Fritsch E. F., Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1998. [Google Scholar]
- Sciaky N., Presley J., Smith C., Zaal K. J., Cole N., Moreira J. E., Terasaki M., Siggia E., Lippincott-Schwartz J. Golgi tubule traffic and the effects of brefeldin A visualized in living cells. J. Cell Biol. 1997;139:1137–1155. doi: 10.1083/jcb.139.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shur B. D., Evans S., Lu Q. Cell surface galactosyltransferase: current issues. Glycoconj. J. 1998;15:537–548. doi: 10.1023/a:1006951407168. [DOI] [PubMed] [Google Scholar]
- Storrie B. Maintenance of Golgi apparatus structure in the face of continuous protein recycling to the endoplasmic reticulum: making ends meet. In: Kwang W. J., editor. A Survey of Cell Biology. Academic Press: New York; 2005. pp. 69–94. [DOI] [PubMed] [Google Scholar]
- Strous G., Berger E. G., van Kerkhof P., Bosshart H., Berger B., Geuze H. J. Brefeldin A induces a microtubule-dependent fusion of galactosyltransferase-containing vesicles with the rough endoplasmic reticulum. Biol. Cell. 1990;71:25–31. doi: 10.1016/0248-4900(91)90048-r. [DOI] [PubMed] [Google Scholar]
- Strous G. J. Golgi and secreted galactosyltransferase. CRC Crit. Rev. Biochem. 1986;21(2):119–151. doi: 10.3109/10409238609113610. [DOI] [PubMed] [Google Scholar]
- Strous G. J., Berger E. G. Biosynthesis, intracellular transport, and release of the Golgi enzyme galactosyltransferase (lactose synthetase A protein) in HeLa cells. J. Biol. Chem. 1982;257:7623–7628. [PubMed] [Google Scholar]
- Strous G. J., Kerkhof P., Fallon R. J., Schwartz A. L. Golgi galactosyltransferase contains serine-linked phosphate. Eur. J. Biochem. 1987;169:307–311. doi: 10.1111/j.1432-1033.1987.tb13613.x. [DOI] [PubMed] [Google Scholar]
- Strous G. J., Van Kerkhof P., Berger E. G. In vitro biosynthesis of two human galactosyltransferase polypeptides. Biochem. Biophys. Res. Commun. 1988;151:314–319. doi: 10.1016/0006-291x(88)90595-5. [DOI] [PubMed] [Google Scholar]
- Strous G. J., van Kerkhof P., van Meer G., Rijnboutt S., Stoorvogel W. Differential effects of brefeldin A on transport of secretory and lysosomal proteins. J. Biol. Chem. 1993;268:2341–2347. [PubMed] [Google Scholar]
- Strous G. J., Van Kerkhof P., Willemsen R., Slot J. W., Geuze H. J. Effect of monensin on the metabolism, localization, and biosynthesis of N- and O-linked oligosaccharides of galactosyltransferase. Eur. J. Cell Biol. 1985;36:256–262. [PubMed] [Google Scholar]
- Taatjes D. J., Roth J., Weinstein J., Paulson J. C., Shaper N. L., Shaper J. H. Codistribution of galactosyl- and sialyltransferase: reorganization of trans Golgi apparatus elements in hepatocytes in intact liver and cell culture. Eur. J. Cell Biol. 1987;44:187–194. [PubMed] [Google Scholar]
- Tartakoff A. M. Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell. 1983;32:1026–1028. doi: 10.1016/0092-8674(83)90286-6. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watzele G., Bachofner R., Berger E. G. Immunocytochemical localization of the Golgi apparatus using protein-specific antibodies to galactosyltransferase. Eur. J. Cell Biol. 1991;56:451–458. [PubMed] [Google Scholar]
- Watzele G., Berger E. G. Near identity of HeLa cell galactosyltransferase with the human placental enzyme. Nucleic Acids Res. 1990;18:7174. doi: 10.1093/nar/18.23.7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youakim A., Dubois D. H., Shur B. D. Localization of the long form of beta-1,4-galactosyltransferase to the plasma membrane and Golgi complex of 3T3 and F9 cells by immunofluorescence confocal microscopy. Proc. Natl. Acad. Sci. USA. 1994;91:10913–10917. doi: 10.1073/pnas.91.23.10913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaal K. J., et al. Golgi membranes are absorbed into and reemerge from the ER during mitosis. Cell. 1999;99:589–601. doi: 10.1016/s0092-8674(00)81548-2. [DOI] [PubMed] [Google Scholar]
- Zhang G. F., Driouich A., Staehelin L. A. Effect of monensin on plant Golgi: re-examination of the monensin-induced changes in cisternal architecture and functional activities of the Golgi apparatus of sycamore suspension-cultured cells. J. Cell Sci. 1993;104:819–831. doi: 10.1242/jcs.104.3.819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.