Abstract
Wnts are lipid-modified secreted glycoproteins that regulate diverse biological processes. We report that Wnt5a, which functions in noncanonical Wnt signaling, has activity on endothelial cells. Wnt5a is endogenously expressed in human primary endothelial cells and is expressed in murine vasculature at several sites in mouse embryos and tissues. Expression of exogenous Wnt5a in human endothelial cells promoted angiogenesis. Wnt5a induced noncanonical Wnt signaling in endothelial cells, as measured by Dishevelled and ERK1/2 phosphorylation, and inhibition of canonical Wnt signaling, a known property of Wnt5a. Wnt5a induced endothelial cell proliferation and enhanced cell survival under serum-deprived conditions. The Wnt5a-mediated proliferation was blocked by Frizzled-4 extracellular domain. Wnt5a expression enhanced capillary-like network formation, whereas reduction of Wnt5a expression decreased network formation. Reduced Wnt5a expression inhibited endothelial cell migration. Screening for Wnt5a-regulated genes in cultured endothelial cells identified several encoding angiogenic regulators, including matrix metalloproteinase-1, an interstitial collagenase, and Tie-2, a receptor for angiopoietins. Thus, Wnt5a acts through noncanonical Wnt signaling to promote angiogenesis.
INTRODUCTION
The Wnt family of proteins comprises a group of secreted, lipid-modified glycoproteins that play crucial roles in the regulation of developmental patterning, cell proliferation, differentiation, polarity, and morphogenetic movement (Logan and Nusse, 2004). Wnts trigger intracellular responses through various signaling pathways, referred to as canonical or noncanonical, utilizing the Frizzled family of receptors (Veeman et al., 2003). Although canonical Wnts signal by blocking degradation of cytosolic β-catenin and hence activating β-catenin/TCF transcriptional responses, noncanonical Wnts are thought to activate the Wnt/Ca2+, Wnt/planar cell polarity (PCP) or other less well-defined pathways. However, a consistent response to noncanonical Wnt signals is the phosphorylation of Dishevelled (Dvl) proteins (Gonzalez-Sancho et al., 2004). Wnt5a has been shown to function in noncanonical Wnt signaling in several systems. For example, expression of Wnt5a in zebra fish or Xenopus embryos can stimulate intracellular Ca2+ fluxes (Slusarski et al., 1997), and Wnt5a regulates morphogenetic movements during gastrulation independently of canonical signaling (Kilian et al., 2003). Consistent with noncanonical signaling, ectopic overexpression of Wnt5a in human primary endothelial cells did not stabilize cytosolic β-catenin or activate β-catenin/TCF-mediated transcription (Wright et al., 1999; Masckauchan et al., 2005).
Wnt5a is essential for proper skeletal development as nullizygous mice exhibit perinatal lethality and appendicular structures outgrowing from the primary body-axis fail to extend (Yamaguchi et al., 1999). This defect in morphogenesis is associated with decreased cell proliferation in tissues crucial to outgrowing structures. In addition, Wnt5a may also participate in hematopoietic stem cell fate decisions (Murdoch et al., 2003). Wnt5a can also negatively influence proliferation, for instance in B cells (Liang et al., 2003). Increased expression of Wnt5a has been reported in malignant melanoma, primary human breast cancer, and colon cancer, as well as cancer from other tissues, suggesting a pathological effect on tumorigenesis that may be either positive or negative (Iozzo et al., 1995; Lejeune et al., 1995; Jonsson et al., 2002; Weeraratna et al., 2002; Dejmek et al., 2005).
The signaling cascades that are activated by Wnt5a to regulate proliferation are not well defined. Wnt5a can interact with Wnt receptors Frizzled-2 (Slusarski et al., 1997), Frizzled-4 (Chen et al., 2003), Frizzled-5 (Sen et al., 2001), and Frizzled-7 (Umbhauer et al., 2000). In different systems, Wnt5a has been shown to activate protein kinase C (Weeraratna et al., 2002), C-Jun N-terminal kinase (Yamanaka et al., 2002) and induce phosphorylation of Dishevelled (Gonzalez-Sancho et al., 2004; Schulte et al., 2005). Signal activation by Wnt5a can also negatively influence the Wnt/β-catenin pathway by several different mechanisms (Saneyoshi et al., 2002; Ishitani et al., 2003; Topol et al., 2003).
Expression of Wnt5a has been reported in human endothelial cells (Wright et al., 1999; Masckauchan et al., 2005), yet its function in endothelium has remained largely unknown. Wnt signaling has been linked to a human vascular disorder by the finding that Frizzled-4, which might be involved in signaling via both canonical and noncanonical pathways, is critical for proper retinal vascular development (Robitaille et al., 2002; Xu et al., 2004). Whether one or both of these two pathways is critical for retinal angiogenesis is still not clear. Because Wnt5a is a known inducer of noncanonical signaling, we ectopically expressed Wnt5a in endothelial cells in order to ask whether noncanonical signaling promotes angiogenesis. To address this question, we utilized a variety of in vitro assays to assess the activity of Wnt5a on proliferation, survival, and morphogenesis of endothelial cells and searched for regulation of angiogenic genes via Wnt5a.
MATERIALS AND METHODS
Cells and Reagents
Human umbilical venous endothelial cells (HUVEC) were isolated from human umbilical vein as previously described (Jaffe et al., 1973) and grown in EGM2 (BioWhittaker, Walkersville, MD) with VEGF, bFGF, EGF, IGF-1, and 2% FBS on dishes coated with type I rat tail collagen (Upstate Biotechnology, Lake Placid, NY). Human microvascular endothelial cells (HMVEC) from human neonatal forskin and their media were obtained from BioWhittaker. Adenoviruses encoding for Wnts or mutant β-catenin were prepared as described (Young et al., 2003). Purified Wnt5a protein was obtained from R&D Systems (Minneapolis, MN). Antibodies were used to recognize human β-catenin (BD Transduction Laboratories, Lexington, KY), Dishevelled2 or Dishevelled3 (Santa Cruz Biotechnology, Santa Cruz, CA), matrix metalloproteinase-1 (MMP-1) and Tie2 (R&D Systems), total and phospho-p44/p42 (ERK1/2; Cell Signaling Technology, Beverly, MA) or α-tubulin antibody (Sigma-Aldrich Life Sciences, St. Louis, MO). SuperTopFlash and SuperFopFlash Tcf-luciferase reporter constructs were generously provided by Dr. Randall Moon (University of Washington School of Medicine, Seattle, WA).
RT-PCR Analysis
Low passage human primary endothelial cells were cultured in EGM-2 BulletKit (BioWhittaker). Total RNA was isolated using RNeasy Protect kit (Qiagen, Hilden, Germany), and reverse transcription reaction was performed using Omniscript Reverse Transcriptase as described by manufacturer's instructions (Qiagen) and PCR was done using Platinum Taq DNA Polymerase (Invitrogen, Carlsbad, CA). Conditions for Wnt5a and Wnt-5b PCR were 94°C for 3 min followed by 35 cycles of 94°C for 1 min, 58°C for 1 min, and 72°C for 1 min, plus 2 min at 72°C. Primers used were 5′GTGCAATGTCTTCCAAGTTCTTC 3′ forward and 5′ GGCACAGTTTCTTCTGTCCTTG 3′ reverse for Wnt5a, and 5′ GACGCCAACTCCTGGTGGC 3′ forward and 5′GCATGACTCTCCCAAAGACAGATG 3′ reverse for Wnt-5b, and product sizes were 195 and 258 base pairs, respectively. PCR for MMP-1 was performed using an MMP-1 primer pair (R&D Systems), following manufacturer's instructions. Primers for Tie2 were as described (Hewett et al., 1998). To correct for sample variations in RT-PCR efficiency, β-actin expression was used to normalize the samples using the following primers: 5′ CGAGGCCCAGAGCAAGAGAG 3′, upper primer; 5′ CTCGTAGATGGGCACAGTGTG 3′, lower primer with a product size of 336 base pairs. Conditions for β-actin PCR were 94°C for 2 min followed by 20 cycles of 94°C for 45 s, 60°C for 1 min, and 72°C for 1 min for 20 cycles, plus 5 min at 72°C.
Immunohistochemistry Staining and In Situ Hybridization
In situ hybridization was performed as described (Schaeren-Wiemers and Gerfin-Moser, 1993) using the Dig labeling method for preparation of in situ probes, provided by the manufacturer (Roche Applied Science, Indianapolis, IN). Probes used were mouse Wnt5a (entire coding region) and mouse Wnt-5b (3′UTR). Plasmids were digested with XhoI, and in vitro transcription was performed with SP6 polymerase. The detection of hybridized mRNA in sections was performed using the NBT/BCIP developing system (Roche Applied Science). Immunohistochemistry staining was performed as described (Vorontchikhina et al., 2005) using antibodies recognizing PECAM at 1:500 dilution (PharMingen, San Diego, CA), Wnt5a at 1:100 dilution (R&D Systems) or Frizzled-4 at 1:100 (R&D Systems).
Reporter Assays
Cells were seeded on collagen-coated 24-well plates (33,000 cells per well). The next day, cells were transfected with either Tcf/Lef transcriptional activation reporter construct SuperTopFlash containing Tcf responsive elements or SuperFopFlash with mutated elements (control) and a renilla-luciferase construct. Transfections were performed in triplicates using 0.18 μg of reporter plasmid, 0.02 μg of renilla-luciferase plasmid and 0.45 μg of inducer plasmid in total with 1.3 μl of Lipofectine in OptiMEM media (Invitrogen) per well, following manufacturer's instructions for procedure. Cells were incubated at 37°C for 5 h with transfection cocktail and then were incubated overnight with fresh full endothelial culture media. Cell lysates were prepared the next day and both firefly and renilla luciferase activities were evaluated using Dual-Luciferase Reporter Assay System (Promega, Madison, WI). Samples were read in a luminometer and values were normalized for transfection efficiency using renilla-luciferase activity. The ratio SuperTop (sTop)/SuperFop (sFop) was calculated and charted to evaluate induction.
Gene Transfer into HUVEC
To infect cells, 4 × 105 passage 3–5 cells were trypsinized and resuspended in 300 μl full culture medium. Adenovirus stock was added at 30 MOI, unless indicated otherwise, for each experiment, and cells were incubated at 37°C for 1 h with gentle shaking every 10 min. Then, cells were seeded onto type I collagen-coated plates and harvested 24–48 h later, depending on the assay. For retroviral gene transfer, the retroviral vector pHyTCX was used. GP293 packaging cells, 5.0 × 106 (Clontech, Palo Alto, CA), were seeded and transfected with 10 μg pHyTC-genes and pVSVG, and retroviral-containing supernatants were collected 48 h later. Retroviruses were added to passage 3 HUVEC, and 48 h later cells were selected for 5 d with hygromycin B at 300 μg/ml and then maintained with hygromycin B at 100 μg/ml. An expression vector encoding the HA-tag (pHyTC-HA) was used as a negative control.
Proliferation and Survival Assays In Vitro
Low-passage primary HUVEC were split and suspended in full endothelial culture media. Cells were infected with Wnt-adenoviruses, as described above, using adenovirus encoding for the LacZ gene as a negative control. Next, cells were seeded at 10,000 cells/well on 24-well plates that were previously collagen-coated containing full endothelial culture media. Cells were allowed to seed overnight, and the medium was then replaced with basal endothelial cell medium EBM-2 supplemented with bFGF. Cell numbers at 48 h after incubation with basal medium were assessed with the Cell Counting Kit-8 assay (Dojindo Molecular Technologies, Gaithersburg, MD). Frizzled-4-CRD competition experiments were performed using purified protein (R&D Systems) at 1 μg/ml. Survival assays were performed in a similar manner to that of proliferation assays, except cells were seeded at 100,000 cells per well in 48-well plates. All assays were performed in quadruplicate.
Wnt5a Gene Silencing
A series of six different target sequences in the human Wnt5a gene were chosen for screening of adequate silencing of this gene. Synthesized oligos were designed using these sequences and were cloned into pSIREN retroviral vector (BD Biosciences, San Jose, CA). Obtained plasmids were used to screen for successful silencing by transfecting 293 cells in culture. Levels of Wnt5a expression were evaluated after reverse transcription of the RNA samples, followed by PCR using primers for Wnt5a in conditions described above. The target sense sequence selected was 5′ AGTGCAATGTCTTCCAAGT 3′. Preparation of HUVEC retroviral lines was performed as described above, and successful silencing of the Wnt5a gene was confirmed by semiquantitative PCR.
Endothelial Network Formation and Migration Assay
Retrovirally selected HUVEC expressing Wnt5a-HA or control gene (HA tag) were analyzed by Western blot to confirm the overexpression of the desired protein. Matrigels were prepared using 24-well plates with 0.3 ml pure Growth Factor-reduced Matrigel (BD Biosciences) per well and incubating plates at 37°C for 1 h. Retrovirally selected HUVEC were seeded at 100,000 cells per well on top of Matrigel in the presence of 0.8 ml of full EC culture medium. Pictures were taken with 4× magnification after 18–19 h of incubation at 37°C. All experiments were performed in duplicates and repeated twice to confirm results. For endothelial migration experiments, cells were seeded at confluence and 1 d later a wound was made to the confluent cell monolayer, and migration of cells into the wounded area was documented 10 h later.
DNA Microarray Analysis
DNA array analysis was performed with cDNA obtained from HUVEC adeno-infected to express Wnt5a, Wnt-1, or LacZ gene (control) at 48 h after infection. Hu95Av2 GeneChips were purchased from Affymetrix (Santa Clara, CA). Microarray and probes were prepared and used as described before (Li et al., 2002), with three different samples per overexpressed gene. To analyze the GeneChip data from the Wnt5a, Wnt1, and LacZ control cells, we used the GeneSpring software package (Agilent Technologies, Wilmington, DE), first filtering to obtain all genes (Affymetrix probe sets) with Affymetrix presence calls in at least three experimental samples, then further filtering to obtain all genes with expression at least 1.5-fold greater than the experiment mean in at least two samples, and lastly carrying out ANOVA with a p value cutoff of 0.01 to find all genes that differed in their mRNA expression as a function of experimental condition (Wnt5a vs. Wnt1 vs. LacZ). The resulting group of 297 probe sets was subjected to hierarchical clustering using the GeneTree function of GeneSpring. This procedure grouped the genes into three clades: 1) high in LacZ but low in Wnt1 and Wnt5a; 2) high in Wnt5a but low or intermediate in LacZ and Wnt1; and 3) high in Wnt1 and low in LacZ and Wnt5a.
Activity Assay for MMP-1
Activity levels of secreted MMP-1 from conditioned media of HUVEC were evaluated using a Biotrak Activity Assay (Amersham Biosciences, Piscataway, NJ). Samples consisted of collected cell culture media of adeno-infected endothelial cells diluted 1:10 in 1× calibrator diluent. All samples were run in triplicates and compared with a standard curve performed according to the manufacturer's instructions.
Immunoblotting and Cell Surface Biotinylation
Wnt5a protein expression in HUVEC was evaluated by immunoblotting using antibodies to the HA epitope. Cells were lysed in TENT buffer (20 mM Tris-HCl, pH 8.0, 2 mM EDTA, 150 mM NaCl, 1% Triton X-100, added with 1 tablet of Complete Mini protease inhibitors [Roche Diagnostics, Mannheim, Germany] per 50 ml of buffer), as described (Shimizu et al., 1997). For Dishevelled immunoblots, lysates were prepared using cold RIPA buffer (1% Igepal CA-630, 1% sodium deoxycholate, 0.1% SDS, 150 mM NaCl, 10 mM Na2HPO4) pH 7.2, 50 mM NaF, and 2 mM EDTA added freshly with protease and phosphatase inhibitors 100 μg/ml PMSF, 1 mM sodium vanadate, 2 μM dithiothreitol, 10 μg/ml aprotinin, and 2 μg/ml leupeptin). Lysates were cleared by centrifugation and frozen immediately after preparation. For ERK1/2, cells were washed with cold PBS buffer and lysed using 1× SDS-PAGE sample buffer. Lysates were obtained by scraping cells from the plate, transferring to a microcentrifuge tube, and sonicating four times for 20 s to shear DNA and reduce viscosity. All lysates were heated at 100°C for 3 min and cooled on ice. Samples were fractionated using SDS-PAGE, transferred to nitrocellulose membrane, and incubated with antibodies. Proteins were visualized by Enhanced Chemiluminescence (Amersham Pharmacia Biotech) in conjunction with a horseradish peroxide (HRP)-tagged secondary antibody. To detect Tie2 receptor by Western blot, cell surface biotinylation followed by Western immunoblot was performed. Forty-eight hours after infection, adenovirus-infected HMVEC overexpressing Wnt5a, β-cateninS37A, or LacZ (control) grown in plates were washed three times with PBS and incubated with 0.5 mg/ml EZ-Link Sulfo-NHS-Biotin reagent (Pierce, Rockford, IL) in PBS for 30 min at 4°C. Cells were then incubated with cold DMEM for 10 min at 4°C and rinsed with PBS to wash off excess biotin. Cells were then lysed in TENT buffer as described above. Protein levels in lysates were evaluated by Bradford assay, and equal amounts of protein were adjusted to same volumes with TENT buffer. Lysates were then incubated overnight with 50 μL of streptavidin-conjugated beads (Pierce). Streptavidin beads were washed three times with TENT buffer, and proteins were eluted in boiling sample buffer. All Western blots were performed by separation on SDS-PAGE gels and transferring to PVDF membrane (Millipore, Bedford, MA). Membranes were probed with the corresponding primary antisera in presence of 2% BSA. Protein bands were visualized by enhanced chemiluminescence (Amersham Pharmacia Biotech) in conjunction with goat anti-mouse IgG-HRP.
RESULTS
Wnt5a Is Expressed in Isolated Human Endothelial Cells and Murine Vasculature
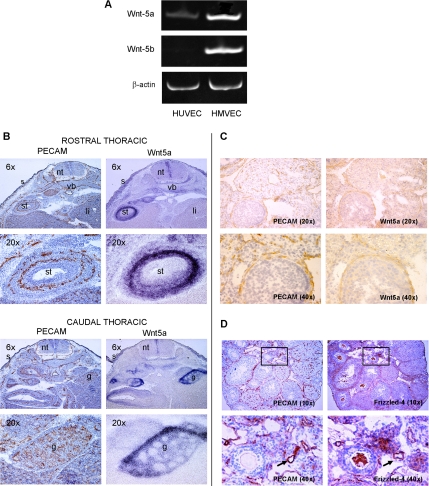
Endogenous expression of Wnt ligands in human and mouse endothelial cells has been previously analyzed (Wright et al., 1999; Cheng et al., 2003), yet their role in angiogenesis is still unclear. Wnt5a transcripts were detected in human primary endothelial cells from both large vessels (HUVEC) and microvasculature (HMVEC), as evaluated by RT-PCR (Figure 1A). In contrast, Wnt-5b transcripts were only observed in HMVEC (Figure 1A). In situ hybridization analysis for Wnt5a expression in mouse embryos was compared with the expression of the endothelial marker PECAM and revealed that Wnt5a is expressed either in the endothelium or in the vicinity of the endothelium, although not in all vascular structures (Figure 1B). Specifically, Wnt5a transcripts were found in developing skin, gonad, vertebral body, stomach, liver, and other organs. In the mouse ovary, a highly vascularized organ, immunohistochemical analysis of Wnt5a and PECAM confirmed that Wnt5a is expressed in ovarian vasculature surrounding follicles (Figure 1C). Frizzled-4, a candidate receptor for Wnt5a, was also expressed in ovary at several sites but notably in ovarian vasculature surrounding follicles (Figure 1D) and on major vessels (Figure 1D, bottom panel). We have also seen Frizzled-4 expression in murine retinal vasculature (Masckauchan and Kitajewski, 2006).
Figure 1.
Wnt5a and Frizzled-4 expression in vascular cells. (A) Wnt5a and Wnt-5b RT-PCR products from human primary endothelial cells, HUVEC and HMVEC, normalized to β-actin transcript levels. (B) In situ hybridization in mouse embryonic tissue at E13.5 for PECAM and Wnt5a. nt, neural tube; li, liver; st, stomach; vb, vertebral body; g, gonad; s, skin. (C) Immunohistochemistry with PECAM (left) and in situ hybridization with Wnt5a mRNA probe (right) in mouse embryonic tissues at E13.5. Brown color represents PECAM protein (left panels), and the nuclei are counterstained in blue. Wnt5a signal is seen in blue color on the in situ (right) panels. Both immunohistochemistry and in situ hybridizations were performed using adjacent serial sections. (D) Adult mouse ovaries were immunostained for either PECAM or Frizzled-4 to compare expression. Top, insets indicate the area shown at higher magnification in the bottom panels; bottom, arrows indicate blood vessels.
Wnt5a Induces Dishevelled Phosphorylation and ERK Phosphorylation and Inhibits Canonical Wnt Signaling in Human Endothelial Cells
Wnt5a signaling is known to induce phosphorylation of Dishevelled in several cell types, and this can be monitored by a mobility shift of Dvl proteins on Western blots (Gonzalez-Sancho et al., 2004; Schulte et al., 2005). This effect is independent of the canonical Wnt signaling coreceptors LRP5 and LRP6 and is thus a manifestation of noncanonical Wnt signaling (Gonzalez-Sancho et al., 2004). Analysis of endothelial cells treated with purified Wnt5a protein led to demonstrably increased phosphorylation of Dvl2 and Dvl3 (Figure 2A). To further analyze signaling downstream of Wnt5a in human endothelial cells, we developed a HA-tagged Wnt5a-expressing adenovirus vector (Ad-Wnt5a) that allows Wnt5a production in cultured primary endothelial cells (Figure 2B). Ectopically expressing Wnt5a also caused an increase in Dishevelled phosphorylation (not shown). Expression of Wnt5a via adenovirus also led to induction of ERK1/2 phosphorylation without affecting levels of total ERK1/2, as evaluated by Western blot (Figure 2C). Thus, Wnt5a induces Dvl phosphorylation and activates ERK1/2 in primary endothelial cells.
Figure 2.
Wnt5a induces Dishevelled and ERK1/2 phosphorylation and antagonizes canonical Wnt signaling in human endothelial cells. (A) Mobility shift assay for Dishevelled phosphorylation. HUVEC were treated with purified Wnt5a protein (75 ng/ml) for 2 h, after which lysates were prepared. Western blot to detect Dishevelled2 or Dishevelled3 was carried out using GSK3β as loading control. Rat2 cells overexpressing Wnt5a or not were used as a positive and negative controls, respectively. (B) Western blot assay showing ectopic expression of adenovirus-infected HUVEC overexpressing control LacZ gene versus Wnt5a-HA. (C) Western blot assay for total ERK1/2 and phosphorylated ERK1/2. HUVEC were adenovirus-infected to overexpress Wnt5a or control (LacZ) gene, and lysates were prepared 48 h after infection. (D) Wnt-5a antagonizes canonical signaling in endothelial cells. Tcf-responsive constructs SuperTop- or control SuperFop-luciferase were cotransfected into HUVEC, together with constant amounts (0.34 μg/well) of wild type β-catenin plasmid in presence of increasing amounts of Wnt5a plasmid (represented in fold excess). Empty vector plasmid was used to equalize total amount of plasmid used.
Wnt5a noncanonical signaling has been shown to antagonize the canonical β-catenin/TCF pathway (Topol et al., 2003). We asked whether this was also the case for human endothelial cells. HUVEC were cotransfected with plasmids encoding either Wnt5a, β-cateninS37A (a stabilized form of β-catenin), or LacZ genes and TCF/Lef reporter constructs. Included in the transfections was a renilla-luciferase plasmid to normalize readings for transfection efficiency. Twenty-four hours after transfection, luciferase activity showed that increasing amounts of Wnt5a antagonized β-cateninS37A–mediated activation of the TCF/Lef reporter in human endothelial cells, in a dose-responsive manner (Figure 2D). Thus, Wnt5a signals via noncanonical pathways in HUVEC, as measured by suppression of canonical Wnt signaling.
Wnt5a Induces Endothelial Cell Proliferation and Promotes Cell Survival
To investigate mitogenic effects of Wnt5a on endothelial cells, cultured HUVEC were infected with Ad-Wnt5a and incubated for 48 h in the presence of serum-free medium supplemented with bFGF. Expression of Wnt5a led to a dose-dependent increase in HUVEC proliferation, when compared with Ad-LacZ control infected cells (Figure 3A). This proliferative response was also observed when cultured HUVEC were incubated with purified Wnt5a protein in a dose-dependent manner, up to 100 ng/ml (Figure 3B). The proliferative responses of endothelial cells to Wnt5a signal activation depended on the absence of VEGF or serum, as the effect was barely observed when cells were cultured in complete endothelial medium, instead of minimal medium containing bFGF.
Figure 3.
Wnt5a induces endothelial cell proliferation and survival. (A) Proliferation assay using adenoviral infected HUVEC to overexpress Wnt5a-HA or negative control gene LacZ at different infection doses. Proliferation was evaluated after 48 h of incubation in basal endothelial media containing bFGF. Bottom, ectopic expression of Wnt5a-HA in lysates from infected cells, as evaluated by Western blot using an anti-HA antibody. (B) Proliferation of HUVEC in presence of increasing concentrations of purified Wnt5a protein. (C) Inhibition of Wnt5a-induced endothelial cell proliferation. HUVEC were incubated in presence (■) or absence (□) of purified Frizzled-4-CRD protein and assayed for proliferation after 48 h of culture. (D) RT-PCR analysis of total cDNA from retroviral selected HUVEC expressing shRNA control or shRNA targeting Wnt5a (shWnt5a) to block gene expression. β-actin expression was used to normalize samples. (E) Retroviral line of HUVEC expressing shRNA to silence the Wnt5a gene showed delayed cell growth when compared with control cells after 3 d of culture. (F) Endothelial cells overexpressing Wnt5a showed enhanced survival when compared with LacZ control cells. Values are expressed as percentage of positive control infection (VEGF). Cell number was evaluated after 48 h of incubation.
As Wnt5a has been shown to participate in Frizzled-4 endocytosis (Chen et al., 2003), and we previously reported that Frizzled-4 is expressed in HUVEC (Masckauchan et al., 2005), we used the Frizzled-4 extracellular cysteine-rich domain (Frizzled-4-CRD) as a competitive inhibitor of Wnt5a. We found that addition of purified Frizzled-4CRD reduced the proliferative stimulation provided by Ad-Wnt5a infection of HUVEC (Figure 3C). Thus, Wnt5a stimulates proliferation of endothelial cells, and this can be blocked with a Frizzled-4 antagonist. In complimentary experiments, shRNA directed against endogenous Wnt5a in HUVEC showed that reduced Wnt5a expression (Figure 3D) leads to reduced endothelial cell proliferation (Figure 3E). In the experiment shown, proliferation was reduced by 38.7% in shWnt5a cells compared with controls (p < 0.001) when measured using a WST8 cell counting assay; a similar reduction in proliferation is seen when determined using direct cell counting.
Wnt5a also promoted endothelial cell survival as Ad-Wnt5a infection of HUVEC enhanced survival of endothelial cells grown in the absence of survival factors (serum-free media), when compared with control Ad-LacZ infected cells (Figure 3F). The effect of Wnt5a expression in HUVEC is less effective than that of adding a known endothelial mitogen, vascular endothelial growth factor (VEGF).
Wnt5a Promotes Endothelial Network Formation and Endothelial Cell Migration
The ability of endothelial cells to form a network of capillaries after they migrate from a pre-existing blood vessel is a crucial property in both normal and tumor angiogenesis. We further analyzed the ability of HUVEC cultured on top of extracellular matrix components to form a network-like structure when they were either overexpressing Wnt5a or when endogenous expression of this gene was decreased by shRNA. HUVEC were programmed to overexpress HA-tagged Wnt5a via retroviral vectors (Wnt5a-HA-HUVEC) and used in a matrigel assay for cord formation. After 18 h of growth on matrigel, Wnt5a-HA HUVEC formed more extensive networks of cords than control, HA-expressing HUVEC (Figure 4A, top panel). The most visible change noticed was a decrease in incomplete cords, defined as cords that are not attached to two separate cords. Fewer incomplete cords are clearly visible in Wnt5a-HA expressing HUVEC than HA-control and quantification of this change revealed a decrease of 58.3% (SD of 11.7%) of incomplete cords in Wnt5a-HA versus control. Consistent with this observation, we observed reduced networks of endothelial cells when we performed the same experiment using retrovirally infected cells with shRNA-mediated reduction of Wnt5a, as compared with control (Figure 4A, bottom panel). In this case, there was an increase of 67.5% (SD of 10.6%) of incomplete cords in shWnt5a expressing HUVEC versus shControl HUVEC, indicating that loss of Wnt5a expression leads to incomplete cord formation in the matrigel assay.
Figure 4.
Wnt5a participates in capillary-like networks of endothelial cells and endothelial cell migration. (A) Retroviral-infected HUVEC either overexpressing Wnt5a-HA, compared with control (HA), showed enhanced formation of networks (top pictures). HUVEC overexpressing shRNA to target Wnt5a gene expression showed reduced ability to form networks when compared with control shRNA cells. (B) Retroviral infected HUVEC overexpressing shRNA targeting Wnt5a gene expression showed reduced ability to migrate, measured 10 h after a wound was made on a confluent plate, when compared with control cells. A representative experiment is shown. Black bars indicate cell front.
Reduction of Wnt5a expression via shRNA also caused reduced migration of endothelial cells into wounded areas of HUVEC monolayers, as monitored 10 h after wounding (Figure 4B). Ten hours after HUVEC monolayers were wounded, only 33 ± 4.5% of the wounded area was filled in with migrating endothelial cells in shWnt5a-HUVEC, whereas 54 ± 5% of the wounded area was filled in by sh-Control HUVEC. However, we did not observe enhanced cell motility when Wnt5a was ectopically overexpressed. Experiments were repeated three times to confirm results.
Noncanonical Wnt Signaling Induces MMP-1 and Tie-2 Expression in Endothelial Cells
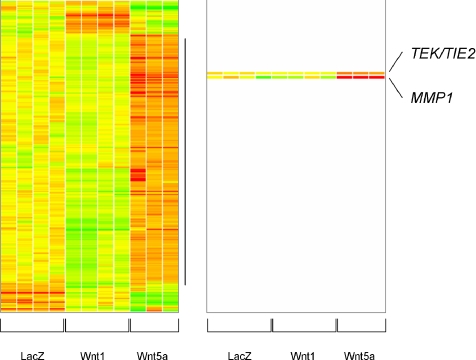
To better understand the potential mechanisms by which Wnt5a signaling promoted endothelial cell proliferation, survival, and network formation, we screened for genes differentially regulated by Wnt5a in endothelial cells. HUVEC were infected with either Ad-Wnt5a or Ad-LacZ and ectopic expression of Wnt5a was confirmed by Western blot. The expression profile of cells overexpressing Wnt5a was compared with Wnt1 and Lacz (control) overexpressing cells by conducting DNA microarray analysis as described (Figure 5). For the current study, we were most interested in the largest clade, in which genes were highly expressed in Wnt5a but low or intermediate in LacZ and Wnt1. The identities and fold regulation of some of these genes, which are predicted to be direct or indirect downstream targets activated by Wnt5a are listed on Table 1. Two differentially expressed genes, encoding the angiogenic regulators MMP-1 and Tie-2 (or TEK), were selected for further analysis in order to confirm they were regulated by overexpression of Wnt5a in primary endothelial cells.
Figure 5.
DNA array analysis of HUVEC ectopically overexpressing LacZ (control), Wnt-1 or Wnt5a, after adenoviral infection. Data analysis produced a set of 297 probe sets, which were subjected to hierarchical clustering. Of these, 239 probe sets were in the well-defined clade (vertical line) with the highest expression in Wnt5a expressing cells, the lowest expression in Wnt-1 cells, and the intermediate expression in LacZ. Colors represent a range of gene expression: low (green), intermediate (yellow), and high (red) relative to the experiment mean.
Table 1.
DNA microarray analysis of gene expression in endothelial cells ectopically overexpressing Wnt-5a
| Symbol | Annotations | Fold |
|---|---|---|
| MMP1 | Matrix metalloproteinase 1 (interstitial collagenase) | 245.0 |
| APOE | Apolipoprotein E | 22.4 |
| ELN | Elastin (supravalvular aortic stenosis, Williams-Beuren syndrome) | 13.0 |
| CDC25B | Cell division cycle 25B | 5.7 |
| PLCG2 | Phospholipase C, gamma 2 (phosphatidylinositol-specific) | 5.2 |
| RBP1 | Retinol-binding protein 1, cellular | 2.9 |
| ARHGAP4 | Rho GTPase activating protein 4 | 2.7 |
| TEK | TEK tyrosine kinase, endothelial (venous malformations, multiple cutaneous and mucosal) | 2.3 |
| PEPD | Peptidase D | 2.1 |
| NOS3 | Nitric oxide synthase 3 (endothelial cell) | 2.1 |
Shown are select genes with the highest fold change in expression in Wnt5a expressing cells, lowest expression in Wnt-1 cells, and intermediate expression in LacZ. Genes are listed according to their fold change compared with LacZ control infection.
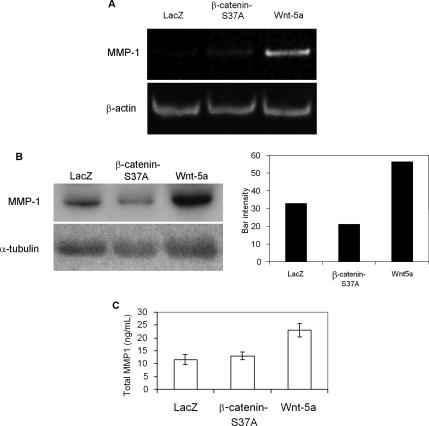
MMP-1, an interstitial collagenase overexpressed in many tumors (Giambernardi et al., 1998), was identified as a candidate Wnt5a target gene. Semiquantitative RT-PCR analysis, Western blot, and an ELISA-based assay (Figures 6, A, B, and C, respectively) showed that MMP-1 was up-regulated in endothelial cells overexpressing Wnt5a compared with control cells, but not in cells in which canonical Wnt signaling was activated by stabilized mutant β-catenin. As evaluated by Western blot, Wnt5a increased MMP-1 expression 1.7- and 2.7-fold when compared with LacZ and β-cateninS37A, respectively (Figure 6B). An ELISA-based assay showed a 2.0- and 1.8-fold increase of total MMP-1 in culture medium, when compared with LacZ and β-cateninS37A, respectively (Figure 6C). We noted that MMP1 was induced to a much higher degree in the initial microarray analysis than after validation by PCR or Western blot analysis. Although microarray analysis is not generally considered an adequate means of quantification of fold effect of a given factors affect on gene expression, the difference may also reflect the use of different batches of isolated primary HUVEC that were used for microarray versus validation experiments.
Figure 6.
Wnt5a can induce MMP-1 expression in endothelial cells. Adenoviral infected HMVEC to overexpress control LacZ, β-cateninS37A, or Wnt5a gene were used. (A) MMP-1 transcript levels analyzed by semiquantitative RT-PCR. β-actin expression was used to normalize samples. (B) Western blot analysis of secreted MMP-1 and band densitometry. Culture medium was collected 48 h after infection of HMVEC. α-tubulin Western blot was used to normalize protein load. (C) MMP-1 activity assay to quantify total secreted enzyme in culture medium from endothelial cells.
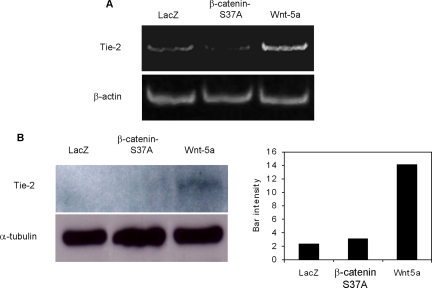
Tie-2, a receptor of angiopoietin 1 and 2 and a key regulator of angiogenesis, was shown to be moderately up-regulated in these cells, as confirmed by RT-PCR and Western blot analysis (Figure 7, A and B, respectively). As evaluated by Western blot, Wnt5a increased Tie-2 expression by 5.9-fold and 4.5-fold when compared with LacZ and β-cateninS37A, respectively (Figure 7B). Although endogenous levels for Tie-2 were detected by PCR, Tie-2 protein was only apparent after longer exposures of Western blots in control-infected cells, thus the PCR analysis appeared to be more sensitive for detecting endogenous Tie-2. Thus, Wnt5a signaling leads endothelial cells to increased production of known angiogenic regulators and this may aid in the proangiogenic activities of Wnt5a.
Figure 7.
Wnt5a induces expression of Tie-2 in HMVEC. Ectopic expression of control LacZ, β-cateninS37A, or Wnt5a gene was performed by adenoviral infections. (A) Tie-2 expression analysis by RT-PCR. β-actin was used to normalize samples. (B) Western blot analysis using an anti-Tie-2 antibody on plasma membrane proteins purified after surface biotinylation of cells and band densitometry. α-tubulin Western blot was performed from cell lysates to normalize protein content.
DISCUSSION
Angiogenesis is driven by VEGF but the process likely occurs in concert with other angiogenic factors. Defining those factors has provided greater insight into how several angiogenic cascades coordinate the building of new vessels. In that context, we here report a novel angiogenic signaling factor, Wnt5a. We demonstrate that Wnt5a promotes some, but not all, of the steps of angiogenesis. Both members of the Wnt5 class, Wnt5a and Wnt5b, were detected in primary human endothelial cells. Wnt5a in two types of endothelial cells, HUVEC and HMVEC, and thus we asked whether Wnt5a act on endothelial cells.
We first documented that Wnt5a activates noncanonical signaling in endothelial cells. Noncanonical Wnt signaling acts via several distinct pathways, including Ca2+, PKC, JNK, and pathways that antagonize canonical Wnt signaling. Noncanonical Wnt signaling also leads to hyper-phosphorylation of the intracellular protein dishevelled (Gonzalez-Sancho et al., 2004). We found that Wnt5a addition to human primary endothelial cells induces phosphorylation of Dvl-2 and Dvl-3. Because we previously established that Wnt5a does not activate Wnt/β-catenin signaling in endothelial cells (Masckauchan et al., 2005), we conclude that Wnt5a acts on endothelial cells in a noncanonical manner. We were unable to detect significant changes in neither NFAT, PKC, nor JNK signaling in response to Wnt5a expression in endothelial cells. However, Wnt5a promoted ERK1/2 phosphorylation in endothelial cells. Previous studies showed that Wnt5a promotes ERK1/2 phosphorylation in serum-starved MC3T3-E1 cells, where Wnt5a was able to protect cells from apoptosis via ERK signaling (Almeida et al., 2005). Wnt3a, a canonical signaling Wnt, can also promote ERK1/2 phosphorylation, and this signal was proposed to mediate proliferation of NIH3T3 cells (Yun et al., 2005). Concomitant with this, we previously reported that increased levels of cytosolic β-catenin in endothelial cells can lead to cell proliferation and survival (Masckauchan et al., 2005). In our study, we found that Wnt5a expression in endothelial cells blocks canonical Wnt signaling, a known function of Wnt5a in other cell types (Topol et al., 2003).
Gene expression analysis in response to Wnt5a showed regulation of a mostly distinct set of genes than that found with Wnt-1, a Wnt that stimulates Wnt/β-catenin signaling in endothelial cells (Masckauchan et al., 2005). This observation supports the hypothesis of an antagonistic role for noncanonical Wnt signaling on canonical signaling in endothelial cells. The existence of a common pathway like ERK1/2 activated by both canonical and noncanonical Wnt signaling, the antagonistic role of Wnt5a on canonical Wnt signaling, and the fact that the genes regulated by either of these pathways differ in endothelial cells point to the notion that Wnt5a has at least one other mechanism of signal transduction in angiogenesis. Ectopic expression of Wnt5a or addition of purified Wnt5a protein induced proliferation of endothelial cells and reducing expression of endogenous Wnt5a led to reduction of endothelial cell proliferation. Ectopic expression of Wnt5a provided survival signals to endothelial cells. Reduced Wnt5a expression mitigated endothelial cell migration and capillary-like network formation. Several cellular steps in angiogenesis, including endothelial proliferation, survival, migration, and capillary-like network formation are promoted by Wnt5a. We thus conclude that Wnt5a functions as an angiogenic factor.
The Wnt receptors, Frizzled-4, Frizzled-5, and Frizzled-6 are expressed in human primary endothelial cells (Masckauchan et al., 2005). Frizzled-4 can directly interact with Wnt5a (Umbhauer et al., 2000), and endocytosis of Frizzled-4 is increased upon addition of Wnt5a protein (Chen et al., 2003). Frizzled-4 is expressed on murine retinal vasculature (Masckauchan and Kitajewski, 2006), and this expression is required for proper retinal vascular development (Xu et al., 2004). In addition, we found that Frizzled-4 is expressed in the vasculature that surrounds murine ovaries and in major vessels in the ovary. Thus, Frizzled-4 is expressed in several vascular beds. We investigated the hypothesis that Wnt5a can act through Frizzled-4 to regulate endothelial cell proliferation. The mitogenic function of Wnt5a on endothelial cells is blocked by addition of the extracellular cysteine-rich domain (CRD) of Frizzled-4 tagged with Fc. Purified Frizzled-2-CRD and Frizzled-5-CRD, also Fc-tagged, and sFRP1 (soluble Frizzled-related protein 1) failed to block Wnt5a-induced proliferation of endothelial cells. Mutations in the Frizzled-4 gene are correlated with angiogenesis-related disorders in humans. Mutations in Frizzled-4 have been linked to familial exudative vitreoretinopathy (FEVR), a disorder of the retinal vasculature (Robitaille et al., 2002). Similar disruption of the vasculature is seen in patients with mutations in a novel Frizzled ligand, Norrin, and retinal vascular development likely depends on Norrin/Frizzled-4 interactions (Xu et al., 2004). Frizzled-4 can activate the noncanonical Wnt pathway through a mechanism involving calcium/calmodulin kinase II (CamKII) and PKC activation (Robitaille et al., 2002). We speculate that Frizzled-4 may also utilize Wnt5a as a ligand to regulate vascular development. It has been noted that the phenotype due to loss of Frizzled-4 is more severe than the phenotype due to loss of Norrin, supporting the hypothesis that there are other important ligands in play (Luhmann et al., 2005). Thus, ligands other than Norrin, such as Wnt5a, may activate Frizzled-4 signaling. Wnt5a mutant mice die perinatally (Yamaguchi et al., 1999), precluding an analysis of retinal vasculature in the mutant pups, which develops after birth in mice.
To further understand the role of Wnt5a in angiogenesis, we screened for genes whose expression is regulated by Wnt5a in endothelial cells and found induction of two known angiogenic regulators. MMP-1, a secreted protease that degrades fibrillar collagens, was induced by Wnt5a. When endothelial cells are induced to grow, they respond by secreting proteases, such as matrix metalloproteinases, that degrade the basement membrane surrounding the vessel (Giambernardi et al., 1998). In the past, Wnt5a has been linked to regulation of a different matrix metalloproteinase, MMP-3, as overexpression of Wnt5a and rat Frizzled-2 in C57MG mammary epithelial cells is able to up-regulate expression of stromelysin-1 (MMP-3) (Prieve and Moon, 2003). Thus, Wnt5a may regulate expression of several MMPs, with MMP-1 regulated in endothelial cells.
Wnt5a induced the expression of the endothelial specific receptor tyrosine kinase, Tie2, that responds to ligands, angiopoietins 1 and 2 (Ang-1 and Ang-2). The role of Ang-2 is thought to be destabilization of vessels and dissociation of pericytes (Holash et al., 1999), which sets the stage for stimulating the sprouting of new blood vessels by VEGF. Of note, Ang-2 has been shown to up-regulate MMP-1 expression in the human gastric cancer cell line MKN-7 (Etoh et al., 2001). Thus, the regulation of MMP-1 by Wnt5a might be indirect via the Ang-2/Tie-2 axis.
It is thought that angiogenesis requires coordination of several angiogenic signaling cascades and that novel factors will be discovered that participate in this process. The precise balance of angiogenic factor activity is crucial in modulating physiological angiogenesis and is likely derailed in pathological angiogenesis. Here we provide the first evidence of an active angiogenic role for Wnt5a in human endothelial cells. We also provide new insights on the intracellular signaling mediated by Wnt-5a and identify novel genes, Tie2 and MMP-1, that are regulated by noncanonical signaling. To date, few genes have been described as targets of noncanonical signaling. Finally, we define a new physiological process, angiogenesis, which is dependent on noncanonical Wnt signaling. The identification of Wnt5a as a novel angiogenic factor suggests investigation of a potential role for noncanonical Wnt signaling in pathological angiogenesis.
ACKNOWLEDGMENTS
We thank Yasuhiro Funahashi, Eunice Kim, and Anshula Sharma for their invaluable technical support. T.N.H.M. is supported by DOD Breast Cancer Research Program fellowship DAMD17-03-1-0293. This work was supported by National Institutes of Health Grants RO1 HL076411 to J.K., RO1 CA47207 to A.M.C.B., and EY T32 013933 to N.L.P.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-04-0320) on October 11, 2006.
REFERENCES
- Almeida M., Han L., Bellido T., Manolagas S. C., Kousteni S. Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by beta-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J. Biol. Chem. 2005;280:41342–41351. doi: 10.1074/jbc.M502168200. [DOI] [PubMed] [Google Scholar]
- Chen W., ten Berge D., Brown J., Ahn S., Hu L. A., Miller W. E., Caron M. G., Barak L. S., Nusse R., Lefkowitz R. J. Dishevelled 2 recruits beta-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science. 2003;301:1391–1394. doi: 10.1126/science.1082808. [DOI] [PubMed] [Google Scholar]
- Cheng C. W., Smith S. K., Charnock-Jones D. S. Wnt-1 signaling inhibits human umbilical vein endothelial cell proliferation and alters cell morphology. Exp. Cell Res. 2003;291:415–425. doi: 10.1016/j.yexcr.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Dejmek J., Leandersson K., Manjer J., Bjartell A., Emdin S. O., Vogel W. F., Landberg G., Andersson T. Expression and signaling activity of Wnt-5a/discoidin domain receptor-1 and Syk plays distinct but decisive roles in breast cancer patient survival. Clin. Cancer Res. 2005;11:520–528. [PubMed] [Google Scholar]
- Etoh T., Inoue H., Tanaka S., Barnard G. F., Kitano S., Mori M. Angiopoietin-2 is related to tumor angiogenesis in gastric carcinoma: possible in vivo regulation via induction of proteases. Cancer Res. 2001;61:2145–2153. [PubMed] [Google Scholar]
- Giambernardi T. A., Grant G. M., Taylor G. P., Hay R. J., Maher V. M., McCormick J. J., Klebe R. J. Overview of matrix metalloproteinase expression in cultured human cells. Matrix Biol. 1998;16:483–496. doi: 10.1016/s0945-053x(98)90019-1. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Sancho J. M., Brennan K. R., Castelo-Soccio L. A., Brown A. M. Wnt proteins induce dishevelled phosphorylation via an LRP5/6- independent mechanism, irrespective of their ability to stabilize beta-catenin. Mol. Cell. Biol. 2004;24:4757–4768. doi: 10.1128/MCB.24.11.4757-4768.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewett P. W., Daft E. L., Murray J. C. Cloning and partial characterization of the human tie-2 receptor tyrosine kinase gene promoter. Biochem. Biophys. Res. Commun. 1998;252:546–551. doi: 10.1006/bbrc.1998.9690. [DOI] [PubMed] [Google Scholar]
- Holash J., Wiegand S. J., Yancopoulos G. D. New model of tumor angiogenesis: dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene. 1999;18:5356–5362. doi: 10.1038/sj.onc.1203035. [DOI] [PubMed] [Google Scholar]
- Iozzo R. V., Eichstetter I., Danielson K. G. Aberrant expression of the growth factor Wnt-5A in human malignancy. Cancer Res. 1995;55:3495–3499. [PubMed] [Google Scholar]
- Ishitani T., Kishida S., Hyodo-Miura J., Ueno N., Yasuda J., Waterman M., Shibuya H., Moon R. T., Ninomiya-Tsuji J., Matsumoto K. The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/beta-catenin signaling. Mol. Cell. Biol. 2003;23:131–139. doi: 10.1128/MCB.23.1.131-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins: Indentification by morphological and immunological criteria. J. Clin. Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson M., Dejmek J., Bendahl P. O., Andersson T. Loss of Wnt-5a protein is associated with early relapse in invasive ductal breast carcinomas. Cancer Res. 2002;62:409–416. [PubMed] [Google Scholar]
- Kilian B., Mansukoski H., Barbosa F. C., Ulrich F., Tada M., Heisenberg C. P. The role of Ppt/Wnt5 in regulating cell shape and movement during zebrafish gastrulation. Mech. Dev. 2003;120:467–476. doi: 10.1016/s0925-4773(03)00004-2. [DOI] [PubMed] [Google Scholar]
- Lejeune S., Huguet E. L., Hamby A., Poulsom R., Harris A. L. Wnt5a cloning, expression, and up-regulation in human primary breast cancers. Clin. Cancer Res. 1995;1:215–222. [PubMed] [Google Scholar]
- Li C. M., Guo M., Borczuk A., Powell C. A., Wei M., Thaker H. M., Friedman R., Klein U., Tycko B. Gene expression in Wilms' tumor mimics the earliest committed stage in the metanephric mesenchymal-epithelial transition. Am. J. Pathol. 2002;160:2181–2190. doi: 10.1016/S0002-9440(10)61166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H., Chen Q., Coles A. H., Anderson S. J., Pihan G., Bradley A., Gerstein R., Jurecic R., Jones S. N. Wnt5a inhibits B cell proliferation and functions as a tumor suppressor in hematopoietic tissue. Cancer Cell. 2003;4:349–360. doi: 10.1016/s1535-6108(03)00268-x. [DOI] [PubMed] [Google Scholar]
- Logan C. Y., Nusse R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Luhmann U. F., Meunier D., Shi W., Luttges A., Pfarrer C., Fundele R., Berger W. Fetal loss in homozygous mutant Norrie disease mice: a new role of Norrin in reproduction. Genesis. 2005;42:253–262. doi: 10.1002/gene.20141. [DOI] [PubMed] [Google Scholar]
- Masckauchan T. N., Kitajewski J. Wnt/Frizzled signaling in the vasculature: new angiogenic factors in sight. Physiology (Bethesda) 2006;21:181–188. doi: 10.1152/physiol.00058.2005. [DOI] [PubMed] [Google Scholar]
- Masckauchan T. N., Shawber C. J., Funahashi Y., Li C. M., Kitajewski J. Wnt/beta-catenin signaling induces proliferation, survival and interleukin-8 in human endothelial cells. Angiogenesis. 2005;8:43–51. doi: 10.1007/s10456-005-5612-9. [DOI] [PubMed] [Google Scholar]
- Murdoch B., Chadwick K., Martin M., Shojaei F., Shah K. V., Gallacher L., Moon R. T., Bhatia M. Wnt-5A augments repopulating capacity and primitive hematopoietic development of human blood stem cells in vivo. Proc. Natl. Acad. Sci. USA. 2003;100:3422–3427. doi: 10.1073/pnas.0130233100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieve M. G., Moon R. T. Stromelysin-1 and mesothelin are differentially regulated by Wnt-5a and Wnt-1 in C57mg mouse mammary epithelial cells. BMC Dev. Biol. 2003;3:2. doi: 10.1186/1471-213X-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille J. Mutant frizzled-4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nat. Genet. 2002;32:326–330. doi: 10.1038/ng957. [DOI] [PubMed] [Google Scholar]
- Saneyoshi T., Kume S., Amasaki Y., Mikoshiba K. The Wnt/calcium pathway activates NF-AT and promotes ventral cell fate in Xenopus embryos. Nature. 2002;417:295–299. doi: 10.1038/417295a. [DOI] [PubMed] [Google Scholar]
- Schaeren-Wiemers N., Gerfin-Moser A. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry. 1993;100:431–440. doi: 10.1007/BF00267823. [DOI] [PubMed] [Google Scholar]
- Schulte G., Bryja V., Rawal N., Castelo-Branco G., Sousa K. M., Arenas E. Purified Wnt-5a increases differentiation of midbrain dopaminergic cells and dishevelled phosphorylation. J. Neurochem. 2005;92:1550–1553. doi: 10.1111/j.1471-4159.2004.03022.x. [DOI] [PubMed] [Google Scholar]
- Sen M., Chamorro M., Reifert J., Corr M., Carson D. A. Blockade of Wnt-5A/frizzled 5 signaling inhibits rheumatoid synoviocyte activation. Arthritis Rheum. 2001;44:772–781. doi: 10.1002/1529-0131(200104)44:4<772::AID-ANR133>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Shimizu H., Julius M. A., Giarre M., Zheng Z., Brown A. M., Kitajewski J. Transformation by Wnt family proteins correlates with regulation of beta-catenin. Cell Growth Differ. 1997;8:1349–1358. [PubMed] [Google Scholar]
- Slusarski D. C., Corces V. G., Moon R. T. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature. 1997;390:410–413. doi: 10.1038/37138. [DOI] [PubMed] [Google Scholar]
- Topol L., Jiang X., Choi H., Garrett-Beal L., Carolan P. J., Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J. Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbhauer M., Djiane A., Goisset C., Penzo-Mendez A., Riou J. F., Boucaut J. C., Shi D. L. The C-terminal cytoplasmic Lys-thr-X-X-X-Trp motif in frizzled receptors mediates Wnt/beta-catenin signalling. EMBO J. 2000;19:4944–4954. doi: 10.1093/emboj/19.18.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeman M. T., Axelrod J. D., Moon R. T. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev. Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- Vorontchikhina M. A., Zimmermann R. C., Shawber C. J., Tang H., Kitajewski J. Unique patterns of Notch1, Notch4 and Jagged1 expression in ovarian vessels during folliculogenesis and corpus luteum formation. Gene Expr. Patterns. 2005;5:701–709. doi: 10.1016/j.modgep.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Weeraratna A. T., Jiang Y., Hostetter G., Rosenblatt K., Duray P., Bittner M., Trent J. M. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell. 2002;1:279–288. doi: 10.1016/s1535-6108(02)00045-4. [DOI] [PubMed] [Google Scholar]
- Wright M., Aikawa M., Szeto W., Papkoff J. Identification of a Wnt-responsive signal transduction pathway in primary endothelial cells. Biochem. Biophys. Res. Commun. 1999;263:384–388. doi: 10.1006/bbrc.1999.1344. [DOI] [PubMed] [Google Scholar]
- Xu Q. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116:883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T. P., Bradley A., McMahon A. P., Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- Yamanaka H., Moriguchi T., Masuyama N., Kusakabe M., Hanafusa H., Takada R., Takada S., Nishida E. JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep. 2002;3:69–75. doi: 10.1093/embo-reports/kvf008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C. S., Masckauchan T. N., Kitajewski J. Beta-catenin/Tcf activation partially mimics the transforming activity of Wnt-1 in Rat-1 fibroblasts. Differentiation. 2003;71:477–485. doi: 10.1046/j.1432-0436.2003.7108002.x. [DOI] [PubMed] [Google Scholar]
- Yun M. S., Kim S. E., Jeon S. H., Lee J. S., Choi K. Y. Both ERK and Wnt/beta-catenin pathways are involved in Wnt3a-induced proliferation. J. Cell Sci. 2005;118:313–322. doi: 10.1242/jcs.01601. [DOI] [PubMed] [Google Scholar]