Abstract
Extracellular signal-regulated kinase (ERK)1/2 activity is reported to be required in mammalian cells for timely entry into and exit from mitosis (i.e., the G2-mitosis [G2/M] and metaphase-anaphase [M/A] transitions). However, it is unclear whether this involvement reflects a direct requirement for ERK1/2 activity during these transitions or for activating gene transcription programs at earlier stages of the cell cycle. To examine these possibilities, we followed live cells in which ERK1/2 activity was inhibited through late G2 and mitosis. We find that acute inhibition of ERK1/2 during late G2 and through mitosis does not affect the timing of the G2/M or M/A transitions in normal or transformed human cells, nor does it impede spindle assembly, inactivate the p38 stress-activated checkpoint during late G2 or the spindle assembly checkpoint during mitosis. Using CENP-F as a marker for progress through G2, we also show that sustained inhibition of ERK1/2 transiently delays the cell cycle in early/mid-G2 via a p53-dependent mechanism. Together, our data reveal that ERK1/2 activity is required in early G2 for a timely entry into mitosis but that it does not directly regulate cell cycle progression from late G2 through mitosis in normal or transformed mammalian cells.
INTRODUCTION
The extracellular signal-regulated (ERK)1/2 pathway consists of Raf1, mitogen-activated protein kinase kinase (MEK)1/2, and ERK1/2 kinases, which upon pathway activation are sequentially and specifically phosphorylated (Seger and Krebs, 1995). Once activated, ERK1/2 phosphorylates numerous cytoplasmic and nuclear substrates that lead to diverse cellular responses, including proliferation and differentiation, via both transcription-dependent and independent mechanisms (Pearson et al., 2001; Yoon and Seger, 2006). Basically, ERK1/2 activity is essential for cell growth; it mediates the G1/S transition by facilitating nucleotide and protein syntheses as well as the transcription of cell cycle regulators, including cyclins D and E (Widmann et al., 1999; Roovers and Assoian, 2000). Not unexpectedly, constitutive activation of ERK1/2 via mutations in Raf or its upstream Ras G protein leads to uncontrolled cell growth, whereas the inability to activate ERK1/2 is lethal in utero (Wojnowski et al., 1998; Giroux et al., 1999) and inhibits the growth of cultured cells (Pages et al., 1993).
In addition to its essential role in promoting the G1/S transition, enhanced ERK1/2 activity is also required in mammalian cells for a timely G2-mitosis (G2/M) transition. Long-term (hours to days) suppression of ERK1/2 by using pharmacological inhibitors of MEK1/2, dominant-negative MEK1, or RNA interference (RNAi) produces a delay in “G2/M” (Wright et al., 1999; Hayne et al., 2000; Roberts et al., 2002; Liu et al., 2004; Knauf et al., 2006). Gene expression profiling of nontransformed mammary epithelial cells also reveals that constitutive activation of ERK1/2 via MEK1 leads to increased levels of mRNAs that encode mitotic proteins such as Cyclin B, CDK1, CENP-E, Bub1, Mad2, and Aurora A (Grill et al., 2004). Among these, Cyclin B is under the control of the FoxM1 transcriptional factor (Alvarez et al., 2001; Laoukili et al., 2005), and the ERK1/2 pathway has recently been shown to activate one of its isoforms, FoxM1c (Ma et al., 2005). Together, these data suggest that ERK1/2 activity plays an upstream role in regulating the G2/M transition in mammalian cells by activating specific gene expression pathways, and this regulation likely occurs during early–mid-G2 before the transcriptional silencing seen in late G2 and mitosis.
The notion that ERK1/2 also regulates progression through the G2/M transition and mitosis in a transcription-independent manner comes primarily from studies on Xenopus oocyte extracts. In this system, ERK1/2 activity is sufficient for activating cyclin B/Cdk1, which induces the first meiotic division (Ferrell, 1999), and once in meiosis ERK1/2 seems to regulate spindle bipolarity via its (direct or indirect) effects on microtubule dynamics (Gotoh et al., 1991; Guadagno and Ferrell, 1998; Horne and Guadagno, 2003). ERK1/2 activity is also required in Xenopus oocyte extracts for a functional spindle assembly checkpoint (SAC) (Minshull et al., 1994; Takenaka et al., 1997; Chung and Chen, 2003) and likely also in Xenopus tadpole cells (Wang et al., 1997).
As in Xenopus oocytes, there is evidence that ERK1/2 also plays a nontranscriptional role in the G2/M transition in transformed mammalian somatic cells. In this regard, several biochemical and/or fluorescence-activated cell sorting studies report that the activity of ERK1/2 is enhanced during G2/M (e.g., Tamemoto et al., 1992; Wright et al., 1999; Hayne et al., 2000; Roberts et al., 2002). The exact meaning of this conclusion is, however, vague because the temporal resolution of these studies is not sufficient to reveal whether the enhancement of ERK1/2 activity occurs during late G2, mitosis, or both. Indeed, although some workers report that ERK1/2 pathway activity increases as cells transit into and/or exit mitosis (Edelmann et al., 1996; Roberts et al., 2002), others conclude that the activity of MEK (Laird et al., 1999; Hayne et al., 2004) and ERK1/2 are depressed during mitosis (Newberry and Pike, 1995; Klein et al., 1997; Gomez-Cambronero, 1999; Harding et al., 2003) and that Cdk1 participates in this inhibitory regulation (Kiyokawa et al., 1997; Dangi and Shapiro, 2005). The situation is further complicated by the fact that past studies on ERK1/2 activity at the G2/M transition often used treatments with microtubule inhibitors to obtain enriched fractions of mitotic cells, and after treatment with these drugs some cell lines show elevated levels of ERK1/2 activity (Schmid-Alliana et al., 1998; Hayne et al., 2000), whereas others do not (Tamemoto et al., 1992; Takenaka et al., 1998; Gomez-Cambronero, 1999). Thus, the questions of whether activation of ERK1/2 near the end of G2 and/or M is biologically relevant in mammals and thus whether ERK1/2 plays a direct role in the G2/M transition remain controversial.
Finally, there are also reports that ERK1/2 activity is required during mitosis in mammalian somatic cells, as it is during meiosis in Xenopus oocytes, for proper spindle formation and thus a timely metaphase-anaphase (M/A) transition (Willard and Crouch, 2001; Horne and Guadagno, 2003). In this regard, activated (phosphorylated) MEK1/2 and ERK1/2 are reported by immunofluorescence (IMF) to be present during mitosis in centrosomes/spindle poles (Shapiro et al., 1998; Liu et al., 2004; Lou et al., 2004) and kinetochores (Shapiro et al., 1998; Zecevic et al., 1998) where it is proposed to play a role in the SAC (Shapiro et al., 1998; Willard and Crouch, 2001; Horne and Guadagno, 2003). However, there is little data to support a functional role at these locations and that which does is indirect and open to different interpretations. Even the presence of active ERK1/2 on kinetochores is suspect, because not all IMF studies on the distribution of active ERK1/2 during mitosis report it in this location (Willard and Crouch, 2001), and popular antibodies used to detect active MEK1/2 during mitosis (i.e., phosphorylated MEK1/2) can cross-react with another phosphoprotein (Hayne et al., 2004). Also, a recent proteomic analysis did not find ERK1/2 on isolated human metaphase chromosomes, even though it identified 209 other chromosome-associated proteins (Uchiyama et al., 2005). Thus, the question of whether active ERK1/2 is a component of kinetochores, and, if so, whether its activity is required for SAC function, also remains controversial.
To address the nontranscriptional role of ERK1/2 in the G2/M and M/A transitions, we rapidly inhibited (or enhanced) ERK1/2 activity during late G2, just before mammalian cells become committed to mitosis, and followed the cells by video light microscopy. This allowed us to directly examine, for the first time, how inhibiting ERK1/2 during late G2 affects the kinetics of the G2/M and M/A transitions, independently of its role in initiating gene transcription pathways earlier in G2. At the same time, we also explored the requirement of ERK1/2 activity for the late G2 p38-mediated “stress” checkpoint as well as the SAC. We also conducted a series of microinjection, small interfering RNA (siRNA), and ERK1/2 overexpression studies in an effort to determine whether active ERK1/2 is really present at kinetochores and centrosomes. Finally, using a unique assay for G2 progression we asked whether the delay in the G2/M transition (i.e., entering mitosis) seen by others in indirect assays, in response to inhibiting ERK1/2, is due to a requirement for ERK1/2 activity during the early stages of G2.
MATERIALS AND METHODS
Cell Culture, Drug Treatment, and Live Cell Imaging
Telomerase-immortalized human retinal pigment epithelia 1 (RPE1), HeLa, and rat kangaroo (PtK) cells were cultured in DMEM or Eagle's minimal essential medium (for PtK) supplemented with 10% fetal bovine serum (FBS). BJ-ELB cells were cultured in a mixture of DMEM and Medium 1999 (4:1) supplemented with 10% FBS. Human mammary epithelial cells (HMECs) were maintained in MEGM (Clonetics BulletKits; Cambrex Bio Science, Walkersville, MD) supplemented with 5 μg/ml transferrin (Sigma-Aldrich, St. Louis, MO) and 10−5 M isoproterenol (Sigma-Aldrich). U0126 (Promega, Madison, WI), U0124 (Calbiochem, San Diego, CA), CI-1040 (Pfizer, Holland, MI), nocodazole (Calbiochem), anisomycin (Calbiochem), and dimethyl sulfoxide (DMSO) (Sigma-Aldrich) were added 15–30 min before each experiments. To activate ERK1/2 with 12-O-tetradecanoylphorbol-13-acetate (TPA) (Cell Signaling Technology, Danvers, MA), cells were pretreated with 10 nM TPA for 1 h and then with 50–100 nM TPA for 15 min according to the manufacturer's instructions. Our basic procedures for live cell imaging are detailed in Khodjakov and Rieder (2006). Briefly, cells were cultured on 25-mm2 coverslips to 70 to 80% confluence, and the coverslips were assembled into Rose Chambers 2 h before the start of each recording. Mid-to-late G2 cells, in which chromosome condensation is just evident by phase-contrast light microscopy, were located and followed on a shuttered Diaphot 200 (Nikon, Melville, NY) microscope equipped with a Micromax camera (Roper Scientific, Trenton, NJ). Images were acquired every 2–10 min and processed by Image-Pro Plus (Media Cybernetics, Silver Spring, MD) and National Institutes of Health ImageJ. The Rose chamber and microscope system were maintained at 37°C throughout recording.
In some experiments, we used low magnification (10×) phase-contrast optics to follow fields of HeLa and NIH 3T3 cells for extended periods, before and after treatment with ERK1/2 inhibitors, at an imaging rate of one image every 5 min. These time-lapse sequences were then analyzed frame by frame, at the individual cell level, to determine the number of cells within the field that entered mitosis every 30 min (i.e., that exhibited nuclear envelope breakdown; NEB) as well as the duration of mitosis (NEB to the initiation of cytokinesis). These data were then entered into the Excel spreadsheet program (Microsoft, Redmond, WA) for statistical calculations. Each graph thus generated incorporated data from at least four experiments (total number of cells in all fields at least 2000).
Immunoblotting and Immunofluorescence
For Western blotting, cells were washed in ice-cold phosphate-buffered saline (PBS), pH 7.4, containing 1 mM Na3VO4, lysed in cold lysis buffer (1% SDS, 20 mM HEPES, pH 7.4, 2 mM EGTA, 50 mM β-glycerophosphate, 2 mM EDTA, 137.5 mM NaCl, 10% glycerol, 1 mM dithiothreitol, 1 mM Na3VO4, 40 μM phenylmethylsulfonyl fluoride, and Complete protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN), incubated for 10 min on ice, and centrifuged at 14,000 rpm at 4°C. Equal amounts of protein (25 or 50 μg) were separated on SDS-PAGE gels, immunoblotted, and detected by enhanced chemiluminescence.
For IMF analysis of CENP-F, cells were grown on coverslips to 50% confluence, washed with PEM (100 mM PIPES, 2.5 mM EGTA, and 2 mM MgCl2, pH 6.9), permeabilized with 0.2% Triton X-100, and fixed with 2% paraformaldehyde. For double IMF analysis of hemagglutinin (HA)-tag and CREST, cells were washed with ice-cold PBS, pH 7.4, containing 1 mM Na3VO4, fixed with 3% paraformaldehyde for 15 min on ice, and permeabilized with 0.1% Triton-100 in PBS. For staining microtubules (MTs), cells were washed with PEM, permeabilized with 1% Triton X-100, and fixed with 1% glutaraldehyde in PEM. After fixation, the cultures were reduced by two 10-min treatments with 1% sodium borohydride twice and then blocked with PBS containing 3% bovine serum albumin (BSA). They were then incubated with primary antibodies in PBS containing 0.05% Tween 20 and 1% BSA, pH 7.4, for 1 h at 37°C and then with secondary antibodies labeled with Alexa Fluor 488 or 546 (Invitrogen, Carlsbad, CA) for 30 min at 37°C. DNA was stained with Hoechst 33342. All cells were imaged as a Z-series (200 nM apart) on an IX70 microscope (Olympus America, Melville, NY), deconvolved as necessary using Delta Vision 2.1 (Applied Precision, Issaquah, WA), and presented as maximal intensity projections.
The primary antibodies used in this study included rabbit Anti-ACTIVE mitogen-activated protein kinase (MAPK) recognizing dually phosphorylated ERK1/2 (pTEpY; Promega), mouse p-ERK antibody against ERK1/2 phosphorylated at Tyr-204 (E-4; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit p44/p42 MAPK antibody (Cell Signaling Technology) for detection of total ERK1/2, rabbit anti-CENP-F (Calbiochem), His-tag antibody (Cell Signaling), mouse anti-HA antibody (Roche Diagnostics, Indianapolis, IN), human anti-kinetochore serum (CREST), p38 MAP kinase antibody (Cell Signaling Technology), anti-ACTIVE p38 (Promega), phospho-p53 antibodies (Ser15 and Ser20; Cell Signaling Technology), p53 antibody (Cell Signaling Technology), p21 Waf1/Cip1 antibody (clone DCS60; Cell Signaling Technology), Cyclin B1 antibody (sc-594; Santa Cruz Biotechnology), rabbit anti-α-tubulin (Sigma-Aldrich), and mouse anti-γ-tubulin (Sigma-Aldrich).
Isolation of Mitotic Cells
HeLa cells were grown in 100-mm dishes, and mitotic cells were separated from those in interphase by mitotic shake off. Mitotic cells from five to 10 dishes were pooled and centrifuged. Cell pellets were rinsed with ice-cold PBS, pH 7.4, containing 1 mM Na3VO4, lysed in cold lysis buffer, and used for immunoblotting. To determine the percentage of mitotic cells in the mitotic and interphase fractions, cell pellets were pipetted in hypotonic solution (0.075 M KCl), incubated for 20 min, and fixed with methanol/acetic acid (3:1). Cells were concentrated by centrifugation and resuspended in fixative, spread onto glass slides and stained with Hoechst 33342.
Purification and Microinjection of Histidine-tagged Wild-Type ERK2 in RPE1
His-tagged wild-type ERK2 (from Dr. Melanie Cobb, University of Texas Southwestern Medical Center, Dallas, TX) was expressed in BL21 strain of Escherichia coli and purified using His-Bind kits (Novagen, Madison, WI). Purified ERK2 protein was dialyzed and concentrated to 1–10 μg/μl in injection buffer (pH 7.7, 10 mM HEPES, 100 mM KCl, and 10% glycerol). The kinase activity of purified His-ERK2 was confirmed by the nonradioactive p44/42 MAP kinase assay kit (Cell Signaling Technology). RPE1 cells in late prophase were microinjected with tagged ERK2. The cells were subsequently fixed for IMF at various times after injection.
Transfection and Expression of HA-tagged ERK2 in RPE1
RPE1 cells were grown on coverslips in 35-mm culture dishes and transfected with 1 μg/dish of vector DNA containing HA-tagged ERK2 (Mainiero et al., 1997) by using FuGENE 6 transfection reagent (Roche Diagnostics, Basel, Switzerland). The activity of HA-ERK2 expressed in human cell lines has been described previously (Aguirre Ghiso et al., 1999). After 24 or 48 h, cells were fixed for IMF by using antibodies against HA and CREST as described above.
siRNA Knockdown of ERK1/2 in BJ-ELB Cells
BJ-ELB cells were kindly provided by Dr. Robert Weinberg (Whitehead Institute for Biomedical Research, Cambridge, MA) and used with his permission. These cells were grown on glass coverslips (for IMF) or without coverslips (for Western blotting) and transfected with a mixture of SignalSilence p42MAPK siRNA and p44MAPK siRNA (Cell Signaling Technology) according to the manufacturer's instructions. Control transfections were performed with SignalSilence Control siRNA (fluorescein conjugate; Cell Signaling Technology).
RESULTS
ERK1/2 Activity Is Not Required during Late G2 for Normal Entry into or Exit from Mitosis
To determine how inhibiting ERK1/2 activity during late G2 affects the G2/M and M/A transitions, we used a live cell assay to study these transitions in nontransformed mammalian cell lines, RPE1 and PtK. For these studies, we defined mitosis as the period from NEB to anaphase onset (chromatid disjunction). Because chromosome condensation actually begins during early G2 (Hendzel et al., 1997), and because this process is fully reversible in mammals up until near the time of NEB, we consider G2 and prophase as a continuous phase of cell cycle that precedes mitosis (Pines and Rieder, 2001). In this assay, progress through prophase equals progress through late G2 (Figure 1A). The advantage of this live cell approach is that it provides direct qualitative (chromosome structure and behavior) and quantitative (the duration of late G2 and mitosis) data on how inhibiting or activating ERK1/2 during late G2 influences the timing of the G2/M and M/A transitions.
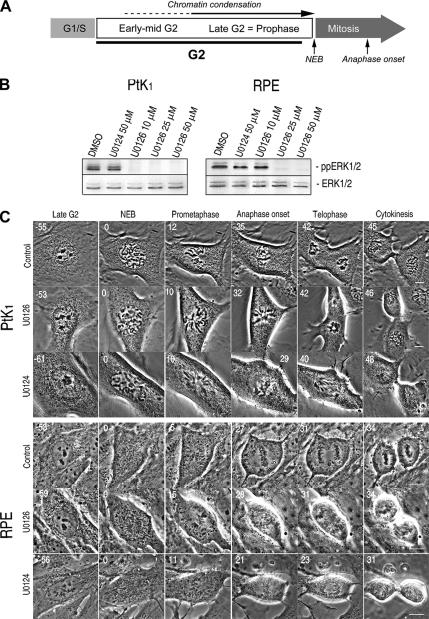
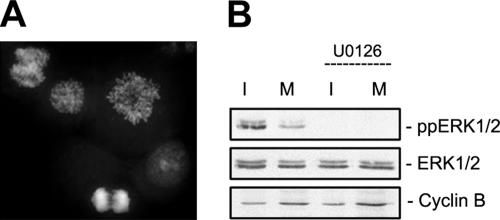
Figure 1.
U0126 potently inhibits ERK1/2 activity but does not delay the G2/M or M/A transitions in PtK or RPE1 cells. (A) We define prophase as the terminal stage of G2, because chromosome condensation can be reversed before NEB. (B) A 15-min treatment with 10–50 μM U0126, but not with DMSO or U0124 (inactive analogue of U0126), was sufficient to prevent ERK1/2 activation in PtK and RPE1 cells. (C) Selected images from time lapse recordings of PtK and RPE1 cells as they progressed from late G2 through mitosis after treatment with DMSO (control), 50 μM U0124 (control), or U0126. Under all conditions RPE1 and PtK cells transition from G2 into mitosis (as evidenced by NEB) after ∼60 min and then transited from metaphase to anaphase 20–35 min later. See Table 1 for mean durations. Time is indicated in minutes before or after nuclear envelope breakdown. Bar, 10 μm.
Initially, we asked how these transitions are affected when ERK1/2 activation is prevented by U0126, a small molecule inhibitor that prevents phosphorylation of ERK1/2 by allosteric binding to MEK1/2 (English and Cobb, 2002). To avoid potential effects on gene expression, U0126 was added to cultures of RPE1 and PtK cells 15–30 min before initiating observations on cells in which chromosome condensation was just evident. Western blotting of whole RPE1 and PtK cell lysates revealed that a 15-min treatment with 25–50 μM U0126 effectively prevented ERK1/2 activation (phosphorylation) in both cell types (Figure 1B). Under these conditions the duration of late G2 and mitosis was similar in both U0126-treated and control cells (untreated or treated with 50 μM U0124; Figure 1C and Table 1). In the absence of ERK1/2 activity, RPE1 and PtK cells also exhibited timely and normal chromosome condensation, spindle formation, chromosome congression, sister chromatid separation, and cytokinesis (Figure 1C).
Table 1.
Mean duration (in minutes) of late G2 and/or mitosis in control, U0124, U0126, and CI-1040 treated PtK, RPE1, HeLa, and NIH 3T3 cells
| PtKab |
RPE1b |
HeLabc Mitosis | NIH3T3bc Mitosis | |||
|---|---|---|---|---|---|---|
| Late G2 | Mitosis | Late G2 | Mitosis | |||
| Controld | 56.8 ± 15.1 (n = 13) | 26.8 ± 7.5 (n = 76) | 66.5 ± 20.0 (n = 32) | 21.4 ± 5.3 (n = 32) | 60.17 ± 6.7 (n = 179) | 18.9 ± 1.5 (n = 71) |
| U0124 (50 μM) | 52.7 ± 6.1 (n = 9) | 29.8 ± 8.3 (n = 20) | 70.8 ± 27.0 (n = 16) | 21.2 ± 6.4 (n = 20) | N/A | N/A |
| U0126 (50 μM) | 50.3 ± 14.9 (n = 13) | 27.2 ± 6.5 (n = 20) | 59.9 ± 20.2 (n = 7) | 26.4 ± 4.4 (n = 17) | 58.42 ± 6.3 (n = 78) | 19.9 ± 2.02 (n = 96) |
| CI-1040 (100–300 nM) | 55.7 ± 3.7 (n = 3) | 24.7 ± 2.9 (n = 6) | 73.7 ± 19.3 (n = 23) | 22.0 ± 7.7 (n = 27) | 68.64 ±15.4 (n = 131) | 19.1 ± 1.39 (n = 96) |
a PtK cells include both PtK1 and PtK2.
b For PtK and RPE1, the duration of late G2 equals the period from the earliest visible sign of chromosome condensation to NEB, whereas the duration of mitosis equals the period from NEB to the onset of anaphase. For HeLa and NIH 3T3, the duration of mitosis equals the period from NEB to the onset of cytokinesis. Neither late G2 nor mitosis was delayed by inhibiting ERK1/2 with U0126 or CI-1040. U0124 is the inactive analogue of U0126. Data are expressed as mean ± SD.
c Data are expressed as mean ± SEM of indicated number (n) of cells.
d Control cells were either untreated or treated with 2.5 μl/ml DMSO.
To confirm that inhibiting ERK1/2 has no specific effect on mitotic progression, we repeated our experiments with CI-1040 (PD184352), a more selective and potent inhibitor of MEK. Although U0126 has been reported to also inhibit ERK5 (Davies et al., 2000; Mody et al., 2001), CI-1040 selectively inactivates ERK1/2 via MEK without inhibiting other protein kinases, even at micromolar concentrations (Squires et al., 2002). After a 15- to 30-min exposure to 100–300 nM of this inhibitor, ERK1/2 activity was completely suppressed in whole cell lysates of both PtK and RPE1 cells (Supplemental Figure S1), yet this inhibition did not affect the duration of late G2, mitosis, or disrupt chromosome segregation (Supplemental Figure S1 and Table 1). Together, our live cell assays reveal that ERK1/2 activity is not required during late G2 for timely entry into or progression through mitosis in normal mammalian somatic cells.
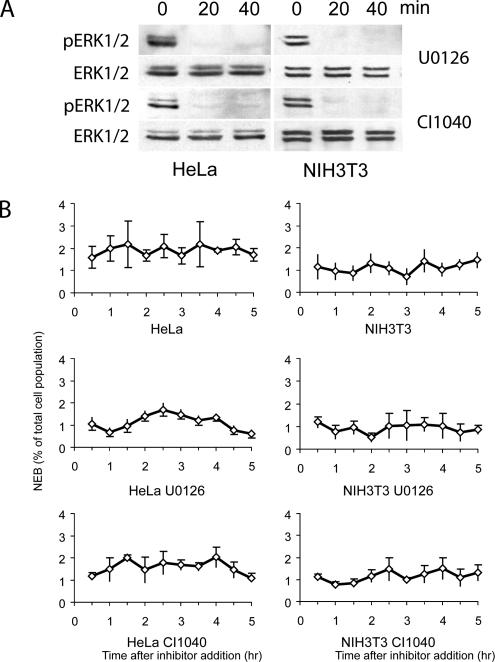
We next asked whether inhibiting ERK1/2 during late G2 delays entry into mitosis in mouse (NIH 3T3) and human transformed cell lines (HeLa) previously used by others to study the effects of long-term ERK1/2 inhibition on the cell cycle. These cells are not readily amenable to live cell observations on the changes of chromatin structure during late G2. As a result, for these experiments, we counted the number of cells entering mitosis and determined the duration of mitosis in low-magnification video sequences 30 min after inhibiting ERK1/2 activity with U0126 or CI-1040. The rationale here was that if ERK1/2 activity is required during mid-to-late G2 for entry into or progression through mitosis, then MEK inhibitors should immediately cause a noticeable decrease in the rate at which cells enter mitosis and/or a noticeable increase in the duration of mitosis. Although these drugs completely inhibited ERK1/2 activity in HeLa and 3T3 cells within 20 min (Figure 2A), they had no effect during the first 4 h of treatment on the rate at which cells entered mitosis, i.e., on the number of cells within the viewing field that underwent NEB (Figure 2B). In addition, we saw no delay in completing mitosis, defined in this assay as the period from NEB to the first signs of cytokinesis, in 3T3 or HeLa cells that entered mitosis in the absence of ERK activity (Table 1). These findings on transformed cells are consistent with our findings on normal cells (see above), and they reveal that ERK1/2 activity is not needed during late G2 for timely entry into or exit from mitosis in normal or transformed cells.
Figure 2.
Inhibiting ERK1/2 activity during late G2 in HeLa and NIH 3T3 cultures does not retard entry into mitosis for at least 4 h. (A) Western blots demonstrating that treating HeLa or NIH 3T3 cultures with 50 μM U0126 or 300 nM CI-1040 inhibits ERK1/2 activity within 20 min. Time in minutes after inhibitor addition is shown above the blots. (B) Graphs that plot the percentage of total cells within a low power field of view (y-axis) that enter mitosis every 30 min (i.e., that undergo nuclear envelope breakdown), after inhibiting ERK1/2 activity with U0126 or CI-1040, in HeLa and NIH 3T3 cultures. x-axis, time in hours after addition of the inhibitor. Note that the rate at which HeLa and NIH 3T3 cells enter mitosis does not change, relative to control cultures, during the first 4 h after inhibiting ERK1/2.
ERK1/2 Activity Is Required for Timely Progress through Early–Mid-G2
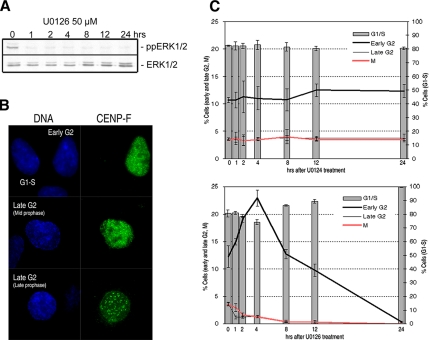
It is well established that a sustained inhibition of ERK1/2 activity induces a delay in the G2/M transition (see Introduction). However, in these studies cell cycle analyses were based on the DNA content and Western blotting that does not distinguish between early/mid-G2, late G2 (prophase) or even mitosis. To determine more precisely when ERK1/2 activity is required during these periods, we treated growing unsynchronized RPE1 cultures with either 50 μM U0126 (Figure 3A) or with 50 μM U0124, an inactive analogue of U0126. We then fixed RPE1 cultures at various time points and immunostained them for centromere protein-F (CENP-F; Liao et al., 1995). This kinetochore protein begins to accumulate in the nucleus during early G2 (and possibly late S), well before chromosome condensation is microscopically evident. By late G2, it is concentrated in kinetochores and the nuclear periphery (Liao et al., 1995). Using CENP-F as a differential marker for the stages of G2, we counted RPE1 cells that were in early/mid-G2 (CENP-F positive, but no visible chromatin condensation; Figure 3B), late G2 (chromosome condensation evident, CENP-F in the nucleus or localized to the kinetochore and/or nuclear periphery) and mitosis (prometaphase through telophase). To expand our analysis to the whole cell cycle, we also counted the number of cells in G1 and S (with basal CENP-F staining).
Figure 3.
Inhibiting ERK1/2 activity delays progression through early–mid-G2. (A) Exposing unsynchronized RPE1 cells to 50 μM U0126 inhibited phosphorylation (activation) of ERK1/2 (ppERK1/2) for up to 24 h, whereas the level of ERK1/2 expression remains unaffected. (B) Cultures of RPE1 cells were immunostained for CENP-F to differentiate among G1-S (basal CENP-F expression, top row), early/midG2 (CENP-F positive, but chromatin condensation is not evident by Hoechst staining, top row), and late G2 populations. In late G2 (prophase) cells, the chromosomes are visibly condensing, and, depending on this progress, CENP-F is either in the nucleus (middle row) or concentrated on the kinetochores and in nuclear periphery (bottom row). (C) RPE1 cultures were treated with 50 μM U0126 or 50 μM U0124 (control); fixed after 0, 1, 2, 4, 8, 12 and 24 h; and then stained for CENP-F. The percentage of cells in G1-S, early–mid-G2, late G2, and mitosis (prometaphase through telophase) was then determined and plotted. The percentage of G1-S (CENP-F negative) cells is presented as bars, and, relative to U0124-treated controls, progressively increases after 4 h in response to inhibiting ERK1/2 until it reaches 100%. In the same cultures, the number of early–mid-G2 cells doubles after 4 h in U0126, after which it progressively decreases as the cells overcome the delay and G1 cells fail to transit into S. At least 6000 cells were counted per slide over three experiments, and values are given as the percentage cells ± SD.
Although control cells showed similar cell cycle population distributions at all time points (Figure 3C, top), we found that the percentage of early G2 cells started to increase shortly after treatment with U0126, and by 4 h it had doubled (Figure 3C, bottom). This increase coincided with a decrease in the percentage of G1-S, late G2 and mitotic cells. After 4 h the percentage of G1-S cells started to increase, no doubt because ERK1/2 activation is required for the G1/S transition (Roovers and Assoian, 2000). Finally, after 24 h most if not all of the cells were in G1 or S (or possibly in G0), and no cells were in mitosis. The delay we observed in early–mid-G2 is, however, transient, and after several hours the cells resume cycling into mitosis and the next G0/G1/S, where they are arrested. Thus, ERK1/2 activity is necessary for timely progress through early/mid-G2, and in less direct studies this delay is manifested as a delay in the “G2/M transition.”
The Early G2 Delay Seen in Response to Inhibiting ERK1/2 Is Mediated by p53
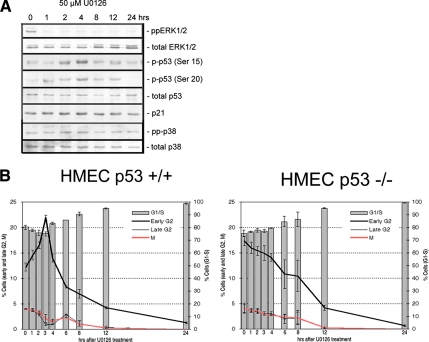
As a first step toward elucidating the molecular basis for the early G2 delay seen after inhibiting the ERK1/2 pathway for 2–4 h, we examined the activity of negative regulators of the cell cycle after inhibiting ERK1/2. We found that the level of p38 expression and activity did not change over time after ERK inhibition in RPE1 cells but that the level of active p53 (phosphorylated at Ser15 and Ser20) was elevated at 2 and 4 h after ERK1/2 inhibition by U0126 (Figure 4A). This suggests that the early G2 delay in response to ERK1/2 inhibition is mediated by p53. To test this, we inhibited ERK1/2 in growing cultures of HMECs that were p53+/+ or p53 deficient, and then we examined the distribution of cells in the cycle with time. These studies revealed that, as expected from the RPE1 work, cultures of p53+/+ HMEC cells showed a significant increase in early G2 cells after U0126 treatment (Figure 4B). By contrast, cultures of HMEC cells lacking p53 showed no elevation in the number of G2 cells in response to U0126 treatment (Figure 4B). Together, these data reveal that in the absence of ERK1/2 activity cells are delayed in early/mid-G2 in a p53-dependent manner. Because p21 expression did not increase in RPE1 cells after ERK1/2 inhibition (Figure 4A), p53 seems to delay early/mid-G2 progression independent of p21 induction.
Figure 4.
The early–mid-G2 delay in response to ERK inhibition is mediated by p53. (A) In RPE1 cells, the level of phosphorylated p53 (at Ser15 and Ser20) increased after 2–4 h of U0126 treatment, whereas expression of p21 and active p38 (pp-p38) remained constant. (B) As in RPE1 cells (Figure 3), the early–mid-G2 cell population transiently increased in response to inhibiting ERK1/2 withU0126 in p53+/+ HMECs. By contrast, this response was not seen in HMEC cells lacking p53. Note that HMECs showed more rapid response to U0126 than RPE1, which may be due to a faster cell cycle. At least 3000 cells were counted per slide, over three experiments, and values are given as the percentage cells ± SD.
Inhibiting ERK1/2 in Late G2 Does Not Impede Spindle Formation
During our live cell studies, we saw no prolongation of mitosis or enhanced abnormalities in chromosome motion or distribution in response to the short-term inhibition of ERK1/2 (via MEK1/2). After a 1-h treatment with 50 μM U0124 or 50 μM U0126, PtK and RPE1 cultures, fixed and stained for α-tubulin and γ-tubulin IMF, contained normal looking prometaphase, metaphase, anaphase, and telophase figures, with no evidence of problems in chromosome segregation (data not shown; Figure 1C). We also found that normal bipolar spindles form in RPE1 and PtK cultures after exposure to U0126 for much longer periods (2, 6, 12, and 24 h; Supplemental Figure S2). As with untreated cultures, <1% of the spindles seen in RPE1 cultures exposed up to 12 h to ERK1/2 inhibitors were abnormal (i.e., monopolar or multipolar), and all of the cultures contained normal anaphase and telophase cells as well as cells that had completed cytokinesis (data not shown). We also found no delay in entering mitosis or exiting mitosis, or an increased incidence of mitotic abnormalities, in NIH 3T3 or HeLa cells that entered mitosis during the first 4–5 h after inhibiting ERK1/2 (Figure 2B and Table 1; also see Supplemental Figure S5). Thus, ERK1/2 activity is not required during mid-to-late G2 in mammalian cells for centrosome separation, bipolar spindle assembly, chromosome segregation, or cytokinesis.
ERK1/2 Localization and Activity during Mitosis
The question of whether ERK1/2 activity is enhanced during mitosis, relative to interphase, remains controversial (see Introduction). To explore this issue, we isolated mitotic fractions of HeLa cells in asynchronously growing cultures by shake-off (Figure 5A) and compared the ERK1/2 activity by Western blotting in mitotic and interphase cells. As shown in Figure 5B, the mitotic cell fraction showed a significantly lower level of phosphorylated ERK1/2 than the interphase fraction. This finding is consistent with the reports of others (see Introduction) that relative to interphase ERK1/2 activity is diminished during mitosis.
Figure 5.
ERK1/2 activity is not enhanced during mitosis relative to interphase. Mitotic cells were isolated from nonsynchronized HeLa cultures by mitotic shake-off. The ERK1/2 activity in these cells was then compared with the remaining interphase cells within the culture by Western blots. (A) A representative example of mitotic HeLa cells obtained by shake-off. In three separate experiments, the mitotic fraction contained 75–85% mitotic cells. (B) Relative to interphase cells, ERK1/2 activity was depressed during mitosis in HeLa. Note also that ERK1/2 activity can be totally suppressed during mitosis (and interphase) by a pretreatment with U0126.
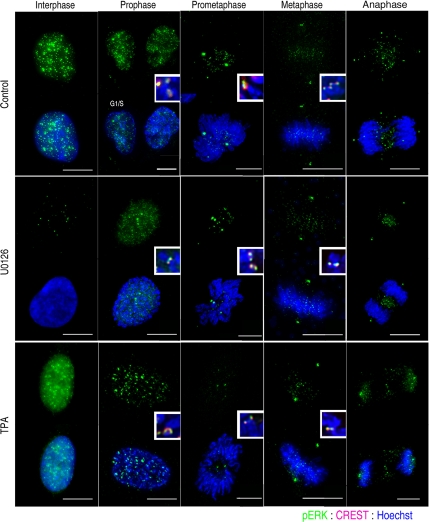
Similarly, as reported previously (see Introduction), we found that antibodies against singly (Santa Cruz Biotechnology) or dually (Promega) phosphorylated ERK1/2 targeted many (but by no means all) centromeric regions, centrosomes, and midbodies when RPE1, PtK, and HeLa cells were stained for IMF (Figure 6, top). Surprisingly, however, when present the fluorescence signal was still strong on these structures in cells treated with 50 μM U0126 for 1, 2, 6, and 12 h, even though adjacent interphase cells showed only a basal level of phosphorylated ERK1/2 (Figure 6, middle). Likewise, when we treated cells with a pharmacological activator of the ERK1/2 pathway (TPA; Supplemental Figure S3A) before IMF staining with the same antibodies against active ERK1/2, the fluorescence signal was only enhanced in interphase but not in mitotic cells (Figure 6, bottom). This inability to pharmacologically regulate ERK1/2 activity during mitosis, combined with the known cross-reactivity of many singly specific phosphorylation site antibodies to other mitotic phosphoproteins, prompted us to ask whether active ERK1/2 staining at kinetochores is an artifact of IMF staining.
Figure 6.
Active ERK1/2 staining on centrosomes, centromeres, and midbodies does not change when MEK1/2 is inhibited or activated. Immunofluorescence of untreated control RPE cells, by using an antibody against singly phorphorylated ERK1/2 (E-4; Santa Cruz Biotechnology), showed a nuclear signal in interphase cells. By contrast in prophase through anaphase cells active ERK1/2 was concentrated in centromeres (inset, with CREST staining), centrosomes, and midzones. After a 2-h treatment with 50 μM U0126, active ERK1/2 was no longer detected in the interphase cells, but it was still seen in late prophase through anaphase cells. When cells were treated with 50 nM TPA for 30 min, to activate ERK1/2, the fluorescence signal was notably increased in the nuclei of interphase cells relative to control and U0126 treated cultures, but not in mitotic cells. Bar, 10 μm.
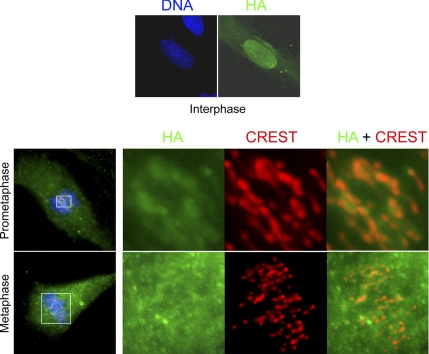
Our initial approach to this question was to microinject histidine-tagged active (as determined from kinase assays) wild-type ERK2 into RPE1 cells during mid-prophase and then fix the cells 15–30 min later (during prometaphase) for IMF with an anti-His antibody. The rationale here was that if ERK1/2 is a bona fide centromere and/or centrosome component, the exogenous histidine-tagged ERK2 should become incorporated into these structures. We found that, although microinjected ERK2 was distributed throughout the cytoplasm, it did not target centromeres or centrosomes (Supplemental Figure S4). However, it may be that more than 30-min is required for the His-tagged ERK2 to become incorporated into kinetochores and centrosomes. To test this possibility, we expressed a functional and activatable HA-tagged ERK2 (Mainiero et al., 1997; Aguirre Ghiso et al., 1999; Aguirre Ghiso, 2002) in RPE1 cells for 48 h (2 cell cycles) and then examined its distribution by IMF. Again, we found that although HA-ERK2 was present on the cytoskeleton and in nuclei during interphase, as it should be (Gonzalez et al., 1993; Reszka et al., 1995), it was not concentrated on centromeres or centrosomes during mitosis (Figure 7).
Figure 7.
HA-ERK2 does not localize to centrosomes and centromeres. Top, after 24 h of transfection in RPE1 cells, HA-ERK2 were expressed in >80% cells and associated predominantly with the nucleus and the cytoskeleton during interphase. Bottom, after 48 h (1–2 cell cycles), cells were fixed and stained for HA (green) and centromeres (CREST; red). Note that in prometaphase and metaphase cells, HA-ERK2 was distributed throughout the cytoplasm and intracellular spaces between chromosomes, but it was not concentrated on centrosomes or centromeres. Images were presented as z-stack maximal intensity projections.
Finally, we studied the distribution of phosphorylated ERK (pERK) during mitosis after knocking down the expression of ERK1 and -2 by using siRNA methods. To ensure that the cells continued to cycle into mitosis during the siRNA treatment, we performed these experiments in BJ-ELB fibroblasts lacking functional pRb and p53 (Hahn et al., 1999). Cells in this line continued to cycle without ERK1/2 expression (Supplemental Figure S5D). As in other cell types, immunostaining of BJ-ELB fibroblasts with antibodies to dually phosphorylated ERK produced a dot-like staining concentrated in the chromosome area, with many dots colocalizing on centromeres (i.e., CREST-stained regions; Supplemental Figure S5B, left). Forty-eight hours after transfecting cultures with siRNAs targeting ERK1 and ERK2, the expression of ERK1/2 was significantly reduced, with the level of pERK falling below 90% of that in control cells (Supplemental Figure S5A). However, many of the centrosome and centromere regions of mitotic cells in cultures depleted of ERK1/2 continued to stain positive for pERK (Supplemental Figure S5B and C; 48 h). In these cultures, the immunoreactivity pattern of the active pERK antibody persisted, at the same level seen in controls, in spite of a significant reduction in the expression level and activity of the target kinase.
The p38-mediated G2 and Spindle Assembly Checkpoints Remain Functional in the Absence of ERK1/2 Activity
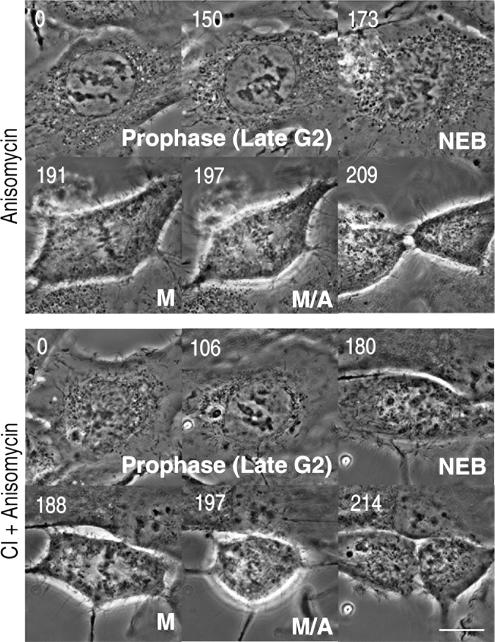
Although we found that ERK1/2 activity is not required during late G2 for normal progression into and through mitosis, it could still be involved in a cell cycle checkpoint that is only apparent under defined conditions. G2 checkpoint pathways function until the cell becomes committed to mitosis during late prophase. These pathways are not constitutively active but are quickly activated in response to various stresses, including DNA damage and osmotic shocks. Many of these insults delay cells in G2 by activating p38 (Mikhailov et al., 2005). This prompted us to ask whether ERK1/2 activity is needed for the p38-mediated checkpoint. To answer this, we determined whether inhibiting ERK1/2 overrides the late G2 (prophase) delay induced by 18.8 nM anisomycin, a potent p38 activator. As reported previously, for PtK cells (Mikhailov et al., 2004), a 15-min treatment with 18.8 nM anisomycin prolongs the duration of late G2 by >3 h in RPE1 cells (Figure 8 and Table 2). However, when we inhibited ERK1/2 activity with 300 nM of CI-1040 for 30 min and then activated p38 with 18.8 nM anisomycin for 15 min, the duration of prophase in RPE1 cells was similar to that seen after anisomycin treatment alone (Figure 8 and Table 2). These results reveal that ERK1/2 activity is not required for activation of the p38-mediated checkpoint during G2.
Figure 8.
Inhibiting ERK1/2 activity during late G2 does not prevent activation of the p38 stress checkpoint. Top, treating RPE cells with 18.8 nM anisomycin delayed progression through late G2 for 3–4 h, but cells ultimately entered and completed a normal mitosis. Bottom, inhibiting ERK1/2 activity with 300 nM CI-1040, before treatment with anisomycin, did not prevent the delay in late G2 induced by activating p38. Bar, 10 μm.
Table 2.
ERK1/2 is not involved in the p38-mediated stress checkpoint
| Control | TPA (50–100 nM) | Anisomycin (18.8 nM) | CI-1040 (300 nM) + anisomycin (18.8 nM) | |
|---|---|---|---|---|
| Late G2 | 66.5 ± 20.0 (n = 32) | 69.1 ± 24.0 (n = 10) | 185.8 ± 72.7 (n = 5) | 170.2 ± 62.5 (n = 13) |
| Mitosis | 21.4 ± 5.3 (n = 32) | 23.7 ± 3.6 (n = 15) | 18.3 ± 2.8 (n = 4) | 19.5 ± 2.4 (n = 15) |
RPE1 cells were treated with 18.8 nM anisomycin that prolongs late G2 by activating p38. Inhibiting ERK1/2 with 300 nM CI-1040 before anisomycin treatment did not prevent this delay, implying that ERK1/2 activity is dispensable for the p-38-mediated G2 checkpoint pathway. When RPE1 cells were treated with 50 or 100 nM TPA to activate ERK1/2, the duration of late G2 and mitosis was similar to that of control cells. Data are expressed as mean ± SD of indicated number (n) of cells.
Next, we asked whether activating ERK1/2 during late G2 delays entry into mitosis, as does activating the p38 kinase. For this study, we treated RPE1 cultures with 50 or 100 nM TPA, which rapidly activates ERK1/2 via a growth factor receptor-independent pathway, and then followed late G2 (early prophase) cells. Under this condition, we found that TPA-treated RPE1 cells entered and exited mitosis with the same timing as controls (Supplemental Figure S3 and Table 2). Thus, activating ERK1/2 does not delay progression through late G2 or impede the G2/M transition.
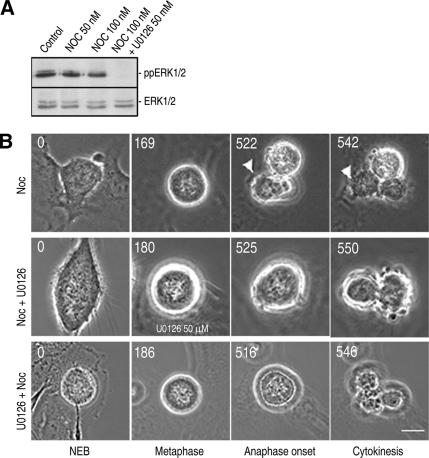
The SAC acts constitutively during mitosis to ensure proper spindle–kinetochore attachments, and agents that destabilize MTs (like nocodazole) significantly prolong mitosis by impeding satisfaction of the SAC (Rieder and Maiato, 2004). To determine whether ERK1/2 activity is required for the SAC, we treated RPE1 cells with nocodazole alone, or with nocodazole and the MEK inhibitor U0126, and then followed them to determine the duration of mitosis. Immunoblotting revealed that 100 nM nocodazole for 3 h alone did not change ERK1/2 activity in unsynchronized RPE1 cells and also that U0126 suppressed ERK1/2 activity in the presence of nocodazole (Figure 9A). We found that when RPE1 cells were treated with 100 nM nocodazole, they remained in mitosis for 6–7 h or occasionally >12 h (Figure 9B, top, and Table 3). When ERK1/2 was inhibited 1 h before nocodazole treatment and well before NEB (i.e., U0126 was added before kinetochore assembly), RPE1 cells were also arrested in mitosis for 6–7 h or in some cases >12 h (Figure 9B, bottom, and Table 3). In these cells, ERK1/2 activity was inhibited well before the cell entered mitosis, yet the SAC remained functional. Finally, when ERK1/2 was inhibited after cells were arrested in mitosis for 3 h, the total duration of mitosis was similar to that of cells treated with nocodazole alone (Figure 9B, middle). From these observations, we conclude that ERK1/2 activity is not required for generating or sustaining the SAC.
Figure 9.
The spindle assembly checkpoint remains functional in the absence of ERK1/2 activity. (A) Western blots showing that nocodazole treatment (3 h; 50–100 nM) did not change ERK1/2 activity in RPE cells and that ERK1/2 activity was inhibited in nocodazole-treated cells by U0126. (B) Top, 100 nM nocodazole alone delays RPE cells in mitosis for >7 h (see Table 3). Middle, treating cells delayed for 3 h in mitosis by nocodazole, with the ERK1/2 inhibitor, did not accelerate exit from mitosis. Bottom, cells that enter mitosis after a 1-h treatment with both 100 nM nocodazole and U0126 were still delayed in mitosis.
Table 3.
ERK1/2 activity is not required for a functional spindle assembly checkpoint
| Control | Nocodazole (100 nM) | U0126 (50 μ M) + nocodazole (100 nM) | |
|---|---|---|---|
| Mitosis | 21.4 ± 5.3 | 397.9 ± 148.4 (n = 9) | 359.8 ± 147.0 (n = 24) |
| (n = 32) | >720 (n = 4) | >720 (n = 5) |
RPE1 cultures were treated with either nocodazole (100 nM) or with 50 μM U0126 before nocodazole treatment. They were then followed by time-lapse microscopy to determine the duration (in minutes) of mitosis (NEB to the anaphase onset). Nocodazole treatment prolonged the duration of mitosis in the presence and absence of ERK1/2 activity by 6–7 h, or in some cells >12 h (720 min). Data are expressed as mean ± SD of indicated number (n) of cells.
DISCUSSION
Unlike previous studies on the role of ERK1/2 in the cell cycle, ours was designed to determine specifically if this kinase plays a nontranscriptional role in controlling entry into and exit from mitosis, i.e., whether ERK1/2 activity is required directly during late G2 and/or mitosis in mammalian somatic cells for, respectively, a timely G2/M and M/A transition. To answer these questions, it was necessary to rapidly and selectively inhibit the pathway at defined points in the cell cycle, because prolonged suppression affects gene expression. The two small molecule inhibitors of MEK1/2 (U0126 and CI-1040) used in our study were ideal for this purpose, because they selectively reduce ERK1/2 activity to undetectable levels on Western blots within 15–20 min (Figures 1B and 2A and Supplemental Figure S1A).
Unlike previous studies, we conducted our experiments on both normal and transformed human cells, and we collected data relevant to two separate issues: the requirement for ERK1/2 activity during late G2 for a timely entry into mitosis; and once in mitosis, its subsequent requirement for proper spindle formation and the SAC. We have therefore divided our discussion into two parts that reflect these separate problems. However, before this discussion it must be noted that some of the apparently conflicting views on the role of ERK1/2 in progression through G2 and mitosis come from a less than precise use of already vague terms. A number of studies, for example, conclude from indirect (i.e., flow cytometry and Western blotting) data that ERK1/2 activity is required for “mitotic progression.” However, in these studies the term mitotic progression is used not in the apparent and accepted meaning that it is required for timely progress through mitosis (i.e., the M/A transition), but rather that it is required for timely progress through the “mitotic cycle” (through G2 into mitosis). Likewise, conclusions that ERK1/2 activity is required for a normal G2/M transition is interpreted by many to mean that ERK1/2 activity is needed during late G2 to activate the cyclin B/Cdk1 kinase, which quickly leads to nuclear envelope breakdown (entry into mitosis). However, in reality such studies simply show that the G2/M transition is delayed, relative to controls, in the absence of ERK1/2 activity. As a result, studies concluding that inhibiting ERK1/2 delays/disrupts the G2/M transition or mitotic progression often really show only that inhibiting ERK1/2 during G2 delays entry into mitosis. One final cautionary note: many fluorescence-activated cell sorting-based studies on the cell cycle use, as a specific marker for mitosis, the anti-phosphohistone H3 antibody. It should be understood, however, that 1) mammalian cells are not “in mitosis” until they become committed to the process at the very end of G2 (i.e., during very late prophase; Pines and Rieder, 2001); and 2) H3 phosphorylation begins not during mitosis but instead during very early G2 (Hendzel et al., 1997; Crosio et al., 2002). As a result, those conclusions that inhibiting ERK1/2 affects the duration of (or progression through) mitosis that are based solely on fluorescence-activated cell sorting of phosphorylated H3 should be evaluated in light of these facts.
ERK1/2 Activity Is Required for Normal Progress through Early-to-Mid- but Not Late G2
It is clear that inhibiting ERK1/2 during G2, by expressing dominant-negative MEK or by RNAi, delays entry into mitosis (see Introduction). However, because these approaches take many hours to days to inactivate ERK1/2, a profound impact on gene expression is unavoidable. Thus, it is possible that ERK1/2 activity is required only during early G2 to initiate a transcriptional program necessary for normal progression through G2 (and/or mitosis). Alternatively, it is just as possible that ERK1/2 kinase activity is required for a timely entry into mitosis because it is needed during late G2 to directly phosphorylate substrates involved in the G2/M transition. Here, we show, by using live PtK, RPE1, HeLa, and NIH 3T3 cells, that ERK1/2 activity is not required during late G2 for a timely entry into mitosis. With the exception of the statement in Horne and Guadagno (2003) (p. 1024) that “the addition of the MEK inhibitor to [3T3] cells synchronized at late G2 had little affect on entry into mitosis as measured by the mitotic index (unpublished results),” this is a novel finding. Our data further reveal that when ERK1/2 is suddenly inactivated in a culture of asynchronously growing cells, the mitotic index gradually falls over a 4- to 5-h period (Figures 3C and 4B). This observation implies that ERK1/2 activity is required (at least in RPE and HMEC P53+/+ cells) 4–5 h before NEB for normal cell cycle (early G2) progression, but not after this time.
Progression through G2 is guarded by several cell cycle checkpoints, mediated by the ataxia telangiectasia mutated/ATM and Rad-3 related (ATM/ATR) and p38 kinases that are triggered, respectively, by DNA damage or stress (for review, see Mikhailov et al., 2005). Based on our live cell studies, we conclude that ERK1/2 activity is not required for a functional p38 checkpoint pathway: in the absence of ERK1/2 activity, early prophase cells are still delayed from entering mitosis when p38 is activated with anisomycin (Figure 8 and Table 2). Several studies have, however, suggested that ERK1/2 activity is required for the cell cycle arrest, and subsequent fate (survival/apoptosis) of cells, when DNA is damaged during G2 (Abbott and Holt, 1999; Tang et al., 2002; Yan et al., 2005). We are currently exploring the possibility that ERK1/2 interfaces with the ATM/ATR complex during DNA damage in late G2.
The dispensability of ERK1/2 activity during late G2 for a timely G2/M transition suggests that the delay in entering mitosis seen in longer term ERK1/2 inhibition studies is due to a requirement for ERK1/2 activity during earlier G2. To explore this idea, we used an assay for progression through G2, based on CENP-F staining (Liao et al., 1995), to determine where cells are delayed in G2 when ERK1/2 is acutely inhibited. We found that that inhibiting ERK1/2 in normal human RPE1 and HMEC cultures causes a transient (4- to 5-h) delay in early–mid-G2, which is then manifested as a retardation of the mitotic index 4–5 h later (Figure 3). This too is also a novel finding, which could not be obtained from less direct population studies that lack the ability to discriminate between early–mid- and late G2 (or even mitotic) cells. Finally, using biochemical assays and isogenic cell lines, we found that this transient delay is mediated by p53 but apparently not via the p21 pathway (Figure 4).
Our conclusion that ERK1/2 activity is required during early–mid-G2 for timely progress toward mitosis is actually consistent with the conclusion of previous studies reporting that ERK1/2 activity is needed for a timely G2/M transition (see Introduction and above). However, we find that the requirement for ERK1/2 activity occurs during early–mid-G2, and not at the G2/M border, and that it is this requirement that is manifested in less direct studies as a delay in the G2/M transition. The reason why ERK1/2 activity is required during early–mid-G2 for normal cell cycle progression remains vague. Although active MEK and ERK are thought to be necessary for Golgi disassembly in preparation for mitosis (Jesch et al., 2001), recent observations suggest this process is really mediated by ERK1c, a spliced isoform of ERK1 (Shaul and Seger, 2006). Furthermore, Golgi dispersion occurs during very late G2, near the time the cell becomes committed to entering mitosis. ERK1/2 is also reported to activate topoisomerase IIα (Shapiro et al., 1999) and the SWI/SNF complex (Sif et al., 1998), both of which are required during G2 for normal structural changes in chromatin. Thus, it is possible modifications in the activation of these complexes lead to a temporary delay in early–mid-G2. A more attractive explanation, however, is that the transient delay seen during early–mid-G2 in response to inhibiting ERK1/2 arises from a requirement for this kinase in mitosis-activating factors, such as FoxM1, that ignite gene transcription programs required for normal cell cycle progression (Laoukili et al., 2005). However, regardless of the mechanism, our data clearly reveal that the activity of ERK1/2 during early–mid-G2 is not an absolute requirement for entry into mitosis, i.e., after 4–5 h the cell “adapts” to the absence of ERK1/2 function and resumes cycling into mitosis. Thus, unlike for the G1/S transition, ERK1/2 activity is not an absolute requirement for progression through G2.
ERK1/2 Activity Is Not Required during Mitosis for Normal Spindle Assembly or for a Functional Spindle Assembly Checkpoint
We found that when ERK1/2 activity is inhibited during late G2, normal and transformed human cells form normal bipolar spindles, segregate sister chromatids, and exit mitosis with normal timing (Figures 1C and 2B, Table 1, and Supplemental Figure S1B). Indeed, relative to interphase cells, we found as others have (Newberry and Pike, 1995; Kiyokawa et al., 1997; Klein et al., 1997; Dangi and Shapiro, 2005) that ERK1/2 activity is diminished during mitosis (Figure 5). In our study, we found no evidence that inhibiting ERK1/2 activity during late G2 or M disrupts spindle assembly and function in NIH 3T3, HeLa, PtK, or RPE1 cells, even in those cells that entered mitosis after a 4- to 5-h delay in early–mid-G2 due to a lack of ERK1/2 activity (Supplemental Figure S2). This conclusion conflicts with that of Horne and Guadagno (2003) who report that a short-term (2- to 3-h) treatment with U0126 leads to a higher incidence of “abnormal spindles” in NIH 3T3 cells. However, the phenotypes reported by these researchers resemble normal intermediates in bipolar spindle assembly, and no attempt was made to compare the numbers of prometaphase, metaphase, anaphase, and multipolar spindles seen after drug treatment with those of nondrug-treated control cultures. In this regard, we found that inhibiting ERK1/2 with U0126, in the absence of nocodazole treatment, had no effect on the duration of mitosis in 3T3 (or any other cell type examined). As in our study, Roberts et al. (2002) also reported that inhibiting MEK1/2 (ERK1/2) with U0126 or PD184352 has no deleterious long-term effects on spindle assembly in HeLa or RPE1 cells.
Given our conclusions that inhibiting ERK1/2 activity during G2 does not prolong mitosis or disrupt spindle assembly in mammalian cells, we were not surprised to find that inhibiting ERK1/2 activity during late G2 or mitosis did not affect the workings of the SAC (Figure 9 and Table 3). Again, this conclusion seems to conflict with reports that inhibiting ERK1/2 in NIH 3T3 (Willard and Crouch, 2001) or HeLa (Roberts et al., 2002) cells delays the M/A transition. In the case of NIH 3T3, this conclusion was based on the observation that mitotic cells do not recover from a 12-h nocodazole block over a 2-h period after being released directly into U0126. However, no controls were run to show that the M/A delay seen in response to the second drug (U0126) was due to the specific inhibition of MEK1/2 and not to toxicity (or to a requirement for ERK1/2 activity in ridding the cells of nocodazole via p-glycoprotein drug pumps). We repeated the Willard and Crouch study, in the absence of a nocodazole pretreatment, and found that inhibiting ERK1/2 activity during late G2 (also with U0126) had no effect on the duration of mitosis (Table 1). In the case of HeLa (Roberts et al., 2002), the conclusion that inhibiting ERK1/2 delays the M/A transition was based on comparing the mitotic index of control and drug (PD184352)-treated cultures, fixed at various time intervals after releasing from a thymidine block. We note, however, that the transient delay in early–mid-G2 in response to inhibiting ERK1/2 seen in our study produces a decrease in the mitotic index in HMEC cultures 3- to 4-h postdrug treatment, which is then followed by a sudden conspicuous increase at the 6-h time point (Figure 4B). This exact same behavior is seen 2.5 and 5 h after releasing synchronized HeLa cells into U0126 (Figure 6D in Roberts et al., 2002), and at the 5-h interval, there are actually many more cells in mitosis (including prophase) than in nondrug-treated controls. We suggest that this increased mitotic index arises not from a delayed progression through mitosis as concluded by Roberts et al. (2002), but instead from a bolus of cells entering mitosis after a transient delay in early–mid-G2 due to a lack of ERK1/2 activity.
ERK1/2 is constitutively present throughout the cell cycle and at any given time the extent of its activity is defined by a balance between regulatory kinases and phosphatases (Widmann et al., 1999). Based on our biochemical results, we were surprised to find that we could not pharmacologically regulate ERK1/2 activity (i.e., the phospho ERK1/2 level) at centromeres or centrosomes during mitosis, even when we applied inhibitors or activators before active ERK1/2 staining is apparent at these structures (in early-to-mid-prophase; see Shapiro et al., 1998; Zecevic et al., 1998). The reason for this is unknown, and there are at least two possibilities. First, it may be that once ERK1/2 becomes associated with centromeres and centrosomes it can no longer be pharmacologically regulated, e.g., its activation may become MEK-independent or the protein may not be accessible to regulatory phosphatases. Alternatively, as noted in the Introduction the active ERK1/2 signal seen by IMF at centromeres and centrosomes may be an immunological artifact. This possibility is consistent with our biochemical data that phosphorylated ERK1/2 is not detectable both in interphase and mitotic cells treated with U0126 (Figure 5B), and with our finding that ERK1/2 does not play a role during mitosis in spindle assembly, the M/A transition or the SAC. It is also supported by our His–ERK2 microinjection, HA–ERK2 overexpression and siRNA studies that, although individually consistent, are admittedly not without potential sources of error. Indeed, in spite of our efforts there is an inherent problem with proving that a protein does not reside at a specific location, because the proof must rely on negative data—and evidence of absence is not proof of absence.
Finally, the constitutive activation ERK1/2, via its upstream activator Ras, leads to a high incidence of errors during mitosis (Saavedra et al., 1999; Knauf et al., 2006). Yet, we found that aberrant activation of ERK1/2 during late G2 has no immediate effect on spindle formation or chromosome segregation. This suggests that the genetic instability associated with constitutive ERK1/2 activation arises primarily from a requirement for ERK activity during or before early G2. In this regard, unscheduled entry into S phase leads to replication stress, which activates the DNA damage checkpoint. In turn, this leads to a block in S and G2, which then generates aberrant DNA contents (Bartkova et al., 2005). Because mutational activation of Raf is seen in many tumor cells (Davies et al., 2002), ERK1/2 pathway inhibitors have been extensively studied as potential therapeutic options. Indeed, MEK inhibitors have proven effective at suppressing proliferation, and CI-1040 used in our study entered phase II clinical trials (Sebolt-Leopold and Herrera, 2004). Our data imply that these drugs delay the cell cycle in noncancerous cells predominantly at G1/S and that they do not induce aberrant mitosis when applied during G2. It remains to be seen whether nontumor cells become genetically unstable after prolonged exposures to these inhibitors.
Supplementary Material
ACKNOWLEDGMENTS
We are indebted to Melanie Cobb (University of Texas Southwestern Medical Center) for providing the recombinant ERK2 construct, Pfizer for providing CI-1040, Martha R. Stampfer (Lawrence Berkeley National Laboratory, Life Sciences Division, Berkeley, CA) for providing the HMEC cell lines, and Robert Weinberg (Whitehead Institute for Biomedical Research, Cambridge, MA) for providing BJ-ELB cells. We also express sincere gratitude to Drs. Alexey Khodjakov, Erasmus Schneider (Wadsworth Center), and Donald Porter (Ordway Research Institute, Albany, NY) for helpful suggestions and discussions. This research was supported by National Institutes of Health Grant GMS-40198 to C.L.R., by National Institutes of Health/National Cancer Institute Grant CA109182, and Samuel Waxman Cancer Research Foundation grants to J.A.A.-G.
Abbreviations used:
- ERK
extracellular signal-regulated kinase
- G2/M
G2-mitosis transition
- IMF
immunofluorescence
- M/A
metaphase-anaphase
- NEB
nuclear envelope breakdown
- SAC
spindle assembly checkpoint.
Footnotes
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-04-0284) on October 11, 2006.
REFERENCES
- Abbott D. W., Holt J. T. Mitogen-activated protein kinase kinase 2 activation is essential for progression through the G2/M checkpoint arrest in cells exposed to ionizing radiation. J. Biol. Chem. 1999;274:2732–2742. doi: 10.1074/jbc.274.5.2732. [DOI] [PubMed] [Google Scholar]
- Aguirre Ghiso J. A. Inhibition of FAK signaling activated by urokinase receptor induces dormancy in human carcinoma cells in vivo. Oncogene. 2002;21:2513–2524. doi: 10.1038/sj.onc.1205342. [DOI] [PubMed] [Google Scholar]
- Aguirre Ghiso J. A., Kovalski K., Ossowski L. Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J. Cell Biol. 1999;147:89–104. doi: 10.1083/jcb.147.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez B., Martinez A. C., Burgering B. M., Carrera A. C. Forkhead transcription factors contribute to execution of the mitotic programme in mammals. Nature. 2001;413:744–747. doi: 10.1038/35099574. [DOI] [PubMed] [Google Scholar]
- Bartkova J. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- Chung E., Chen R. H. Phosphorylation of Cdc20 is required for its inhibition by the spindle checkpoint. Nat. Cell Biol. 2003;5:748–753. doi: 10.1038/ncb1022. [DOI] [PubMed] [Google Scholar]
- Crosio C., Fimia G. M., Loury R., Kimura M., Okano Y., Zhou H., Sen S., Allis C. D., Sassone-Corsi P. Mitotic phosphorylation of histone H 3, spatio-temporal regulation by mammalian Aurora kinases. Mol. Cell. Biol. 2002;22:874–885. doi: 10.1128/MCB.22.3.874-885.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangi S., Shapiro P. Cdc2-mediated inhibition of epidermal growth factor activation of the extracellular signal-regulated kinase pathway during mitosis. J. Biol. Chem. 2005;280:24524–24531. doi: 10.1074/jbc.M414079200. [DOI] [PubMed] [Google Scholar]
- Davies H. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Davies S. P., Reddy H., Caivano M., Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann H. M., Kuhne C., Petritsch C., Ballou L. M. Cell cycle regulation of p70 S6 kinase and p42/p44 mitogen-activated protein kinases in Swiss mouse 3T3 fibroblasts. J. Biol. Chem. 1996;271:963–971. doi: 10.1074/jbc.271.2.963. [DOI] [PubMed] [Google Scholar]
- English J. M., Cobb M. H. Pharmacological inhibitors of MAPK pathways. Trends Pharmacol Sci. 2002;23:40–45. doi: 10.1016/s0165-6147(00)01865-4. [DOI] [PubMed] [Google Scholar]
- Ferrell J. E., Jr Xenopus oocyte maturation: new lessons from a good egg. Bioessays. 1999;21:833–842. doi: 10.1002/(SICI)1521-1878(199910)21:10<833::AID-BIES5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Giroux S. Embryonic death of Mek1-deficient mice reveals a role for this kinase in angiogenesis in the labyrinthine region of the placenta. Curr. Biol. 1999;9:369–372. doi: 10.1016/s0960-9822(99)80164-x. [DOI] [PubMed] [Google Scholar]
- Gomez-Cambronero J. P42-MAP kinase is activated in EGF-stimulated interphase but not in metaphase-arrested HeLa cells. FEBS Lett. 1999;443:126–130. doi: 10.1016/s0014-5793(98)01685-8. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. A., Seth A., Raden D. L., Bowman D. S., Fay F. S., Davis R. J. Serum-induced translocation of mitogen-activated protein kinase to the cell surface ruffling membrane and the nucleus. J. Cell Biol. 1993;122:1089–1101. doi: 10.1083/jcb.122.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh Y., Nishida E., Matsuda S., Shiina N., Kosako H., Shiokawa K., Akiyama T., Ohta K., Sakai H. In vitro effects on microtubule dynamics of purified Xenopus M phase-activated MAP kinase. Nature. 1991;349:251–254. doi: 10.1038/349251a0. [DOI] [PubMed] [Google Scholar]
- Grill C., Gheyas F., Dayananth P., Jin W., Ding W., Qiu P., Wang L., Doll R. J., English J. M. Analysis of the ERK1,2 transcriptome in mammary epithelial cells. Biochem. J. 2004;381:635–644. doi: 10.1042/BJ20031688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadagno T. M., Ferrell J. E., Jr Requirement for MAPK activation for normal mitotic progression in Xenopus egg extracts. Science. 1998;282:1312–1315. doi: 10.1126/science.282.5392.1312. [DOI] [PubMed] [Google Scholar]
- Hahn W. C., Counter C. M., Lundberg A. S., Beijersbergen R. L., Brooks M. W., Weinberg R. A. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- Harding A., Giles N., Burgess A., Hancock J. F., Gabrielli B. G. Mechanism of mitosis-specific activation of MEK1. J. Biol. Chem. 2003;278:16747–16754. doi: 10.1074/jbc.M301015200. [DOI] [PubMed] [Google Scholar]
- Hayne C., Tzivion G., Luo Z. Raf-1/MEK/MAPK pathway is necessary for the G2/M transition induced by nocodazole. J. Biol. Chem. 2000;275:31876–31882. doi: 10.1074/jbc.M002766200. [DOI] [PubMed] [Google Scholar]
- Hayne C., Xiang X., Luo Z. MEK inhibition and phosphorylation of serine 4 on B23 are two coincident events in mitosis. Biochem. Biophys. Res. Commun. 2004;321:675–680. doi: 10.1016/j.bbrc.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Hendzel M. J., Wei Y., Mancini M. A., Van Hooser A., Ranalli T., Brinkley B. R., Bazett-Jones D. P., Allis C. D. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106:348–360. doi: 10.1007/s004120050256. [DOI] [PubMed] [Google Scholar]
- Horne M. M., Guadagno T. M. A requirement for MAP kinase in the assembly and maintenance of the mitotic spindle. J. Cell Biol. 2003;161:1021–1028. doi: 10.1083/jcb.200304144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesch S. A., Lewis T. S., Ahn N. G., Linstedt A. D. Mitotic phosphorylation of Golgi reassembly stacking protein 55 by mitogen-activated protein kinase ERK2. Mol. Biol. Cell. 2001;12:1811–1817. doi: 10.1091/mbc.12.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov A., Rieder C. L. Imaging the division process in living tissue culture cells. Methods. 2006;38:2–16. doi: 10.1016/j.ymeth.2005.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyokawa N., Lee E. K., Karunagaran D., Lin S. Y., Hung M. C. Mitosis-specific negative regulation of epidermal growth factor receptor, triggered by a decrease in ligand binding and dimerization, can be overcome by overexpression of receptor. J. Biol. Chem. 1997;272:18656–18665. doi: 10.1074/jbc.272.30.18656. [DOI] [PubMed] [Google Scholar]
- Klein S., Kaszkin M., Barth H., Kinzel V. Signal transduction through epidermal growth factor receptor is altered in HeLa monolayer cells during mitosis. Biochem. J. 1997;322:937–946. doi: 10.1042/bj3220937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf J. A., Ouyang B., Knudsen E. S., Fukasawa K., Babcock G., Fagin J. A. Oncogenic RAS induces accelerated transition through G2/M and promotes defects in the G2 DNA damage and mitotic spindle checkpoints. J. Biol. Chem. 2006;281:3800–3809. doi: 10.1074/jbc.M511690200. [DOI] [PubMed] [Google Scholar]
- Laird A. D., Morrison D. K., Shalloway D. Characterization of Raf-1 activation in mitosis. J. Biol. Chem. 1999;274:4430–4439. doi: 10.1074/jbc.274.7.4430. [DOI] [PubMed] [Google Scholar]
- Laoukili J., Kooistra M. R., Bras A., Kauw J., Kerkhoven R. M., Morrison A., Clevers H., Medema R. H. FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat. Cell Biol. 2005;7:126–136. doi: 10.1038/ncb1217. [DOI] [PubMed] [Google Scholar]
- Liao H., Winkfein R. J., Mack G., Rattner J. B., Yen T. J. CENP-F is a protein of the nuclear matrix that assembles onto kinetochores at late G2 and is rapidly degraded after mitosis. J. Cell Biol. 1995;130:507–518. doi: 10.1083/jcb.130.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Yan S., Zhou T., Terada Y., Erikson R. L. The MAP kinase pathway is required for entry into mitosis and cell survival. Oncogene. 2004;23:763–776. doi: 10.1038/sj.onc.1207188. [DOI] [PubMed] [Google Scholar]
- Lou Y., Xie W., Zhang D. F., Yao J. H., Luo Z. F., Wang Y. Z., Shi Y. Y., Yao X. B. Nek2A specifies the centrosomal localization of Erk2. Biochem. Biophys. Res. Commun. 2004;321:495–501. doi: 10.1016/j.bbrc.2004.06.171. [DOI] [PubMed] [Google Scholar]
- Ma R. Y., Tong T. H., Cheung A. M., Tsang A. C., Leung W. Y., Yao K. M. Raf/MEK/MAPK signaling stimulates the nuclear translocation and transactivating activity of FOXM1c. J. Cell Sci. 2005;118:795–806. doi: 10.1242/jcs.01657. [DOI] [PubMed] [Google Scholar]
- Mainiero F., Murgia C., Wary K. K., Curatola A. M., Pepe A., Blumemberg M., Westwick J. K., Der C. J., Giancotti F. G. The coupling of alpha6beta4 integrin to Ras-MAP kinase pathways mediated by Shc controls keratinocyte proliferation. EMBO J. 1997;16:2365–2375. doi: 10.1093/emboj/16.9.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhailov A., Shinohara M., Rieder C. L. Topoisomerase II and histone deacetylase inhibitors delay the G2/M transition by triggering the p38 MAPK checkpoint pathway. J. Cell Biol. 2004;166:517–526. doi: 10.1083/jcb.200405167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhailov A., Shinohara M., Rieder C. L. The p38-mediated stress-activated checkpoint. A rapid response system for delaying progression through antephase and entry into mitosis. Cell Cycle. 2005;4:57–62. doi: 10.4161/cc.4.1.1357. [DOI] [PubMed] [Google Scholar]
- Minshull J., Sun H., Tonks N. K., Murray A. W. A MAP kinase-dependent spindle assembly checkpoint in Xenopus egg extracts. Cell. 1994;79:475–486. doi: 10.1016/0092-8674(94)90256-9. [DOI] [PubMed] [Google Scholar]
- Mody N., Leitch J., Armstrong C., Dixon J., Cohen P. Effects of MAP kinase cascade inhibitors on the MKK5/ERK5 pathway. FEBS Lett. 2001;502:21–24. doi: 10.1016/s0014-5793(01)02651-5. [DOI] [PubMed] [Google Scholar]
- Newberry E. P., Pike L. J. Cell-cycle-dependent modulation of EGF-receptor-mediated signaling. Biochem. Biophys. Res. Commun. 1995;208:253–259. doi: 10.1006/bbrc.1995.1331. [DOI] [PubMed] [Google Scholar]
- Pages G., Lenormand P., L'Allemain G., Chambard J. C., Meloche S., Pouyssegur J. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc. Natl. Acad. Sci. USA. 1993;90:8319–8323. doi: 10.1073/pnas.90.18.8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson G., Robinson F., Beers Gibson T., Xu B. E., Karandikar M., Berman K., Cobb M. H. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Pines J., Rieder C. L. Re-staging mitosis: a contemporary view of mitotic progression. Nat. Cell Biol. 2001;3:E3–E6. doi: 10.1038/35050676. [DOI] [PubMed] [Google Scholar]
- Reszka A. A., Seger R., Diltz C. D., Krebs E. G., Fischer E. H. Association of mitogen-activated protein kinase with the microtubule cytoskeleton. Proc. Natl. Acad. Sci. USA. 1995;92:8881–8885. doi: 10.1073/pnas.92.19.8881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder C. L., Maiato H. Stuck in division or passing through: what happens when cells cannot satisfy the spindle assembly checkpoint. Dev. Cell. 2004;7:637–651. doi: 10.1016/j.devcel.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Roberts E. C., Shapiro P. S., Nahreini T. S., Pages G., Pouyssegur J., Ahn N. G. Distinct cell cycle timing requirements for extracellular signal-regulated kinase and phosphoinositide 3-kinase signaling pathways in somatic cell mitosis. Mol. Cell. Biol. 2002;22:7226–7241. doi: 10.1128/MCB.22.20.7226-7241.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roovers K., Assoian R. K. Integrating the MAP kinase signal into the G1 phase cell cycle machinery. Bioessays. 2000;22:818–826. doi: 10.1002/1521-1878(200009)22:9<818::AID-BIES7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Saavedra H. I., Fukasawa K., Conn C. W., Stambrook P. J. MAPK mediates RAS-induced chromosome instability. J. Biol. Chem. 1999;274:38083–38090. doi: 10.1074/jbc.274.53.38083. [DOI] [PubMed] [Google Scholar]
- Schmid-Alliana A., Menou L., Manie S., Schmid-Antomarchi H., Millet M.-A., Giuriato S., Ferrua B., Rossi B. Microtubule integrity regulates Src-like and extracellular signal-regulated kinase activities in human pro-monocytic cells. J. Biol. Chem. 1998;273:3394–3400. doi: 10.1074/jbc.273.6.3394. [DOI] [PubMed] [Google Scholar]
- Sebolt-Leopold J. S., Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat. Rev. Cancer. 2004;4:937–947. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- Seger R., Krebs E.G. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- Shapiro P. S., Vaisberg E., Hunt A. J., Tolwinski N. S., Whalen A. M., McIntosh J. R., Ahn N. G. Activation of the MKK/ERK pathway during somatic cell mitosis: direct interactions of active ERK with kinetochores and regulation of the mitotic 3F3/2 phosphoantigen. J. Cell Biol. 1998;142:1533–1545. doi: 10.1083/jcb.142.6.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro P. S., Whalen A. M., Tolwinski N. S., Wilsbacher J., Froelich-Ammon S. J., Garcia M., Osheroff N., Ahn N. G. Extracellular signal-regulated kinase activates topoisomerase IIalpha through a mechanism independent of phosphorylation. Mol. Cell. Biol. 1999;19:3551–3560. doi: 10.1128/mcb.19.5.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul Y. D., Seger R. ERK1c regulates Golgi fragmentation during mitosis. J. Cell Biol. 2006;172:885–897. doi: 10.1083/jcb.200509063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sif S., Stukenberg P. T., Kirschner M. W., Kingston R. E. Mitotic inactivation of a human SWI/SNF chromatin remodeling complex. Genes Dev. 1998;12:2842–2851. doi: 10.1101/gad.12.18.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires M. S., Nixon P. M., Cook S. J. Cell-cycle arrest by PD184352 requires inhibition of extracellular signal-regulated kinases (ERK) 1/2 but not ERK5/BMK1. Biochem. J. 2002;366:673–680. doi: 10.1042/BJ20020372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka K., Gotoh Y., Nishida E. MAP kinase is required for the spindle assembly checkpoint but is dispensable for the normal M phase entry and exit in Xenopus egg cell cycle extracts. J. Cell Biol. 1997;136:1091–1097. doi: 10.1083/jcb.136.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka K., Moriguchi T., Nishida E. Activation of the protein kinase p38 in the spindle assembly checkpoint and mitotic arrest. Science. 1998;280:599–602. doi: 10.1126/science.280.5363.599. [DOI] [PubMed] [Google Scholar]
- Tamemoto H., Kadowaki T., Tobe K., Ueki K., Izumi T., Chatani Y., Kohno M., Kasuga M., Yazaki Y., Akanuma Y. Biphasic activation of two mitogen-activated protein kinases during the cell cycle in mammalian cells. J. Biol. Chem. 1992;267:20293–20297. [PubMed] [Google Scholar]
- Tang D., Wu D., Hirao A., Lahti J. M., Liu L., Mazza B., Kidd V. J., Mak T. W., Ingram A. J. ERK activation mediates cell cycle arrest and apoptosis after DNA damage independently of p53. J. Biol. Chem. 2002;277:12710–12717. doi: 10.1074/jbc.M111598200. [DOI] [PubMed] [Google Scholar]
- Uchiyama S. Proteome analysis of human metaphase chromosomes. J. Biol. Chem. 2005;280:16994–17004. doi: 10.1074/jbc.M412774200. [DOI] [PubMed] [Google Scholar]
- Wang X. M., Zhai Y., Ferrell J. E., Jr A role for mitogen-activated protein kinase in the spindle assembly checkpoint in XTC cells. J. Cell Biol. 1997;137:433–443. doi: 10.1083/jcb.137.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmann C., Gibson S., Jarpe M. B., Johnson G. L. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol. Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- Willard F. S., Crouch M. F. MEK, ERK, and p90RSK are present on mitotic tubulin in Swiss 3T3 cells: a role for the MAP kinase pathway in regulating mitotic exit. Cell Signal. 2001;13:653–664. doi: 10.1016/s0898-6568(01)00185-1. [DOI] [PubMed] [Google Scholar]
- Wojnowski L., Stancato L. F., Zimmer A. M., Hahn H., Beck T. W., Larner A. C., Rapp U. R., Zimmer A. Craf-1 protein kinase is essential for mouse development. Mech. Dev. 1998;76:141–149. doi: 10.1016/s0925-4773(98)00111-7. [DOI] [PubMed] [Google Scholar]
- Wright J. H., Munar E., Jameson D. R., Andreassen P. R., Margolis R. L., Seger R., Krebs E. G. Mitogen-activated protein kinase kinase activity is required for the G(2)/M transition of the cell cycle in mammalian fibroblasts. Proc. Natl. Acad. Sci. USA. 1999;96:11335–11340. doi: 10.1073/pnas.96.20.11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Spieker R. S., Kim M., Stoeger S. M., Cowan K. H. BRCA1-mediated G2/M cell cycle arrest requires ERK1/2 kinase activation. Oncogene. 2005;24:3285–3296. doi: 10.1038/sj.onc.1208492. [DOI] [PubMed] [Google Scholar]
- Yoon S., Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24:21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]
- Zecevic M., Catling A. D., Eblen S. T., Renzi L., Hittle J. C., Yen T. J., Gorbsky G. J., Weber M. J. Active MAP kinase in mitosis: localization at kinetochores and association with the motor protein CENP-E. J. Cell Biol. 1998;142:1547–1558. doi: 10.1083/jcb.142.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.