Abstract
Synaptotagmin I, a synaptic vesicle protein required for efficient synaptic transmission, contains a highly conserved polylysine motif necessary for function. Using Drosophila, we examined in which step of the synaptic vesicle cycle this motif functions. Polylysine motif mutants exhibited an apparent decreased Ca2+ affinity of release, and, at low Ca2+, an increased failure rate, increased facilitation, and increased augmentation, indicative of a decreased release probability. Disruption of Ca2+ binding, however, cannot account for all of the deficits in the mutants; rather, the decreased release probability is probably due to a disruption in the coupling of synaptotagmin to the release machinery. Mutants exhibited a major slowing of recovery from synaptic depression, which suggests that membrane trafficking before fusion is disrupted. The disrupted process is not endocytosis because the rate of FM 1-43 uptake was unchanged in the mutants, and the polylysine motif mutant synaptotagmin was able to rescue the synaptic vesicle depletion normally found in sytnull mutants. Thus, the polylysine motif functions after endocytosis and before fusion. Finally, mutation of the polylysine motif inhibits the Ca2+-independent ability of synaptotagmin to accelerate SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor)-mediated fusion. Together, our results demonstrate that the polylysine motif is required for efficient Ca2+-independent docking and/or priming of synaptic vesicles in vivo.
INTRODUCTION
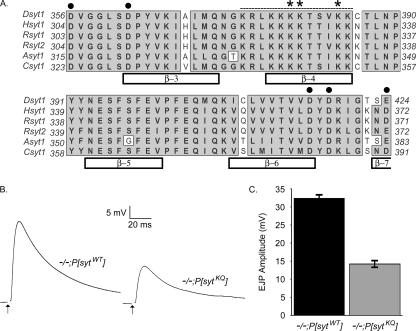
Synaptotagmin I is a synaptic vesicle protein whose cytoplasmic region contains two C2 domains, C2A and C2B (Perin et al., 1991; Brose et al., 1992). Although synaptotagmin is a Ca2+ sensor for synaptic vesicle exocytosis (Brose et al., 1992; Geppert et al., 1994), primarily via its C2B Ca2+-binding motif (Figure 1A, circles; Mackler et al., 2002; Nishiki and Augustine, 2004), it likely mediates other molecular interactions via additional functional motifs.
Figure 1.
Synaptotagmin C2B polylysine region is highly conserved throughout evolution and is required for efficient synaptic transmission. (A) ClustalW sequence alignment of 56 highly conserved amino acids from the C2B domain of synaptotagmin. Bars indicate β-sheets within the C2B domain. Circles mark the Ca2+-binding motif. Dotted line denotes the polybasic region. Asterisks mark the polylysine motif. (B) Representative traces showing evoked release during 0.05-Hz stimulation in HL3 saline containing 1.5 mM Ca2+ in transgenic controls (−/−;P[sytWT]) and polylysine motif mutants (−/−;P[sytKQ]). Each trace is an average of 10–12 individual traces from a single muscle fiber. Stimulus artifact has been removed for clarity. (C) The mean EJP amplitude in saline containing 1.5 mM Ca2+ was 14.2 ± 0.82 mV (mean ± SEM; n = 34 fibers) for polylysine motif mutants and 32.4 ± 0.99 mV (mean ± SEM; n = 42 fibers) for transgenic wild-type controls (p ≪ 0.001).
The C2B domain of synaptotagmin contains a highly conserved polybasic region (Figure 1A, dashes; Rickman et al., 2004). Three lysines within this region (the polylysine motif; Figure 1A, asterisks) are required for full synaptotagmin function. In Drosophila, when these lysines are mutated to glutamines (sytKQ) and the sytKQ transgene is expressed in a sytnull background (−/−;P[sytKQ]), evoked transmitter release is decreased compared with transgenic wild-type controls (−/−;P[sytWT]) (Mackler and Reist, 2001). The function of the polylysine motif within the synaptic vesicle cycle remains controversial. Results from in vitro experiments suggest this motif may mediate three steps in the synaptic vesicle cycle: 1) endocytosis, 2) Ca2+-triggered vesicle fusion, and 3) Ca2+-independent docking and/or priming of synaptic vesicles.
Functional studies demonstrate that synaptotagmin I is required for synaptic vesicle endocytosis (von Poser et al., 2000; Jarousse and Kelly, 2001; Jarousse et al., 2003; Poskanzer et al., 2003; Llinás et al., 2004; Nicholson-Tomishima and Ryan, 2004), and in vitro studies suggest that the C2B domain of synaptotagmin may mediate this role (von Poser et al., 2000; Jarousse and Kelly, 2001; Littleton et al., 2001; Jarousse et al., 2003; Llinás et al., 2004). Specifically, synaptic vesicle endocytosis may be mediated by the polylysine motif in the C2B domain of synaptotagmin (Takei and Haucke, 2001; but see Poskanzer et al., 2006) via Ca2+-independent interactions with the clathrin adaptor protein AP-2 (Zhang et al., 1994; Chapman et al., 1998; Haucke and De Camilli, 1999; Haucke et al., 2000; Littleton et al., 2001; Grass et al., 2004) and/or with phosphatidylinositol bisphosphate (PIP2) (Bai et al., 2004). Both AP-2 and PIP2 play a role in clathrin-mediated endocytosis (Cremona and De Camilli, 2001; Hurley and Wendland, 2002).
A second proposed function for the polylysine motif of synaptotagmin is regulation of Ca2+-dependent processes (Chapman et al., 1998; Wu et al., 2003; Borden et al., 2005; Araç et al., 2006; Li et al., 2006). Polylysine motif mutations decrease the apparent Ca2+ affinity of synaptotagmin (Borden et al., 2005; Li et al., 2006). Furthermore, in vitro studies have implicated this motif in three Ca2+-dependent processes: the ability of synaptotagmin to oligomerize (Chapman et al., 1998; Wu et al., 2003), the ability of synaptotagmin to simultaneously bind to two membranes (Araç et al., 2006), and the ability of synaptotagmin to bind to negatively charged phospholipids (Li et al., 2006). The Ca2+-dependent oligomerization and phospholipid binding of synaptotagmin have been proposed to regulate the opening, dilation, or stability of the fusion pore (Wang et al., 2001; Bai and Chapman, 2004; Li et al., 2006). The simultaneous binding of synaptotagmin to two membranes has been proposed to accelerate membrane fusion by bringing the synaptic vesicle and plasma membrane into proximity (Araç et al., 2006).
Finally, the polylysine motif may participate in a Ca2+-independent interaction that either holds synaptic vesicles at release sites (docking) and/or subsequently increases their probability of release (priming). Priming likely consists of multiple maturation steps that transform vesicles from a docked but not releasable state, through multiple releasable states with differing release properties (Martin, 2003). In the absence of Ca2+, the polylysine motif of synaptotagmin preferentially binds to PIP2-containing membranes, potentially mediating vesicle docking via a trans-interaction between the synaptic vesicle and the plasma membrane (Bai et al., 2004; Li et al., 2006) where PIP2 is predominantly located (Holz et al., 2000; Micheva et al., 2001). This interaction could also mediate Ca2+-independent vesicle priming, because it increases the rate of synaptotagmin penetration into lipid membranes in vitro upon Ca2+ influx (Bai et al., 2004), which is postulated to be important for fusion (Bai et al., 2000, 2002; Wang et al., 2003; Rhee et al., 2005). Additionally, the polylysine motif of synaptotagmin binds to target membrane-soluble N-ethylmaleimide-sensitive factor attachment protein receptor (t-SNARE) heterodimers (syntaxin/synaptosome-associated protein 25 kDa [SNAP-25]) in the absence of Ca2+ (Rickman et al., 2004, 2006). This Ca2+-independent interaction may prime vesicles by bringing the synaptic vesicle (v)-SNARE (vesicle-associated membrane protein [VAMP] or synaptobrevin) into proximity with the t-SNARE heterodimer, preparing the vesicle for Ca2+-triggered fusion via formation or stabilization of the SNARE complex (VAMP/syntaxin/SNAP-25; Xu et al., 1999; Rickman et al., 2004, 2006; Bhalla et al., 2006). Indeed, inclusion of synaptotagmin in v-SNARE–containing vesicles directly accelerates SNARE-mediated membrane fusion in a Ca2+-independent manner (Mahal et al., 2002).
Using Drosophila mutants, we show that the polylysine motif plays a role during synaptic vesicle recycling after endocytosis and before fusion. This role serves to increase the rate of recovery from synaptic depression and increase the efficacy of synaptic vesicle fusion in vivo. Using a fluorescent lipid mixing assay, we show that the polylysine motif mediates the Ca2+-independent ability of synaptotagmin to accelerate SNARE-mediated fusion. We discuss these results in the context of a single deficit hypothesis where the polylysine motif plays an important role in Ca2+-independent docking/priming of synaptic vesicles.
MATERIALS AND METHODS
Fly Stocks
The polylysine motif (Figure 1A, asterisks) of Drosophila synaptotagmin was mutated (K379,380,384Q), and the mutant synaptotagmin was expressed from a transgene in a synaptotagmin null (sytAD4) line. The experimental flies (−/−;P[sytKQ]) had the genotype yw; sytAD4 P[elav-Gal4]/sytAD4;P[UAS sytK379,380,384Q]/+ and were generated as described previously (Mackler and Reist, 2001). The control flies (−/−;P[sytWT]) expressed wild-type synaptotagmin I from a transgene (Mackler and Reist, 2001) and had the genotype yw; sytAD4 P[elav-Gal4]/sytAD4;P[UAS sytWT]/+. Two independent transgenic wild-type synaptotagmin lines and two independent transgenic C2B polylysine motif lines were generated. Because both lines of a given genotype had similar levels of evoked release (control lines [our unpublished data]; mutant lines, Mackler and Reist, 2001), one control line (2C) and one mutant line (#59) were used in subsequent experiments.
Solutions
Standard saline for these experiments is composed of 5 mM KCl, 2 mM CaCl2, 130 mM NaCl, 2 mM MgCl2, 36 mM sucrose, and 5 mM HEPES, pH 7.3 (Jan and Jan, 1976). In Ca2+-free saline, the CaCl2 was replaced by additional MgCl2. Stimulating saline contained 90 mM KCl, 5 mM CaCl2, 45 mM NaCl, 2 mM MgCl2, 36 mM sucrose, and 5 mM HEPES, pH 7.3. HL3 saline contained 5 mM KCl, 70 mM NaCl, 20 mM MgCl2, 10 mM NaHCO3, 5 mM HEPES, 115 mM sucrose, 5 mM trehalose, and 1.5 mM CaCl2 (Stewart et al., 1994). For the Ca2+ dependence curves, the CaCl2 concentration in the HL3 saline varied (0.3, 0.5, 0.6, 0.8, 1.0, 1.5, 2.5, 3.5, 5.0, or 6.0 mM), whereas the MgCl2 concentration was held constant at 20 mM. Hyperosmotic saline consisted of HL3 saline containing 0.8 mM Ca2+ and 0.5 M sucrose.
Dye Uptake Assay
Synaptic boutons in Drosophila third instars were labeled with the activity-dependent, fluorescent dye FM 1-43 (Invitrogen, Carlsbad, CA). Larvae were dissected in Ca2+-free saline, stimulated for 6 min with stimulating saline, and then the stimulating saline was replaced with standard saline containing 3 μM tetrodotoxin (TTX) for a variable length of time (Δt = 0, 30, 60, or 180 s). TTX was added to block spontaneous activity coming from the attached CNS. After Δt, the preparation was incubated in standard saline + 3 μM TTX + 4 μm FM 1-43 dye for 6 min to load endocytosing vesicles, and then it was washed for 15 min with three changes of Ca2+-free saline to remove excess extracellular dye.
Fluorescence Microscopy and Image Processing
FM 1-43–labeled preparations were viewed using a 40×/0.80 numerical aperture water immersion objective lens (Leica, Bannockburn, IL) on a DMRA light microscope (Leica, Nussloch, Germany) equipped with epifluorescence optics (51019 EGFP/DsRed filter; Chroma Technology, Brattleboro, VT) and fitted with a microstepping servomotor in the z-axis. Images were captured with a Hamamatsu charge-coupled device camera (C4742–95). A through focal series by using 0.4-μm steps was taken of each labeled neuromuscular junction. All imaging was accomplished within 2 min of first exposure to light. Images were acquired, stored, and processed using Open Lab 2.2.0 software (Improvision, Boston, MA) on a Mac G-4 platform.
The average fluorescence intensity of 12–15 brightly stained boutons with diameters ≥2 μm were measured at each neuromuscular junction. Only boutons on muscle fibers 6 and 7 from segment 3 or 4 were used. Only one neuromuscular junction per preparation was imaged. Nine neuromuscular junctions (from 9 larvae) were analyzed for each genotype. Each bouton was imaged in its optimal focal plane. Images were imported into the public domain Object Image program (http://simon.bio.uva.nl/object-image.html). Background fluorescence was measured over nearby muscle nuclei and was subtracted from the fluorescence intensity of each bouton. Statistical comparisons between genotypes were tested with a mixed model analysis of variance (ANOVA) by using SAS software 6.12 (SAS Institute, Cary, NC).
Electrophysiology
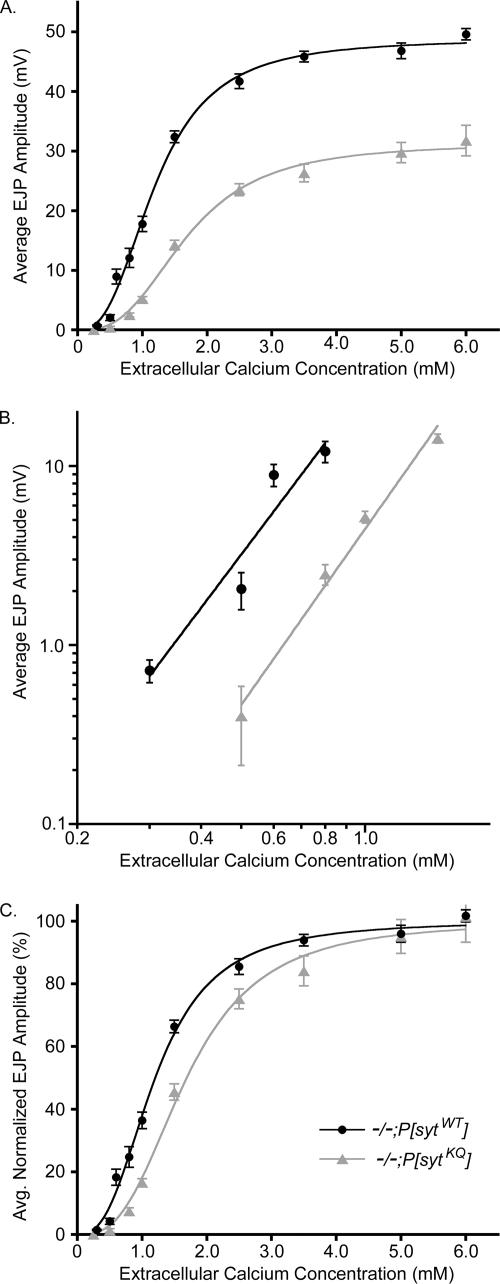
Electrophysiology experiments were performed at room temperature in HL3 saline (Stewart et al., 1994) containing variable levels of Ca2+ (see above). Recordings were from muscle fiber 6 from abdominal segments 3 and 4 of third instar fillet preparations. Fibers were impaled with 15- to 45-MΩ electrodes filled with a solution of 3 parts 2 M potassium citrate to 1 part 3 M potassium chloride or a 4 M potassium acetate solution. Segmental nerves were stimulated with a 5- to 10-μm diameter glass micropipette filled with HL3 to evoke excitatory junctional potentials. Nerves were stimulated with 1-ms pulses at the indicated frequency, and voltage traces were collected using an AxoClamp 2B (Molecular Devices, Sunnyvale, CA) and digitized using a MacLab4s A/D converter (Chart or Scope software; ADInstruments, Colorado Springs, CO). The resting membrane potential of each muscle fiber was normally between −65 and −45 mV but was maintained at about −55 mV by passing a bias current. KaleidaGraph software (Synergy Software, Reading, PA) was used to fit linear regression lines to the data in Figure 3B and the Hill equation [(Max × [Ca2+]n)/(EC50n + [Ca2+]n)] to the data in Figure 3, A and C.
Figure 3.
Calcium cooperativity of release is unchanged in the polylysine motif mutants, but the apparent calcium affinity and efficacy of release are decreased. EJPs were evoked by 0.05-Hz stimulation, and 10–15 sweeps were averaged for each fiber at each [Ca2+]. (A) EJP amplitudes at various extracellular Ca2+ concentrations for both polylysine motif mutants (gray triangles), and transgenic controls (black circles). For both genotypes, at 0.3–0.6 mM extracellular [Ca2+], n = 5–15 fibers; at 1.5 mM extracellular [Ca2+], n = 34–42 fibers; and at all other extracellular [Ca2+], n = 15–32 fibers. (B) Mean EJP amplitudes versus extracellular Ca2+ concentration were graphed on a double log plot. The slope (n) was determined by fitting the data points with linear regression lines (−/−;P[sytKQ]: n = 3.3, R = 0.99; −/−;P[sytWT]: n = 3.1, R = 0.95). (C) EJP amplitudes in A were normalized to the maximum value predicted by the Hill equation for each genotype and replotted to illustrate the shift in EC50 (−/−;P[sytKQ]: EC50 = 1.69 ± 0.08 mM; −/−;P[sytWT]: EC50 = 1.20 ± 0.05 mM). Error bars are SEM.
Hyperosmotic saline was pressure injected with a PLI-100 PICO-INJECTOR (Medical Systems, Greenvale, NY) onto the neuromuscular junction for 10 s with an injection pressure of 1.0 psi by using a glass micropipette with a tip diameter <1 μm. The recording electrode impaled the muscle at the extreme posterior end of the muscle, outside the area of hyperosmotic saline application, to reduce nonspecific leak current (Aravamudan et al., 1999). Voltage traces were collected as described above. In a blind analysis, the number of quantal events within 1-s intervals was binned during the 10-s hyperosmotic puff as well as during the 10 s before and after the puff.
Immunohistochemistry
Experimental and control third instars were dissected in the same dish in cold Ca2+-free HL3 saline; fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS); rinsed in PBS containing 0.1% Triton X-100 and 0.02% azide (PBST); and incubated overnight at 4°C in monoclonal antibody (mAb) nc82 (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), diluted 1:100 in PBST containing 5% normal goat serum (PBST-NGS) and anti-GluRIII antibody (Marrus et al., 2004), diluted 1:200 in PBST-NGS. The preparations were washed in PBST, incubated for 1 h in Alexa Fluor 488 goat anti-mouse IgG (Invitrogen), diluted 1:500 in PBST-NGS and Texas Red goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA), diluted 1:200 in PBST-NGS. Preparations were washed in PBST and mounted in Citifluor AF-1 (Ted Pella, Redding, CA).
Neuromuscular junctions from abdominal segments 3 or 4 were imaged on a Zeiss LSM 510Meta confocal microscope (Carl Zeiss Microimaging, Thornwood, NY) equipped with argon and HeNe1 lasers. Emissions were collected using a band pass 505–530 emission filter (for Alexa Fluor 488) or a long pass 585 emission filter (for Texas Red). Images were collected at 40 and 63× (pinhole set for 1 Airy unit for each), and confocal settings were adjusted to optimize the intensity range of the signal. For all images used for quantitative analysis, the settings were held constant between control and experimental preparations. Eight-bit images were acquired and z-stacks were converted to projections using Zeiss LM510 software.
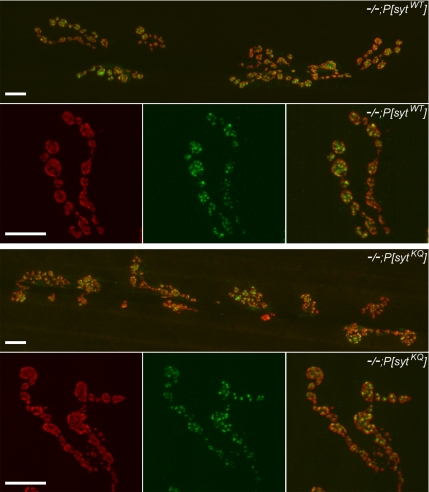
For analysis, a region of interest (ROI) of constant area was drawn around each synaptic arborization as well as around a nearby area that contained only background staining. The histogram function of the software was used to determine the absolute number of pixels at every intensity (0–255) in these ROIs. To determine the intensity range of the signal (i.e., the range where the number of pixels in the signal was consistently greater than the number of pixels in the background), the number of pixels in the background ROI was subtracted from the number in the ROI containing the stained arborization for each intensity level. Once the intensity range of the signal was determined, the area of the signal was calculated by summing the number of pixels in the signal range. For Figure 2, the gamma setting of a Codonics NP-1600 Photographic Network Printer was adjusted to match the image appearance on the screen.
Figure 2.
GluRIII and nc82 antibody staining was unchanged in polylysine motif mutants. Representative 40 and 63× images of neuromuscular junctions from transgenic controls (−/−;P[sytWT], top) and polylysine motif mutants (−/−;P[sytKQ], bottom). Junctions were stained with an anti-GluRIII antibody (red) to show postsynaptic receptors, and they were stained with mAb nc82 (green) to show active zones. Bar, 10 μm.
Electron Microscopy
Third instars were dissected in cold Ca2+-free HL3 saline and then embedded, sectioned, and stained as described previously (Reist et al., 1998). Electron micrographs of neuromuscular junctions on muscle fiber number 6 and 7 from abdominal segments 3 or 4 were taken at 15,000× magnification on a JEOL JEM 2000 EX-II transmission electron microscope operated at 100 kV. Images were scanned using an Agfa DuoScan T2500 and adjusted for contrast using Adobe Photoshop software (Adobe Systems, Mountain View, CA).
Plasmid Construction and Site-directed Mutation for Fusion Proteins
Syntaxin 1A (amino acids 4-288), SNAP-25 (amino acids 1-206), and VAMP2 (amino acids 1-116) from rat were all inserted into pGEX-KG (between EcoRI and HindIII sites) as glutathione S-transferase (GST) fusion proteins. Four native cysteines in SNAP-25, one in VAMP2 and three in syntaxin 1A, were replaced with alanines. The plasmid for rat synaptotagmin I with a truncated luminal domain (amino acids 57-421) was inserted into pET-28 (b) (between NcoI and XhoI) as a C-terminal His6-tagged protein. Among six native cysteines of synaptotagmin I, five cysteines located in the transmembrane domain were changed to alanines. The triple mutant (K326,327,331Q) was made using a QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). The DNA sequences were confirmed by DNA sequencing (Iowa State University DNA Sequencing Facility, Ames, IA).
Protein Expression and Purification
The details of protein expression and purification were described previously (Lu et al., 2005; Xu et al., 2005). Briefly, GST-tagged proteins were expressed in Escherichia coli Rosetta (DE3) pLysS (Novagen, Madison, WI). GST fusion proteins were purified by affinity chromatography using glutathione-agarose beads (Sigma-Aldrich, St. Louis, MO). To purify proteins, the cell pellet was resuspended in 10 ml of PBST buffer [phosphate-buffered saline, pH 7.4, with 0.5% (vol/vol) Triton X-100] with the final concentrations of 1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF), 1 mM EDTA, and 5 mM dithiothreitol (DTT). n-Lauroyl sarcosine (0.5%) was added to the solution. Cells were broken by sonication on the ice bath and centrifuged at 13,000 × g for 20 min at 4°C. The supernatant was mixed with 5 ml of glutathione-agrose beads (50%) in PBST buffer and incubated in the cold room for 2 h. During incubation for binding, 20 μg/ml DNase I and 4 μg/ml RNase were added. The beads were then washed with PBST buffer 10 times. The GST-tagged proteins were cleaved by bovine thrombin (Calbiochem, San Diego, CA) ng 1% n-octyl-d-glucopyranoside (OG).
The His-tagged synaptotagmin I was expressed in E. coli condon plus (BL21) pLysS (Stratagene) and induced by 0.3 mM isopropyl-d-galactopyranoside. The cell pellet was sonicated and centrifuged in 10 ml of lysis buffer (25 mM HEPES, 400 mM KCl, 10 mM imidazole, 2 mM EDTA, 2 mM AEBSF, 2 mM DTT, 0.5% Triton X-100, and 0.5% n-lauryl sarcosine, pH 7.4). The supernatant was mixed with 2 ml of nickel-nitrilotriacetic acid (Ni-NTA) agarose beads (QIAGEN, Valencia, CA). DNase I and RNase were added to the supernatant and the Ni-NTA beads mixture. After binding for 2 h at 4°C, Ni-NTA beads were washed with wash buffer six times. Synaptotagmin I was eluted in elution buffer (25 mM HEPES, 400 mM KCl, 400 mM imidazole, and 1% OG, pH 8.0). Purified proteins were examined with 15% SDS–PAGE, and the purity was at least 90% for all proteins.
Membrane Reconstitution
The procedure was described previously (Lu et al., 2005, 2006). Briefly, syntaxin 1A was incubated with SNAP-25 for 1 h at room temperature to allow the formation of t-SNARE complex. The liposomes containing 1-palmitoyl-2-dioleoyl-sn-glycero-3-phosphatidylcholine (POPC) and 1,2-dioleoyl-sn-glycero-3-phosphatidylserine (DOPS) (molar ratio 85:15; 50 mM) were reconstituted with the preformed t-SNARE complex in a lipid/protein ratio of 200:1. The fluorescent liposomes containing POPC, DOPS, 1,2-dioleoyl-sn-glycero-3-phosphoserine-N-(7-nitro-2–1,3-benzoxadiazol-4-yl) (NBD-PS), and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (rhodamine-PE) (molar ratio of 83:15:1:1; 10 mM) were reconstituted with VAMP2 in 200:1 lipid/protein ratio. Synaptotagmin I was reconstituted with VAMP2 (molar ratio 1:1). To remove OG, the samples were diluted two times with dialysis buffer (25 mM HEPES and 100 mM KCl, pH 7.4) and then dialyzed against 2 liters of dialysis buffer at 4°C overnight. After dialysis, the solution was centrifuged at 10,000 × g to remove protein and lipid aggregates. The reconstitution efficiencies were determined using SDS-PAGE and were at least 70%.
Total Fluorescence Lipid Mixing Assay
To measure the lipid mixing, v-SNARE liposomes with or without synaptotagmin I were mixed with t-SNARE liposomes in the ratio of 3:7. The final solution for each reaction contained ∼1 mM lipids in HEPES buffer (25 mM HEPES and 100 mM KCl, pH 7.4, and 5% glycerol) with the total volume of 100 μl. Fluorescence intensity was monitored with the excitation and emission wavelengths of 465 and 530 nm, respectively. The fluorescence signal was recorded using a Varian Cary Eclipse model fluorescence spectrophotometer with a quartz cell of 100 μl with a 2-mm path length. After 4000 s, 0.25% n-dodecylmaltoside was added to obtain the maximum fluorescence intensity. All of the lipid mixing experiments were carried out at 35°C.
RESULTS
Synaptic Transmission Is Disrupted in Synaptotagmin Polylysine Motif Mutants
The C2B polylysine motif of synaptotagmin I is required for efficient synaptic transmission (Figures 1, B and C, and 3; Mackler and Reist, 2001; Borden et al., 2005; Li et al., 2006). We examined excitatory junctional potentials (EJPs) in Drosophila synaptotagmin null third instars that expressed either a wild-type synaptotagmin transgene (−/−;P[sytWT]) or a C2B-polylysine motif mutant transgene (−/−;P[sytKQ]). Figure 1B shows representative traces recorded in saline containing 1.5 mM Ca2+. Because both the polylysine motif transgene and the control transgene exhibit similar levels of synaptotagmin expression (Mackler and Reist, 2001), the only difference between these lines is the mutation in the C2B polylysine motif of synaptotagmin. Figure 1C illustrates that the average EJP amplitude at third instar neuromuscular junctions in saline containing 1.5 mM Ca2+ was 14.2 ± 0.82 mV for polylysine motif mutants (−/−;P[sytKQ]; n = 34 fibers) and 32.4 ± 0.99 mV for transgenic wild-type controls (−/−;P[sytWT]; n = 42 fibers). Thus, similar to what has been reported previously for this synapse (Mackler and Reist, 2001), evoked transmitter release in 1.5 mM Ca2+ was decreased in polylysine motif mutants to ∼45% of transgenic controls.
The Postsynaptic Responsiveness Is Not Changed in Synaptotagmin Polylysine Motif Mutants
To verify that the decrease in the evoked response was not due to a developmental disruption that resulted in a reduced number of postsynaptic glutamate receptors or presynaptic active zones, we examined GluRIII (Marrus et al., 2004) and nc82 (an active zone marker; Wucherpfennig et al., 2003; Qin et al., 2005) staining in polylysine motif mutants and controls (Figure 2). The area of GluRIII staining [polylysine motif mutants, 49,500 ± 6600; transgenic controls, 47,000 ± 5500 pixels at 40× magnification (mean ± SEM)] was not significantly different in polylysine motif mutants compared with transgenic controls (p > 0.78, Student's t test; n = 6 junctions for each genotype). In addition, the amplitude of spontaneous fusion events is unchanged in the mutants (Mackler and Reist, 2001), demonstrating that the density of glutamate receptors is unchanged. Because neither the area nor density of postsynaptic receptors is changed, the postsynaptic response to neurotransmitter release is unaltered in the mutants. Furthermore, the area of nc82 staining [polylysine motif mutants: 65,200 ± 7500; transgenic controls: 63,800 ± 7600 pixels at 40× (mean ± SEM)] is also not significantly different in the mutants (p > 0.78, Student's t test; n = 6 junctions for each genotype). Thus, the decrease in the evoked response in polylysine motif mutants likely results from a defect in vesicle availability or release probability, because it does not result from a decrease in the ability of the postsynaptic cell to respond to neurotransmitter nor a decrease in the active zone area.
The Calcium Cooperativity of Release Is Unchanged in the Polylysine Motif Mutants, but the Apparent Calcium Affinity of Release Is Decreased
During synaptic transmission, the amount of neurotransmitter release triggered by nerve stimulation depends on some power (n) of the extracellular Ca2+ concentration. In 1967, Dodge and Rahamimoff showed at frog neuromuscular junctions that the dependence of release on extracellular Ca2+ can be explained by assuming a cooperative action of ∼4 Ca2+ ions (Dodge and Rahamimoff, 1967). Support for the hypothesis that the binding of three to five Ca2+ ions to a Ca2+ sensor(s) triggers neurotransmitter release has subsequently been demonstrated at other synapses as well, such as goldfish retinal bipolar synapses (Heidelberger et al., 1994), the rat Calyx of Held (Bollmann et al., 2000), and Drosophila neuromuscular junctions (Littleton et al., 1994; Stewart et al., 2000; Yoshihara and Littleton, 2002; Okamoto et al., 2005). To determine whether a disruption of Ca2+ binding by the polylysine motif mutant synaptotagmin contributed to the mutants' decreased release, we examined the Ca2+ dependence of release in both the polylysine motif mutants and the transgenic controls (Figure 3).
To estimate the Ca2+ dependence of release, we measured the mean EJP amplitude at various extracellular Ca2+ concentrations for both polylysine motif mutants and transgenic controls (Figure 3A). To examine the Ca2+ cooperativity of release, we graphed the mean EJP amplitude versus extracellular Ca2+ concentration on a double log plot (Figure 3B), and we determined that the slope for the polylysine motif mutants was 3.3 in nonsaturating Ca2+ ranges. This is similar to the cooperativity of 3.1 that was measured in our transgenic wild-type controls and to values (3.0–3.6) reported previously for Drosophila neuromuscular junctions (Littleton et al., 1994; Stewart et al., 2000; Yoshihara and Littleton, 2002; Okamoto et al., 2005). Thus, the polylysine motif mutation does not alter the number of Ca2+ ions necessary to trigger neurotransmitter release.
Although the Ca2+ cooperativity of release was not changed in the polylysine motif mutants, the EC50 was changed. To illustrate the increase in EC50 in the polylysine motif mutants, the EJP amplitudes were normalized to the predicted maximum value of the Hill equation for each genotype and replotted in Figure 3C [EC50 = 1.69 ± 0.08 mM for −/−;P[sytKQ] and EC50 = 1.20 ± 0.05 for −/−;P[sytWT] (mean ± SEM)]. Thus, the polylysine motif mutants exhibit an ∼40% increase in EC50. Because the cooperativity of release is unchanged in these mutants, the increase in EC50 corresponds to an ∼40% decrease in the apparent Ca2+ affinity of release. Similar results have been reported for cultured, hippocampal neurons expressing synaptotagmin with a mutation in the polylysine motif (Borden et al., 2005; Li et al., 2006).
Synaptotagmin Polylysine Motif Mutants Have a Decreased Release Probability
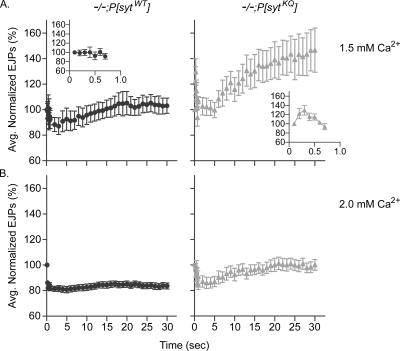
The finding that the mutants exhibit less release at every Ca2+ concentration indicates that the polylysine motif mutants have a decreased release probability and/or a decrease in the number of readily releasable vesicles. To examine release probability, we measured synaptic facilitation and augmentation during high frequency stimulation. Although the mechanisms underlying short-term plasticity are not completely understood, generally a decrease in release probability results in greater synaptic augmentation and facilitation (Atwood and Karunanithi, 2002; Zucker and Regehr, 2002). EJPs were evoked by 10-Hz stimulation for 30 s. Polylysine motif mutants exhibited larger synaptic augmentation than transgenic control larvae in 1.5 mM extracellular Ca2+ (Figure 4A) and still exhibited synaptic augmentation at 2.0 mM extracellular Ca2+, a concentration where control larvae no longer augment (Figure 4B). In addition, polylysine motif mutants exhibited facilitation during the first few pulses of 10 Hz stimulation in 1.5 mM Ca2+, but transgenic control larvae did not (Figure 4A, insets). Examination of the Ca2+ concentration where evoked release failed in the mutants also indicates a decrease in release probability. At 0.5 mM extracellular Ca2+, the failure rate in controls was 21 ± 5%, whereas that of the polylysine motif mutants was 64 ± 14% (p < 0.05). Thus, polylysine motif mutants exhibit a decreased release probability compared with transgenic control larvae.
Figure 4.
Polylysine motif mutants have a decreased release probability compared with transgenic controls. EJPs were evoked by 30 s of 10-Hz stimulation in 1.5 mM (A) or 2.0 mM (B) extracellular Ca2+. EJP amplitudes were normalized to the amplitude of the EJP evoked by the first pulse and then averaged. After the first nine points, the points plotted are averages of 10 EJPs. Polylysine motif mutants: gray triangles, n = 6 fibers for 1.5 mM Ca2+ and n = 9 fibers for 2.0 mM Ca2+. Transgenic controls: black circles, n = 6 fibers for 1.5 mM Ca2+ and n = 10 fibers for 2.0 mM Ca2+. Error bars are SEM.
The Readily Releasable Pool of Synaptic Vesicles Is Unaltered in Synaptotagmin Polylysine Motif Mutants
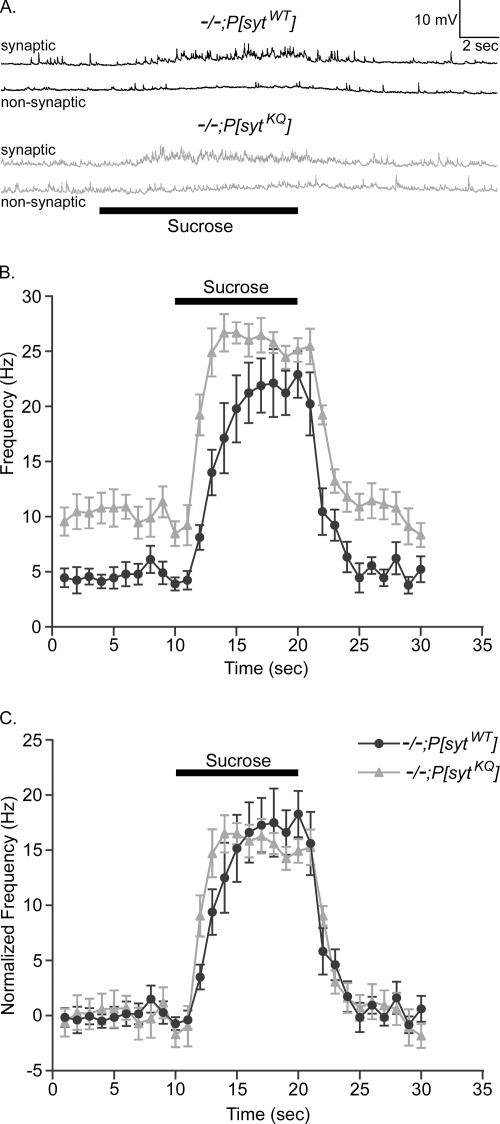
To determine whether there was also a change in the number of readily releasable vesicles, we elicited synaptic vesicle fusion with hypertonic sucrose. In Drosophila (Aravamudan et al., 1999; Suzuki et al., 2002a, b) as in frog (Fatt and Katz, 1952; Chen and Grinnell, 1997; Kashani et al., 2001) and in cultured neuronal cells (Stevens and Tsujimoto, 1995; Rosenmund and Stevens, 1996; Mochida et al., 1998), application of a hypertonic sucrose solution dramatically increases the frequency of miniature synaptic potentials. External Ca2+ is not needed for this response (Rosenmund and Stevens, 1996; Mochida et al., 1998; Suzuki et al., 2002a, b); however, the response is blocked when neuronal synaptobrevin (n-syb, also known as VAMP), SNAP-25, or syntaxin (syx) is cleaved by clostridial neurotoxin (Capogna et al., 1997), and it is greatly reduced in n-syb, syx, or Unc-13 Drosophila knockouts (Aravamudan et al., 1999). Thus, the hypertonic sucrose response is thought to bypass the Ca2+-sensing step of vesicle fusion and to trigger the fusion of docked vesicles (Rosenmund and Stevens, 1996; Schikorski and Stevens, 2001) by using the same basic fusion machinery that underlies Ca2+-triggered fusion. Furthermore, at Drosophila neuromuscular junctions, the size of the hypertonic response correlates with the size of the readily releasable pool of synaptic vesicles (Aravamudan et al., 1999; Suzuki et al., 2002a, b; Okamoto et al., 2005).
During a puff-application of 500 mM sucrose for 10 s, the frequency of miniature synaptic potentials (minis) dramatically increased in both polylysine motif mutants and transgenic controls (Figure 5). Figure 5A shows sample recordings from mutant and control larvae in both synaptic and nonsynaptic regions of the muscle fiber. The basal frequency of minis is elevated in each genotype only when sucrose is puffed on the synaptic region. The frequency before, during, and after the sucrose puff was determined and is plotted in Figure 5B. The basal frequency was higher in polylysine motif mutants than in controls (Figure 5B; Mackler and Reist, 2001). Therefore, to facilitate comparison between genotypes, the average frequency during the 10 s before the sucrose puff was subtracted from the calculated frequencies within each genotype (Figure 5C). The sucrose response in the polylysine motif mutants was comparable to that of controls. Thus, the decrease in the Ca2+-evoked response recorded in the polylysine motif mutants (Figure 3) is not due to a decrease in the size of the readily releasable pool of synaptic vesicles (Figure 5), but rather to a decrease in the release probability (Figure 4).
Figure 5.
Size of the readily releasable pool of synaptic vesicles is unchanged in synaptotagmin polylysine motif mutants. (A) Sample traces showing that application of a hypertonic sucrose solution (bar) to synaptic regions for 10 s increases the frequency of miniature synaptic potentials. (B) Frequency of miniature synaptic potentials is plotted versus time from 10 s before to 10 s after the application of sucrose. (C) Because the basal rate of spontaneous release is greater in the mutants, the frequency of release was normalized to the basal rate within each genotype for comparison. The sucrose-induced, increased frequency in polylysine motif mutants (gray triangles, n = 9 fibers) is comparable to that in transgenic controls (black circles, n = 9 fibers). Error bars are SEM.
The Polylysine Motif Mutation Slows Synaptotagmin·SNARE-mediated Lipid Mixing In Vitro
A decreased release probability could result from disruption of either Ca2+ sensing or synaptic vesicle docking/priming. The decrease in apparent Ca2+ affinity of release (Figure 3C) suggests a disruption in Ca2+ sensing. Yet, decreased Ca2+ affinity could only account for a decrease in the maximum amplitude of release (Figure 3A) if the Ca2+ affinity has decreased to such an extent that the intracellular Ca2+ concentration can never reach a level sufficient to trigger maximal release. Although at high extracellular Ca2+ concentrations, the Ca2+ influx through voltage-gated Ca2+ channels is expected to saturate (Hagiwara and Takahashi, 1967), at Drosophila neuromuscular junctions, this saturation is only reached when extracellular Ca2+ is ≥10 mM (Okamoto et al., 2005). Because the maximal response recorded in our polylysine motif mutants occurs around 5 mM (Figure 3A), well below saturation levels for Ca2+ channels, the decrease in maximal release recorded in these mutants is not due to the decrease in apparent Ca2+ affinity. Indeed, a decrease in the efficacy of release is more likely due to a disruption in the coupling of synaptotagmin to the release machinery (Borden et al., 2005).
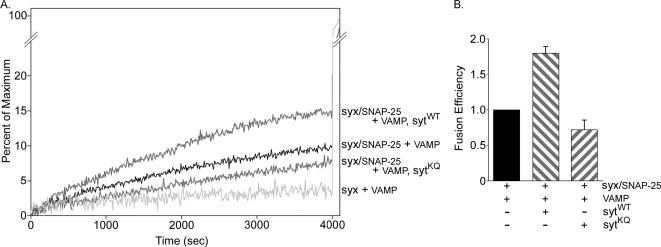
On Ca2+ influx, the interaction of synaptotagmin with the presynaptic membrane is thought to be important for fusion (Bai et al., 2000, 2002; Wang et al., 2003; Rhee et al., 2005). The Ca2+-independent interaction of the polylysine motif with t-SNARE heterodimers (Rickman et al., 2004) may play an important role in coupling synaptotagmin to the release machinery by positioning the Ca2+-binding site of the C2B domain near the plasma membrane. This Ca2+-independent interaction would thus prime synaptic vesicles for efficient fusion. To directly test the importance of the C2B polylysine motif in Ca2+-independent facilitation of fusion, we examined the rate of fusion in a fluorescence lipid mixing assay. When v-SNARE–containing liposomes are mixed with liposomes containing t-SNARE heterodimers in the absence of Ca2+, SNARE complex-specific fusion of the two populations occurred (Figure 6A; Weber et al., 1998; Parlati et al., 1999). Addition of membrane-bound synaptotagmin into the v-SNARE–containing liposomes directly accelerated the rate of fusion in a Ca2+-independent manner (Figure 6, A and B; Mahal et al., 2002) consistent with the hypothesis that a Ca2+-independent interaction between synaptotagmin and t-SNARE heterodimers facilitates SNARE-dependent fusion. Mutation of the polylysine motif, which has been shown to block the Ca2+-independent interaction between synaptotagmin and t-SNARE heterodimers (Rickman et al., 2004), abolished the ability of synaptotagmin to accelerate the fusion reaction (Figure 6, A and B). These results indicate that the polylysine motif of synaptotagmin plays a critical role in Ca2+-independent vesicle docking/priming and/or fusion in vitro.
Figure 6.
Polylysine motif mutation abolishes synaptotagmin's Ca2+-independent ability to accelerate SNARE-mediated fusion. Fusion of v-SNARE liposomes (VAMP) with t-SNARE liposomes (syx/SNAP-25) was monitored via a fluorescence lipid mixing assay in the absence of Ca2+. (A) Sample traces showing the increase in fluorescence when t-SNARE liposomes are mixed with v-SNARE liposomes that do not contain synaptotagmin (syx/SNAP-25 + VAMP), with v-SNARE liposomes that contain wild-type synaptotagmin (syx/SNAP-25 + VAMP, sytWT) or with v-SNARE liposomes that contain the polylysine motif mutant synaptotagmin (syx/SNAP-25 + VAMP, sytKQ). Light gray trace shows the background rate of lipid mixing when SNAP-25 is omitted from the t-SNARE liposomes (syx + VAMP). Detergent is added at 4000 s to determine the maximum fluorescence intensity (Weber et al., 2000). (B) The final values before the addition of detergent were used. After subtraction of background fusion (syx + VAMP), values were normalized to fusion in the absence of synaptotagmin and plotted as fusion efficiency. n = 3; error bars are SD.
Synaptic Vesicle Recycling Is Slowed in Polylysine Motif Mutants In Vivo
If the polylysine motif were important for synaptic vesicle docking/priming before Ca2+-triggered fusion in vivo, then the polylysine motif mutants should exhibit a defect in synaptic vesicle availability, a process upstream of Ca2+ sensing. First, we investigated whether the polylysine motif functions in maintaining the supply of synaptic vesicles available for Ca2+-triggered fusion by examining the ability of the mutants to sustain neurotransmitter release at the neuromuscular junction during high-frequency stimulation. When the rate of resupply is slower than the rate of synaptic vesicle exocytosis, the result is a net loss of synaptic vesicles available for release, which is reflected in decreasing EJP amplitudes. Thus, the degree of synaptic depression reflects the balance between synaptic vesicle exocytosis and synaptic vesicle resupply, which would include both synaptic vesicle mobilization and recycling (Delgado et al., 2000). Second, because recovery from synaptic depression occurs by synaptic vesicle recycling rather than vesicle mobilization from a reserve pool (Pyle et al., 2000; Richards et al., 2003), we investigated whether the polylysine motif functions specifically during synaptic vesicle recycling by examining the mutants' ability to recover from synaptic depression.
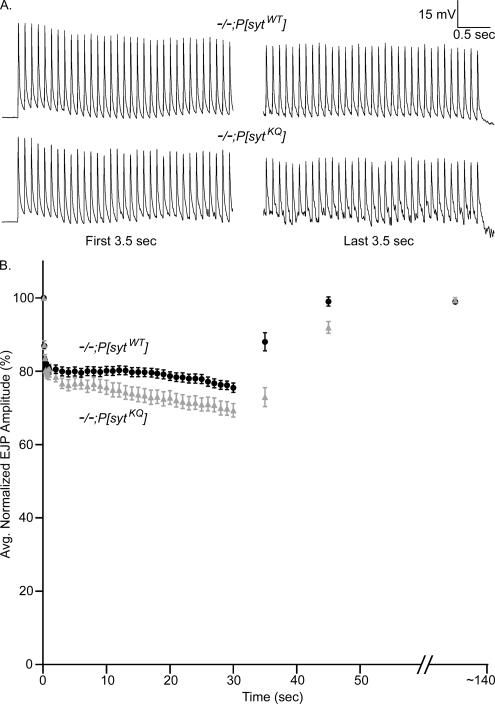
During 30 s of 10-Hz stimulation in 5 mM Ca2+, the larval neuromuscular junction exhibited synaptic depression (Figure 7). Both the sample traces in Figure 7A and the averaged data graphed in Figure 7B illustrate that during 30 s of stimulation, evoked release decreased to a greater extent in polylysine motif mutants than in transgenic controls. To compare the magnitude of depression in mutant and control transgenic lines, EJP amplitudes were normalized to the EJP amplitude elicited by the first pulse in the train. Despite that the polylysine motif mutants have a decreased release probability and release fewer vesicles with each stimulus (Figure 3, 5 mM Ca2+), they exhibited a small (∼6%) increase in the magnitude of depression (Figure 7B). However, this apparent increase in synaptic depression might be an artifact of nonlinear summation. Because the EJP amplitudes are larger in the controls (Figures 3A and 7A), they would be more affected by nonlinear summation, which would underestimate their true degree of synaptic depression relative to the mutants. To explore this possibility, we applied the equation of Martin (1955) as an estimate to correct for nonlinear summation (our unpublished data). Indeed, using this correction factor, the polylysine motif mutants exhibited slightly less (∼4%) synaptic depression than controls. However, because this formula is an imperfect correction (McLachlan and Martin, 1981), the true degree of synaptic depression is likely somewhere between that calculated from the noncorrected and corrected EJP amplitudes. Thus, the degree of synaptic depression is likely quite similar between mutants and controls.
Figure 7.
Polylysine motif mutants recover more slowly from synaptic depression than transgenic controls. Synaptic depression was elicited by stimulating fibers at 10 Hz for 30 s in 5 mM extracellular Ca2+. Recovery was monitored by recording the EJP amplitude of a single shock either 5 or 15 s after the 10-Hz stimulation stopped. Each fiber was also stimulated at an extended time point (∼140 s) to verify full recovery. (A) Representative traces from polylysine motif mutants (−/−;P[sytKQ]) and transgenic controls (−/−;P[sytWT]) showing the first and last 3.5 s of stimulation at 10 Hz for 30 s in saline containing 5 mM Ca2+. (B) EJP amplitudes were normalized to the amplitude of the first shock. After the first nine points, the points plotted during the 10-Hz stimulation are averages of 10 EJPs. Transgenic controls: black circles, n = 10 fibers for 5-s recovery and n = 14 fibers for 15-s recovery. Polylysine motif mutants: gray triangles, n = 10 fibers for 5-s recovery and n = 14 fibers for 15-s recovery. Error bars are SEM.
Recovery from synaptic depression was examined either 5 or 15 s after the 30 s of 10-Hz stimulation ceased (Figure 7B). Only larvae that exhibited ≥95% recovery at an extended time point were included. Recovery from synaptic depression was dramatically slower in polylysine motif mutants than in transgenic controls. After 5 s, the mutants showed 41% less recovery than controls and by 15 s the mutants were still depressed 24%, whereas controls had already completely recovered. The slower rate of recovery in polylysine motif mutants in vivo demonstrates that a major defect caused by the polylysine motif mutation occurs during synaptic vesicle recycling before the Ca2+-dependent fusion step; if a disruption of Ca2+-dependent fusion were the primary defect in these mutants, they should recover from synaptic depression as quickly as controls. It also suggests that the polylysine motif functions in a Ca2+-independent process, because Ca2+ levels drop rapidly upon the cessation of stimulation (within a few seconds; Wu and Betz, 1996; Karunanithi et al., 1997; Suzuki et al., 2000; Macleod et al., 2002).
Synaptic Vesicle Endocytosis Is Not Impaired in Polylysine Motif Mutants
Our in vitro studies demonstrate a Ca2+-independent role for the polylysine motif in docking/priming and/or fusion, whereas our in vivo studies demonstrate a Ca2+-independent role before Ca2+-triggered fusion. Together, these studies support a role for the polylysine motif in docking/priming; however, they do not exclude additional disruptions during synaptic vesicle recycling. Recycling consists of many steps that include, but are not limited to, endocytosis, clathrin uncoating, refilling with neurotransmitter, docking, and priming of vesicles. Because functional studies demonstrate that synaptotagmin I is required during endocytosis (von Poser et al., 2000; Jarousse and Kelly, 2001; Jarousse et al., 2003; Poskanzer et al., 2003; Llinás et al., 2004; Nicholson-Tomishima and Ryan, 2004) and the polylysine motif of the C2B domain of synaptotagmin is postulated to mediate this role (Takei and Haucke, 2001; but see Poskanzer et al., 2006), we wanted to determine whether endocytosis was disrupted in our mutants. However, the rate of endocytosis is difficult to assess in mutants with defects in exocytosis. Therefore, we investigated the importance of the C2B polylysine motif for endocytosis by using three approaches.
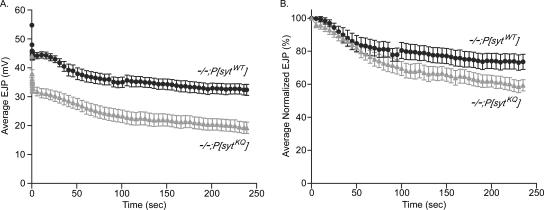
As a first step in examining endocytosis, the ability of the mutants to maintain a supply of synaptic vesicles for release was further challenged by stimulating preparations at 10 Hz for 4 min in 5 mM Ca2+. The averaged data (Figure 8A) illustrate that during the 4 min of stimulation evoked release decreased to a greater extent in polylysine motif mutants than in transgenic controls. To compare the magnitude of depression in mutant and control transgenic lines, EJP amplitudes were binned at 5-s intervals and normalized to the first EJP amplitude bin (Figure 8B). EJP amplitudes from polylysine motif mutants did decrease to a greater extent than the transgenic controls. Although this result seems to suggest that the rate of synaptic vesicle resupply relative to the rate of exocytosis is slowed in the polylysine motif mutants compared with controls, again this apparent difference is probably exaggerated by nonlinear summation. Indeed, when the correction factor of Martin (Martin, 1955) is applied, the polylysine motif mutants again show less depression (∼4%) than controls (our unpublished data). Even without the correction factor, the increased synaptic depression in the polylysine motif mutants is not severe (5% more depression after 60 s and 15% more after 4 min). Thus, the polylysine motif mutants are able to maintain release relatively well for at least 4 min of 10-Hz stimulation, an unexpected result if the polylysine motif were critical for endocytosis.
Figure 8.
Polylysine motif mutants maintain significant levels of evoked release during prolonged high-frequency stimulation. (A) Average EJP amplitudes evoked during 4 min of 10-Hz stimulation in saline containing 5 mM Ca2+. Except for the first nine points and the last point, the EJP amplitudes were binned at 5-s intervals, and the average of the bin is plotted. The first nine points are not binned. The last point is the average EJP amplitude of a 3-s bin. Transgenic controls: black circles, n = 8 fibers. Polylysine motif mutants: gray triangles, n = 10 fibers. (B) Same EJP amplitudes as in A but binned at 5-s intervals and normalized to the first 5-s bin.
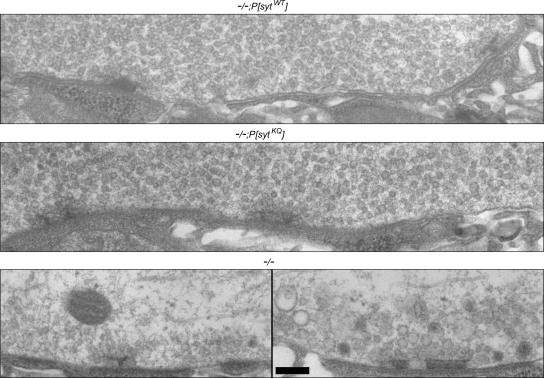
A common ultrastructural phenotype seen at neuromuscular junctions in endocytic mutants is severe synaptic vesicle depletion (Koenig et al., 1989; Fernandez et al., 1998; Verstreken et al., 2002, 2003). Indeed, sytnull mutants in Drosophila exhibit such depletion, along with an accumulation of larger membranous structures (Figure 9, bottom; Reist et al., 1998; Loewen et al., 2006). Therefore, our second approach was to examine the synaptic ultrastructure of polylysine motif mutants to see whether they exhibited synaptic vesicle depletion, indicative of an endocytic defect.
Figure 9.
Synaptic vesicles are abundant in nerve terminals of polylysine motif mutants. Electron micrographs showing the synaptic ultrastructure of neuromuscular junctions in transgenic controls (top), polylysine motif mutants (middle), and sytnull mutants (bottom). Bar, 200 nm.
The boutons of transgenic control larvae (Figure 9, top) and polylysine motif mutants (Figure 9, middle) contain numerous synaptic vesicles located at active zones and throughout the nerve terminal, and no accumulation of larger membranous structures (compare to sytnull mutants, Figure 9, bottom). Thus, both the polylysine motif mutant transgene and the wild-type transgene are able to rescue the ultrastructural deficits seen in sytnull mutant terminals. Again, this finding suggests that the polylysine motif of synaptotagmin does not mediate the role of synaptotagmin in synaptic vesicle endocytosis. Furthermore, it is consistent with our finding that the readily releasable pool size is not changed in polylysine motif mutants (Figure 5).
Finally, to examine the rate of endocytosis in polylysine motif mutants directly, we used an FM 1-43 dye uptake assay. FM 1-43 fluoresces when it inserts into lipid membranes. If it is present when synaptic vesicle membrane is internalized, these synaptic vesicles will be fluorescently labeled. Subsequently, the dye can be washed from the outside of cells, leaving only the internalized synaptic vesicles labeled. Thus, FM 1-43 makes it possible to examine the process of endocytosis directly (Betz et al., 1992).
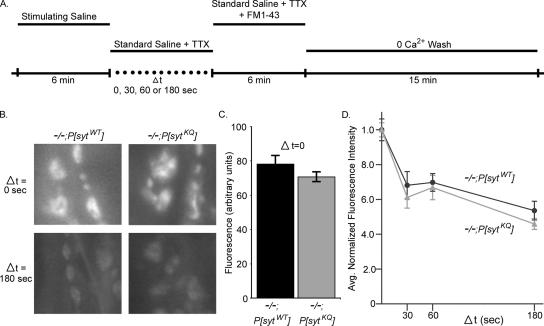
Figure 10A illustrates the endocytic assay we used to test the involvement of the polylysine motif in endocytosis [adapted from the assays of (Ryan et al., 1996; Kuromi and Kidokoro, 1998; Stimson et al., 2001; Kim et al., 2002)]. Drosophila third instars were stimulated for 6 min with a stimulating saline containing high potassium. Stimulation was terminated when the stimulating saline was replaced by standard saline plus 3 μM TTX. TTX was included to block any spontaneous action potentials coming from the attached CNS. The standard saline + TTX was left on the preparation for a variable amount of time (Δt = 0, 30, 60, or 180 s). After Δt, the preparation was bathed in standard saline containing TTX and FM 1-43 for 6 min to label endocytosing vesicles. Finally the preparation was washed for 15 min with 3 changes of Ca2+-free saline to remove extracellular FM 1-43 from the plasma membrane. Ca2+-free saline was used to minimize vesicle fusion during the washing stage. After washing, the fluorescence intensity of the FM 1-43-labeled synaptic boutons was measured.
Figure 10.
Rate of endocytosis is not significantly different in synaptotagmin polylysine motif mutants. (A) Schematic of endocytic assay using FM 1-43 dye. (B) Synaptic boutons on muscle 6 and 7 in polylysine motif mutants (−/−;P[sytKQ]) and transgenic controls (−/−;P[sytWT]) were labeled with FM 1-43 dye as described in A. Labeling is shown for Δt = 0 s (top) and Δt = 180 (bottom). (C) At Δt = 0, the average fluorescence intensity of boutons from polylysine motif mutants was ∼10% less than controls. (D) Fluorescence intensity of synaptic boutons is graphed as a function of the time between stimulus termination and application of the FM 1-43 dye (Δt). Polylysine motif mutants: gray triangles, n = 132–135 boutons from nine larvae for each Δt. Transgenic controls: black circles, n = 135 boutons from nine larvae for each Δt. To account for differences between genotypes in the absolute number of synaptic vesicles released during stimulation, fluorescence values for each genotype were normalized to the fluorescence intensity of that genotype at Δt = 0 s. 95% CI = −11.6 to 2.75.
Because FM 1-43 is present after stimulation ceases, and the signal is subsequently normalized to its maximum (see below), the above-mentioned protocol allowed us to determine the rate of internalization independent of the rate of exocytosis (Ryan et al., 1996; Stimson et al., 2001; Kim et al., 2002). However, only those synaptic vesicles that remain on the surface at the end of the stimulation can be labeled. Thus, a strong stimulus was needed to provide sufficient fluorescent signals for analysis. In standard saline containing 1.5 mM Ca2+, evoked release is only 14.2 ± 0.82 mV in the polylysine mutants (Figures 1B and 3A) and the FM 1-43 labeling achieved by the above-mentioned protocol was quite dim. Because our goal in these experiments was to determine whether endocytosis is a major defect in the polylysine motif mutants, we increased the Ca2+ in the stimulating saline to 5 mM to maximize release in these mutants and thus to increase FM 1-43 labeling.
During the delay period between the end of stimulation and addition of FM 1-43 dye, Δt, some fraction of the synaptic vesicle membrane remaining on the plasma membrane undergoes endocytosis. As Δt increases, more and more vesicle membrane is internalized, leaving less vesicle membrane on the surface to be labeled when FM 1-43 is subsequently applied. Therefore, as Δt increases, less FM 1-43 is internalized and nerve terminal labeling becomes progressively dimmer. This is illustrated in Figure 10B, which shows FM 1-43-labeled synaptic boutons on muscle 6 and 7 in both transgenic control and polylysine motif mutant larvae after Δt = 0 and 180 s. In both polylysine motif mutant and transgenic control larvae, labeling was brightest at Δt = 0 s and dimmest at Δt = 180 s. Loading was dependent on both stimulation and Ca2+ (our unpublished data).
At all time points, the mean fluorescence of polylysine motif mutant boutons was somewhat lower than the mean fluorescence for controls. This is most likely because the mutants exocytose fewer vesicles than the controls during the stimulation. Figure 10C shows the mean level of FM 1-43 labeling at Δt = 0 s for both mutants and controls. Under these stimulation conditions (high K+, high Ca2+ for 6 min), the amount of membrane awaiting endocytosis after stimulation ceased was only ∼10% less in the polylysine motif mutants. Because exocytosis is ∼40% less in the mutants in 5 mM Ca2+ (Figure 3A), this relative buildup of vesicle membrane stranded in the plasma membrane could indicate that endocytosis is slowed in the mutants. However, a single time point cannot accurately measure the rate of endocytosis because other factors, such as the size of the releasable pools and saturation of the endocytic machinery, can also influence the amount of membrane awaiting endocytosis. So, to determine the kinetics of synaptic vesicle internalization independent of this ∼10% difference, we normalized our data according to previous methods used to compare endocytic rates between preparations with different amounts of exocytosis (Ryan et al., 1996; Stimson et al., 2001; Kim et al., 2002). Thus, the fluorescence value of each bouton was normalized to the mean fluorescence value of boutons of the corresponding genotype at Δt = 0 s.
If the rate of endocytosis were impaired in polylysine motif mutant larvae, then during any given Δt, they should not be able to endocytose as large a fraction of the remaining synaptic vesicle membrane as controls. As a result, a larger proportion of synaptic vesicle membrane would remain in the plasma membrane when the FM 1-43 was applied, leading to proportionally more FM 1-43 internalization. Thus, if polylysine motif mutants had endocytic deficits, then after any given Δt, polylysine motif terminals should exhibit a normalized fluorescence that was brighter than controls. Indeed, stoned mutants, which also exhibit a decrease in evoked release, were shown to have an endocytic defect using a similar assay (Stimson et al., 2001). C2B polylysine motif mutants, in contrast, do not (Figure 10D). At each time point, the normalized fluorescence of the mutant terminals was slightly lower than that of controls. Lower fluorescence values would suggest a faster rate of endocytosis in the mutants, not an impaired rate. However, the differences between polylysine motif mutants and transgenic controls were not statistically significant. The mixed model ANOVA used to generate a 95% confidence interval for the true difference between mutants and controls included 0 (−11.6, 2.75). Because the rate of endocytosis was not disrupted in the mutants, the relatively small difference in labeling at Δt = 0 s (Figure 10C) suggests that endocytosis was the rate-limiting step and that our strong stimulation protocol successfully drove the majority of the cycling vesicle membrane (which is approximately equal in mutants and controls; Figure 5C) to the surface.
DISCUSSION
The highly conserved C2B polylysine motif plays a functional role in the synaptic vesicle cycle. Mutations in this motif decrease the amplitude of the evoked response at all Ca2+ concentrations; increase the EC50 of Ca2+ for release; increase augmentation, facilitation, and failure rate at low Ca2+ concentrations; cause a marked decrease in the rate of recovery from synaptic depression; block Ca2+-independent interactions with t-SNARE heterodimers (Rickman et al., 2004); and abolish the ability of synaptotagmin to accelerate Ca2+-independent, SNARE-mediated fusion. However, polylysine motif mutations do not alter postsynaptic receptor area, the size of minis (Mackler and Reist, 2001), active zone area, the Ca2+ cooperativity of release, the size of the hypertonic response, or the rate of endocytosis, and they do not result in synaptic vesicle depletion. These findings are discussed in terms of a single deficit hypothesis, namely, disruption of Ca2+-independent synaptic vesicle docking/priming (Figure 11).
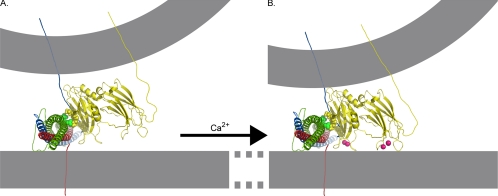
Figure 11.
Model of the role of the polylysine motif in Ca2+-independent synaptic vesicle docking/priming. Nuclear magnetic resonance structures of C2A (PDB file 1BYN) and C2B (PDB file 1K5W) domains (yellow) of synaptotagmin, the crystal structure of the core complex (PDB file 1SFC, containing VAMP [blue], SNAP-25 [green], and syntaxin [red]), and Ca2+ (pink) are shown to scale by using the PyMOL Molecular Graphics System (DeLano, 2002). The N termini of the SNARE proteins are darker and the C termini are paler to indicate depth. The membranes, the link between C2A and C2B domains of synaptotagmin, as well as transmembrane links for synaptotagmin, VAMP, syntaxin, and SNAP-25 were subsequently added. (A) Ca2+-independent interactions between the polylysine motif lysines in C2B (yellow, space-filled residues) with negatively charged residues of SNAP-25 (green, space-filled residues; Rickman et al., 2004, 2006) could hold the C2B Ca2+ binding site in the immediate vicinity of negatively charged phospholipids of the presynaptic membrane. (B) On Ca2+ influx, Ca2+-binding pockets of synaptotagmin insert into the plasma membrane (Bai et al., 2000, 2002, Wang et al., 2003; Rhee et al., 2005) allowing these negatively charged phospholipids to complete the coordination sphere for Ca2+ (Fernández-Chacón et al., 2002). The opposite end of the C2B domain interacts with the vesicle membrane (Araç et al., 2006) pulling the vesicle and plasma membranes closer together.
Neither the response to a single quantum of neurotransmitter (Mackler and Reist, 2001) nor the area occupied by the postsynaptic receptors has decreased. Therefore, the postsynaptic responsiveness is unchanged and the decrease in the evoked response must be due to a presynaptic deficit; either the size of the readily releasable pool and/or the release probability of synaptic vesicles must be decreased in the polylysine motif mutants. Because neither the area of presynaptic active zones nor the response to a hypertonic solution is decreased, the size of the readily releasable pool has not changed in the mutants. Together with the finding that facilitation, augmentation, and failure rate are increased in the mutants, these data provide strong support for the hypothesis that the polylysine motif regulates the release probability of synaptic vesicles.
Of the previously hypothesized functions for the polylysine motif, a decrease in either Ca2+-triggered fusion and/or Ca2+-independent docking/priming could result in a decrease in vesicle release probability. We discuss each possibility in turn below.
Although a defect in Ca2+-triggered fusion could result in a decrease in release probability, it cannot account for all of the deficits seen in polylysine motif mutants. Ca2+-dependent binding to negatively charged phospholipids (PC/PS) is reportedly unchanged by a polylysine motif mutation when a GST-pull-down assay is used (Bai et al., 2004), suggesting that Ca2+ sensing is not disrupted by this mutation in vitro. However, recent results using a centrifugation assay and PC/PE/PS/PI (phosphatidylinositol)/cholesterol lipids suggest otherwise (Li et al., 2006). In our polylysine motif mutants, the apparent Ca2+ affinity of release has decreased, suggestive of a Ca2+-sensing defect. However, the mutants exhibit their maximal response at a Ca2+ concentration well below the level thought to saturate Ca2+ channels (Okamoto et al., 2005). Therefore, the decrease in maximal response cannot be fully accounted for by the decrease in the apparent Ca2+ affinity. Instead, the decrease in maximal response suggests that the decrease in release probability is due to disrupted coupling between the mutant synaptotagmin and the release machinery (Borden et al., 2005). Although disrupted coupling could disrupt fusion, our data demonstrate that the polylysine motif must play a role in the synaptic vesicle cycle at a step other than fusion. If the primary defect in the polylysine motif mutants were in Ca2+-triggered fusion, then the mutants should recover from synaptic depression as quickly as controls, but they do not. Recovery from depression is dramatically slower in mutants than in controls. Thus, the defect in the polylysine motif mutants cannot be solely in the Ca2+-triggered fusion step.
In contrast, a defect in Ca2+-independent docking/priming can account for all of the deficits seen in the polylysine motif mutants, even the decrease in the apparent Ca2+ affinity of release. It has been shown that negatively charged phospholipids increase the apparent Ca2+ affinities of the C2 domains of synaptotagmin by completing the coordination spheres for Ca2+ (Fernández-Chacón et al., 2001). It may be that the Ca2+-independent interaction between the C2B polylysine motif and t-SNARE heterodimers primes vesicles for Ca2+-triggered fusion by positioning the C2B Ca2+-binding pocket near the negatively charged phospholipids of the presynaptic membrane (Figure 11A). Thus, upon Ca2+ influx, these lipids are immediately at hand to complete the coordination sphere of calcium (Figure 11B), thereby effectively increasing the affinity of synaptotagmin for Ca2+. It should be noted that the Ca2+-independent interaction between the polylysine motif and PIP2-containing membranes could also mediate such a function (Bai et al., 2004). The decreased affinity in the polylysine motif mutants (Figure 3; Borden et al., 2005; Li et al., 2006) may result from the lack of a Ca2+-independent interaction that normally strengthens Ca2+-dependent phospholipid binding. Thus, although our data do not preclude a defect in Ca2+ sensing, direct disruption of Ca2+ binding is not required to account for the decrease in Ca2+ affinity seen in the polylysine motif mutants.
Further evidence in support of the hypothesis that the polylysine motif mediates Ca2+-independent docking/priming comes from our experiments where we monitored recovery from synaptic depression. The ability of the mutants to maintain neurotransmitter release during 30 s of high-frequency stimulation is less affected than their ability to recover from this synaptic depression (Figure 7). This difference may be because intracellular Ca2+ is higher during stimulation compared with after stimulation (Wu and Betz, 1996; Karunanithi et al., 1997; Suzuki et al., 2000; Macleod et al., 2002). Elevated intracellular Ca2+ during a stimulus train is thought to accelerate the rate of recovery from synaptic depression during the train compared with the rate of recovery poststimulation (Zucker and Regehr, 2002). Our observation that the polylysine motif mutants have greater difficulty recovering from depression (when intracellular Ca2+ levels have fallen) than maintaining neurotransmitter release (when intracellular Ca2+ levels are elevated) also indicates that the defect in these mutants is in a Ca2+-independent process. This finding suggests that during the stimulus train, Ca2+-dependent processes that are not impaired by the mutation may partially rescue the Ca2+-independent defect.
We provide direct support for the hypothesis that Ca2+-independent docking/priming is impaired in polylysine motif mutants with our finding that the mutation abolished the Ca2+-independent ability of synaptotagmin to accelerate the fusion of v-SNARE–containing vesicles to t-SNARE heterodimer-containing vesicles (Figure 6). Other in vitro studies also support this hypothesis. The C2B polylysine motif exhibits Ca2+-independent interactions with t-SNARE heterodimers (Rickman et al., 2004), suggesting that the polylysine motif may dock vesicles by bringing the synaptic vesicle v-SNARE into proximity with the t-SNAREs (Rickman et al., 2004, 2006). Such an interaction could also prime vesicles by positioning the C2B Ca2+-binding pocket near the presynaptic membrane, thereby facilitating Ca2+-triggered fusion (Figure 11). Ca2+-independent docking/priming by the polylysine motif could also be mediated by its Ca2+-independent interaction with PIP2 (Bai et al., 2004), which is located predominately in the plasma membrane (Holz et al., 2000; Micheva et al., 2001). This interaction may facilitate a trans-interaction between synaptotagmin in the synaptic vesicle membrane and PIP2 in the plasma membrane. This Ca2+-independent interaction has been proposed to mediate docking/priming of vesicles because it increases the rate of Ca2+-dependent penetration of synaptotagmin into lipid membranes (Bai et al., 2004), a step thought to be important for fusion. Alternatively, a very recent report indicates that the polylysine motif does not increase the rate of vesicle fusion, but instead increases the number of vesicles capable of fusing with a fast time constant (Li et al., 2006). This finding is consistent with the polylysine motif facilitating synaptic vesicle docking/priming. In this scenario, the polylysine motif mutation would result in fewer synaptic vesicles existing in a fully primed state. Fewer primed synaptic vesicles could explain the selective decrease in the amount of fast release (Li et al., 2006), and the decreased release probability (Figure 4; Borden et al., 2005; Li et al., 2006) seen in polylysine motif mutants. Furthermore, if the polylysine motif did indeed facilitate synaptic vesicle priming, then priming would be slower in its absence, and recovery from synaptic depression would be slower in polylysine motif mutants (Figure 7). Thus, disruption of either of these interactions (with t-SNARE heterodimers, as indicated by Figure 6, or with PIP2) could impair Ca2+-independent docking/priming while leaving many interactions proposed to mediate Ca2+-dependent docking/priming intact (Augustine, 2001).
Ultrastructural studies have demonstrated that synaptotagmin is important for synaptic vesicle docking. In synaptotagmin null mutants, the number of morphologically docked vesicles is specifically decreased (Reist et al., 1998; Loewen et al., 2006). A detailed ultrastructural analysis would be required to determine whether the C2B polylysine motif participates in the function of synaptotagmin in vesicle docking. However, in random electron micrographs of neuromuscular junctions from transgenic controls (Loewen et al., 2006) and polylysine motif mutants (our unpublished data), vesicles located immediately adjacent to the presynaptic membrane are common, unlike synaptotagmin null mutants (Reist et al., 1998; Loewen et al., 2006). In addition, because the readily releasable pool maybe correlated with the number of docked synaptic vesicles (Rosenmund and Stevens, 1996; Schikorski and Stevens, 2001), our observation that the readily releasable pool is unchanged would be consistent with no disruption in synaptic vesicle docking. Thus, although we cannot exclude vesicle docking, it is more likely that the polylysine motif functions primarily after docking to prime vesicles for rapid, efficient fusion upon the influx of Ca2+.
The marked slowing of recovery from synaptic depression after high-frequency stimulation demonstrates that the polylysine motif is necessary for a membrane trafficking step of the synaptic vesicle cycle in vivo. Although our in vitro data demonstrate a role for the polylysine motif in Ca2+-independent docking/priming, they do not exclude additional defects such as in endocytosis. However, further analysis of our mutants ruled out a major role for the polylysine motif in synaptic vesicle endocytosis. Electrophysiological, ultrastructural, and FM 1-43 studies are all inconsistent with the hypothesis that the polylysine motif functions during endocytosis. In known endocytic mutants (e.g., synaptojanin and endophilin), evoked release during low-frequency stimulation is not different from controls. However, during 4 min of 10-Hz stimulation, these mutants exhibit severe synaptic depression (Verstreken et al., 2002, 2003). In contrast, polylysine motif mutants have a significant defect in evoked release during low frequency stimulation (Figures 1 and 3; Mackler and Reist, 2001; Borden et al., 2005; Li et al., 2006), yet they exhibit only modest if any increase in synaptic depression during 4 min of 10-Hz stimulation (Figure 8). Thus, the electrophysiological phenotype of the polylysine motif mutants is inconsistent with an endocytic defect.
Ultrastructural analysis of nerve terminals in endocytic mutants show severe synaptic vesicle depletion (Koenig et al., 1989; Fernandez et al., 1998; Guichet et al., 2002; Verstreken et al., 2002, 2003). Indeed, sytnull mutants in Drosophila and Caenorhabditis elegans also show severe synaptic vesicle depletion (Figure 9, bottom; Jorgensen et al., 1995; Reist et al., 1998; Loewen et al., 2006), consistent with the role of synaptotagmin in endocytosis (von Poser et al., 2000; Jarousse and Kelly, 2001; Jarousse et al., 2003; Poskanzer et al., 2003; Llinás et al., 2004; Nicholson-Tomishima and Ryan, 2004). However, nerve terminals in polylysine motif mutants do not exhibit synaptic vesicle depletion (Figure 9, middle). On the contrary, the polylysine motif mutant synaptotagmin transgene rescues the sytnull ultrastructural phenotype, again inconsistent with an endocytic defect.
We directly measured the rate of synaptic vesicle internalization in polylysine motif mutants and controls using an FM 1-43 assay and found that it was not significantly different (Figure 10D). Previous studies indicate that the rate of endocytosis may decrease as more and more membrane is internalized (Wu and Betz, 1996; Sankaranarayanan and Ryan, 2001). Thus, the trend toward faster internalization in the polylysine motif mutants may be due to the fact that they have ∼10% less membrane to endocytose after stimulation than do their controls; the boutons of polylysine motif mutants are ∼90% as bright as control boutons at Δt = 0 (Figure 10C).
The finding that polylysine motif mutants have only ∼10% less membrane to endocytose than controls at Δt = 0 (Figure 10C), whereas their evoked release is ∼40% less in 5 mM Ca2+ (Figure 3A), may seem in conflict with our finding that endocytosis is not disrupted. However, that endocytosis is much slower than exocytosis and our finding that the size of the readily releasable vesicle pool is similar in polylysine motif mutants and controls (Figure 5B) could account for this apparent discrepancy. If the strong stimulation protocol used in the dye uptake assay (90 mM K+ and 5 mM Ca2+ for 6 min) fully mobilized the readily releasable pool in both genotypes and endocytosis were the rate limiting step, then when stimulation stopped a major fraction of the cycling membrane in both genotypes would remain incorporated in the plasma membrane awaiting endocytosis. Because evoked release is decreased in polylysine motif mutants, it is likely that for the mutants, the total time required to fully mobilize the readily releasable pool would be longer than for controls. However, if this time were <6 min, then at Δt = 0 both genotypes would have fully mobilized their readily releasable pool. Because the size of this pool is similar in both genotypes (Figure 5B), both would have similar amounts of vesicle membrane awaiting endocytosis at Δt = 0.
Multiple studies have indicated a functional role for synaptotagmin I in synaptic vesicle endocytosis in vivo (Figure 9, bottom; Jorgensen et al., 1995; Reist et al., 1998; Poskanzer et al., 2003; Llinás et al., 2004; Nicholson-Tomishima and Ryan, 2004; Loewen et al., 2006). Whereas the C2B domain has been implicated in endocytic function of synaptotagmin (Jarousse and Kelly, 2001; Littleton et al., 2001; Jarousse et al., 2003; Llinás et al., 2004), our studies clearly demonstrate that the polylysine motif within the C2B domain is not involved. Our results do not, however, rule out a role for other motifs within the C2B domain of synaptotagmin in synaptic vesicle endocytosis. Indeed, the conserved WHXL motif in the C2B domain of synaptotagmin has been shown to be critical for internalization of synaptotagmin in PC12 cells (Jarousse et al., 2003).
Three molecular mechanisms have been proposed to mediate the role of the polylysine motif in the synaptic vesicle cycle: 1) synaptic vesicle endocytosis, 2) regulation of Ca2+-triggered fusion, and 3) Ca2+-independent docking and/or priming of synaptic vesicles. Our data demonstrate that the polylysine motif mutation decreases the release probability of synaptic vesicles. Electron micrographs show that the polylysine motif mutant larvae do not exhibit synaptic vesicle depletion and an FM 1-43 assay demonstrates that the rate of endocytosis is not impaired in these mutants. The mutant larvae maintain release relatively well during high-frequency stimulation when Ca2+ levels are elevated. However, they are slow to recover from synaptic depression, when intracellular Ca2+ levels are low, indicating that a Ca2+-independent process is likely disrupted in these larvae. The decreased probability of release observed in polylysine motif mutants, coupled with their slow rate of recovery from depression, is consistent with the hypothesis that Ca2+-independent docking/priming of synaptic vesicles is disrupted in these mutants. The fact that the polylysine mutation abolished the ability of synaptotagmin to accelerate Ca2+-independent, SNARE-mediated fusion provides direct support for this hypothesis. Thus, we propose that the major role of the polylysine motif of synaptotagmin during the synaptic vesicle cycle in vivo is to facilitate Ca2+-independent priming of synaptic vesicles at release sites.
ACKNOWLEDGMENTS
We thank Dr. Suzanne Royer for technical assistance with electron microscopy and Brie Paddock for assistance with PyMOL. This work was supported by National Science Foundation Grant 9982862 and National Institute of Health Grant NS-045865.
Abbreviations used:
- t-SNARE heterodimers
syntaxin/SNAP-25
- v-SNARE
VAMP or synaptobrevin
- SNARE complex
VAMP/syntaxin/SNAP-25
- minis
miniature synaptic potentials.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-07-0622) on September 20, 2006.
REFERENCES
- Araç D., Chen X., Khant H. A., Ubach J., Ludtke S. J., Kikkawa M., Johnson A. E., Chiu W., Südhof T. C., Rizo J. Close membrane-membrane proximity induced by Ca(2+)-dependent multivalent binding of synaptotagmin-1 to phospholipids. Nat. Struct. Mol. Biol. 2006;13:209–217. doi: 10.1038/nsmb1056. [DOI] [PubMed] [Google Scholar]
- Aravamudan B., Fergestad T., Davis W. S., Rodesch C. K., Broadie K. Drosophila UNC-13 is essential for synaptic transmission. Nat. Neurosci. 1999;2:965–971. doi: 10.1038/14764. [DOI] [PubMed] [Google Scholar]
- Atwood H. L., Karunanithi S. Diversification of synaptic strength: presynaptic elements. Nat. Rev. Neurosci. 2002;3:497–516. doi: 10.1038/nrn876. [DOI] [PubMed] [Google Scholar]
- Augustine G. J. How does calcium trigger neurotransmitter release? Curr. Opin. Neurobiol. 2001;11:320–326. doi: 10.1016/s0959-4388(00)00214-2. [DOI] [PubMed] [Google Scholar]
- Bai J., Chapman E. R. The C2 domains of synaptotagmin–partners in exocytosis. Trends Biochem. Sci. 2004;29:143–151. doi: 10.1016/j.tibs.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Bai J., Earles C. A., Lewis J. L., Chapman E. R. Membrane-embedded synaptotagmin penetrates cis or trans target membranes and clusters via a novel mechanism. J. Biol. Chem. 2000;275:25427–25435. doi: 10.1074/jbc.M906729199. [DOI] [PubMed] [Google Scholar]
- Bai J., Tucker W. C., Chapman E. R. PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nat. Struct. Mol. Biol. 2004;11:36–44. doi: 10.1038/nsmb709. [DOI] [PubMed] [Google Scholar]
- Bai J., Wang P., Chapman E. R. C2A activates a cryptic Ca(2+)-triggered membrane penetration activity within the C2B domain of synaptotagmin I. Proc. Natl. Acad. Sci. USA. 2002;99:1665–1670. doi: 10.1073/pnas.032541099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W. J., Mao F., Bewick G. S. Activity-dependent fluorescent staining and destaining of living vertebrate motor nerve terminals. J. Neurosci. 1992;12:363–375. doi: 10.1523/JNEUROSCI.12-02-00363.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla A., Chicka M. C., Tucker W. C., Chapman E. R. Ca(2+)-synaptotagmin directly regulates t-SNARE function during reconstituted membrane fusion. Nat. Struct. Mol. Biol. 2006;13:323–330. doi: 10.1038/nsmb1076. [DOI] [PubMed] [Google Scholar]
- Bollmann J. H., Sakmann B., Borst J. G. Calcium sensitivity of glutamate release in a calyx-type terminal. Science. 2000;289:953–957. doi: 10.1126/science.289.5481.953. [DOI] [PubMed] [Google Scholar]
- Borden C. R., Stevens C. F., Sullivan J. M., Zhu Y. Synaptotagmin mutants Y311N and K326/327A alter the calcium dependence of neurotransmission. Mol. Cell. Neurosci. 2005;29:462–470. doi: 10.1016/j.mcn.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Brose N., Petrenko A. G., Südhof T. C., Jahn R. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science. 1992;256:1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- Capogna M., McKinney R. A., O'Connor V., Gähwiler B. H., Thompson S. M. Ca2+ or Sr2+ partially rescues synaptic transmission in hippocampal cultures treated with botulinum toxin A and C, but not tetanus toxin. J. Neurosci. 1997;17:7190–7202. doi: 10.1523/JNEUROSCI.17-19-07190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman E. R., Desai R. C., Davis A. F., Tornehl C. K. Delineation of the oligomerization, AP-2 binding, and synprint binding region of the C2B domain of synaptotagmin. J. Biol. Chem. 1998;273:32966–32972. doi: 10.1074/jbc.273.49.32966. [DOI] [PubMed] [Google Scholar]
- Chen B. M., Grinnell A. D. Kinetics, Ca2+ dependence, and biophysical properties of integrin-mediated mechanical modulation of transmitter release from frog motor nerve terminals. J. Neurosci. 1997;17:904–916. doi: 10.1523/JNEUROSCI.17-03-00904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremona O., De Camilli P. Phosphoinositides in membrane traffic at the synapse. J. Cell Sci. 2001;114:1041–1052. doi: 10.1242/jcs.114.6.1041. [DOI] [PubMed] [Google Scholar]
- DeLano W. L. The PyMOL Molecular Graphics System. 2002 http://www.pymol.org.
- Delgado R., Maureira C., Oliva C., Kidokoro Y., Labarca P. Size of vesicle pools, rates of mobilization, and recycling at neuromuscular synapses of a Drosophila mutant, shibire. Neuron. 2000;28:941–953. doi: 10.1016/s0896-6273(00)00165-3. [DOI] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action of calcium ions in transmitter release at the neuromuscular junction. J. Physiol. 1967;193:419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatt P., Katz B. Spontaneous subthreshold activity at the motor nerve endings. J. Physiol. 1952;117:109–128. [PMC free article] [PubMed] [Google Scholar]
- Fernandez I., Ubach J., Dulubova I., Zhang X., Südhof T. C., Rizo J. Three-dimensional structure of an evolutionarily conserved N-terminal domain of syntaxin 1A. Cell. 1998;94:841–849. doi: 10.1016/s0092-8674(00)81742-0. [DOI] [PubMed] [Google Scholar]
- Fernández-Chacón R., Königstorfer A., Gerber S. H., García J., Matos M. F., Stevens C. F., Brose N., Rizo J., Rosenmund C., Südhof T. C. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- Fernández-Chacón R., Shin O. H., Königstorfer A., Matos M. F., Meyer A. C., Garcia J., Gerber S. H., Rizo J., Südhof T. C., Rosenmund C. Structure/function analysis of Ca2+ binding to the C2A domain of synaptotagmin 1. J. Neurosci. 2002;22:8438–8446. doi: 10.1523/JNEUROSCI.22-19-08438.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert M., Goda Y., Hammer R. E., Li C., Rosahl T. W., Stevens C. F., Südhof T. C. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- Grass I., Thiel S., Höning S., Haucke V. Recognition of a basic AP-2 binding motif within the C2B domain of synaptotagmin is dependent on multimerization. J. Biol. Chem. 2004;279:54872–54880. doi: 10.1074/jbc.M409995200. [DOI] [PubMed] [Google Scholar]
- Guichet A., Wucherpfennig T., Dudu V., Etter S., Wilsch-Bräuniger M., Hellwig A., González-Gaitán M., Huttner W. B., Schmidt A. A. Essential role of endophilin A in synaptic vesicle budding at the Drosophila neuromuscular junction. EMBO J. 2002;21:1661–1672. doi: 10.1093/emboj/21.7.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. Surface density of calcium ions and calcium spikes in the barnacle muscle fiber membrane. J. Gen. Physiol. 1967;50:583–601. doi: 10.1085/jgp.50.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haucke V., De Camilli P. AP-2 recruitment to synaptotagmin stimulated by tyrosine-based endocytic motifs. Science. 1999;285:1268–1271. doi: 10.1126/science.285.5431.1268. [DOI] [PubMed] [Google Scholar]
- Haucke V., Wenk M. R., Chapman E. R., Farsad K., De Camilli P. Dual interaction of synaptotagmin with mu2- and alpha-adaptin facilitates clathrin-coated pit nucleation. EMBO J. 2000;19:6011–6019. doi: 10.1093/emboj/19.22.6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger R., Heinemann C., Neher E., Matthews G. Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature. 1994;371:513–515. doi: 10.1038/371513a0. [DOI] [PubMed] [Google Scholar]
- Holz R. W., Hlubek M. D., Sorensen S. D., Fisher S. K., Balla T., Ozaki S., Prestwich G. D., Stuenkel E. L., Bittner M. A. A pleckstrin homology domain specific for phosphatidylinositol 4,5-bisphosphate (PtdIns-4,5-P2) and fused to green fluorescent protein identifies plasma membrane PtdIns-4,5-P2 as being important in exocytosis. J. Biol. Chem. 2000;275:17878–17885. doi: 10.1074/jbc.M000925200. [DOI] [PubMed] [Google Scholar]
- Hurley J. H., Wendland B. Endocytosis: driving membranes around the bend. Cell. 2002;111:143–146. doi: 10.1016/s0092-8674(02)01044-9. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Properties of the larval neuromuscular junction in Drosophila melanogaster. J. Physiol. 1976;262:189–214. doi: 10.1113/jphysiol.1976.sp011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarousse N., Kelly R. B. The AP2 binding site of synaptotagmin 1 is not an internalization signal but a regulator of endocytosis. J. Cell Biol. 2001;154:857–866. doi: 10.1083/jcb.200103040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarousse N., Wilson J. D., Araç D., Rizo J., Kelly R. B. Endocytosis of synaptotagmin 1 is mediated by a novel, tryptophan-containing motif. Traffic. 2003;4:468–478. doi: 10.1034/j.1600-0854.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- Jorgensen E. M., Hartwieg E., Schuske K., Nonet M. L., Jin Y., Horvitz H. R. Defective recycling of synaptic vesicles in synaptotagmin mutants of Caenorhabditis elegans. Nature. 1995;378:196–199. doi: 10.1038/378196a0. [DOI] [PubMed] [Google Scholar]
- Karunanithi S., Georgiou J., Charlton M. P., Atwood H. L. Imaging of calcium in Drosophila larval motor nerve terminals. J. Neurophysiol. 1997;78:3465–3467. doi: 10.1152/jn.1997.78.6.3465. [DOI] [PubMed] [Google Scholar]
- Kashani A. H., Chen B. M., Grinnell A. D. Hypertonic enhancement of transmitter release from frog motor nerve terminals: Ca2+ independence and role of integrins. J. Physiol. 2001;530:243–252. doi: 10.1111/j.1469-7793.2001.0243l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W. T., Chang S., Daniell L., Cremona O., Di Paolo G., De Camilli P. Delayed reentry of recycling vesicles into the fusion-competent synaptic vesicle pool in synaptojanin 1 knockout mice. Proc. Natl. Acad. Sci. USA. 2002;99:17143–17148. doi: 10.1073/pnas.222657399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig J. H., Kosaka T., Ikeda K. The relationship between the number of synaptic vesicles and the amount of transmitter released. J. Neurosci. 1989;9:1937–1942. doi: 10.1523/JNEUROSCI.09-06-01937.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuromi H., Kidokoro Y. Two distinct pools of synaptic vesicles in single presynaptic boutons in a temperature-sensitive Drosophila mutant, shibire. Neuron. 1998;20:917–925. doi: 10.1016/s0896-6273(00)80473-0. [DOI] [PubMed] [Google Scholar]
- Li L., Shin O. H., Rhee J. S., Araç D., Rah J. C., Rizo J., Südhof T., Rosenmund C. Phosphatidylinositol phosphates as co-activators of Ca2+ binding to C2 domains of synaptotagmin 1. J. Biol. Chem. 2006;281:15845–15852. doi: 10.1074/jbc.M600888200. [DOI] [PubMed] [Google Scholar]
- Littleton J. T., Bai J., Vyas B., Desai R., Baltus A. E., Garment M. B., Carlson S. D., Ganetzky B., Chapman E. R. synaptotagmin mutants reveal essential functions for the C2B domain in Ca2+-triggered fusion and recycling of synaptic vesicles in vivo. J. Neurosci. 2001;21:1421–1433. doi: 10.1523/JNEUROSCI.21-05-01421.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littleton J. T., Stern M., Perin M., Bellen H. J. Calcium dependence of neurotransmitter release and rate of spontaneous vesicle fusions are altered in Drosophila synaptotagmin mutants. Proc. Natl. Acad. Sci. USA. 1994;91:10888–10892. doi: 10.1073/pnas.91.23.10888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R. R., Sugimori M., Moran K. A., Moreira J. E., Fukuda M. Vesicular reuptake inhibition by a synaptotagmin I C2B domain antibody at the squid giant synapse. Proc. Natl. Acad. Sci. USA. 2004;101:17855–17860. doi: 10.1073/pnas.0408200101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewen C. A., Royer S. M., Reist N. E. Drosophila synaptotagmin I null mutants show severe alterations in vesicle populations but calcium-binding motif mutants do not. J. Comp. Neurol. 2006;496:1–12. doi: 10.1002/cne.20868. [DOI] [PubMed] [Google Scholar]
- Lu X., Xu Y., Zhang F., Shin Y. K. Synaptotagmin I and Ca(2+) promote half fusion more than full fusion in SNARE-mediated bilayer fusion. FEBS letters. 2006;580:2238–2246. doi: 10.1016/j.febslet.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Lu X., Zhang F., McNew J. A., Shin Y. K. Membrane fusion induced by neuronal SNAREs transits through hemifusion. J. Biol. Chem. 2005;280:30538–30541. doi: 10.1074/jbc.M506862200. [DOI] [PubMed] [Google Scholar]
- Mackler J. M., Drummond J. A., Loewen C. A., Robinson I. M., Reist N. E. The C2B Ca2+-binding motif of synaptotagmin is required for synaptic transmission in vivo. Nature. 2002;418:340–344. doi: 10.1038/nature00846. [DOI] [PubMed] [Google Scholar]
- Mackler J. M., Reist N. E. Mutations in the second C2 domain of synaptotagmin disrupt synaptic transmission at Drosophila neuromuscular junctions. J. Comp. Neurol. 2001;436:4–16. [PubMed] [Google Scholar]
- Macleod G. T., Hegström-Wojtowicz M., Charlton M. P., Atwood H. L. Fast calcium signals in Drosophila motor neuron terminals. J. Neurophysiol. 2002;88:2659–2663. doi: 10.1152/jn.00515.2002. [DOI] [PubMed] [Google Scholar]
- Mahal L. K., Sequeira S. M., Gureasko J. M., Söllner T. H. Calcium-independent stimulation of membrane fusion and SNAREpin formation by synaptotagmin I. J. Cell Biol. 2002;158:273–282. doi: 10.1083/jcb.200203135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrus S. B., Portman S. L., Allen M. J., Moffat K. G., DiAntonio A. Differential localization of glutamate receptor subunits at the Drosophila neuromuscular junction. J. Neurosci. 2004;24:1406–1415. doi: 10.1523/JNEUROSCI.1575-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. R. A further study of the statistical composition of the endplate potential. J. Physiol. 1955;130:114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T. F. Tuning exocytosis for speed: fast and slow modes. Biochim. Biophys. Acta. 2003;1641:157–165. doi: 10.1016/s0167-4889(03)00093-4. [DOI] [PubMed] [Google Scholar]
- McLachlan E. M., Martin A. R. Non-linear summation of end-plate potentials in the frog and mouse. J. Physiol. 1981;311:307–324. doi: 10.1113/jphysiol.1981.sp013586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheva K. D., Holz R. W., Smith S. J. Regulation of presynaptic phosphatidylinositol 4,5-biphosphate by neuronal activity. J. Cell Biol. 2001;154:355–368. doi: 10.1083/jcb.200102098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida S., Yokoyama C. T., Kim D. K., Itoh K., Catterall W. A. Evidence for a voltage-dependent enhancement of neurotransmitter release mediated via the synaptic protein interaction site of N-type Ca2+ channels. Proc. Natl. Acad. Sci. USA. 1998;95:14523–14528. doi: 10.1073/pnas.95.24.14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson-Tomishima K., Ryan T. A. Kinetic efficiency of endocytosis at mammalian CNS synapses requires synaptotagmin I. Proc. Natl. Acad. Sci. USA. 2004;101:16648–16652. doi: 10.1073/pnas.0406968101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiki T., Augustine G. J. Dual roles of the C2B domain of synaptotagmin I in synchronizing Ca2+-dependent neurotransmitter release. J. Neurosci. 2004;24:8542–8550. doi: 10.1523/JNEUROSCI.2545-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T., Tamura T., Suzuki K., Kidokoro Y. External Ca2+ dependency of synaptic transmission in Drosophila synaptotagmin I mutants. J. Neurophysiol. 2005;94:1574–1586. doi: 10.1152/jn.00205.2005. [DOI] [PubMed] [Google Scholar]
- Parlati F., Weber T., McNew J. A., Westermann B., Söllner T. H., Rothman J. E. Rapid and efficient fusion of phospholipid vesicles by the alpha-helical core of a SNARE complex in the absence of an N-terminal regulatory domain. Proc. Natl. Acad. Sci. USA. 1999;96:12565–12570. doi: 10.1073/pnas.96.22.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin M. S., Brose N., Jahn R., Südhof T. C. Domain structure of synaptotagmin (p65) J. Biol. Chem. 1991;266:623–629. [PubMed] [Google Scholar]
- Poskanzer K. E., Fetter R. D., Davis G. W. Discrete residues in the c(2)b domain of synaptotagmin I independently specify endocytic rate and synaptic vesicle size. Neuron. 2006;50:49–62. doi: 10.1016/j.neuron.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Poskanzer K. E., Marek K. W., Sweeney S. T., Davis G. W. Synaptotagmin I is necessary for compensatory synaptic vesicle endocytosis in vivo. Nature. 2003;426:559–563. doi: 10.1038/nature02184. [DOI] [PubMed] [Google Scholar]
- Pyle J. L., Kavalali E. T., Piedras-Rentería E. S., Tsien R. W. Rapid reuse of readily releasable pool vesicles at hippocampal synapses. Neuron. 2000;28:221–231. doi: 10.1016/s0896-6273(00)00098-2. [DOI] [PubMed] [Google Scholar]
- Qin G., Schwarz T., Kittel R. J., Schmid A., Rasse T. M., Kappei D., Ponimaskin E., Heckmann M., Sigrist S. J. Four different subunits are essential for expressing the synaptic glutamate receptor at neuromuscular junctions of Drosophila. J. Neurosci. 2005;25:3209–3218. doi: 10.1523/JNEUROSCI.4194-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reist N. E., Buchanan J., Li J., DiAntonio A., Buxton E. M., Schwarz T. L. Morphologically docked synaptic vesicles are reduced in synaptotagmin mutants of Drosophila. J. Neurosci. 1998;18:7662–7673. doi: 10.1523/JNEUROSCI.18-19-07662.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee J. S., Li L. Y., Shin O. H., Rah J. C., Rizo J., Südhof T. C., Rosenmund C. Augmenting neurotransmitter release by enhancing the apparent Ca2+ affinity of synaptotagmin 1. Proc. Natl. Acad. Sci. USA. 2005;102:18664–18669. doi: 10.1073/pnas.0509153102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards D. A., Guatimosim C., Rizzoli S. O., Betz W. J. Synaptic vesicle pools at the frog neuromuscular junction. Neuron. 2003;39:529–541. doi: 10.1016/s0896-6273(03)00405-7. [DOI] [PubMed] [Google Scholar]
- Rickman C., Archer D. A., Meunier F. A., Craxton M., Fukuda M., Burgoyne R. D., Davletov B. Synaptotagmin interaction with the syntaxin/SNAP-25 dimer is mediated by an evolutionarily conserved motif and is sensitive to inositol hexakisphosphate. J. Biol. Chem. 2004;279:12574–12579. doi: 10.1074/jbc.M310710200. [DOI] [PubMed] [Google Scholar]
- Rickman C., Jiménez J. L., Graham M. E., Archer D. A., Soloviev M., Burgoyne R. D., Davletov B. Conserved prefusion protein assembly in regulated exocytosis. Mol. Biol. Cell. 2006;17:283–294. doi: 10.1091/mbc.E05-07-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C., Stevens C. F. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron. 1996;16:1197–1207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- Ryan T. A., Li L., Chin L. S., Greengard P., Smith S. J. Synaptic vesicle recycling in synapsin I knock-out mice. J. Cell Biol. 1996;134:1219–1227. doi: 10.1083/jcb.134.5.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan S., Ryan T. A. Calcium accelerates endocytosis of vSNAREs at hippocampal synapses. Nat. Neurosci. 2001;4:129–136. doi: 10.1038/83949. [DOI] [PubMed] [Google Scholar]
- Schikorski T., Stevens C. F. Morphological correlates of functionally defined synaptic vesicle populations. Nat. Neurosci. 2001;4:391–395. doi: 10.1038/86042. [DOI] [PubMed] [Google Scholar]
- Stevens C. F., Tsujimoto T. Estimates for the pool size of releasable quanta at a single central synapse and for the time required to refill the pool. Proc. Natl. Acad. Sci. USA. 1995;92:846–849. doi: 10.1073/pnas.92.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart B. A., Atwood H. L., Renger J. J., Wang J., Wu C. F. Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J. Comp. Physiol. A. 1994;175:179–191. doi: 10.1007/BF00215114. [DOI] [PubMed] [Google Scholar]
- Stewart B. A., Mohtashami M., Trimble W. S., Boulianne G. L. SNARE proteins contribute to calcium cooperativity of synaptic transmission. Proc. Natl. Acad. Sci. USA. 2000;97:13955–13960. doi: 10.1073/pnas.250491397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimson D. T., Estes P. S., Rao S., Krishnan K. S., Kelly L. E., Ramaswami M. Drosophila stoned proteins regulate the rate and fidelity of synaptic vesicle internalization. J. Neurosci. 2001;21:3034–3044. doi: 10.1523/JNEUROSCI.21-09-03034.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Grinnell A. D., Kidokoro Y. Hypertonicity-induced transmitter release at Drosophila neuromuscular junctions is partly mediated by integrins and cAMP/protein kinase A. J. Physiol. 2002a;538:103–119. doi: 10.1113/jphysiol.2001.012901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Okamoto T., Kidokoro Y. Biphasic modulation of synaptic transmission by hypertonicity at the embryonic Drosophila neuromuscular junction. J. Physiol. 2002b;545:119–131. doi: 10.1113/jphysiol.2002.026898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Osanai M., Murase M., Suzuki N., Ito K., Shirasaki T., Narita K., Ohnuma K., Kuba K., Kijima H. Ca2+ dynamics at the frog motor nerve terminal. Pflueg. Arch. Eur. J. Physiol. 2000;440:351–365. doi: 10.1007/s004240000278. [DOI] [PubMed] [Google Scholar]
- Takei K., Haucke V. Clathrin-mediated endocytosis: membrane factors pull the trigger. Trends Cell Biol. 2001;11:385–391. doi: 10.1016/s0962-8924(01)02082-7. [DOI] [PubMed] [Google Scholar]
- Verstreken P., Kjaerulff O., Lloyd T. E., Atkinson R., Zhou Y., Meinertzhagen I. A., Bellen H. J. Endophilin mutations block clathrin-mediated endocytosis but not neurotransmitter release. Cell. 2002;109:101–112. doi: 10.1016/s0092-8674(02)00688-8. [DOI] [PubMed] [Google Scholar]
- Verstreken P., Koh T. W., Schulze K. L., Zhai R. G., Hiesinger P. R., Zhou Y., Mehta S. Q., Cao Y., Roos J., Bellen H. J. Synaptojanin is recruited by endophilin to promote synaptic vesicle uncoating. Neuron. 2003;40:733–748. doi: 10.1016/s0896-6273(03)00644-5. [DOI] [PubMed] [Google Scholar]
- von Poser C., Zhang J. Z., Mineo C., Ding W., Ying Y., Südhof T. C., Anderson R. G. Synaptotagmin regulation of coated pit assembly. J. Biol. Chem. 2000;275:30916–30924. doi: 10.1074/jbc.M005559200. [DOI] [PubMed] [Google Scholar]
- Wang C. T., Grishanin R., Earles C. A., Chang P. Y., Martin T. F., Chapman E. R., Jackson M. B. Synaptotagmin modulation of fusion pore kinetics in regulated exocytosis of dense-core vesicles. Science. 2001;294:1111–1115. doi: 10.1126/science.1064002. [DOI] [PubMed] [Google Scholar]
- Wang P., Wang C. T., Bai J., Jackson M. B., Chapman E. R. Mutations in the effector binding loops in the C2A and C2B domains of synaptotagmin I disrupt exocytosis in a nonadditive manner. J. Biol. Chem. 2003;278:47030–47037. doi: 10.1074/jbc.M306728200. [DOI] [PubMed] [Google Scholar]
- Weber T., Parlati F., McNew J. A., Johnston R. J., Westermann B., Sèollner T. H., Rothman J. E. SNAREpins are functionally resistant to disruption by NSF and alphaSNAP. J. Cell Biol. 2000;149:1063–1072. doi: 10.1083/jcb.149.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T., Zemelman B. V., McNew J. A., Westermann B., Gmachl M., Parlati F., Sèollner T. H., Rothman J. E. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- Wu L. G., Betz W. J. Nerve activity but not intracellular calcium determines the time course of endocytosis at the frog neuromuscular junction. Neuron. 1996;17:769–779. doi: 10.1016/s0896-6273(00)80208-1. [DOI] [PubMed] [Google Scholar]
- Wu Y., He Y., Bai J., Ji S. R., Tucker W. C., Chapman E. R., Sui S. F. Visualization of synaptotagmin I oligomers assembled onto lipid monolayers. Proc. Natl. Acad. Sci. USA. 2003;100:2082–2087. doi: 10.1073/pnas.0435872100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig T., Wilsch-Bräuninger M., González-Gaitán M. Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J. Cell Biol. 2003;161:609–624. doi: 10.1083/jcb.200211087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T., Rammner B., Margittai M., Artalejo A. R., Neher E., Jahn R. Inhibition of SNARE complex assembly differentially affects kinetic components of exocytosis. Cell. 1999;99:713–722. doi: 10.1016/s0092-8674(00)81669-4. [DOI] [PubMed] [Google Scholar]
- Xu Y., Zhang F., Su Z., McNew J. A., Shin Y. K. Hemifusion in SNARE-mediated membrane fusion. Nat. Struct. Mol. Biol. 2005;12:417–422. doi: 10.1038/nsmb921. [DOI] [PubMed] [Google Scholar]
- Yoshihara M., Littleton J. T. Synaptotagmin I functions as a calcium sensor to synchronize neurotransmitter release. Neuron. 2002;36:897–908. doi: 10.1016/s0896-6273(02)01065-6. [DOI] [PubMed] [Google Scholar]
- Zhang J. Z., Davletov B. A., Südhof T. C., Anderson R. G. Synaptotagmin I is a high affinity receptor for clathrin AP-2, implications for membrane recycling. Cell. 1994;78:751–760. doi: 10.1016/s0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]
- Zucker R. S., Regehr W. G. Short-term synaptic plasticity. Annu. Rev. Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]