Abstract
The peripheral Golgi protein golgin-160 is induced during 3T3L1 adipogenesis and is primarily localized to the Golgi cisternae distinct from the trans-Golgi network (TGN) in a general distribution similar to p115. Small interfering RNA (siRNA)-mediated reduction in golgin-160 protein resulted in an increase accumulation of the insulin-responsive amino peptidase (IRAP) and the insulin-regulated glucose transporter (GLUT4) at the plasma membrane concomitant with enhanced glucose uptake in the basal state. The redistribution of GLUT4 was rescued by expression of a siRNA-resistant golgin-160 cDNA. The basal state accumulation of plasma membrane GLUT4 occurred due to an increased rate of exocytosis without any significant effect on the rate of endocytosis. This GLUT4 trafficking to the plasma membrane in the absence of golgin-160 was independent of TGN/Golgi sorting, because it was no longer inhibited by the expression of a dominant-interfering Golgi-localized, γ-ear–containing ARF-binding protein mutant and displayed reduced binding to the lectin wheat germ agglutinin. Moreover, expression of the amino terminal head domain (amino acids 1–393) had no significant effect on the distribution or insulin-regulated trafficking of GLUT4 or IRAP. In contrast, expression of carboxyl α helical region (393–1498) inhibited insulin-stimulated GLUT4 and IRAP translocation, but it had no effect on the sorting of constitutive membrane trafficking proteins, the transferrin receptor, or vesicular stomatitis virus G protein. Together, these data demonstrate that golgin-160 plays an important role in directing insulin-regulated trafficking proteins toward the insulin-responsive compartment in adipocytes.
INTRODUCTION
GLUT4 is the facilitative glucose transporter that is primarily expressed in insulin-responsive tissues (i.e., striated muscle and adipose) and is responsible for the clearance of circulating glucose in the postprandial state (Mueckler, 1994; Watson and Pessin, 2001). Under basal conditions, the majority of GLUT4 is sequestered in specialized GLUT4 insulin-responsive storage compartments (IRCs). In response to insulin, GLUT4 translocates from these intracellular compartments and is functionally incorporated into the plasma membrane, thereby enhancing glucose uptake (Chang et al., 2004; Watson and Pessin, 2006). Despite the established importance of GLUT4 translocation in the regulation of glucose homeostasis, details of how muscle and adipose tissues maintain an insulin-sensitive IRC remain unknown.
In a current model for GLUT4 compartmentalization, the newly synthesized GLUT4 protein is sorted directly into intracellular IRC (Watson et al., 2004). This process is different from the trafficking of related proteins (e.g., GLUT1) and other constitutively trafficking proteins (vesicular stomatitis virus G protein; VSV-G) that traffic directly to the plasma membrane after biosynthesis. The important initial biosynthetic processing steps that establish an insulin-responsive pool of GLUT4 are thought to take place in the sorting compartments of the trans-Golgi network (TGN). In support of this model, the TGN cargo adaptor Golgi-localized, γ-ear–containing ARF-binding protein (GGA) is required for the initial entry of GLUT4 from the TGN into the insulin-responsive compartment (Watson et al., 2004). More recently, the expression of an amino-terminal deletion mutant of the Golgi tethering protein, p115 was found to inhibit insulin-stimulated GLUT4 translocation to the plasma membrane, suggesting a role for p115 in the intracellular tethering of GLUT4 (Hosaka et al., 2005).
p115 is one member of a large golgin family of Golgi-localized proteins. These proteins have been implicated in the maintenance of proper Golgi structure and function (Barr et al., 2003). It has long been appreciated that the cisternal Golgi membrane stacks of higher eukaryotes function in the processing and sorting of proteins en route from the endoplasmic reticulum to the plasma membrane as well as to other intracellular locations (Fullekrug and Nilsson, 1998). At least some golgins contribute to cargo protein trafficking and sorting in Golgi cisternae, allowing for proper intracellular compartmentalization. The importance of golgins in cargo sorting is highlighted by the fact that disruption of tethering complexes between the golgins GM130 and giantin results in an inhibition of Golgi transport and subsequent accumulation of transport vesicles (Alvarez et al., 2001).
One golgin that has been the focus of current research is the protein golgin-160. Recent studies have demonstrated that golgin-160 is a caspase substrate during programmed cell death, and after cleavage the head domain is required for efficient apoptotic disassembly of the Golgi (Mancini et al., 2000). These results suggest that cleavage of golgin-160 disrupts protein–protein interactions that are important for Golgi function. However, the function of golgin-160 under normal (nonapoptotic) conditions remains unknown. In this regard, golgin-160 has recently been shown to be involved in the trafficking of the ROMK potassium channel (Bundis et al., 2006) and the β-1 adrenergic receptor (Hicks et al., 2006). Golgin-160 has also been observed to directly associate with PIST (also known as GOPC, CAL, and FIG) that seems to be required for the vesicular trafficking of a subset of plasma membrane proteins (Hicks and Machamer, 2005). In particular, PIST was found to associate with syntaxin 6 (Stx6), and this Q-SNARE has been implicated in insulin-stimulated GLUT4 translocation (Charest et al., 2001; Shewan et al., 2003; Perera et al., 2003). Based upon these data, we have examined the potential role of golgin-160 in the regulation of GLUT4 trafficking. Our data demonstrate that golgin-160 is up-regulated during adipogenesis and is required for the proper sorting of GLUT4 to the insulin-responsive compartment at a step before GGA-dependent function.
MATERIALS AND METHODS
Brefeldin A (Sigma-Aldrich, St. Louis, MO) was prepared as a 5-mg/ml stock in methanol and used at a final concentration of 5 μg/ml. SuperSignal enhanced chemiluminescence reagents were purchased from Pierce Chemical (Rockford, IL) and used according to the manufacturer's instructions. The rabbit golgin-160 antibody used in this study was produced in rabbits to a glutathione S-transferase (GST) fusion protein containing residues 1–393 of human golgin-160, generated and purified as described previously (Hicks and Machamer, 2005). The antibody was affinity purified on GST-golgin-160 bound to glutathione beads. The Stx6 and p115 monoclonal antibodies were purchased from BD Transduction Laboratories (Lexington, KY). The monoclonal VSV-G antibody was purchased from Accurate Chemical and Scientific (Westbury, NY). The c-Myc (9E10) antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Horseradish peroxidase-conjugated secondary antibodies were purchased from Pierce Chemical. Insulin, dexamethasone, isobutyl-1-methylxanthine, bovine serum albumin, and donkey serum were purchased from Sigma-Aldrich. VECTASHIELD was obtained from Vector Laboratories (Burlingame, CA).
The carboxy-terminal enhanced green fluorescent protein (EGFP)-tagged GLUT4 (GLUT4-EGFP) and the myc epitope-tagged GLUT4 (myc-GLUT4) constructs were prepared as described previously (Thurmond et al., 1998; Kanzaki et al., 2004). The TR-IRAP construct was prepared as previously described (Subtil et al., 2000; Hou et al., 2006). Myc-tagged full-length golgin-160 and golgin-160 truncation mutants were prepared as described previously (Hicks and Machamer, 2002). VSV-G cDNA was obtained from the University of Iowa DNA Core facility (Iowa City, IA). Transferrin receptor cDNA was obtained from the America Type Culture Collection (Manassas, VA).
Small-interfering double-stranded RNA (siRNA) molecules were generated using the target sequence of CACUUGAUCCUGAGCUCAU (nucleotides 557–575 of mouse golgin-160) and purchased from Dharmacon RNA Technologies (Lafayette, CO). The scrambled control sequence was CCCAUGGACGACAACACAA.
cDNA for siRNA-resistant golgin-160 was generated using QuikChange mutagenesis to convert the wild-type sequence of CGCTTGATCCTGAGCTCAT to CGCTTGACCCCGAACTTAT. This resulted in an alternative codon usage without affecting the protein sequence of golgin-160.
Cell Culture and Transient Transfection of 3T3L1 Adipocytes
Murine 3T3L1 preadipocytes were purchased from the American Type Tissue Culture repository. Cells were cultured in DMEM supplemented with 25 mM glucose and 10% calf serum at 37°C with 8% carbon dioxide. Cells were differentiated into adipocytes with the addition of 1 μg/ml insulin, 1 mM dexamethasone, and 0.5 mM isobutyl-1-methylxanthine. Adipocytes were used in experiments 6–9 d after the initiation of differentiation. Cells were electroporated using the Gene Pulser II (Bio-Rad, Hercules, CA) with settings of 0.16 kV and 960 microfarads. For all experiments, 50 μg of DNA and 1 nmol of siRNA were used for electroporation. After electroporation, cells were plated on glass coverslips and allowed to recover in complete medium.
Immunofluorescence and Image Analysis
Differentiated 3T3L1 adipocytes transfected with the appropriate cDNAs and/or siRNA constructs were grown on coverslips and serum-starved in DMEM for 2 h before each experiment. Cells were then incubated with 100 nM insulin for 30 min or left untreated. Shortly after stimulation, cells were fixed using 4% paraformaldehyde with 0.18% Triton X-100 for 10 min at room temperature. Cells were then blocked using 1% bovine serum albumin solution with 5% donkey serum for 1 h at room temperature. Coverslips were then incubated in primary antibody solution for 1 h at 37°C. After incubation, the coverslips were washed three times with phosphate-buffered saline (PBS) solution, followed by incubation in secondary antibody for 1 h at 37°C. Coverslips were then again washed three times with PBS and mounted with VECTASHIELD medium. Plasma membrane localization was determined by using an LSM510 confocal fluorescence microscope (Carl Zeiss, Thornwood, NY) to score 50 representative cells per condition for the appearance of a continuous plasma membrane ring of GLUT4. The results represent the mean ± SD for three to five independent experiments.
Immunoblotting
Samples were separated by SDS-PAGE and electrophoretically transferred to polyvinylidene difluoride membranes. The samples were immunoblotted with monoclonal or polyclonal-specific antibody as indicated in figure legends. The primary antibodies were detected with horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG antibodies. Specific signals were visualized, scanned, and analyzed by Molecular Imager FX (Bio-Rad).
GLUT4 Endocytosis Assay
Basal golgin-160 knockdown cells and insulin-stimulated control adipocytes were chilled and incubated with the myc monoclonal antibody for 1 h at 4°C to label the GLUT4 at the plasma membrane. Cells were then washed to remove insulin and excess myc antibody as described previously (Kao et al., 1998). The cells were placed at 37°C and incubated for various times to allow myc antibody-bound GLUT4 to internalize. The reactions were stopped by washing once with ice-cold PBS and fixing in 4% paraformaldehyde and 0.2% Triton X-100 in PBS at room temperature for 10 min. The cells were incubated with Alexa584-anti-mouse secondary antibody at 37°C for 1 h. Translocation of myc antibody-bound GLUT4 from plasma membrane to the intracellular pool was examined by immunofluorescence microscopy.
2-Deoxyglucose Assay
3T3L1 adipocytes were seeded in 12-well plates and 2-deoxyglucose uptake determined as described previously (Kanzaki and Pessin, 2001). In brief, cells were serum starved for 3 h before the assay. Cells were then washed twice with KRPH buffer (5 mM Na2HPO4, 20 mM HEPES, pH 7.4, 1 mM MgSO4, 1 mM CaCl2, 136 mM NaCl, 4.7 mM KCl, 0.1% [wt/vol] BSA) and stimulated with 100 nM insulin or left untreated for 30 min. Glucose uptake was measured by incubation with 0.1 mM 2-deoxyglucose containing 1 μCi/ml 2-deoxy-d-glucose, [1,2-3H] at 4°C for 5 min. Transport was terminated by washing the cells three times with ice-cold PBS. Cells were solubilized with 1% Triton X-100, and the radioactivity was detected by scintillation counting. Nonspecific deoxyglucose uptake was measured in the presence of 20 μM cytochalasin B and was subtracted from each determination to obtain specific uptake. Data are representative of three experiments, and each value was corrected for protein content.
Plasma Membrane Sheet Assay
Plasma membrane sheets were prepared from adipocytes by the method described previously (Elmendorf et al., 1998). Briefly, after the appropriate treatment as described in the figure legend, coverslips were rinsed once in ice-cold PBS and incubated with 0.5 mg/ml poly-l-lysine in PBS for 30 s. Cells were then swollen in a hypotonic buffer (23 mM KCl, 10 mM HEPES, pH 7.5, 2 mM MgCl2, and 1 mM EGTA) by three successive rinses. The swollen cells were sonicated for 3 s in sonication buffer (70 mM KCl, 30 mM HEPES, pH 7.5, 5 mM MgCl2, 3 mM EGTA, 1 mM dithiothreitol, and 0.1 mM phenylmethylsulfonyl fluoride). The bound plasma membrane sheets were washed two times with sonication buffer and used for indirect immunofluorescence as described above.
IRAP and GLUT4 Glycosylation
One milligram of total detergent cell extracts (in buffer containing 10% SDS, 20% glycerol, and 0.125 M Tris) from random and golgin-160 siRNA-treated 3T3L1 adipocytes were incubated with 200 μl of wheat germ agglutinin (WGA) immobilized on agarose beads (glycoprotein isolation kit; Pierce Chemical). The WGA-bound glycoproteins were washed and then eluted. Equal percentage aliquots of the total cell lysates incubated with WGA and eluted proteins were then immunoblotted for the GLUT4, IRAP, transferrin receptor, and CD36 as described above.
RESULTS
Localization of Golgin-160 in 3T3L1 Adipocytes
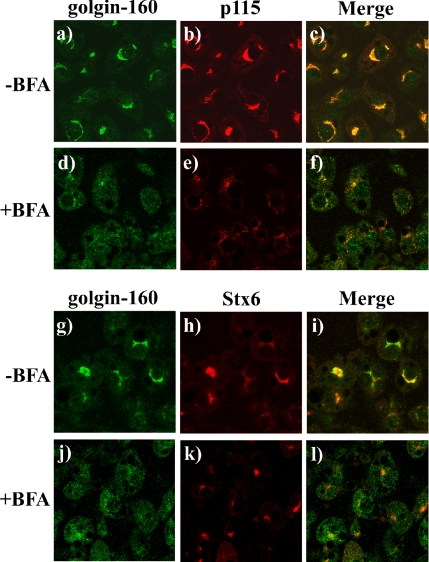
To examine the distribution of golgin-160 in adipocytes, we compared the localization of endogenous golgin-160 with that of the Golgi marker p115 as well as the TGN marker Stx6 by using confocal immunofluorescence microscopy. Golgin-160 was primarily concentrated in the perinuclear region, with low levels of diffuse background staining throughout the cell (Figure 1a). This distribution overlapped well with the localization pattern of p115 (Figure 1, b and c) and displayed partial colocalization with Stx6 (Figure 1, g–i).
Figure 1.
Golgin-160 colocalizes with p115 in the Golgi stacks in 3T3L1 adipocytes. Fully differentiated 3T3L1 adipocytes were either left untreated (a–c, g–i) or incubated with 5 μg/ml BFA (d–f, j–l) for 30 min at 37°C. The cells were fixed and subjected to confocal fluorescent microscopy for the localization of golgin-160 (a, d, g, and j) and p115 (b and e) and stx6 (h and k). Merged images are shown in c, f, i, and l. These are representative image taken from three independent experiments.
To test whether golgin-160 was localized to the Golgi stacks and/or to the TGN, we took advantage of the ability of brefeldin A (BFA) to collapse the Golgi cisternae back into the endoplasmic reticulum (Banting and Ponnambalam, 1997; Lippincott-Schwartz et al., 1991; Reaves and Banting, 1992). Under these conditions, the BFA inhibition of TGN anterograde transport also results in fusion of the TGN with late endosomes that coalesce into a more spherical mass near the microtubule organization center (Chardin and McCormick, 1999). As expected, BFA treatment resulted in the loss of perinuclear p115 labeling that became more diffuse, consistent with dissolution of the Golgi stacks and retrograde transport into the endoplasmic reticulum (Figure 1, e). In contrast, BFA treatment resulted in a more concentrated, spherical distribution of Stx6, consistent with TGN localization (Figure 1k). On BFA treatment, the perinuclear labeling of golgin-160 was disrupted and a more diffuse localization pattern similar to that of p115 was observed (Figure 1, d and j). These effects of BFA are consistent with the localization of golgin-160 to the Golgi cisternae but not to any appreciable extent in the TGN of 3T3L1 adipocytes. This is consistent with more recent immunoelectron microscopy data in HeLa cells demonstrating that the majority of golgin-160 is localized to the Golgi and not to the TGN (Hicks et al., 2006).
Knockdown of Golgin-160 and Its Effects on Golgi Structure
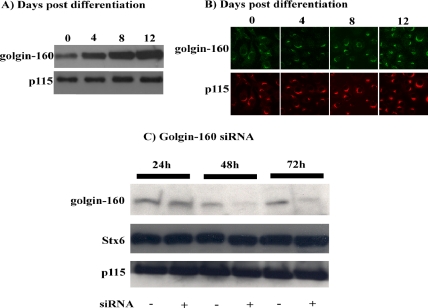
Several proteins that are required for adipocyte-specific function are typically induced during the conversion from preadipocytes (fibroblast-like phenotype) to fully differentiated adipocytes. We therefore examined the protein levels of golgin-160 levels during 3T3L1 adipogenesis (Figure 2A). Although golgin-160 was expressed in preadipocytes (day 0), there was a significant induction during the adipogenic process (days 4–12). In comparison, total p115 protein levels seemed to increase slightly after the differentiation of preadipocytes into adipocytes. Immunofluorescent images of preadipocytes and cells undergoing adipogenesis revealed that golgin-160 maintains a Golgi-associated distribution throughout the differentiation process (Figure 2B). This distribution was very similar to the staining pattern observed for p115 in these cells.
Figure 2.
Golgin-160 protein expression is induced during adipogenesis and is effectively reduced after siRNA transfection. (A) Total cell extracts were prepared from 3T3L1 cells before the initiation of differentiation and at 4, 8, and 12 d postdifferentiation. The cell extracts were then immunoblotted for golgin-160 and p115. (B) 3T3L1 cells were fixed at the initiation of differentiation (0) and at 4, 8, and 12 d postdifferentiation. The cells were then subjected to confocal fluorescence microscopy for the endogenous golgin-160 and p115 proteins. (C) Fully differentiated adipocytes were transfected with a random (−) or golgin-160–specific siRNA (+). Total cell extracts were prepared 24, 48, and 72 h later and immunoblotted for the presence of golgin-160, Stx6, and p115. These are representative experiments independently performed three times.
We next transfected fully differentiated 3T3L1 adipocytes with a golgin-160–specific siRNA to reduce protein expression levels (Figure 2C). Compared with cells treated with a scrambled siRNA sequence, cells treated with siRNA designed to target golgin-160 showed a marked reduction in the levels of golgin-160 protein 48 h after electroporation (Figure 2C). The golgin-160 levels were further decreased after 72 h of siRNA transfection. Importantly, the golgin-160 knockdown was specific, because the levels of Stx6 and p115 were not affected by either random or golgin-160–specific siRNA transfection.
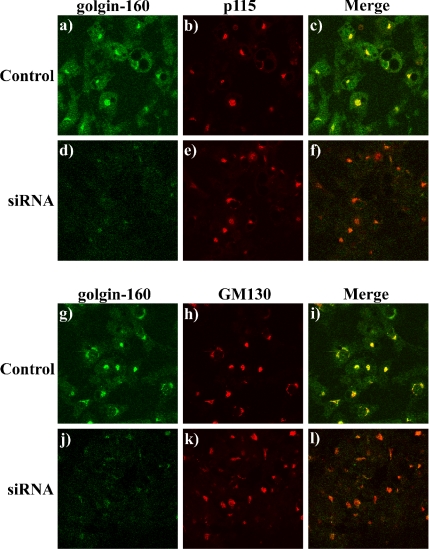
Because caspase-mediated cleavage of golgin-160 in fibroblasts correlated with Golgi stack fragmentation (Mancini et al., 2000), we next investigated whether knockdown of golgin-160 effected the general structural organization of the Golgi apparatus in adipocytes. As observed previously, golgin-160 strongly colocalized with p115 (Figure 3, a–c) as well as another golgin protein GM130 (Figure 3, g–i). Whereas the level of golgin-160 labeling was markedly reduced in the golgin-160 siRNA knockdown adipocytes (Figure 3, d and j), the levels and localization of p115 and GM130 were completely unaffected (Figure 3, e and k). Together, these data demonstrate that knockdown of golgin-160 was specific for golgin-160 and did not result in a gross morphological change in adipocyte Golgi organization.
Figure 3.
Reduction of golgin-160 protein levels does not alter the general Golgi morphology in 3T3L1 adipocytes. Fully differentiated adipocytes were either transfected with a random siRNA (a–c, g–i) or with the golgin-160–specific siRNA (d–f, j–l). Forty-eight hours later, the cells were fixed and subjected to confocal fluorescent microscopy for the localization of golgin-160 (a, d, g, and j), p115 (b and e), and GM130 (h and k). Merged images are shown in c, f, i, and l. These are representative image taken from three independent experiments.
Effect of Golgin-160 Knockdown on GLUT4 Translocation
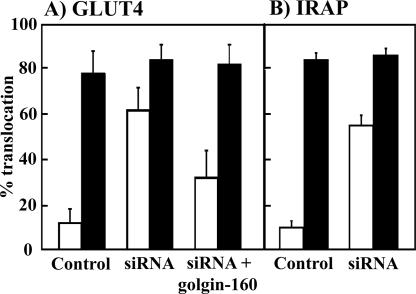
To examine the potential role of golgin-160 in regulated sorting of adipocyte membrane transport, we next determined the effect of golgin-160 knockdown on GLUT4 translocation by using the GLUT4-EGFP reporter construct as described previously (Watson et al., 2004). As expected, treatment of control adipocytes with 100 nM insulin for 30 min resulted in an approximate eightfold increase in the percentage of cells showing a plasma membrane localization of GLUT4 compared with unstimulated cells, indicating a marked increase in the amount of GLUT4 protein translocation (Figure 4). Surprisingly, knockdown of golgin-160 resulted in an approximate sixfold increase in the percentage of cells that showed a plasma membrane localization of GLUT4 in the basal state, compared with control cells transfected with scrambled siRNA. Due to the high basal state localization of GLUT4 at the plasma membrane there was only a small effect of insulin to stimulate translocation. However, this still occurred to the same extent as in the control cells.
Figure 4.
Golgin-160 knockdown results in enhanced GLUT4 and IRAP localization to the plasma membrane. (A) Fully differentiated 3T3L1 adipocytes were cotransfected with a random siRNA (bars 1 and 2) or the golgin-160–specific siRNA (bars 3 and 4) plus the GLUT4-GFP reporter cDNA. The cells were also cotransfected with the murine golgin-160–specific siRNA, GLUT4-GFP reporter and a golgin-160–resistant cDNA (bars 5 and 6). Forty-eight hours later, the cells were either left untreated (open bars) or stimulated with 100 nM insulin (closed bars) for 30 min. The cells were fixed and subjected to confocal fluorescent microscopy for the localization of GLUT4-GFP. These data represent the counting of 50 cells/experiment (from 3 to 6 independent experiments) displaying a continuous plasma membrane rim. (B) Fully differentiated 3T3L1 adipocytes were cotransfected with a random siRNA (bars 1 and 2) and the golgin-160–specific siRNA (bars 3 and 4) plus the TR-IRAP reporter cDNA. Forty-eight hours later, the cells were either left untreated (open bars) or stimulated with 100 nM insulin for 30 min (closed bars). The cells were fixed and subjected to confocal fluorescent microscopy for the localization of TR-IRAP. These data represent the counting of 50 cells/experiment (from 3 independent experiments) displaying a continuous plasma membrane rim.
To ensure that this effect was specific to the loss of golgin-160, we cotransfected the golgin-160 siRNA (to reduce the endogenous golgin-160 protein levels) with a siRNA-resistant golgin-160 cDNA construct. Under basal conditions, knockdown cells overexpressing siRNA-resistant golgin-160 showed a GLUT4 translocation profile that was similar to the random siRNA-transfected control cells. Thus, the expression of siRNA-resistant golgin-160 was able to rescue the effects of golgin-160 siRNA treatment, suggesting that this effect was specific for the loss of golgin-160 protein. These results imply that the knockdown of golgin-160 resulted in a defect in the ability of these cells to properly sequester GLUT4 under basal conditions.
To confirm this effect of golgin-160 depletion on GLUT4 sequestration, we took advantage of insulin-regulated IRAP trafficking. Previous studies have demonstrated that IRAP undergoes identical intracellular sequestration and insulin-stimulated plasma membrane translocation as GLUT4 (Kandror and Pilch, 1994a,b; Kandror et al., 1994; Malide et al., 1997; Martin et al., 1997; Ross et al., 1997). In addition, trafficking can readily be assessed using a transferrin-receptor-IRAP (TR-IRAP) chimera reporter construct (Subtil et al., 2000). Knockdown of golgin-160 also resulted in a significant increase in the percentage of cells that showed a plasma membrane localization of IRAP in the basal state (Figure 4B).
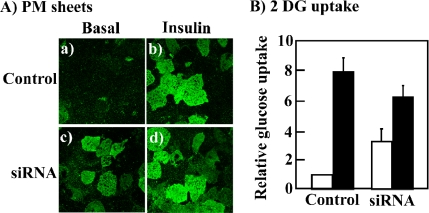
Because the above-mentioned studies were based upon the trafficking of the transfected GLUT4-GFP fusion reporter protein, in parallel we examined the effect of golgin-160 knockdown on the endogenous GLUT4 protein. Plasma membrane sheets were prepared from scrambled and golgin-160 siRNA-transfected cells (Figure 5A). Plasma membranes sheets isolated from scrambled siRNA-treated control cells had a relatively low level of immunoreactive GLUT4 in the basal state (Figure 5A, a). Insulin stimulation resulted in the translocation of the endogenous GLUT4 protein as depicted by the increase in plasma membrane sheet immunofluorescence (Figure 5A, b). In agreement with the results obtained using GLUT4-GFP, knockdown of golgin-160 resulted in an increase in the level of endogenous GLUT4 at the plasma membrane in the basal state (Figure 5A, c). Insulin stimulation slightly increased the level of the endogenous GLUT4 at the plasma membrane to a similar level as the control insulin-stimulated cells, consistent with the effects observed using the GLUT4-GFP reporter (Figure 5A, d).
Figure 5.
Golgin-160 protein knockdown increases the basal state plasma membrane distribution of the endogenous GLUT4 protein. Fully differentiated 3T3L1 adipocytes were transfected with a random siRNA or the golgin-160–specific siRNA. (A) Forty-eight hours later, the cells were either left untreated or stimulated with 100 nM insulin for 30 min. Plasma membrane sheets were prepared and subjected to confocal fluorescent microscopy for the presence of the endogenous GLUT4 protein. These are representative image taken from three independent experiments. (B) The cells treated as described above were assayed for the uptake of [3H]2-deoxyglucose. Open bars represent untreated cells and closed bars represent cells treated with insulin. These are the average values obtained from three independent experiments.
To further confirm the necessary role of golgin-160 function in the intracellular retention of the endogenous GLUT4 protein, we next examined the effect of golgin-160 knockdown on glucose uptake (Figure 5B). Scrambled siRNA-transfected cells displayed an approximate eightfold insulin-stimulated increase in the rate of glucose uptake. In comparison, the knockdown of golgin-160 resulted in an approximate threefold increase in glucose uptake in the basal state and a sixfold increase after insulin stimulation. Although the effect of golgin-160 knockdown on glucose uptake in the basal state was not as robust as that observed for GLUT4 translocation, these data strongly support a requirement for golgin-160 in the sorting of GLUT4 necessary for appropriate intracellular retention.
Golgin-160 Does Not Regulate GLUT4 Endocytosis
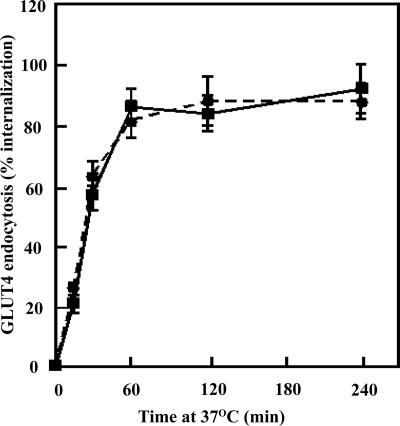
There are only two general mechanisms that can account for increased levels of GLUT4 at the plasma membrane. Either the reduction of golgin-160 increases the rate of GLUT4 exocytosis and/or decreases the rate of plasma membrane endocytosis. To address this issue, we took advantage of an exofacial myc epitope-tagged GLUT4 protein (myc-GLUT4) that has been routinely used to label the cell surface-exposed myc epitope and to subsequently examine the internalization of GLUT4. Control and golgin-160 siRNA knockdown cells were stimulated with insulin and were labeled with a myc antibody at 4°C. After insulin removal, the cells were warmed to 37°C and the time-dependent appearance of intracellular localized myc antibody was determined (Figure 6). In control cells, the t1/2 for endocytosis was ∼28 min in good agreement with previous studies (Satoh et al., 1993; Al-Hasani et al., 2002; Kanzaki et al., 2004; Karylowski et al., 2004). Similarly, in the golgin-160 siRNA knockdown cells essentially an identical rate of GLUT4 endocytosis was obtained.
Figure 6.
Golgin-160 knockdown has no significant effect on the rate of GLUT4 endocytosis. Fully differentiated adipocytes were transfected with a random siRNA (solid squares) or the golgin-160–specific siRNA (solid circles) plus a myc-GLUT4 cDNA reporter. Forty-eight hours later, the cells were stimulated with 100 nM insulin for 30 min and then cooled to 4°C. The exofacial exposed myc epitope was then labeled with a myc antibody, the cells were then washed to remove insulin and excess antibody and subsequently warmed to 37°C. At various times, the cells were fixed, permeabilized, and incubated with a rhodamine-conjugated secondary antibody, and the cells scored for the presence of internalized GLUT4 as described under Experimental Procedures. These data represent the counting of 50 cells/experiment from four independent experiments.
Golgin-160 Functions in a Pre-GGA–dependent Step in the Sorting of GLUT4
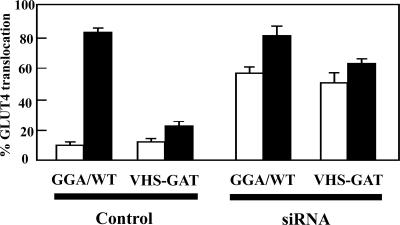
The above-mentioned data demonstrate that the increased cell surface distribution of GLUT4 in the golgin-160 knockdown cells is due to enhanced exocytosis without any significant effect on the rate of plasma membrane endocytosis. To examine the basis for the golgin-160–dependent exocytosis of GLUT4, we took advantage of the dominant-interfering GGA mutant (VHS-GAT) that prevents the TGN sorting of newly synthesized GLUT4 into the insulin-responsive compartment. As reported previously (Watson et al., 2004), compared with cells overexpressing the wild-type GGA protein (GGA/WT), expression of VHS-GAT mutant resulted in an inhibition of insulin-stimulated GLUT4 translocation (Figure 7). As expected, knockdown of golgin-160 in adipocytes overexpressing GGA/WT also increased plasma membrane GLUT4 localization in the basal state. Although expression of VHS-GAT inhibited insulin-stimulated tranlocation of GLUT4, there was no significant effect on the basal state GLUT4 translocation induced by the golgin-160 siRNA knockdown. Interestingly, the expression of VHS-GAT in combination with the golgin-160 protein reduction did attenuate the incremental insulin-stimulated translocation of GLUT4. Regardless, these results clearly demonstrate that the loss of golgin-160 protein alters a GLUT4-sorting step that precedes the function of GGA.
Figure 7.
Golgin-160 knockdown bypasses the ability of the dominant-interfering GGA mutant (VHS-GAT) to inhibit the plasma membrane trafficking of newly synthesized GLUT4. Fully differentiated 3T3L1 adipocytes were cotransfected with the GLUT4-GFP reporter plus a random siRNA or the golgin-160–specific siRNA in addition to either the full-length GGA cDNA or a cDNA encoding for the dominant-interfering GGA mutant (VHS-GAT). Forty-eight hours later, the cells were stimulated with (closed bars) or without (open bars) 100 nM insulin for 30 min, fixed, and subjected to confocal fluorescent microscopy. These data represent the counting of 50 cells/experiment from three independent experiments.
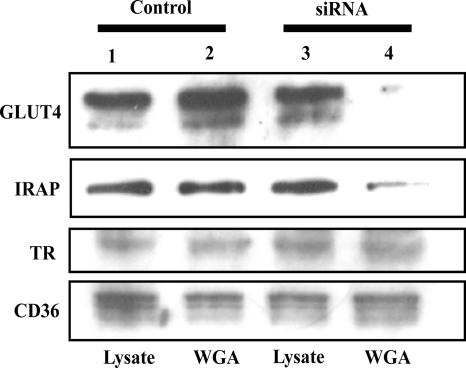
One important function of the TGN is the addition of terminal glycosidic linkages (sialic acids) to maturing glycoproteins (Dunphy and Rothman, 1985). If the loss of golgin-160 functions results in a bypass trafficking of GLUT4 away from the TGN this should be reflective by an alteration in terminal glycosylation. To address this issue, we took advantage of the fact that WGA preferentially binds to terminal sialic acid residues (Bhavanandan and Katlic, 1979; Nizheradze, 2000). We therefore examined the ability of GLUT4 and IRAP to bind to a WGA-agarose affinity column. Equal proportions of whole cell extracts (lysates) from control and golgin-160 siRNA-treated cells displayed similar levels of endogenous GLUT4, IRAP, TR, and CD36 proteins (Figure 8, lanes 1 and 3). The relative amount of GLUT4, IRAP, transferrin receptor, and CD36 eluted from the WGA-agarose column was also similar to the amount in the whole cell extracts (Figure 8, lanes 1 and 2). These data demonstrate that these glycoproteins were efficiently bound and eluted from the WGA column. In contrast, although the amount of transferrin receptor and CD36 bound and eluted from the WGA column was essentially identical in golgin-160 knockdown cells, the amount of GLUT4 and IRAP was markedly reduced (Figure 8, lanes 3 and 4). These data are consistent with a specific reduction in terminal glycosylation of GLUT4 and IRAP in golgin-160 siRNA-treated adipocytes.
Figure 8.
Knockdown of golgin-160 results in a loss of GLUT4 and IRAP terminal glycosylation. Fully differentiated adipocytes were transfected with a random (lanes 1 and 2) or golgin-160–specific siRNA (lanes 3 and 4). Total cell extracts were prepared 48 h later. Cell lysates were incubated with WGA-agarose beads, and the bound proteins eluted as described under Experimental Procedures. Equal proportions of the total cell extracts (lysates, lanes 1 and 3) and WGA-agarose bead eluants (lanes 2 and 4) were immunoblotted for the presence of GLUT4, IRAP, TR, and CD36. These are representative experiments independently performed three times.
Selectivity of Golgin-160 Function
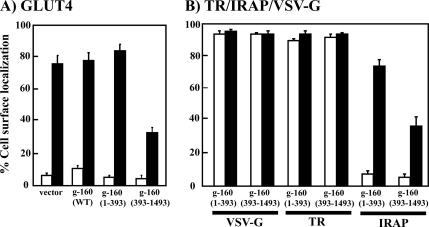
Golgin-160 is composed of a 393-amino acid head domain and a long carboxy-terminal α-helical coiled-coil domain structure. The head domain contains Golgi-targeting information and is responsible for its association with the Golgi-associated proteins (Hicks and Machamer, 2002, 2005). Having established that reduction of golgin-160 protein levels resulted in a missorting of GLUT4 to the plasma membrane, we next assessed the effects of the golgin-160 amino terminal head domain (1–393) and the carboxy-terminal α-helical domain (393–1498) on GLUT4 plasma membrane accumulation. As observed for the siRNA golgin-160 rescue (Figure 4), overexpression of the wild-type golgin-160 protein had no significant effect on the basal state distribution or insulin-stimulated GLUT4 translocation (Figure 9A). Similarly, expression of the golgin-160 head domain (amino acids 1–393) was without effect on basal or on the insulin-stimulated distribution of GLUT4 compared with control cells. In contrast, overexpression of carboxy-α helical coil domain (393–1498) resulted in an inhibition of insulin-stimulated GLUT4 translocation.
Figure 9.
Expression of the carboxy-terminal domain of golgin-160 specifically inhibits the trafficking of insulin-regulated but not constitutive trafficking proteins. (A) Fully differentiated 3T3L1 adipocytes were cotransfected with cDNAs encoding the wild-type (WT), amino-terminal (1–393), or carboxy-terminal (393–1498) domains of golgin-160 plus the GLUT4-GFP reporter. Twenty-four hours later, the cells were stimulated with or without 100 nM insulin for 30 min, fixed, and subjected to confocal fluorescent microscopy. (B) Fully differentiated 3T3L1 adipocytes were cotransfected with cDNAs encoding the amino terminal (1–393) or carboxy-terminal (393–1498) domains of golgin-160 plus either VSV-G, TR, or the TR-IRAP reporter. Twenty-four hours later, the cells were stimulated with (closed bars) or without (open bars) 100 nM insulin for 30 min, fixed, and subjected to confocal fluorescent microscopy. These data represent the counting of 50 cells/experiment from three independent experiments.
As controls for specificity, we also examined the α-helical coil domain effect on the distribution of IRAP and the constitutively trafficking proteins, the TR, and VSV-G. Consistent with the effects on GLUT4, expression of the carboxy-terminal golgin-160 domain (393–1498) inhibited insulin-stimulated translocation of the IRAP-TR construct (Figure 9B). In contrast, golgin-160 (393–1498) had no significant effect on the constitutive trafficking of the transferrin receptor itself or VSV-G protein to the plasma membrane. Thus, the inhibitory effect of golgin-160 (393–1498) is consistent with golgin-160 being involved in a specific Golgi membrane sorting step require to direct GLUT4 and IRAP in the correct transport pathway leading to the specialized insulin-responsive storage compartment.
DISCUSSION
It is generally accepted that the TGN functions as the key-sorting compartment from which transmembrane and luminal cargo proteins are targeted to post-Golgi destinations (Keller and Simons, 1997). However, it has been suggested that vesicular exit from transcisternae of the Golgi stack also can occur, which provides alternative exit routes for various trafficking and sorting proteins (Ladinsky et al., 2002). In adipocytes, it has been reported that constitutive as well as regulated secretion of adipokines can occur independently from TGN trafficking. For example, it has been reported that leptin is directly secreted from the endoplasmic reticulum and adiponection from the Golgi cisternae (Barr et al., 1997; Xie et al., 2006). Although these latter two proteins are soluble luminal proteins, adipocytes also display complex patterns of integral membrane protein transport with several proteins undergoing various degrees of insulin responsiveness.
Previously, we have observed that the newly synthesized GLUT4 and IRAP proteins, but not constitutive trafficking proteins, undergo a GGA-dependent sorting step into the IRC (Watson et al., 2004; Hou et al., 2006). Because GGA is thought to primarily function as a cargo adaptor for TGN exit, these data implicate a specific TGN exit pathway for proteins destined for sequestration into the IRC. This is also consistent with a GLUT4 plasma membrane recycling study that demonstrate the endocytosis of GLUT4 back to the TGN and subsequent processing and acquisition of insulin responsiveness (Shewan et al., 2003). We therefore wondered whether there could also be a pre-TGN (Golgi cisternae) decision step that directs GLUT4 toward a specific pathway required for appropriate TGN sorting. While our study was in progress Hosaka et al. (2005) provided further support for this speculation by demonstrated that the p115 golgin protein could directly bind to IRAP (Hosaka et al., 2005). Regardless, we elected to examine the role of golgin-160 on the trafficking of GLUT4 due to the observations that golgin-160 can directly interact with the protein PIST/GOPC/CAL/FIG (Hicks and Machamer, 2005). PIST has been reported to interact with the TGN Q-SNARE Stx6 that has also been suggested to be involved in the TGN sorting of GLUT4 (Charest et al., 2001; Perera et al., 2003; Shewan et al., 2003). Moreover, PIST was found to interact with the small Rho family GTP binding protein TC10 that has also been implicated in the insulin-regulated trafficking of GLUT4 (Chiang et al., 2001; Neudauer et al., 2001; Watson et al., 2001; Chiang et al., 2002).
Our data clearly demonstrate that golgin-160 is required for the intracellular sequestration of endogenous as well as the exogenously expressed GLUT4 and IRAP proteins. This was based upon siRNA knockdown of golgin-160 that induced the basal state appearance of GLUT4 and IRAP at the plasma membrane. Importantly, this could be reversed by coexpression of a siRNA resistant golgin-160 cDNA. Moreover, golgin-160 was not globally required for all trafficking events, because the distribution of Stx6 was not altered by siRNA-mediated knockdown of golgin-160 under the same conditions that missorted GLUT4 and IRAP. Similarly, constitutive membrane protein trafficking (transferrin receptor and VSV-G protein) was unaffected by expression of a dominant-interfering golgin-160 truncation mutant, whereas insulin-regulated trafficking proteins (GLUT4 and IRAP) were inhibited.
It should be noted that golgin-160 knockdown also resulted in a basal state increase in glucose uptake consistent with a redistribution of GLUT4 to the plasma membrane. However, the effect of the golgin-160 knockdown was not as robust as that observed for GLUT4 translocation. This could have resulted from a lack of insulin-stimulated activation of the intrinsic glucose transport activity of GLUT4 (Sweeney et al., 1999; Somwar et al., 2001; Yamaguchi et al., 2005). Alternatively, appropriate GLUT4 glycosylation may be required for efficient glucose uptake, because terminal GLUT4 glycosylation was reduced in the golgin-160 knockdown cells. In contrast, it has been suggested that GLUT1 contributes a large proportion of glucose transport activity in 3T3L1 adipocytes (Liao et al., 2006). Under such conditions, the high level of GLUT1-dependent glucose uptake would reduce the relative effect of GLUT4 dependent (insulin-stimulated) glucose uptake. Nevertheless, these data strongly support a requirement for golgin-160 in the sorting of GLUT4 necessary for appropriate intracellular retention.
The necessary requirement for golgin-160 begs the question how can Golgi function be required for GLUT4 intracellular retention in the IRC? One possible explanation is schematically represented in Figure 10 based upon the finding that the rate of GLUT4 endocytosis was identical in both control and golgin-160 knockdown cells. Because there is no significant difference in the rate of endocytosis this necessarily requires an increase rate of GLUT4 exocytosis. However, the increased exocytosis probably occurs from the Golgi complex and not the TGN, because GLUT4 trafficking occurred even in the presence of the dominant-interfering GGA mutant. Consistent with this model, terminal glycosylation occurs in the TGN and both GLUT4 and IRAP had marked reduction in terminal glycosylation, detected by WGA binding, in the golgin-160 knockdown cells. However, these data do not exclude the possibility that the golgin-dependent missorting occurs in a specialized domain and/or at the TGN/Golgi transition.
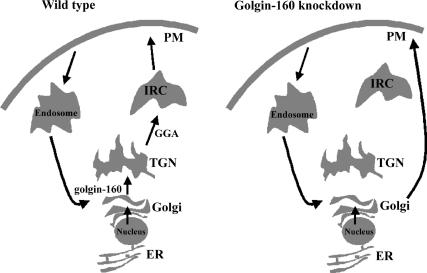
Figure 10.
Schematic model for golgin-160 function in the regulation of GLUT4 and IRAP protein trafficking in adipocytes. Under normal conditions, GLUT4 and IRAP undergo a biosynthetic sorting from endoplasmic reticulum (ER) to the IRC via the Golgi and TGN. Once at the plasma membrane, endocytosis and recycling result in the trafficking back to the Golgi/TGN and retention in the IRC. In the absence of golgin-160 function, the GLUT4 and IRAP proteins default to the plasma membrane, bypassing the GGA-dependent sorting to the IRC. The plasma membrane GLUT4 and IRAP then undergo recycling back to the Golgi/TGN, but again they are missorted away from the IRC in the absence of golgin-160.
Once at the plasma membrane, GLUT4 undergoes endocytosis and recycles back to the Golgi complex. In the absence of golgin-160, GLUT4 will then undergo constitutive exit and cycle back to the plasma membrane. This accounts for the apparent steady-state accumulation at the plasma membrane, yet with a normal rate of endocytosis. In contrast, in the presence of golgin-160, GLUT4 will then recycle back to the Golgi but in this case, it undergoes normal trafficking to the IRC.
This model would account for the effect of golgin-160 knockdown on both the endogenous and exogenously expressed GLUT4. In transfected GLUT4, after endoplasmic reticulum cotranslational insertion and trafficking to the Golgi, the protein will then default to the plasma membrane and undergo multiple rounds of recycling. Because it continually bypasses the IRC, the steady-state accumulation at the plasma membrane is observed. In endogenous GLUT4 protein, it is well established that there is a slow basal rate of trafficking to the plasma membrane and recycling back to the IRC. Because the knockdown of golgin-160 takes between 48 and 72 h and the basal state t1/2 of plasma membrane trafficking is ∼4 h (Karylowski et al., 2004), this is more than sufficient time for the majority of GLUT4 to have transited the plasma membrane and undergone intracellular recycling. The fact that the recycled GLUT4 defaults back to the plasma membrane in the absence of golgin-160 strongly supports the hypothesis that GLUT4 recycles back to the Golgi. This is further consistent with a study demonstrating that after cell surface desialylation, the IRAP protein recycles and becomes a substrate for sialyltransferase, which is a TGN localized glycosyltransferase (Shewan et al., 2003).
Together, these data demonstrate that following biosynthesis the GLUT4 protein enters a sorting pathway within the Golgi cisternae that is dependent on golgin-160 function. This pathway directs GLUT4 along a specific trafficking route leading to the TGN and a GGA-dependent step necessary for the entry into the insulin-responsive compartment. Moreover, in the absence of golgin-160–dependent Golgi processing, the GLUT4 protein defaults to the cell surface and undergoes continuous unregulated recycling.
ACKNOWLEDGMENTS
We thank Jeffery Smith and Bintou Diouf for assistance during this study. This study was supported by National Institutes of Health Grants DK-33823, DK-55811, and GM-42522.
Abbreviations used:
- BFA
brefeldin A
- IRC
insulin-responsive compartment
- IRAP
insulin-responsive aminopeptidase
- TGN
trans-Golgi network
- TR
transferrin receptor
- VSV-G
vesicular stomatitis virus G
- WGA
wheat germ agglutinin
- GGA
Golgi-localized γ-ear–containing ARF-binding protein.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-05-0386) on October 18, 2006.
REFERENCES
- Al-Hasani H., Kunamneni R. K., Dawson K., Hinck C. S., Muller-Wieland D., Cushman S. W. Roles of the N- and C-termini of GLUT4 in endocytosis. J. Cell Sci. 2002;115:131–140. doi: 10.1242/jcs.115.1.131. [DOI] [PubMed] [Google Scholar]
- Alvarez C., Garcia-Mata R., Hauri H. P., Sztul E. The p115-interactive proteins GM130 and giantin participate in endoplasmic reticulum-Golgi traffic. J. Biol. Chem. 2001;276:2693–2700. doi: 10.1074/jbc.M007957200. [DOI] [PubMed] [Google Scholar]
- Banting G., Ponnambalam S. TGN38 and its orthologues: roles in post-TGN vesicle formation and maintenance of TGN morphology. Biochim. Biophys. Acta. 1997;1355:209–217. doi: 10.1016/s0167-4889(96)00146-2. [DOI] [PubMed] [Google Scholar]
- Barr F. A., Short B. Golgins in the structure and dynamics of the Golgi apparatus. Curr. Opin. Cell Biol. 2003;15:405–413. doi: 10.1016/s0955-0674(03)00054-1. [DOI] [PubMed] [Google Scholar]
- Barr V. A., Malide D., Zarnowski M. J., Taylor S. I., Cushman S. W. Insulin stimulates both leptin secretion and production by rat white adipose tissue. Endocrinology. 1997;138:4463–4472. doi: 10.1210/endo.138.10.5451. [DOI] [PubMed] [Google Scholar]
- Bhavanandan V. P., Katlic A. W. The interaction of wheat germ agglutinin with sialoglycoproteins. The role of sialic acid. J. Biol. Chem. 1979;254:4000–4008. [PubMed] [Google Scholar]
- Bundis F., Neagoe I., Schwappach B., Steinmeyer K. Involvement of Golgin-160 in cell surface transport of renal ROMK channel: co-expression of Golgin-160 increases ROMK currents. Cell Physiol. Biochem. 2006;17:1–12. doi: 10.1159/000091454. [DOI] [PubMed] [Google Scholar]
- Chang L., Chiang S. H., Saltiel A. R. Insulin signaling and the regulation of glucose transport. Mol. Med. 2004;10:65–71. doi: 10.2119/2005-00029.Saltiel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chardin P., McCormick F. Brefeldin A: the advantage of being uncompetitive. Cell. 1999;97:153–155. doi: 10.1016/s0092-8674(00)80724-2. [DOI] [PubMed] [Google Scholar]
- Charest A., Lane K., McMahon K., Housman D. E. Association of a novel PDZ domain-containing peripheral Golgi protein with the Q-SNARE (Q-soluble N-ethylmaleimide-sensitive fusion protein (NSF) attachment protein receptor) protein syntaxin 6. J. Biol. Chem. 2001;276:29456–29465. doi: 10.1074/jbc.M104137200. [DOI] [PubMed] [Google Scholar]
- Chiang S. H., Baumann C. A., Kanzaki M., Thurmond D. C., Watson R. T., Neudauer C. L., Macara I. G., Pessin J. E., Saltiel A. R. Insulin-stimulated GLUT4 translocation requires the CAP-dependent activation of TC10. Nature. 2001;410:944–948. doi: 10.1038/35073608. [DOI] [PubMed] [Google Scholar]
- Chiang S. H., Hou J. C., Hwang J., Pessin J. E., Saltiel A. R. Cloning and functional characterization of related TC10 isoforms, a subfamily of Rho proteins involved in insulin-stimulated glucose transport. J. Biol. Chem. 2002;277:13067–13073. doi: 10.1074/jbc.M109471200. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Rothman J. E. Compartmental organization of the Golgi stack. Cell. 1985;42:13–21. doi: 10.1016/s0092-8674(85)80097-0. [DOI] [PubMed] [Google Scholar]
- Elmendorf J. S., Chen D., Pessin J. E. Guanosine 5′-O-(3-thiotriphosphate) (GTPγS) stimulation of GLUT4 translocation is tyrosine kinase-dependent. J. Biol. Chem. 1998;273:13289–13296. doi: 10.1074/jbc.273.21.13289. [DOI] [PubMed] [Google Scholar]
- Fullekrug J., Nilsson T. Protein sorting in the Golgi complex. Biochim. Biophys. Acta. 1998;1404:77–84. doi: 10.1016/S0167-4889(98)00048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks S. W., Horn T. A., McCaffery J. M., Zuckerman D. M., Machamer C. E. Golgin-160 promotes cell surface expression of the beta-1 adrenergic receptor. Traffic. 2006 doi: 10.1111/j.1600-0854.2006.00504.x. (in press) [DOI] [PubMed] [Google Scholar]
- Hicks S. W., Machamer C. E. The NH2-terminal domain of Golgin-160 contains both Golgi and nuclear targeting information. J. Biol. Chem. 2002;277:35833–35839. doi: 10.1074/jbc.M206280200. [DOI] [PubMed] [Google Scholar]
- Hicks S. W., Machamer C. E. Isoform-specific interaction of golgin-160 with the Golgi-associated protein PIST. J. Biol. Chem. 2005;280:28944–28951. doi: 10.1074/jbc.M504937200. [DOI] [PubMed] [Google Scholar]
- Hosaka T., Brooks C. C., Presman E., Kim S. K., Zhang Z., Breen M., Gross D. N., Sztul E., Pilch P. F. p115 Interacts with the GLUT4 vesicle protein, IRAP, and plays a critical role in insulin-stimulated GLUT4 translocation. Mol. Biol. Cell. 2005;16:2882–2890. doi: 10.1091/mbc.E05-01-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J. C., Suzuki N., Pessin J. E., Watson R. T. A specific di-leucine motif is required for the GGA-dependent entry of newly synthesized insulin-responsive aminopeptidase into the insulin-responsive compartment. J. Biol. Chem. 2006;281:33457–33466. doi: 10.1074/jbc.M601583200. [DOI] [PubMed] [Google Scholar]
- Kandror K., Pilch P. F. Identification and isolation of glycoproteins that translocate to the cell surface from GLUT4-enriched vesicles in an insulin-dependent fashion. J. Biol. Chem. 1994a;269:138–142. [PubMed] [Google Scholar]
- Kandror K. V., Pilch P. F. gp160, a tissue-specific marker for insulin-activated glucose transport. Proc. Natl. Acad. Sci. USA. 1994b;91:8017–8021. doi: 10.1073/pnas.91.17.8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandror K. V., Yu L., Pilch P. F. The major protein of GLUT4-containing vesicles, gp160, has aminopeptidase activity. J. Biol. Chem. 1994;269:30777–30780. [PubMed] [Google Scholar]
- Kanzaki M., Furukawa M., Raab W., Pessin J. E. Phosphatidylinositol 4,5-bisphosphate regulates adipocyte actin dynamics and GLUT4 vesicle recycling. J. Biol. Chem. 2004;279:30622–30633. doi: 10.1074/jbc.M401443200. [DOI] [PubMed] [Google Scholar]
- Kanzaki M., Pessin J. E. Insulin-stimulated GLUT4 translocation in adipocytes is dependent upon cortical actin remodeling. J. Biol. Chem. 2001;276:42436–42444. doi: 10.1074/jbc.M108297200. [DOI] [PubMed] [Google Scholar]
- Kao A. W., Ceresa B. P., Santeler S. R., Pessin J. E. Expression of a dominant interfering dynamin mutant in 3T3–L1 adipocytes inhibits GLUT4 endocytosis without affecting insulin signaling. J. Biol. Chem. 1998;273:25450–25457. doi: 10.1074/jbc.273.39.25450. [DOI] [PubMed] [Google Scholar]
- Karylowski O., Zeigerer A., Cohen A., McGraw T. E. GLUT4 is retained by an intracellular cycle of vesicle formation and fusion with endosomes. Mol. Biol. Cell. 2004;15:870–882. doi: 10.1091/mbc.E03-07-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller P., Simons K. Post-Golgi biosynthetic trafficking. J. Cell Sci. 1997;110:3001–3009. doi: 10.1242/jcs.110.24.3001. [DOI] [PubMed] [Google Scholar]
- Ladinsky M. S., Wu C. C., McIntosh S., McIntosh J. R., Howell K. E. Structure of the Golgi and distribution of reporter molecules at 20 degrees C reveals the complexity of the exit compartments. Mol. Biol. Cell. 2002;13:2810–2825. doi: 10.1091/mbc.01-12-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W., Nguyen M. T., Imamura T., Singer O., Verma I. M., Olefsky J. M. Lentiviral short hairpin ribonucleic acid-mediated knockdown of GLUT4 in 3T3-L1 adipocytes. Endocrinology. 2006;147:2245–2252. doi: 10.1210/en.2005-1638. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L., Tipper C., Amherdt M., Orci L., Klausner R. D. Brefeldin A's effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structures and membrane traffic. Cell. 1991;67:601–616. doi: 10.1016/0092-8674(91)90534-6. [DOI] [PubMed] [Google Scholar]
- Malide D., St-Denis J. F., Keller S. R., Cushman S. W. Vp165 and GLUT4 share similar vesicle pools along their trafficking pathways in rat adipose cells. FEBS Lett. 1997;409:461–468. doi: 10.1016/s0014-5793(97)00563-2. [DOI] [PubMed] [Google Scholar]
- Mancini M., Machamer C. E., Roy S., Nicholson D. W., Thornberry N. A., Casciola-Rosen L. A., Rosen A. Caspase-2 is localized at the Golgi complex and cleaves golgin-160 during apoptosis. J. Cell Biol. 2000;149:603–612. doi: 10.1083/jcb.149.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S., Rice J. E., Gould G. W., Keller S. R., Slot J. W., James D. E. The glucose transporter GLUT4 and the aminopeptidase vp165 colocalise in tubulo-vesicular elements in adipocytes and cardiomyocytes. J. Cell Sci. 1997;110:2281–2291. doi: 10.1242/jcs.110.18.2281. [DOI] [PubMed] [Google Scholar]
- Mueckler M. Facilitative glucose transporters. Eur. J. Biochem. 1994;219:713–725. doi: 10.1111/j.1432-1033.1994.tb18550.x. [DOI] [PubMed] [Google Scholar]
- Neudauer C. L., Joberty G., Macara I. G. PIST: a novel PDZ/coiled-coil domain binding partner for the rho-family GTPase TC10. Biochem. Biophys. Res. Commun. 2001;280:541–547. doi: 10.1006/bbrc.2000.4160. [DOI] [PubMed] [Google Scholar]
- Nizheradze K. A. Binding of wheat germ agglutinin to extracellular network produced by cultured human fibroblasts. Folia Histochem. Cytobiol. 2000;38:167–173. [PubMed] [Google Scholar]
- Perera H. K., Clarke M., Morris N. J., Hong W., Chamberlain L. H., Gould G. W. Syntaxin 6 regulates Glut4 trafficking in 3T3-L1 adipocytes. Mol. Biol. Cell. 2003;14:2946–2958. doi: 10.1091/mbc.E02-11-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaves B., Banting G. Perturbation of the morphology of the trans-Golgi network following Brefeldin A treatment: redistribution of a TGN-specific integral membrane protein, TGN38. J. Cell. Biol. 1992;116:85–94. doi: 10.1083/jcb.116.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S. A., Herbst J. J., Keller S. R., Lienhard G. E. Trafficking kinetics of the insulin-regulated membrane aminopeptidase in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 1997;239:247–251. doi: 10.1006/bbrc.1997.7459. [DOI] [PubMed] [Google Scholar]
- Satoh S., Nishimura H., Clark A. E., Kozka I. J., Vannucci S. J., Simpson I. A., Quon M. J., Cushman S. W., Holman G. D. Use of bismannose photolabel to elucidate insulin-regulated GLUT4 subcellular trafficking kinetics in rat adipose cells. Evidence that exocytosis is a critical site of hormone action. J. Biol. Chem. 1993;268:17820–17829. [PubMed] [Google Scholar]
- Shewan A. M., van Dam E. M., Martin S., Luen T. B., Hong W., Bryant N. J., James D. E. GLUT4 recycles via a trans-Golgi network (TGN) subdomain enriched in syntaxins 6 and 16 but not TGN 38, involvement of an acidic targeting motif. Mol. Biol. Cell. 2003;14:973–986. doi: 10.1091/mbc.E02-06-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somwar R., Kim D. Y., Sweeney G., Huang C., Niu W., Lador C., Ramlal T., Klip A. GLUT4 translocation precedes the stimulation of glucose uptake by insulin in muscle cells: potential activation of GLUT4 via p38 mitogen-activated protein kinase. Biochem. J. 2001;359:639–649. doi: 10.1042/0264-6021:3590639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subtil A., Lampson M. A., Keller S. R., McGraw T. E. Characterization of the insulin-regulated endocytic recycling mechanism in 3T3-L1 adipocytes using a novel reporter molecule. J. Biol. Chem. 2000;275:4787–4795. doi: 10.1074/jbc.275.7.4787. [DOI] [PubMed] [Google Scholar]
- Sweeney G., Somwar R., Ramlal T., Volchuk A., Ueyama A., Klip A. An inhibitor of p38 mitogen-activated protein kinase prevents insulin-stimulated glucose transport but not glucose transporter translocation in 3T3–L1 adipocytes and L6 myotubes. J. Biol. Chem. 1999;274:10071–10078. doi: 10.1074/jbc.274.15.10071. [DOI] [PubMed] [Google Scholar]
- Thurmond D. C., Ceresa B. P., Okada S., Elmendorf J. S., Coker K., Pessin J. E. Regulation of insulin-stimulated GLUT4 translocation by Munc18c in 3T3–L1 adipocytes. J. Biol. Chem. 1998;273:33876–33883. doi: 10.1074/jbc.273.50.33876. [DOI] [PubMed] [Google Scholar]
- Watson R. T., Khan A. H., Furukawa M., Hou J. C., Li L., Kanzaki M., Okada S., Kandror K. V., Pessin J. E. Entry of newly synthesized GLUT4 into the insulin-responsive storage compartment is GGA dependent. EMBO J. 2004;23:2059–2070. doi: 10.1038/sj.emboj.7600159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. T., Pessin J. E. Intracellular organization of insulin signaling and GLUT4 translocation. Recent Prog. Horm. Res. 2001;56:175–193. doi: 10.1210/rp.56.1.175. [DOI] [PubMed] [Google Scholar]
- Watson R. T., Pessin J. E. Bridging the GAP between insulin signaling and GLUT4 translocation. Trends Biochem. Sci. 2006;31:215–222. doi: 10.1016/j.tibs.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Watson R. T., Shigematsu S., Chiang S. H., Mora S., Kanzaki M., Macara I. G., Saltiel A. R., Pessin J. E. Lipid raft microdomain compartmentalization of TC10 is required for insulin signaling and GLUT4 translocation. J. Cell Biol. 2001;154:829–840. doi: 10.1083/jcb.200102078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L., Boyle D., Sanford D., Scherer P. E., Pessin J. E., Mora S. Intracellular trafficking and secretion of adiponectin is dependent on GGA-coated vesicles. J. Biol. Chem. 2006;281:7253–7259. doi: 10.1074/jbc.M511313200. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S., et al. Activators of AMP-activated protein kinase enhance GLUT4 translocation and its glucose transport activity in 3T3-L1 adipocytes. Am. J. Physiol. Endocrinol. Metab. 2005;289:E643–E649. doi: 10.1152/ajpendo.00456.2004. [DOI] [PubMed] [Google Scholar]