Abstract
The constituent proteins of gap junctions, called connexins (Cxs), have a short half-life. Despite this, the physiological stimuli that control the assembly of Cxs into gap junctions and their degradation have remained poorly understood. We show here that in androgen-responsive human prostate cancer cells, androgens control the expression level of Cx32—and hence the extent of gap junction formation—post-translationally. In the absence of androgens, a major fraction of Cx32 is degraded presumably by endoplasmic reticulum–associated degradation, whereas in their presence, this fraction is rescued from degradation. We also show that Cx32 and Cx43 degrade by a similar mechanism. Thus, androgens regulate the formation and degradation of gap junctions by rerouting the pool of Cxs, which normally would have been degraded from the early secretory compartment, to the cell surface, and enhancing assembly into gap junctions. Androgens had no significant effect on the formation and degradation of adherens and tight junction–associated proteins. The findings that in a cell culture model that mimics the progression of human prostate cancer, degradation of Cxs, as well as formation of gap junctions, are androgen-dependent strongly implicate an important role of junctional communication in the prostate morphogenesis and oncogenesis.
INTRODUCTION
Epithelial cells interact with their neighbors through junctional complexes such as tight junctions, adherens junctions, desmosomes, and gap junctions (Gumbiner, 2000; Wheelock and Johnson, 2003; Saez et al., 2003; Getsios et al., 2004). Apart from linking the cytoskeletal network of neighboring cells, these complexes have been postulated to transduce signals not only among cells within a population but also from the interior of the cells to the extracellular milieu reciprocally (Braga, 2002; Balda and Matter, 2004). Evidence is mounting that this form of signaling is instrumental in controlling the proliferation and differentiation of epithelial cells and maintaining tissue homeostasis. Although each junctional complex likely triggers a unique signaling cascade, among all the junctional complexes studied so far, gap junctions are highly specialized conglomerations of channels that signal uncanonically, by permitting the direct exchange of small molecules (≤1500 Da) between the cytoplasmic interiors of contiguous cells (Loewenstein, 1981, 1987; Saez et al., 2003). The gap junction channels are formed of proteins called connexins (Cxs), which are designated according to their molecular mass (Saez et al., 2003). Currently ∼20 distinct, but highly related, Cx genes have been cloned from both human and mouse. The Cxs are transmembrane proteins that traverse the membrane four times, share a similar topology, and are expressed both redundantly as well as in a tissue-specific manner (Wei et al., 2004; Sosinsky and Nicholson, 2005). Evidence to date indicates that Cxs are transported to the cell surface as hexamers, also called connexons or hemichannels, by sequential passage through endoplasmic reticulum (ER), Golgi, and trans-Golgi network (VanSlyke and Musil, 2000; Segretain and Falk, 2004). All Cxs, with the exception of Cx26, are phosphoproteins with a relatively short half-life of 2–5 h, suggesting that the assembly and disassembly of gap junctions are highly dynamic processes (VanSlyke and Musil, 2000; Segretain and Falk, 2004; Laird, 2005; Solan and Lampe, 2005).
Gap junctional communication has been postulated to fulfill the roles of intercellular buffering of cytoplasmic ions, synchronization of cellular behavior, and regulation of cell growth (Loewenstein, 1981; Saez et al., 2003). Although these roles were well-supported by several studies, showing attenuation of malignant phenotype upon forced expression of Cx genes in Cx-deficient tumor cell lines (Mehta et al., 1991; Zhu et al., 1991; Rose et al., 1993; Ruch, 1994; Trosko and Ruch, 1998), convincing evidence supporting a key role for gap junctions in regulating diverse physiological processes, such as cell growth, differentiation, and apoptosis in vivo, has recently emerged from the association of specific Cx mutations with human genetic diseases and from tissue-specific Cx knockouts in mice (Wei et al., 2004). For example, Cx32 is abundantly expressed in the hepatocytes, and knockout mice for this Cx exhibit a higher incidence of chemical- and radiation-induced liver tumors than their wild-type counterparts, respectively (Temme et al., 1997; King and Lampe, 2005a, 2005b). Also, Cx32 has been documented to act as a lung tumor suppressor, as assessed by higher bronchioloalveolar tumor incidence (King and Lampe, 2004; King et al., 2005a, 2005b). Distinct dominant mutations in Cx26, a Cx abundantly expressed in the inner ear and skin, have been shown to cause not only hearing loss but also autosomal dominant disorders of epidermal keratinization and differentiation (Kelsell et al., 2001). The above studies lend credence to the long-standing notion that the impaired and aberrant expression of Cxs—and consequently passage of growth regulatory signals from cell-to-cell—can have diverse pathological consequences, including pathogenesis of cancer (Loewenstein, 1981; Trosko and Ruch, 1998).
Of all the known Cxs, Cx43 and Cx32 have been found to be expressed in endocrine and exocrine glands (Meda, 1996; Munari-Silem and Rousset, 1996). The prostate is an exocrine gland that is comprised predominantly of two types of cells: the epithelial cells, which line the ducts and acini, and the mesenchymal cells, which form the stroma (Cunha et al., 1987). Androgens, apart from regulating the development and differentiation of male reproductive system before birth and during puberty, play a major role not only in the development of normal prostate but also in the survival and maintenance of secretory (differentiation-related) function of luminal epithelial cells of normal prostate because androgen ablation, either by surgical or chemical means, induces apoptosis in these cells (Cunha et al., 1987, 1992). Paradoxically, androgens are also required for the initial development of prostate cancer because their removal triggers apoptosis in malignant prostate epithelial cells, resulting in the regression of prostate tumors. Further progression of prostate cancer is accompanied by the emergence of androgen-independent malignant cells that lose their dependence on the androgens for survival and proliferation (Feldman and Feldman, 2001).
Our previous studies showed that the expression as well as trafficking and assembly of Cx43 and Cx32, the 2 Cxs expressed by the well-differentiated epithelial cells of the normal prostate, are impaired during prostate cancer progression both in vitro and in vivo (Mehta et al., 1999; Govindarajan et al., 2002). We have also shown that the basal epithelial cells in normal prostate express Cx43, whereas luminal cells express Cx32, and that the expression of the latter in the acinar epithelial cells coincides with the acquisition of the differentiated state (Habermann et al., 2001, 2002). Moreover, our studies have documented that the reintroduction of Cx32 and Cx43 in an indolent, androgen-responsive human prostate cancer cell line retards growth and induces differentiation, whereas reintroduction of the same Cxs in an invasive, androgen-independent cell line results in intracellular accumulation with no concomitant effect on growth (Mehta et al., 1999; Govindarajan et al., 2002). On the basis of the above studies, we proposed that the differentiated state of epithelial cells in normal prostate and in prostate tumors might depend on the assembly of Cxs into gap junctions and that the pathways that govern the trafficking and assembly of Cxs into gap junctions are gradually impaired during the progression of human prostate cancer from an androgen-dependent state to an androgen-independent state (Mehta et al., 1999; Govindarajan et al., 2002). Although it is well established that the growth and differentiation of normal and malignant prostate epithelial cells are influenced by the androgens, limited knowledge exists regarding androgen-regulated genes that govern cell growth and differentiation during prostate morphogenesis and oncogenesis (Feldman and Feldman, 2001). Because Cx32 is expressed by the well-differentiated luminal cells of the normal prostate and by the epithelial cells of well-differentiated prostate tumors, we investigated whether the formation and degradation of gap junctions composed of Cx32 are regulated by the androgens in an androgen-responsive human prostate cancer cell line LNCaP.
We used LNCaP cells because they express androgen receptor (AR), require androgens for both in vivo and in vitro growth, express epithelial markers such as cytokeratin 8 and 18, and secrete prostate-specific antigen, which is expressed by the normal prostate luminal epithelial cells (for reviews see Webber et al., 1996; Karan et al., 2001; see also Lin et al., 2001; Igawa et al., 2002). Moreover, these cells have been widely used to study the effect of depletion of androgens on cellular growth and differentiation in vivo and in vitro (Cox et al., 2000; Deeble et al., 2001; Wright et al., 2003). Furthermore, we used these cells because expression of Cx43 and Cx32 as well as of AR is heterogeneous in primary cultures established from normal human prostate and prostate tumors and is lost upon cultivation in vitro, as evidenced by several studies (reviewed in Webber et al., 1996), including ours (Mehta et al., 1996). We show here that in this cell culture model, androgens enhance the formation of gap junctions composed of Cx32 post-translationally, by facilitating its trafficking from the ER to the cell surface. Conversely, Cx32 and gap junctions are degraded upon removal of the androgens. We also present evidence that in the absence of the androgens, Cx32 is degraded either from the ER or immediately upon its exit before reaching Golgi, presumably, via endoplasmic reticulum–associated degradation (ERAD). This effect seems to be gap junction–specific, because degradation of the adherens and tight junction proteins is not significantly affected.
MATERIALS AND METHODS
Cell Culture
Androgen-responsive, early passage LNCaP cells (ATCC CRL 1740) and DU-145 (ATCC CRL HTB-81) were grown in RPMI (GIBCO, Grand Island, NY) containing 5% defined fetal bovine serum (Hyclone, Salt Lake City, UT) in an atmosphere of 5% CO2/95% air, and stock cultures were maintained as previously described (Mehta et al., 1999). During the course of these studies, we used three separate lots of fetal bovine sera obtained from HyClone Laboratories (Salt Lake City, UT) that varied considerably with respect to steroid concentration and hence determined the basal level of Cx expression. The retroviral packaging cell lines PA317 (ATCC CRL 9078), PTi-67 (Clontech Laboratories, San Diego, CA) and Phoenix 293 (a gift from Dr. Michel Ouellette, Eppley Institute) were grown in RPMI containing 5% defined fetal bovine serum as described previously (Mehta et al., 1999). We also used phenol red–free RPMI for experiments in which charcoal-stripped serum (HyClone Laboratories) was used (see below).
Antibodies and Immunostaining
Hybridoma M12.13 (a gift from Dr. Dan Goodenough, Harvard University) and a rabbit polyclonal antibody against Cx43 have been described earlier (Mehta et al., 1991, 1992, 1999; Govindarajan et al., 2002). Rabbit anti-Golgi β coatamer protein (PA1-061), rabbit anti-EEA-1 (PA1-063), rabbit anti-calreticulin (PA3-900), and mouse anti-TGN 38 (MA3-063) were from Affinity BioReagents (Golden, CO). Mouse anti-occludin (clone OC-3F10) was from Zymed Laboratories (South San Francisco, CA). Rabbit anti-α-catenin, rabbit anti-β-catenin, rabbit anti-Cx32, mouse anti-β-actin (clone C-15) were from Sigma (St. Louis, MO). Mouse anti-E-cadherin, mouse anti-α-catenin, mouse anti-β-catenin antibodies were generously provided by Drs. Johnson and Wheelock (Eppley Institute). A rabbit polyclonal anti-AR receptor antibody was from Santa Cruz Biotechnology (sc-13062, San Diego, CA).
Cells were immunostained after fixing with 2% para-formaldehyde for 10 min as described previously (Mehta et al., 1991, 1992, 1999; Govindarajan et al., 2002). Briefly, 1.5 × 105 cells, seeded in six-well clusters containing glass cover slips, were allowed to grow to ∼50% confluence. Cells were immunostained at room temperature with various antibodies at appropriately calibrated dilutions. Secondary antibodies (rabbit or mouse) conjugated with Alexa 488 and Alexa 594 were used as appropriate. Images of immunostained cells were acquired with Leica DMRIE microscope (Leica Microsystems, Wetzler, Germany) equipped with Hamamatsu ORCA-ER CCD camera (Hamamatsu City, Japan). For colocalization studies, serial z-sections (0.5 μm) were collected and analyzed using image-processing software (Openlab 4.0; Improvision, Lexington, MA).
Androgen Depletion and Other Treatments
Cells were seeded in six-well clusters containing glass coverslips (1.5–3 × 105), 6-cm (2 × 105), or 10-cm (3.5 × 105) dishes in 2, 4, and 10 ml complete medium, respectively, and treated when ∼50% confluent, by replenishing with fresh medium containing various reagents at the desired concentration added from stock solutions so that the final concentration of the solvent did not exceed 0.3%. To grow cells under androgen-depleted conditions, normal cell culture medium was replaced with androgen-depleted culture medium consisting of phenol red–free RPMI and charcoal-stripped (steroid-depleted) serum (5%, HyClone Laboratories). The controls received fresh medium containing normal serum. Stock solutions of various reagents were prepared as follows: MG132 from BIOMOL (Plymouth Meeting, PA; Lee and Goldberg, 1998; Goldberg and Rock, 2002) at 10 mM in ethanol; leupeptin (Calbiochem, La Jolla, CA; Mehdi, 1991) at 100 mM in water; monensin from Calbiochem (Tartakoff, 1983) at 10 mM in ethanol; mibolerone (MB; BIOMOL) at 1 mM in ethanol. Lactacystin at 10 mM in water and acetyl-leu-leu-norleucinal (ALLN) at 100 mM (BIOMOL) in DMSO. These solutions were stored at −20°C in small aliquots.
Plasmids, Retroviral Vectors, and Other Recombinant DNA Constructs
Retroviral vectors (pLXSN) containing rat Cx32 and Cx43 in sense orientation were constructed as described (Mehta et al., 1991, 1999). A full-length AR cDNA (a kind gift from Dr. K. Burnstein, University of Miami School of Medicine) was subcloned into EcoRI site of retroviral vector, pMSCV-puro (Clontech Laboratories). Carboxy terminus–deleted mutant rat Cx32, truncated at position 220 (rCx32-T-220), was engineered by PCR-based cloning technique using wild-type rCx32-cDNA cloned into pLXSN vector as a template. The PCR amplification was performed using the forward primer: 5′-GCCGAATTCATGAACTGGACAGGTC-3′, and reverse primer containing a premature stop codon (TGA, Umber) at amino acid position 221: 5′-CCGGAATTCTCAACGGCGGGCACAG-3′. Both the forward and reverse primers contained an EcoRI site as underlined. The PCR products were purified with Qiaquick PCR purification kit (Qiagen, Chatsworth, CA), digested with EcoRI and ligated into the EcoRI site of pLXSN. Plasmid pDsRed-Cx32-N1 was generously provided by Dr. Falk (Lehigh University, Bethlehem, PA). Plasmid pEYFP-N1 was purchased from Clontech.
Retrovirus Production and Infection of Cells
Control and recombinant retroviruses harboring wild-type and mutant Cx and AR cDNAs were produced in either amphotropic packaging cell line PTi67 or in Phoenix 293 cells (Govindarajan et al., 2002). LNCaP and DU-145 cells were multiply infected with various recombinant retroviruses and selected in G418 for 2–3 wk as described previously (Mehta et al., 1999). LNCaP cells overexpressing AR were selected in puromycin (4 μg/ml). Glass cylinders were used to isolate individual G418-resistant clones, which were expanded, frozen, and maintained in G418 (200 μg/ml).
Blockage of Intracellular Protein Transport
The transport of proteins from ER to Golgi was blocked by incubating cells, seeded in six-well clusters containing glass coverslips as described above, at 15°C in appropriate medium buffered with HEPES (pH 7) for 24 h in a CO2 free incubator (Bannykh et al., 1996). The transport of proteins between Golgi compartments was blocked by treating cells for 8 h with 10 μM monensin (Tartakoff, 1983).
RNA Isolation and RT-PCR Analysis
Total RNA was isolated from 50% confluent cultures using TRIzol reagent (Invitrogen, Grand Island, NY) as described previously (Mehta et al., 1992). Equal amount of total RNA (2–4 μg) was reverse-transcribed with M-MuLV reverse transcriptase (New England Biolabs, Beverley, MA) using oligodT primers in a reaction volume of 20 μl. The cDNA obtained was diluted to 100 μl, and equal volume (5 μl) from each reaction was used for PCR analysis. The primers used for PCR analysis were as follows: forward primer 5′-GCGCGAAGTGATCCAGAACC-3′ and reverse primer 5′-CTCCTTGGCGTTGTCAGAAATG-3′ for hAR and forward primer 5′-CAACGGCTCCGGCATGTG-3′ and reverse primer 5′-GGCCAGCCAGGTCCAGACG-3′ for human β-actin cDNA as an internal control. Five-microliter aliquots of PCR reaction were size-separated on a 1.5% agarose gel and stained with ethidium bromide. After electrophoresis, the bands obtained were quantified using Quantity One Software (Bio-Rad, Hercules, CA).
Real-Time PCR Analysis of RNA
Total cellular RNA was extracted under various conditions as described above. cDNA was synthesized using 5 μg total RNA, oligo(dT)18 primer and Superscript RT (Invitrogen). Quantitative real-time PCR was performed using 2 μl of a 1:50 dilution of first-strand cDNA in FastStart DNA Master SYBR Green I (Roche Applied System, Indianapolis, IN) using primers specific to Cx32 and GAPDH in iCycler (Bio-Rad, Hercules, CA). The following forward and reverse primers were used: Cx32: 5′-TCCTGCAGCTCATCCTAGT-3′ and 5′-CCTCAAGCCGTAGCATTTTC-3′; GAPDH: 5′- AGCCTCGTCCCGTAGACAAAA-3′ and 5′-GATGACAAGCTTCCCATTCTCG-3′. The efficiency of the PCR reaction and the specificity of primers were determined using serial dilutions of template cDNA and analyzing the melt curve data. Each cDNA sample was used in duplicate, and a corresponding no cDNA sample was included as a negative control. The amount of PCR product was monitored after each cycle from the fluorescent intensity of double-strand specific SYBR Green I. Cx32 mRNA levels were then normalized to the GAPDH mRNA levels, and results were graphically represented as percentage difference in mRNA level in normal and charcoal stripped conditions.
Western Blot Analysis
Preparation of cell lysates, detergent solubility of Cx32 and Cx43, and Western blot analysis were performed as described (Musil and Goodenough, 1991; Mehta et al., 1996, 1999; VanSlyke and Musil, 2000; Govindarajan et al., 2002) except that cells were lysed in buffer SSK (10 mM Tris, 1 mM EGTA, 1 mM PMSF, 10 mM NEM, 10 mM iodoacetamide, 10 mM NaF, 10 mM Na2VO4, 0.5% Triton X-100 [TX100], pH 7.4) supplemented with protease inhibitor cocktail (Sigma, St. Louis, MO). For detergent solubility assay, the concentration of TX100 was raised to 1% before subjecting to centrifugation at 100,000 × g for 60 min on a tabletop Beckman ultracentrifuge (Model TL-100, Fullerton, CA). The detergent-insoluble pellet was dissolved in buffer C (70 mM Tris/HCl, pH 6.8, 8 M urea, 10 mM NEM, 10 mM iodoacetamide, 2.5% SDS, and 0.1 M DTT). Total, TX100-soluble and -insoluble fractions were mixed with 4× Laemmli buffer to a final concentration of 1× and incubated at room temperature for 60 min before SDS-PAGE analysis.
Separation of Cytosolic and Membrane (Vesicular) Fractions
Cx32-expressing LNCaP cells seeded on 10-cm tissue culture dishes were washed twice with ice-cold 1× PBS (pH 7.4) and scraped in 1 ml of cold rinsing buffer (1× PBS, 1 mM EDTA, 1 mM EGTA, 1 mM PMSF, 10 mM NEM, 10 mM NaF, 10 mM Na2VO4, pH 7.4) supplemented with protease inhibitor cocktail (Aldrich-Sigma, St. Louis, MO). Cells were then spun down and resuspended in 1 ml of cold lysis buffer (10 mM Tris, 1 mM EGTA, 1 mM PMSF, 10 mM NEM, 10 mM NaF, 10 mM Na2VO4, pH 7.4) supplemented with protease inhibitor cocktail. The cells were disrupted by passing 10 times through 22-, 25-, and 27-gauge needles sequentially. A 100-μl aliquot of cell lysate was saved for protein estimation. Protein estimation was performed using BCA method (Pierce, Rockford, IL). To the remaining cell lysate iodoacetamide (10 mM) was added, and the lysate was divided into two equal halves (450 μl each). Ten percent SDS was added to one of the aliquots to a final concentration of 1% and was kept aside as a total fraction. To the remaining 450-μl aliquot, 2.5 M NaCl was added to a final concentration of 250 mM, followed by end-to-end rocking for 30 min at 4°C. The lysate was then separated into cytosolic (supernatant) and membrane (pellet) fractions by centrifugation at 100,000 × g (35,000 rpm in analytical Beckman ultracentrifuge; Model l7-65 using a SW50.1 rotor) for 1 h at 4°C. The pellet was dissolved in 450 μl of buffer C, and 2.5 M NaCl was added to a final concentration of 250 mM. Equal volumes of total, cytosolic, and membrane fractions were resolved by 12% SDS-PAGE and immunoblotted with anti-Cx32 antibody. The blots were reprobed with anti-β-actin antibody as a loading control. Ten micrograms of total protein was subjected to the SDS-PAGE.
Determination of Connexin32 Half-life by Metabolic Labeling and Pulse-Chase Analysis
Connexin32-expressing LNCaP were seeded in 60-mm dishes and grown to 50% confluence. The cells were then grown further for 18 h either in normal serum or in androgen-depleted, charcoal-stripped serum containing 1) MB, 2) lactacystin (100 μM), and 3) ALLN (100 μM). The cells were starved for methionine and cysteine for 30 min in labeling medium (RPMI-1640 without methionine and cysteine), after which the medium was then replaced with fresh 2 ml labeling medium containing tran-35S-label (MP Biomedicals, Irvine, CA) at 100 μCi/ml for 1 h. Cells were rinsed once and chased for various times in RPMI-1640 containing 5% FBS or charcoal-stripped serum supplemented with 0.5 mM nonlabeled methionine and cysteine (chase medium). Labeling and chase media contained MB, or lactacystin, and ALLN as appropriate. Cells were harvested at the indicated time points and lysed in SSK buffer containing 0.6% SDS. Percent incorporation of the metabolic label was determined by subjecting 10-μl aliquots (in triplicate) to trichloroacetic acid (TCA) precipitation and counting in TRI-CARB 2900TR Liquid Scintillation Analyzer (Packard Bioscience, Meriden, CT) using Quantasmart Software One.
Equal TCA-insoluble counts per minute (CPMs) were used for immunoprecipitating Cx32 as described below. Cx32 was immunoprecipitated as described (VanSlyke and Musil, 2000) with minor modifications. Briefly, after lysing cells, the SDS concentration was lowered to 0.1% by adding rinsing buffer (1× PBS, pH 7.4, supplemented with 1 mM EGTA, 1 mM EDTA, 1 mM PMSF, 10 mM NEM, 10 mM Na3VO4, 10 mM NaF, and protease inhibitor cocktail). The lysates were precleared with protein A/G PLUS-Agarose beads (40 μl; Santa Cruz Biotechnology) for 1 h at 4°C. Precleared lysates were then used for immunoprecipitation of connexin32 using anti-rabbit Cx32 antibody (1.5 μg) as described by Musil et al. (1990). Immunoprecipitates were eluted with SDS sample buffer at room temperature for 30 min and analyzed on 12% SDS-PAGE. The gels were processed for fluorography using Amplify (Amersham Bioscience, Piscataway, NJ) following the manufacturer's instructions, dried, and imaged on Typhoon 9410 Variable Mode Imager (Amersham Bioscience). The densitometric quantification was done using AlphaDigiDoc 1201 software.
Transient Transfection
LNCaP cells were seeded at a density of 3 × 105 per well on glass coverslips in six-well clusters. After 24 h cells were transfected using FuGene 6 Transfection Reagent (Roche Diagnostics, Alameda, CA) following manufacturer's instructions or as described (Govindarajan et al., 2002). A transfection efficiency of 20–30% was observed with LNCaP cells.
Communication Assays
Gap junctional communication was assayed by microinjecting the following fluorescent tracers: Lucifer Yellow (MW 443 Da; lithium salt); Alexa Fluor 488 (MW 570 Da; A-10436), and Alexa Fluor 594 (MW 760 Da; A-10438). All Alexa dyes were obtained as hydrazide sodium salts from Molecular Probes (Carlsbad, CA), and their stock solutions were prepared in water at 10 mM. Lucifer yellow was microinjected as 5% aqueous stock solution. These fluorescent tracers were microinjected into test cells by Eppendorf InjectMan and FemtoJet microinjection systems (models 5271 and 5242, Brinkmann Instrument, Westbury, NY) mounted on Leica DMIRE2 microscope as described previously (Mehta et al., 1986, 1999; Govindarajan et al., 2002). The microinjected cells were viewed and images captured with the aid of CCD camera (Retiga 2000R, FAST 1394) using QCapture (QImaging, Burnaby, British Columbia, Canada). The captured images were stored as TIFF files and processed using Corel photopaint (Ottawa, Ontario, Canada). Junctional transfer of fluorescent tracer was quantitated by scoring the number of fluorescent cells (excluding the injected one) from the captured TIFF images either at 1 min (Lucifer Yellow) or at 15 min (Alexa 488 and Alexa 594) as described (Mehta et al., 1986, 1991, 1999).
RESULTS
Retroviral-mediated Expression of Cx32 into LNCaP Cells
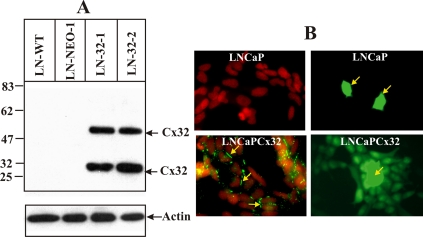
Our previous studies showed that LNCaP cells neither expressed Cx32 and Cx43 nor detectable levels of other known Cxs (Mehta et al., 1996). Because these cells progress from androgen-responsive to androgen-insensitive state upon serial passage in vitro, with the early passage cells responsive to androgens and the late passage cells becoming nonresponsive (Igawa et al., 2002), we introduced Cx32 into early passage LNCaP cells using a Cx32-harboring recombinant retrovirus described previously (Mehta et al., 1999). Expression of Cx32 was confirmed in several randomly isolated G418-resistant clones by Western blot and immunocytochemical and functional analyses, and the representative data from two such clones used in the present study are shown in Figure 1. The data document that these clones express abundant Cx32 (Figure 1A, LN-32-1 and LN-32-2), and assemble it efficiently into functional gap junctions (Figure 1B, bottom panels), compared with the parental cells that neither express Cx32 (Figure 1A, LN-WT and LN-Neo) nor form functional gap junctions (Figure 1B, top panels). Moreover, these clones remain androgen-responsive for at least 100–200 population doublings after their isolation (data not shown).
Figure 1.
Retroviral expression of Cx32 and gap junctional communication in androgen-responsive LNCaP cells. LNCaP cells were infected with the control and Cx32-harboring recombinant retroviruses, and Cx32 expression level and junctional communication were determined by Western blot (A), immunocytochemical (B, left panels) and functional (B, right panels) analyses. (A) Note that only clones infected with Cx32-harboring retroviruses (lanes LN-32-1 and LN-32-2) express Cx32 (a 27-kDa monomer and a 54-kDa dimer), whereas a control clone infected with the control retrovirus (lane LN-NEO-1) and parental, early passage cells (lane LN-WT) do not. (B) Note that Cx32-expressing LNCaP cells form gap junctions (green, bottom left panel, yellow arrows), whereas no gap junctions are detected in control cells (top left panel). The nuclei (red) were stained with DAPI. Note extensive transfer of gap junctional permeable tracer, Lucifer Yellow, in Cx32-expressing LNCaP clone (bottom right panel) and lack of transfer in control cells (top right panel). The microinjected cells are marked by the yellow arrows. For Western blot analysis, 5 μg of total protein was loaded in each lane. After analyzing the expression of Cx32, the blot was stripped and reprobed for β-actin to verify equal loading. The position of the molecular weight markers is indicated on the left.
Androgens Enhance Cx32 Expression Level, Gap Junction Assembly, and Junctional Communication
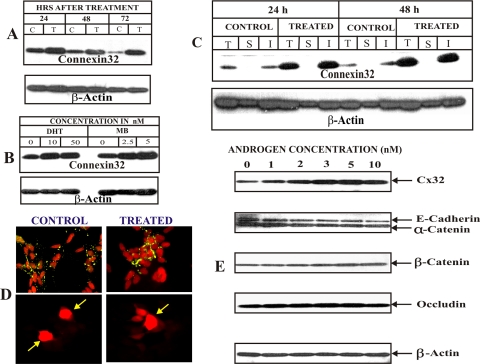
We first investigated the effect of androgens, both natural (testosterone and its more active derivative di-hydrotestosterone, [DHT]) and synthetic androgen, mibolerone (MB), on the expression of endogenous and retrovirally transcribed Cx32 mRNA level by semiquantitative RT-PCR analysis and found that they had no effect both in parental and Cx32-expressing LNCaP cells (data not shown). Previous studies had shown expression of Cx32 to be regulated post-translationally both in vivo and in vitro (Dermietzel et al., 1987; Kren et al., 1993; Kojima et al., 1996), Therefore, we investigated if androgens would regulate gap junction formation and junctional communication by altering Cx32 expression level post-transcriptionally and post-translationally. Figure 2shows the effect of the natural and synthetic analogs of androgens. Testosterone (Figure 2A), DHT and MB (Figure 2B), increased Cx32 expression level in dose-dependent manner, and a significant increase in the expression could be observed up to 72 h after treatment (Figure 2, A and B). Based on three separate experiments, we chose synthetic androgen, MB, for further studies because it increased Cx32 expression level significantly at a much lower, nontoxic concentration (1–5 nM), compared with testosterone and DHT, which produced a significant effect only at higher concentrations (10–50 nM), which seemed cytotoxic (data not shown). Concomitant with an increase in the expression of Cx32, MB significantly increased its assembly into gap junctions as assessed immunocytochemically (Figure 2D, top row, compare right and left panels) and by TX100 insolubility assay (Figure 2C), an assay that reliably measures the assembly of Cxs into gap junctions, as documented by earlier studies (Musil et al., 1990, 1991; VanSlyke and Musil, 2000), including ours (Govindarajan et al., 2002). Moreover, as shown in Figure 2D, enhancement of gap junctional assembly was accompanied by a parallel increase in junctional communication, as assessed by the junctional transfer of three gap junction–permeable fluorescent tracers, Lucifer Yellow, Alexa 488 (MW 570), and Alexa 594 (MW 760). For example, MB increased junctional transfer of Alexa 594 (MW 760) to several neighbors compared with control in which transfer could be barely detected (Figure 2D, see also Table 1).
Figure 2.
Androgens increase Cx32 expression level and gap junction formation. Cx32-expressing LNCaP cells were treated with the androgens as indicated. (A) Kinetics of enhancement of Cx32 level upon testosterone (10 nM) treatment. Note that significant enhancement is observed up to 72 h. (B) Synthetic androgen, mibolerone (MB), is more potent than dihydro-testosterone (DHT) in increasing Cx32 expression level. Cells were treated with various concentrations of DHT and MB for 48 h. Note that MB is at least 4–5 times more potent than DHT on an equimolar basis. (C and D) MB enhances Cx32 expression level and gap junction assembly. Formation of gap junctions was analyzed by detergent insolubility of Cx32 by Western blot analysis (C) and by immunocytochemical analysis (D, top panels) 48 h after treatment with MB (5 nM). Note an increase in size of gap junctions after treatment (compare control and treated, top panels). Note also that increase in Cx32 expression level is accompanied by an increase in detergent-insoluble fraction. (D, bottom) junctional transfer of fluorescent tracer, Alexa 594 (MW 760), was examined as described in Material and Methods. Note extensive junctional transfer to several first-order neighbors upon MB treatment. (E) Androgens have no effect on adherens and tight junction proteins. Cx32-expressing LNCaP cells were treated with various MB concentrations (1–10 nM) for 24 h. Expression of Cx32, adherens junction–associated proteins E-cadherin and α- and β-catenins, and tight junction–associated protein, occludin, was analyzed by Western blot analysis of total cell lysate (5 μg). Detection of E-cadherin and α-catenin was done simultaneously with monoclonal and polyclonal antibodies, respectively. Note that MB increased the expression of only Cx32, but not of adherens and tight junction–associated proteins, at all the concentrations tested.
Table 1.
Effect of androgens and androgen depletion on junctional transfer in Cx32-expressing LNCaP cells
| Junctional tracer | Expt | Junctional transfera | |||
|---|---|---|---|---|---|
| NS | NS+MBb | STRIPc | STRIP+MBc | ||
| LY | 1 | 12.7 ± 3.4 (12) | 24.6 ± 5.7 (16) | ND | ND |
| 2 | 11.2 ± 3.9 (14) | 26.6 ± 4.5 (14) | ND | ND | |
| Alexa 488 | 1 | 13.9 ± 6.3 (15) | 18.1 ± 4.5 (15) | 5.1 ± 4.3 (9) | 28.7 ± 13 (29) |
| 2 | 9.2 ± 4.4 (11) | 15.2 ± 2.5 (12) | 3.1 ± 3.8 (16) | 29.8 ± 09 (29) | |
| Alexa 594 | 1 | 5.7 ± 2.0 (15) | 11.1 ± 2.0 (15) | 0.2 ± 0.7 (9) | 10 ± 4.8 (30) |
| 2 | 2.1 ± 0.7 (10) | 8.3 ± 3.9 (12) | 0.0 ± 00 (16) | 8 ± 4.6 (17) | |
ND, not determined; NS, normal serum. Values are mean ± SD, with the total number of injection trials in parentheses.
a The number of fluorescent cell neighbors 1 min (LY) and 15 min (Alexa 488 and Alexa 594) after injection of the tracer into test cell.
b Cells were treated for 24 h with mibolerone (MB, 5 nM).
c Cells were grown in charcoal-stripped (STRIP) serum for 24 h without or with MB (STRIP+MB).
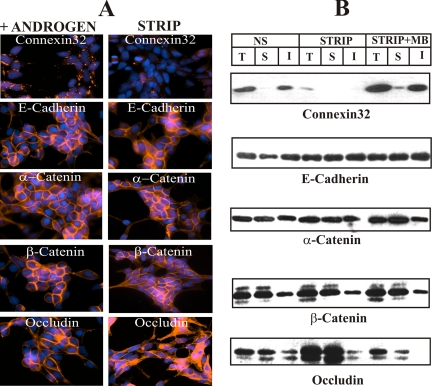
Because recent studies have shown that the protein components of various junctional complexes interact to regulate one another's assembly and disassembly (Braga, 2002; Balda and Matte, 2004), we examined whether MB increased the expression of Cx32 and its assembly into gap junctions indirectly, by altering the expression level of other cell junction–associated proteins. We found that MB had no significant affect on the expression level of occludin and adherens junction proteins E-cadherin and α- and β-catenins (Figure 2E). Taken together, these data suggest that the androgens enhance Cx32 expression level post-translationally, with concomitant increase in gap junction assembly, without significantly altering the expression of adherens and tight junction–associated proteins.
Androgen Depletion Decreases Cx32 Expression Level, Gap Junction Assembly, and Junctional Communication
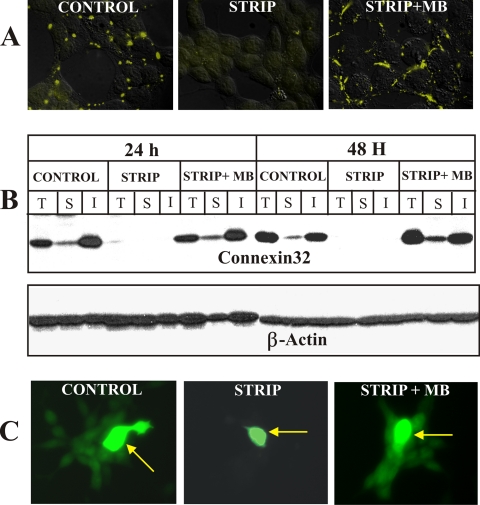
Prompted by the data shown in Figure 2, we next investigated the expression level of Cx32 and its subcellular fate in LNCaP cells upon androgen depletion. Androgens were removed from the serum by charcoal stripping. As assessed immunocytochemically and by Western blot analysis of TX100-soluble and -insoluble fractions, we found that androgen depletion decreased Cx32 expression level and gap junction assembly, which was prevented upon replenishing androgens (Figure 3A, compare left, middle, and right panels, and B). Temporal analysis revealed that a significant decrease was observed as early as 4 h after androgen depletion and was cell density dependent. In sparse cultures, a conspicuous decrease was observed within 8–12 h after androgen removal, whereas in confluent cultures at least 12–16 h were required to observe a significant effect (data not shown). Based on the densitometric scanning analyses of 10 randomly chosen blots, androgen depletion consistently decreased the expression level of Cx32 by 70–90% (see Figure 6C). To examine if decreased expression level of Cx32 and gap junction assembly reduced gap junctional communication, we measured junctional transfer of Lucifer Yellow (MW 443 Da), Alexa 488 (MW 570 Da), and Alexa 594 (MW 760 Da). As shown in Figure 3C, androgen depletion decreased junctional transfer of Lucifer Yellow significantly, which was prevented upon replenishing the charcoal-stripped medium with MB. Androgen depletion also had similar effect on the junctional transfer of both Alexa 488 and Alexa 594 (see Table 1). As a control, depletion of androgens had no effect on the junctional transfer of these tracers in Cx32-lacking parental LNCaP cells (data not shown). These data suggest that androgen depletion decreases Cx32 expression level and gap junction formation, with concomitant decrease in function.
Figure 3.
Androgen depletion decreases Cx32 expression level, gap junctions, and junctional communication. Cx32-expressing LNCaP cells were switched to charcoal-stripped (STRIP) medium. Formation of gap junctions was analyzed by immunocytochemical analysis (A) after 48 h, by Western blot analysis of total, detergent-soluble and -insoluble fractions (B), and by junctional transfer of Lucifer Yellow (C). Note that Cx32 and gap junctions are degraded in charcoal-stripped serum (STRIP), and degradation is prevented upon replenishment of 5 nM MB (STRIP+MB). Note also the larger size of gap junctions in cells treated with MB. Note also lack of junctional transfer of Lucifer Yellow in cells grown under charcoal-stripped serum.
Figure 6.
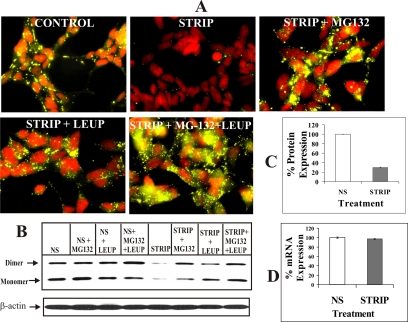
Cx32 degrades via the proteasomal and lysosomal pathway upon androgen depletion. Cx32-expressing LNCaP cells, seeded in six-well clusters containing glass coverslips for immunocytochemical analysis (A), in 10-cm dishes for Western blot analysis (B and C), and in 6-cm dishes for RT-PCR analysis (D), were allowed to grow to 50% confluence. Cells were then further grown for 24 h in normal serum (CONTROL), in charcoal-stripped serum alone (STRIP), or in charcoal-stripped serum supplemented with MG132 (20 μM; STRIP+MG132), leupeptin (50 μM; STRIP+LEUP), and MG132 and leupeptin (STRIP+MG132+LEUP). Degradation and subcellular localization of Cx32 were analyzed by immunocytochemical (A) and Western blot (B) analyses as described in Materials and Methods. (A) Note that Cx32 and gap junctions are degraded in charcoal-stripped serum, and there is no intracellular accumulation, and degradation is prevented by MG132 and leupeptin, with combined treatment resulting in higher intracellular level. (B) Western blot analysis of total cell lysates. Note that Cx32 is degraded in charcoal-stripped serum (STRIP), and degradation is prevented by MG132 (STRIP+MG132) and leupeptin (STRIP+LEUP), and combined treatment is additive (STRIP+MG132+LEUP). (C and D) Cx32 mRNA and protein levels were measured by quantitative real-time PCR analysis (D) and by quantifying Western blots (C) as described in Material and Methods. Total RNA was extracted from Cx32-expressing cells grown in normal serum (NS) or charcoal-stripped serum for 24 h (STRIP). Note that the Cx32 mRNA levels did not change significantly, but protein levels decreased by ∼70%. NS, normal serum; LEUP, leupeptin; STRIP, charcoal-stripped serum.
Androgen Receptor (AR) Regulates Cx32 Expression Level and Gap Junction Formation
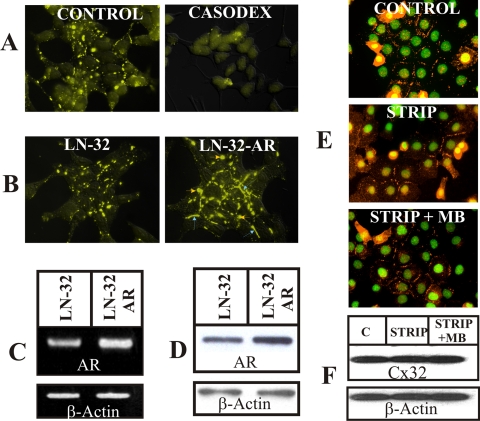
To investigate if androgens enhance Cx32 expression level by acting through AR—and not through nongenotropic mechanisms, we undertook three experimental approaches. In the first approach, we treated Cx32-expressing LNCaP cells with an anti-androgen, bicalutamide (Casodex, AstraZeneca Pharmaceuticals, Newark, DE), which inhibits AR signaling (Iversen, 2002). In the second approach, we overexpressed AR in Cx32-expressing LNCaP cells by infecting them with AR-containing recombinant retrovirus and isolated polyclonal cultures. In the third approach, we introduced Cx32 into another human prostate cancer cell line, DU-145, which lacked AR. Bicalutamide (Casodex) decreased Cx32 expression level and gap junctions in charcoal-stripped medium supplemented with MB (Figure 4A), as well as in normal serum (data not shown). The size of gap junctions increased in polyclonal cultures of Cx32-expressing LNCaP cells that overexpressed AR (Figure 4, B–D). Androgen depletion had no significant effect on Cx32 expression level and gap junction formation in AR-lacking DU-145 cells (Figure 4, E and F). These data suggest that androgen-mediated activation of AR, and not androgen-mediated nongenotropic effect, determined the expression level of Cx32 and gap junction formation.
Figure 4.
Androgen receptor mediates increase in Cx32 expression. (A) Cx32-expressing LNCaP cells were grown in charcoal-stripped serum containing 5 nM MB in the presence (top right panel) and absence (top left panel) of 10 μM Casodex (bicalutamide) for 24 h. Note absence of gap junctions in cells grown in Casodex. (B) Androgen-receptor (AR) was overexpressed in Cx32-expressing LNCaP cells using AR-harboring retrovirus as described in Materials and Methods. Formation of gap junctions (yellow) was studied immunocytochemically. Note increase in size of gap junctions in cells expressing higher level of AR (labeled LN-32-AR, right panel) compared with those expressing basal level (labeled LN-32, left panel). (C and D) AR mRNA (C) and protein (D) were determined semiquantitatively by RT-PCR and Western blot analysis in Cx32-expressing LNCaP cells (labeled LN-32) and in polyclonal cultures after infection with AR containing retrovirus (labeled LN-32-AR). Note the higher level of AR mRNA (C) and protein (D) in infected polyclonal cultures. (E and F) Cx32-expressing, AR-negative, DU-145 cells were grown in charcoal-stripped serum (STRIP) in the presence and absence of MB for 24 h. Cx32 expression level and gap junctions were analyzed immunocytochemically (E) and by Western blot analysis (F). Cells grown in normal serum were used as controls. Note lack of Cx32 and gap junction degradation upon androgen depletion.
Androgen Depletion and Other Junctional Complexes
To test if androgen depletion affected components of other cell junctions, we determined the expression level and detergent solubility of adherens and tight junction–associated proteins. Figure 5 shows that the expression level and detergent solubility of adherens junction–associated proteins (E-cadherin and α- and β-catenins) and the tight junction–associated protein, occludin, did not decrease significantly (Figure 5B). Moreover, these proteins remained localized at cell-to-cell contact areas (Figure 5A). It is worth noticing that although the total level of occludin did not decrease, androgen depletion decreased its detergent-insoluble fraction, with a concomitant increase in the soluble fraction (Figure 5B), leading to its intracellular accumulation (Figure 5A, compare bottom right and left panels). Taken together, the data suggest that among various junctional complexes studied, only the expression level of Cx32 and gap junction formation seemed to decrease significantly upon removal of androgens.
Figure 5.
Androgen depletion does not affect degradation of adherens and tight junction–associated proteins. Cx32-expressing LNCaP cells, grown either in six-well clusters or 10-cm dishes, were switched to charcoal-stripped (STRIP) medium. After 48 h, degradation of Cx32 and formation of gap junctions and that of tight and adherens junction–associated proteins occludin and E-cadherin and α- and β-catenins were assessed immunocytochemically (A) and biochemically by TX100 insolubility assay (B) as described in Materials and Methods. Note that the expression level of only Cx32 decreased in charcoal-stripped (STRIP) serum. Note also that the expression level of E-cadherin and α- and β-catenins as well as of occludin did not decrease in the presence and absence of androgens (B) and these proteins remained localized at cell–cell contact areas (A). T, total; S, TX100-soluble fraction; I, TX100-insoluble fraction.
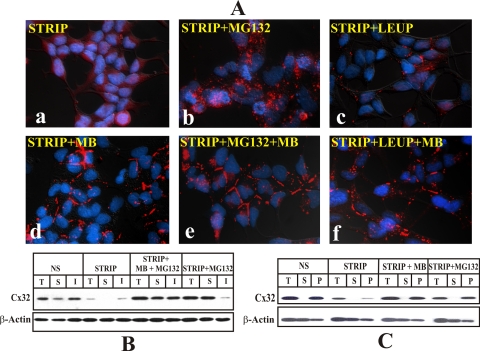
Androgen Depletion Triggers Degradation of Cx32 in the Proteasome and Lysosome
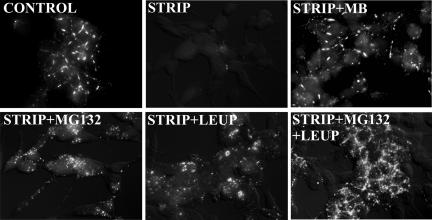
We next investigated the mechanism by which androgen depletion decreased the expression level of Cx32. Because Cxs have been shown to be degraded by the proteasomal and lysosomal pathways (Laing and Beyer, 2000; VanSlyke et al., 2001; VanSlyke and Musil, 2002), we investigated if Cx32 was degraded by these pathways upon androgen depletion. For these analyses, we switched LNCaP cells to charcoal-stripped medium in the presence of MG132, an inhibitor of the proteasomal pathway (Goldberg and Rock, 2002), and leupeptin, an inhibitor of lysosomal pathway (Mehdi, 1991). Treatment with both inhibitors prevented the decline in Cx32 expression level upon androgen depletion as assessed by immunocytochemical (Figure 6A) and Western blot (Figure 6B) analyses. Moreover, a major proportion of Cx32 accumulated intracellularly and no immunocytochemically detectable gap junctions were observed (Figure 6A). Furthermore, combined treatment with MG132 and leupeptin resulted in higher intracellular accumulation than treatment with either inhibitor alone (Figure 6, A and B). These data suggest that upon androgen depletion Cx32 was degraded both by the proteasomal and lysosomal pathways and that the effect of these inhibitors was additive, raising the possibility that the inhibitors acted on different Cx pools. We also determined the level of Cx32 mRNA (Figure 6D) and protein (Figure 6C) in normal medium and in charcoal-stripped medium to test if androgen depletion altered its transcription from retroviral long-terminal repeats (LTRs) by quantitative real-time PCR and Western blot analyses. Figure 6, C and D, shows that whereas the retroviral Cx32 mRNA level was not significantly affected by the androgens, protein levels decreased by ∼70%. Taken together these data suggest that androgen depletion alters the expression level of Cx32 by regulating its degradation in the proteasome and lysosome post-translationally—and not by altering Cx32 mRNA level transcribed from the viral promoter.
Androgen Depletion Degrades Cx32 Before its Assembly into Gap Junctions
The half-life of Cxs has been estimated to be 2–5 h in vivo and in vitro (Musil and Goodenough, 1991; VanSlyke and Musil, 2000; VanSlyke et al., 2001). Previous studies showed that up to 40–50% of newly synthesized pool of Cxs was degraded by ERAD in the proteasome (VanSlyke et al., 2001; VanSlyke and Musil, 2002), whereas the cell surface and gap junction–associated pools were degraded in the lysosome (VanSlyke and Musil, 2005). Therefore, we investigated whether androgen depletion caused degradation of only intracellular pool of Cxs or the cell surface and gap junction–associated pool. For these investigations, Cx32-expressing LNCaP cells were switched to charcoal-stripped serum for 24 h, which depleted most of pre-existing intracellular and the cell surface–associated pools (both junctional and nonjunctional) of Cx32 (Figure 7). Cells were then grown for an additional 24 h as follows: 1) in charcoal-stripped serum alone, 2) in charcoal-stripped serum containing MG132, leupeptin or MB alone, and 3) in charcoal-stripped serum supplemented with both MB and MG132 or leupeptin. Degradation, subcellular localization and detergent solubility of Cx32 were then analyzed by immunocytochemical and Western blot analyses. Figure 7 shows the representative data from one of five such experiments. No significant intracellular or junctional immunostaining was noticed when cells were grown for an additional 24 h in charcoal-stripped serum in the absence (Figure 7Aa) or presence of leupeptin (Figure 7Ac) because Cx32 did not form gap junction or a cell surface–associated pool whose degradation was leupeptin-sensitive. However, substantial intracellular accumulation was observed when cells were grown in charcoal-stripped serum containing MG132 and only then (Figure 7Ab). Moreover, gap junctions were observed only in cells treated with either MB alone or with MB and MG132 or leupeptin (Figure 7A, d–f). Furthermore, the combined effect of MB and MG132 was not additive (Figure 7A, compare gap junctional staining in panel d with panel e), indicating that MB rerouted MG132-sensitive pool of Cx32 to the cell surface for gap junction assembly. Some intracellular accumulation of Cx32 was observed in cells grown in charcoal-stripped serum containing MB and leupeptin (Figure 7Af). This most likely represents a pool of Cx32 that was assembled into gap junctions in the presence of MB to form leupeptin-sensitive pool targeted to lysosomal degradation (VanSlyke and Musil, 2005). Consistent with the immunocytochemical data, a corresponding increase in the Cx32 detergent-insoluble fraction was observed in cells grown in charcoal-stripped serum containing MG132 and MB compared with MG132 alone (Figure 7B). These data clearly indicate that MB facilitates gap junction assembly of MG132-sensitive pool.
Figure 7.
Androgen depletion degrades Cx32 before its assembly into gap junctions. (A and B) Cx32-expressing LNCaP cells were seeded for immunocytochemical (A) and Western blot (B) analyses as in Figure 6. Cells were switched to charcoal-stripped conditions for 24 h to deplete the pre-existing pool of Cx32. Cells were then grown for an additional 24 h either in charcoal-stripped serum alone (STRIP) or in charcoal-stripped serum supplemented with MG132 (STRIP+MG132), leupeptin (STRIP+LEUP), MB alone (STRIP+MB), or with MG132 (STRIP+MG132+MB) or leupeptin (STRIP+LEUP+MB). Degradation, subcellular localization and detergent solubility of Cx32 was studied by immunocytochemical and Western blot analyses as described in Materials and Methods. (A) Note that no significant intracellular Cx32 (red) is observed in cells grown in charcoal-stripped serum (a) or charcoal-stripped serum with leupeptin (c). Note that extensive intracellular accumulation (red puncta) was observed only in cells grown in charcoal-stripped serum containing MG132 (b). Note that gap junctional immunostaining was observed in cells grown in MB in the presence (e) or absence of MG132 (d) and leupeptin (f). Note also that gap junctional staining was observed upon treatment of charcoal-stripped cells with MB (d) or with MB and MG132 (e), and there was no intracellular accumulation. (B) Note also that significant increase in the detergent-insoluble fraction of Cx32 was observed in cells grown in charcoal-stripped serum in the presence of MB and MG132 (STRIP+MB+MG132) compared with MG132 alone (STRIP+MG132). (C) Intracellularly accumulated Cx32 resides in the membrane/vesicular fraction upon proteasomal inhibition. Cx32-expressing LNCaP cells were switched to charcoal-stripped serum for 24 h. Cells were further grown for 24 h in charcoal-stripped serum alone (STRIP) and charcoal-stripped serum supplemented with MB (STRIP+MB) or MG132 (STRIP+MG132). Cells maintained in normal serum were used as controls. Total (T), membrane (P), and cytosolic (S) fractions were subjected to Western blot analysis as described in Materials and Methods.
Because intracellularly accumulated Cx32 appeared as discrete spots of variable sizes that seemed to be distributed throughout the cytoplasm (Figure 7Ab), we examined its subcellular fate. Colocalization experiments revealed that Cx32, which was rescued upon proteasomal inhibition after androgen depletion, did not reside in the ER, lysosome, Golgi, and trans-Golgi network, as assessed by confocal microscopy using specific markers for these compartments (data not shown). Because Cx43 and Cx32 have been recovered in an intact soluble form from the cytosol upon inhibition of proteasomal function (VanSlyke and Musil, 2002, 2005), we investigated if intracellularly accumulated Cx32 resided in the cytosol or in some vesicular compartment. For these investigations, we separated membrane and cytosolic fractions from cells, which had been grown in normal and charcoal-stripped serum in the presence and absence of MB or MG132, by centrifuging the extracts at 100,000 × g and determined the Cx32 level in the pellet and supernatant by Western blot analysis (see Materials and Methods). A representative Western blot is shown in Figure 7C. As assessed by the densitometric scanning analysis of three blots, we found that between 80 and 90% of intracellularly accumulated Cx32 remained in the pellet (membrane/vesicular fraction) and was not present in the supernatant that represented the cytosolic fraction. Taken together, these results show that in the absence of androgens, a major portion of Cx32 is degraded before its assembly into gap junctions, and degradation involves an as yet unidentified intermediate compartment.
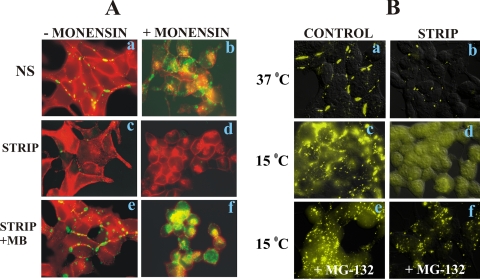
Androgen Depletion Triggers Cx32 Degradation En Route to Golgi
In the next series of experiments, we investigated if Cx32 was degraded directly from the ER or en route to the cell surface from the Golgi or trans-Golgi network upon inhibition of proteasomal function. We used brefeldin, which inhibits ER-to-Golgi transport (Klausner et al., 1992; Chardin and McCormick, 1999), and monensin, which inhibits transport from the cis-Golgi to the medial Golgi compartment (Tartakoff, 1983). The data from several pilot experiments in LNCaP cells showed that brefeldin did not block the transport of Cx32 from ER to Golgi effectively. However, we found that ER-to-Golgi transport could be effectively blocked by incubating cells at 15°C (Bannykh et al., 1996; Hauri et al., 2000), whereas transport from cis-to medial-Golgi could be blocked with monensin.
To determine the site of degradation of Cx32 upon androgen depletion, we first switched LNCaP cells to charcoal-stripped serum for 24 h to deplete its pre-existing intracellular and cell surface–associated (junctional and nonjunctional) pools. The cells were then grown for an additional 24 h in charcoal-stripped serum in the presence and absence of androgens and treated with monensin during the last 8 h. Cells that were continuously grown in normal serum or charcoal-stripped medium supplemented with MB without monensin were used as controls. The degradation and the subcellular localization of Cx32 were analyzed by immunocytochemical analysis. Figure 8A shows the representative data from one of three such independent experiments. We found that in stark contrast to E-cadherin, Cx32 was degraded and did not accumulate intracellularly under androgen-depleted conditions (Figure 8A, c and d) even when intra-Golgi transport was blocked by monensin for 8 h (Figure 8Ad). On the other hand, in control cells (Figure 8A, a and b) or androgen-treated cells (Figure 8A, e and f), substantial intracellular accumulation of both Cx32 and E-cadherin was observed upon monensin treatment (Figure 8A, b and f). These data suggest that Cx32 is degraded before the site of monensin action, i.e., in the early secretory pathway comprised of ER and cis-Golgi, and is rescued from degradation by the androgens.
Figure 8.
Androgen depletion triggers Cx32 degradation en route to Golgi. (A) Cx32-expressing LNCaP cells, seeded on glass coverslips and grown at 37°C for 3 d, were switched to charcoal-stripped serum for 24 h at 37°C to deplete the pre-existing pool of Cx32. Control cells were maintained in normal serum (NS, a and b), and charcoal-stripped cells were further grown for 24 h in charcoal-stripped serum (STRIP, c and d) and in charcoal-stripped serum supplemented with MB (STRIP+ MB, e and f). Monensin (10 μM) was used to block intra-Golgi transport for the last 8 h (+MONENSIN) and the vehicle treated cells were used as control (−MONENSIN). Note that Cx32 (green) does not accumulate in the intra-Golgi compartment under androgen-depleted conditions upon monensin treatment, whereas E-cadherin (red) accumulates (see d). Note also that substantial intra-Golgi accumulation of both Cx32 and E-cadherin is observed in the presence of monensin in control or MB-treated cells (b and f). (B) Cx32-expressing LNCaP cells, seeded on glass coverslips and grown at 37°C for 3 d, were maintained in normal serum (a) or were switched to charcoal-stripped serum for 24 h at 37°C to deplete the pre-existing pool of Cx32. Control and charcoal-stripped cells were then further grown at 37°C (a and b) or at 15°C (c–f) for an additional 24 h in normal serum (c and e) or in charcoal-stripped serum (b, d, and f) in the absence (c and d) and presence of MG132 (e and f). Subcellular localization of Cx32 was studied by immunocytochemical analysis. Note also that at 15°C, no intracellular immunostaining of Cx32 is observed in charcoal-stripped serum (d). The degradation is prevented by MG132 (f).
To determine further the site of degradation, cells were grown in charcoal-stripped serum for 24 h to deplete the pre-existing pool of Cx32 as described above. These cells were then grown for an additional 24 h at 37 or 15°C with or without MG132 in medium containing normal or charcoal-stripped serum. Subcellular localization of Cx32 was determined immunocytochemically. Figure 8B shows the representative data from one of five such experiments. The data show that at 15°C, Cx32 accumulated intracellularly in cells grown in normal serum (Figure 8Bc) but was degraded in cells grown in charcoal-stripped serum for 24 h (Figure 8Bd). The degradation was substantially prevented when cells were grown in the presence of MG132 at 15°C in charcoal-stripped serum (Figure 8Bf). These data suggest that upon androgen depletion, a major fraction of Cx32 is degraded either from the ER itself or from the ER-to-Golgi intermediate compartment.
Androgen-regulated ERAD of Cx32
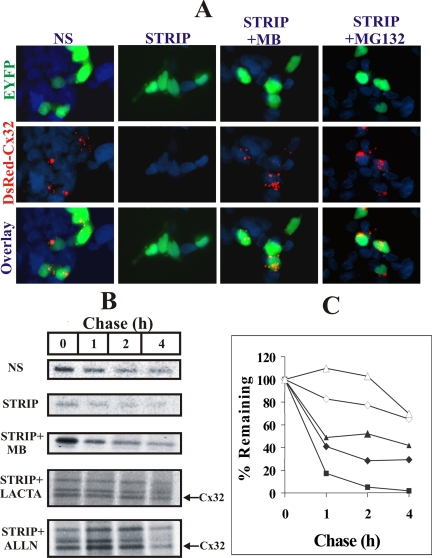
To substantiate the above data, we undertook two kinds of experimental approaches. In the first approach, we transfected Cx32-expressing LNCaP cells transiently with DsRed-Cx32, which remains trapped in the ER (Lauf et al., 2001), together with pEYFP-N1. Twelve-hours after transfection, medium was replaced and cells were switched to normal and charcoal-stripped serum with or without MB or MG132. Degradation of ER-trapped DsRed-Cx32 was assessed 24 h later by immunocytochemical analysis (Figure 9 legend). As shown in Figure 9A, we found that ER-trapped DsRed-Cx32 was degraded effectively upon androgen depletion (Figure 9A, compare column 1 with column 2), and the degradation was prevented by MB (Figure 9A, column 3) or MG132 (Figure 9 A, column 4). In the second approach, we analyzed the metabolic turnover of Cx32 under normal condition as well as androgen-depleted condition in the presence and absence of androgens and proteasomal inhibitors lactacystin and ALLN. Cells were metabolically labeled and chased for 1–4 h in the presence of indicated treatments (see Materials and Methods, Figure 9 legend). As shown in Figure 9, B and C, compared with control conditions (NS), a major fraction (>80%) of Cx32 degraded rapidly under androgen-depleted conditions (STRIP) within 1 h, presumably by ERAD, whereas the remaining fraction (<20%), most likely representing a fraction that escapes ERAD, degraded at a slower rate, consistent with its normal trafficking and degradation. Moreover, a significant fraction of Cx32 destined for ERAD was rescued upon androgen replenishment (STRIP+MB). Furthermore, lactacystin (STRIP+LAC) and ALLN (STRIP+ ALLN) also prevented degradation. We also consistently observed that lactacystin and ALLN treatment caused the emergence of two additional Cx32 forms that migrated slower on SDS-PAGE and whose degradation was also prevented by lactacystin and ALLN. These additional forms of Cx32, most likely resulting from the post-translational modifications, remain to be further characterized. Taken together, these data document that androgen depletion triggers degradation of a major fraction of Cx32 from the ER by ERAD in a proteasomal inhibitor-sensitive manner.
Figure 9.
Androgen-regulated ERAD of connexin32. (A) Wild-type LNCaP cells were transfected transiently with DsRed-Cx32 (DsRed-Cx32) and pEYFP-N1. After 12 h, cells were switched to normal serum (NS) and charcoal-stripped serum (STRIP) containing MB (STRIP+ MB) or MG132 (STRIP+MG132). Degradation of DsRed-Cx32 was assessed 24 h later by immunocytochemical analysis. Note degradation of ER-trapped DsRed-Cx32 upon androgen depletion (compare column 1 with column 2). Note also that the degradation is prevented by MB (column 3) or MG132 (column 4). (B) Metabolic turnover of Cx32 was analyzed in normal serum and in charcoal-stripped serum containing MB, lactacystin (10 μM), and ALLN (100 μM) as described in Materials and Methods. Between 1–5 × 106 TCA-insoluble counts were immunoprecipitated at indicated time points (0–4 h). Note that compared with normal serum (NS, lanes 1–2) a major fraction (>80%) is degraded within 1 h of chase in charcoal-stripped serum (STRIP, lanes 1 and 2). Replenishment of androgens (STRIP+MB, lanes 1–4) decreases the rate of degradation. Also Cx32 levels are not significantly altered in charcoal-stripped serum containing either lactacystin (STRIP+LACTA, lanes 1–4) or ALLN (STRIP+ALLN, lanes 1–4). Note also that two slower migrating forms of Cx32 detected in lactacystin- and ALLN-treated cells most likely represent post-translational modifications that result before inhibition of proteasomal function or immediately thereafter. (C) Plot of the time course of pulse-labeled Cx32 turnover during the 4-h chase period. The plot was drawn from the data obtained from the densitometric analyses of the typical pulse-chased blots using AlphaDigiDoc 1201 software. Each experiment was repeated twice with qualitatively similar results. Note that Cx32 has a significantly shorter half-life in androgen-depleted, charcoal-stripped serum, and degradation is prevented by proteasomal inhibition. ♦, NS; ▴, STRIP+MB; ■, STRIP; ◇, STRIP+LACTA; ▵, STRIP+ALLN.
Androgens and Degradation of Cx43
To investigate if androgens also regulate the degradation of other members of the Cx gene family, androgen-responsive early passage LNCaP cells were infected with the recombinant retrovirus containing Cx43. Connexin43 has a low sequence similarity to Cx32 (Kumar and Gilula, 1996). After isolating several independent clones and characterizing them for the expression, we studied the degradation of Cx43 in one well-characterized clone in the presence and absence of androgens. As shown in Figure 10, androgen depletion degraded Cx43, which was prevented by replenishment of MB and MG132 and leupeptin; moreover, the combined effect was additive. These findings suggest that Cx43 also degrades upon androgen depletion, most likely, by a mechanism similar to that involved in the degradation of Cx32 despite sequence dissimilarity.
Figure 10.
Degradation of Cx43 upon androgen depletion. Cx43-expressing LNCaP cells were seeded in six-well clusters and allowed to grow to confluence as described in Figure 6. Cells were then further grown for 24 h in normal serum (CONTROL, top left), in charcoal-stripped serum (STRIP, top middle), or in charcoal-stripped serum containing MB (STRIP+MB, 5 nM, top right), MG132 (20 μM; STRIP+MG132, bottom left), leupeptin (50 μM; STRIP+LEUP, bottom middle), and MG132 and leupeptin (STRIP+MG132+LEUP, bottom right). Expression levels of Cx43 and gap junctions were analyzed by immunocytochemical analysis as described in Materials and Methods. Note that Cx43 and gap junctions are degraded in charcoal-stripped serum, and there is no intracellular accumulation. Note also that MB prevents the degradation of Cx43 and facilitates gap junction formation. Also, degradation is prevented by MG132 and leupeptin, and combined treatment results in a higher intracellular level.
Cx32 Carboxyl Terminal Tail Does Not Mediate Degradation
Despite a large body of evidence that Cxs degrade via the lysosomal and proteasomal pathways (Laing and Beyer, 2000; VanSlyke et al., 2001; Segretain and Falk, 2004; Laird, 2005), it is as yet unknown which motifs control sorting of Cxs to lysosomes or proteasomes. Because both Cx32 and Cx43 were degraded similarly upon androgen depletion and because carboxyl termini of most members of the Cx gene family are highly divergent, we reasoned that these termini are unlikely to harbor sequence motifs that determine degradation upon androgen depletion. Hence, we examined the degradation pattern of a carboxy terminus–deleted mutant of Cx32, designated as Cx32-T-220 (aa 221–283 deleted) upon androgen depletion. This mutant lacks all the consensus phosphorylation sites as well as 9 of 12 lysines that are likely to be ubiquitinated (Laird, 2005).
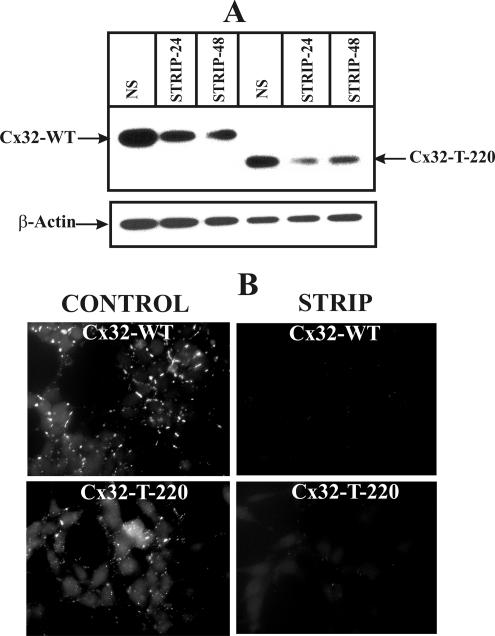
Androgen-responsive early passage LNCaP cells were infected with the recombinant retroviruses containing the wild-type and carboxy terminus–deleted mutant, and several independent clones were isolated and characterized for the expression and degradation upon androgen depletion. As shown in Figure 11, we found that the Cx32-T-220 mutant, like its full-length counterpart, was also degraded upon androgen depletion as assessed by immunocytochemical (Figure 11B) and Western blot analyses (Figure 11A). Gap junctions composed of this mutant, however, appeared to be smaller compared with those formed by the full length Cx32. These data suggest that the carboxy terminus of Cx32 is dispensable for degradation and is unlikely to harbor motifs that determine its degradation upon androgen depletion. Collectively, the degradation of Cx43 and carboxy terminus deleted Cx32 upon androgen depletion indicate that the molecular determinants are likely to reside in the cytoplasmic loops or in domains that are conserved between Cxs. Our ongoing studies are directed toward identifying domains in Cxs that confer sensitivity toward degradation upon androgen depletion.
Figure 11.
The carboxy terminus of Cx32 is not involved in the degradation upon androgen depletion. (A and B) LNCaP cells expressing full length Cx32 (Cx32-WT) and the carboxy terminus deleted mutant (Cx32-T-220) were switched to charcoal-stripped serum for 24–48 h, and degradation of gap junctions was analyzed by immunocytochemical (B, 24 h) and Western blot (A) analyses. Note that, upon androgen depletion, wild-type and mutant Cx32, as well as gap junctions composed of them, are degraded. Note also that gap junctions composed of mutant Cx32 are smaller.
DISCUSSION
In this study we show that androgens control the expression level of Cx32—and hence the extent of gap junction formation—post-translationally in androgen-responsive human prostate cancer cells in which the assembly of Cxs into gap junctions is highly efficient. In the absence of androgens, a major fraction of Cx32 is degraded from ER, presumably by ERAD, whereas in their presence, this fraction is rescued from degradation. Thus, androgens seem to regulate the formation and degradation of gap junctions in a novel way: by rerouting the pool of Cx32, which normally would have been degraded from the early secretory compartment, to the cell surface, and enhancing the cell surface–associated pool amenable for gap junction assembly. Moreover, we also show that Cx32 and Cx43 degrade by a similar mechanism and that degradation does not involve the entire cytoplasmic tail of Cx32. The significance of these data are further underscored by the lack of appreciable effect of androgens on the degradation of adherens junction–associated proteins, E-cadherin and α- and β-catenins, and tight junction–associated protein, occludin. Thus, in this model system, androgen-regulated proteasome—and not the lysosome—mediated degradation of Cx32 appeared to be the major control point in modulating the formation and degradation of gap junctions.
The present study was prompted by three independent lines of inquiry. First, previous findings had shown that Cx32 was expressed by the well-differentiated cells of the liver (Dermietzel et al., 1987; Stutenkemper et al., 1992; Kojima et al., 1996, 2001) and cells derived from other hormone-responsive tissues, such as pancreas and thyroid, where gap junctional communication had been documented to play an important role in regulating differentiation (Munari-Sliem et al., 1991; Munari-Sliem and Rousset, 1996; Meda, 1996; Guerrier et al., 1995; Stock et al., 1998). Second, we had previously shown that Cx32 was expressed by the well-differentiated epithelial cells of the prostate and prostate tumors and that its expression coincided with the morphological, histological and cellular (secretory) differentiation of the prostate (Mehta et al., 1999; Habermann et al., 2001, 2002). Third, consistent with the tumor suppressing and prodifferentiating role of Cx32 in vivo and in vitro (Mehta et al., 1999; King and Lampe, 2004, 2005; King et al., 2005), and on the basis of the prodifferentiating role of androgens in prostate morphogenesis (Cunha et al., 1992), we reasoned that androgens might regulate its assembly into gap junctions.
Multiple lines of evidence suggested that in the absence of androgens a major proportion of Cx32 was degraded from the ER en route to the Golgi in a proteasomal inhibitor-sensitive manner. First, in the absence of androgens, Cx32 was barely detectable by immunocytochemical analysis at 37°C, or at 15°C, when its transport from ER to Golgi was blocked (Figure 8B). Second, inhibition of proteasomal function at 15°C under androgen-depleted conditions enhanced expression level of Cx32 and caused its intracellular accumulation (Figure 8B). Third, ER exit of Cx32 and its subsequent accumulation in the cis-Golgi upon monensin block was observed only when cells were grown in androgen-containing medium (Figure 8A). Fourth, a DsRed-Cx32 chimera that remained trapped in the ER was also degraded upon androgen depletion and its degradation was prevented by MG132 (Figure 9A). Fifth, pulse chase analysis of metabolically labeled Cx32 indicated that it degraded rapidly soon after its synthesis in cells grown in charcoal-stripped (androgen-depleted) serum in stark contrast to cells grown in normal serum and in charcoal-stripped serum supplemented with the androgen or lactacystin or ALLN. Finally, inhibiting lysosomal function under androgen-depleted conditions prevented the degradation of only cell surface and gap junction-associated pool of Cx32 (Figure 7). These results suggest that in the absence of androgens, Cx32 is degraded soon after its synthesis and that only the newly synthesized intracellular pool of Cx32 appears to be affected by the androgens or MG132 under androgen-depleted conditions. The gap junctional plaque associated pool seems to be predominantly degraded in the lysosome both in the presence and absence of androgens. On inhibition of proteasomal function, intracellularly accumulated Cx32 appeared to localize in discrete puncta or aggregates of variable sizes that were found to be scattered through out the cytoplasm. These puncta did not seem to reside in the ER, Golgi, and trans-Golgi network, as assessed by colocalization studies using well-established markers for these organelles and seemed to reside in as yet undefined compartment as assessed by the cell fractionation assay (Figure 7C). Further studies are required to identify the biochemical nature of these aggregates and the subcellular compartments in which they reside.
As assessed by quantitative real-time PCR analysis, androgens neither induced the transcription of endogenous Cx32 gene (data not shown) nor affected Cx32 mRNA level driven by the retroviral promoter (Figure 6D). Moreover, it also seemed unlikely that androgens regulated the expression of Cx32 at the translational level, because inhibition of proteasomal function under androgen-depleted conditions increased steady state expression level and caused substantial intracellular accumulation (Figure 6A). These data indicate that in the absence of androgens, Cx32 protein was synthesized but was degraded immediately after its synthesis. Furthermore, the fact that Cx32 accumulated intracellularly to a similar extent upon inhibition of proteasomal function both in the presence and absence of androgens at 15°C, when ER-to-Golgi transport was blocked (Bannykh et al., 1996; Hauri et al., 2000), argue further against translational control as one of the mechanisms for decreasing the expression level (Figure 8B). Thus, the level of Cx32 seemed to be controlled by the androgens post-translationally. Because Cx43 was also degraded upon androgen depletion, it appears that this may be a common mechanism by which expression level of some Cxs is regulated to permit gap junction assembly. In previous findings a decrease in, or loss of, Cx32 expression was observed upon partial hepatectomy and in primary cultures of rat hepatocytes, without significant changes in the mRNA level, but no rational explanation was put forward (Dermietzel et al., 1987; Kren et al., 1993; Kojima et al., 1996). It is possible that in those studies the expression level of Cx32 was regulated post-translationally by a similar mechanism involving degradation.
Our findings suggest that AR-mediated signaling was required for maintaining Cx32 expression level and preventing its degradation post-translationally. In AR-negative DU-145 cells, in which Cx32 was introduced using recombinant retrovirus, depletion of androgens neither enhanced its expression level nor induced degradation. Moreover, in androgen-responsive LNCaP cells, Cx32 degraded rapidly in androgen-containing medium upon addition of an anti-androgen, bicalutamide, which inhibits AR-mediated signaling by competing with the androgens (Iversen, 2002). These findings document that the depletion of androgens is the predominant factor responsible for the degradation of Cx32 when cells are grown in charcoal-stripped serum—and not other factors that might also be removed upon charcoal stripping. When AR was overexpressed in Cx32-expressing LNCaP cells, the expression level of Cx32 was increased, along with the size of the gap junctions, most likely due to increased level of AR which was available for the hormones to bind. These results also corroborate the androgen dose–response data and concur with the notion that androgens themselves increase AR level by stabilizing it (Dehm and Tindall, 2005; Shen and Coetzee, 2005).
One salient feature of our data is that androgen depletion induced degradation of only Cx32 and had no effect on the degradation of adherens junction–associated proteins E-cadherin and α- and β-catenins and tight junction–associated protein, occludin (Figure 5). Because cadherins had previously been shown to facilitate the trafficking and assembly of Cxs into gap junctions (Musil et al., 1990; Jongen et al., 1991; Hernandez-Blazquez et al., 2001), these data imply that the degradation of Cx32 and gap junctions composed of it was not triggered indirectly by the loss of E-cadherin or disassembly of adherens junctions upon removal of androgens. It is worth noticing that although the total level of occludin did not decrease, androgen depletion decreased its detergent-insoluble fraction, with a concomitant increase in the soluble fraction, causing it to accumulate intracellularly. One possible interpretation of these data are that the trafficking of occludin to the cell surface and its detergent insolubility (that is, incorporation into tight junctions) might be regulated by Cx32. This line of thought is well supported by earlier studies, which showed that in cell lines derived from Cx32 knock out mice, occludin remained in the cytoplasm and reintroduction of Cx32 induced its trafficking and assembly into tight junctions (Kojima et al., 2001, 2002). Thus, it is possible that an increase in the detergent solubility of occludin is indirectly affected by the degradation of Cx32 and gap junctions upon androgen removal. Further studies are required to substantiate this notion.
Although Cx32 and Cx43 have previously been shown to be degraded by the proteasomal and the lysosomal pathways in several cell culture model systems (Laing and Beyer, 1995, 2000; Laing et al., 1997; Musil et al., 2000; VanSlyke and Musil, 2002; Laird, 2005; VanSlyke and Musil, 2005), the physiological stimuli that trigger their degradation or dictate the choice of the pathway have not yet been explored. Using cell lines that differed widely in their ability to assemble Cx43 and Cx32 into functional gap junctions, a substantial portion of newly synthesized Cx43 and Cx32 was shown to be degraded by ERAD; moreover, the degradation was prevented by nontoxic hyperthermic and oxidative stress or by proteasomal inhibitors and was accompanied by enhanced gap junction assembly and function (Musil et al., 2000; VanSlyke and Musil, 2005). Furthermore, similar treatments also increased gap junctions by preventing the degradation of the cell surface–associated pool of Cx43 and by enhancing its recycling (VanSlyke and Musil, 2005). Thus, it has been proposed that regulation of Cx turnover might be an important mechanism to enhance gap junction assembly and function upon physiological demand (Musil et al., 2000; VanSlyke and Musil, 2005). Our findings—that androgens enhanced the expression level of Cx32 by rerouting its presumably ERAD-targeted pool to the cell surface—lend credence to the above notion, and for the first time have identified a novel, hormonally regulated route by which the formation and degradation of gap junctions could be rapidly controlled in response to physiological stimuli from the ER. Although ERAD has been shown to be a common mechanism by which misfolded proteins are degraded, the molecular mechanisms have not yet been elucidated (Arvan et al., 2002; Ellgaard and Helenius, 2003; McCracken and Brodsky, 2003; Meusser et al., 2005). Hence, the mechanism by which androgens reroute the ERAD-targeted pool of Cx32 to the cell surface is likely to be complex and may be related to induction and repression of factors that regulate Cx trafficking and degradation or both. Based on our findings, it is tempting to speculate that degradation of Cxs before their assembly into gap junctions by ERAD itself is a physiologically regulated event.
Requirement for androgens throughout normal prostatic development and prostate cancer progression is well established (Cunha et al., 1987, 1992). Androgen-responsive LNCaP cells have been widely used for studying the progression of human prostate cancer from an androgen-dependent state to an androgen-independent state in vitro (Webber et al., 1996). Although the molecular events involved during the progression of prostate cancer from an indolent, hormone (androgen)-dependent state to an invasive, androgen-independent state are not fully understood, the progression is characterized by the emergence of cells that no longer depend on androgens for survival (Abate-Shen and Shen, 2000; Feldman and Feldman, 2001). Because rescue of Cx32 and Cx43 degradation upon addition of androgens was accompanied by parallel changes in gap junction function, our findings suggest that it is likely to be intimately involved in regulating the physiological functions of the prostate. Moreover, the findings that in a cell culture model that mimics the progression of human prostate cancer (Igawa et al., 2002), degradation of Cxs as well as formation of gap junctions—and not of other junction-associated proteins—is androgen-dependent strongly implicate an important role of junctional communication in the prostate morphogenesis and oncogenesis.
ACKNOWLEDGMENTS
We thank Dr. S. Caplan for his helpful suggestions and discussion through out the course of this study. We also thank Dr. Ming-Fong Lin for providing us with early passage LNCaP cells and other human prostate cancer cell lines, Dr. Rakesh Singh and Anguraj Sadanandam for help with real-time quantitative PCR, and Pallavi Chaturvedi for her friendship. This research was supported by National Institutes of Health Grants CA113903 (P.P.M.) and CA78590 (S.K.B.). We gratefully acknowledge support from the Nebraska Center for Cellular Signaling in the form of graduate fellowship to Shalini Mitra and Souvik Chakraborty.
Abbreviations used:
- Cx
connexin
- MB
mibolerone
- ER
endoplasmic reticulum
- ERAD
endoplasmic reticulum–associated degradation
- AR
androgen receptor
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-04-0280) on October 18, 2006.
REFERENCES
- Abate-Shen C., Shen M. M. Molecular genetics of prostate cancer. Genes Dev. 2000;14:2410–2434. doi: 10.1101/gad.819500. [DOI] [PubMed] [Google Scholar]
- Arvan P., Zhao X., Ramos-Castaneda J., Chang A. Secretory pathway quality control operating in Golgi, plasmalemmal, and endosomal systems. Traffic. 2002;3:771–780. doi: 10.1034/j.1600-0854.2002.31102.x. [DOI] [PubMed] [Google Scholar]
- Balda M. S., Matter K. Epithelial cell adhesion and the regulation of gene expression. Trends Cell Biol. 2004;13:310–318. doi: 10.1016/s0962-8924(03)00105-3. [DOI] [PubMed] [Google Scholar]
- Bannykh S. I., Rowe T., Balch W. E. The organization of endoplasmic reticulum export complexes. J. Cell Biol. 1996;135:19–35. doi: 10.1083/jcb.135.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga V. M. Cell-cell adhesion and signaling. Curr. Opin. Cell Biol. 2002;14:546–556. doi: 10.1016/s0955-0674(02)00373-3. [DOI] [PubMed] [Google Scholar]
- Chardin P., McCormick F. Brefeldin A: the advantage of being uncompetitive. Cell. 1999;97:153–155. doi: 10.1016/s0092-8674(00)80724-2. [DOI] [PubMed] [Google Scholar]
- Cox M. E., Deeble P. D., Bissonette E. A., Parsons S. J. Activated 3′, 5′-cyclic AMP-dependent protein kinase is sufficient to induce neuroendocrine–like differentiation of the LNCaP prostate tumor cell line. J. Biol. Chem. 2000;275:13812–13818. doi: 10.1074/jbc.275.18.13812. [DOI] [PubMed] [Google Scholar]
- Cunha G. R., Alarid E. T., Turner T., Donjacour A. A., Boutin E. E., Foster B. A. Normal and abnormal development of the male urogenital tract. Role of androgens, mesenchymal-epithelial interactions, and growth factors. J. Androl. 1992;13:465–475. [PubMed] [Google Scholar]
- Cunha G. R., Donjacour A. A., Cooke P. S., Mee S., Bigsby R. M., Higgins S. J., Sugimura Y. The endocrinology and developmental biology of the prostate. Endoc. Rev. 1987;8:338–362. doi: 10.1210/edrv-8-3-338. [DOI] [PubMed] [Google Scholar]
- Deeble P. D., Murphy D. J., Parsons S. J., Cox M. E. Interleukin-6- and cyclic AMP-mediated signaling potentiates neuroendocrine differentiation of LNCaP prostate tumor cells. Mol. Cell Biol. 2001;21:8471–8482. doi: 10.1128/MCB.21.24.8471-8482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehm S. M., Tindall D. J. Regulation of androgen receptor signaling in prostate cancer. Expert Rev. Anticancer Ther. 2005;5:63–74. doi: 10.1586/14737140.5.1.63. [DOI] [PubMed] [Google Scholar]
- Dermietzel R., Yancey S. B., Traub O., Willecke K., Revel J. P. Major loss of the 28-KD protein of gap junction in proliferating hepatocytes. J. Cell Biol. 1987;105:1925–1934. doi: 10.1083/jcb.105.4.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellgaard L., Helenius A. Quality control in the endoplasmic reticulum. Nat. Rev. Mol. Cell Biol. 2003;4:181–191. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- Feldman B. J., Feldman D. The development of androgen-independent prostate cancer. Nat. Rev. Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- Getsios S., Huen A. C., Green K. J. Working out the strengths and flexibility of desmosomes. Nat. Rev. Mol. Cell Biol. 2004;5:271–281. doi: 10.1038/nrm1356. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Rock K. Not just research tools—proteasome inhibitors offer therapeutic promise. Nat. Med. 2002;8:338–340. doi: 10.1038/nm0402-338. [DOI] [PubMed] [Google Scholar]
- Govindarajan R., Song X. H., Guo R. J., Wheelock M. J., Johnson K. R., Mehta P. P. Impaired traffcking of connexins in androgen-independent human prostate cancer cell lines and its mitigation by α-catenin. J. Biol. Chem. 2002;277:50087–50097. doi: 10.1074/jbc.M202652200. [DOI] [PubMed] [Google Scholar]
- Guerrier A., Fonlupt P., Morand I., Rabilloud R., Audebet C., Krutovskikh V., Gros D., Rousset B., Munari-Silem Y. Gap junctions and cell polarity: connexin32 and connexin43 expressed in polarized thyroid epithelial cells assemble into gap junctions, which are located in distinct regions of the lateral plasma membrane domain. J. Cell Sci. 1995;108:2609–2617. doi: 10.1242/jcs.108.7.2609. [DOI] [PubMed] [Google Scholar]
- Gumbiner B. M. Regulation of cadherin adhesive-activity. J. Cell Biol. 2000;148:399–404. doi: 10.1083/jcb.148.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann H., Chang W. Y., Birch L., Mehta P., Prins G. S. Developmental exposure to estrogens alters epithelial cell adhesion and gap junction proteins in the adult rat prostate. Endocrinology. 2001;142:359–369. doi: 10.1210/endo.142.1.7893. [DOI] [PubMed] [Google Scholar]
- Habermann H., Ray V., Prins G. S. Alterations in gap junction protein expression in human benign prostatic hyperplasia and prostate cancer. J. Urol. 2002;167:655–660. doi: 10.1016/S0022-5347(01)69118-3. [DOI] [PubMed] [Google Scholar]
- Hauri H. P., Kappeler F., Andersson H., Appenzeller C. ERGIC-53 and traffic in the secretory pathway. J. Cell Sci. 2000;113:587–596. doi: 10.1242/jcs.113.4.587. [DOI] [PubMed] [Google Scholar]
- Hernandez-Blazquez F. J., Joazeiro P. P., Omori Y., Yamasaki H. Control of intracellular movement of connexins by E-cadherin in murine skin papilloma cells. Exp. Cell Res. 2001;270:235–247. doi: 10.1006/excr.2001.5342. [DOI] [PubMed] [Google Scholar]
- Igawa T., Lin F. F., Lee M. S., Karan D., Batra S. K., Lin M. F. Establishment and characterization of androgen-independent human prostate cancer LNCaP cell model. Prostate. 2002;50:222–235. doi: 10.1002/pros.10054. [DOI] [PubMed] [Google Scholar]
- Iversen P. Antiandrogen monotherapy: indications and results. Urology. 2002;60:64–71. doi: 10.1016/s0090-4295(02)01576-5. [DOI] [PubMed] [Google Scholar]
- Jongen W.M.F., Fitzgerald D. J., Asamoto M., Piccoli C., Slaga T. J., Gros D., Takeichi M., Yamasaki H. Regulation of connexin 43-mediated gap junctional intercellular communication by Ca2+ in mouse epidermal cells is controlled by E-cadherin. J. Cell Biol. 1991;114:545–555. doi: 10.1083/jcb.114.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karan D., Schmied B. M., Dave B. J., Wittel U. A., Lin M. F., Batra S. K. Decreased androgen-responsive growth of human prostate cancer is associated with increased genetic alterations. Clin. Cancer Res. 2001;7:3472–3480. [PubMed] [Google Scholar]
- Kelsell D. P., Dunlop J., Hodgins M. B. Human diseases: clues to cracking the connexin code? Trends Cell Biol. 2001;11:2–6. doi: 10.1016/s0962-8924(00)01866-3. [DOI] [PubMed] [Google Scholar]
- King T. J., Lampe P. D. Altered tumor biology and tumorigenesis in irradiated and chemical carcinogen-treated single and combined connexin32/p27Kip1-deficient mice. Communication Cell Adhes. 2005a;12:293–305. doi: 10.1080/15419060500514168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King T. J., Gurley K. E., Prunty J., Shin J. L., Kemp C. J., Lampe P. D. Deficiency in the gap junction protein connexin32 alters p27Kip1 tumor suppression and MAPK activation in a tissue-specific manner. Oncogene. 2005b;24:1718–1726. doi: 10.1038/sj.onc.1208355. [DOI] [PubMed] [Google Scholar]
- King T. J., Lampe P. D. The gap junction protein connexin32 is a mouse lung tumor suppressor. Cancer Res. 2004;64:7191–7196. doi: 10.1158/0008-5472.CAN-04-0624. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Donaldson J. G., Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J. Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima T., Kokai Y., Chiba H., Yamamoto M., Mochizuki Y., Sawada N. Cx32 but not Cx26 is associated with tight junctions in primary cultures of rat hepatocytes. Biochem. Biophys. Res. Comm. 2001;263:193–201. doi: 10.1006/excr.2000.5103. [DOI] [PubMed] [Google Scholar]
- Kojima T., Spray D. C., Kokai Y., Chiba H., Mochizuki Y., Sawada N. Cx32 formation and/or Cx32-mediated intercellular communication induces expression and function of tight junctions in hepatocytic cell line. Exp. Cell Res. 2002;276:40–51. doi: 10.1006/excr.2002.5511. [DOI] [PubMed] [Google Scholar]
- Kojima T., Yamamoto M., Tobioka H., Mizuguchi T., Mitaka T., Mochizuki Y. Changes in cellular distribution of connexins 32 and 26 during formation of gap junctions in primary cultures of rat hepatocytes. Exp. Cell Res. 1996;223:314–326. doi: 10.1006/excr.1996.0087. [DOI] [PubMed] [Google Scholar]
- Kren B. T., Kumar N. M., Wang S. Q., Gilula N. B., Steer C. J. Differential regulation of multiple gap junction transcripts and proteins during rat liver regeneration. J. Cell Biol. 1993;123:707–718. doi: 10.1083/jcb.123.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N. M., Gilula N. B. The gap junction communication channel. Cell. 1996;84:381–388. doi: 10.1016/s0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- Laing J. G., Beyer E. C. The gap junction protein connexin43 is degraded via the ubiquitin proteasome pathway. J. Biol. Chem. 1995;270:26399–26403. doi: 10.1074/jbc.270.44.26399. [DOI] [PubMed] [Google Scholar]
- Laing J. G., Beyer E. C. Degradation of gap junctions and connexins. Curr. Topics Membr. 2000;49:23–41. [Google Scholar]
- Laing J. G., Tadros P. N., Wessels E. C., Beyer E. C. Degradation of connexin43 gap junctions involves both the proteasome and the lysosome. Exp. Cell Res. 1997;236:482–492. doi: 10.1006/excr.1997.3747. [DOI] [PubMed] [Google Scholar]
- Laird D. W. Connexin phosphorylation as a regulatory event linked to gap junction internalization and degradation. Biochim. Biophys. Acta. 2005;1711:172–182. doi: 10.1016/j.bbamem.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Lauf U., Lopez P., Falk M. M. Expression of fluorescently tagged connexins: a novel approach to rescue function of oligomeric DsRed-tagged proteins. FEBS Lett. 2001;498:11–15. doi: 10.1016/s0014-5793(01)02462-0. [DOI] [PubMed] [Google Scholar]
- Lee H. D., Goldberg A. L. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 1998;8:397–403. doi: 10.1016/s0962-8924(98)01346-4. [DOI] [PubMed] [Google Scholar]
- Lin M. F., Lee M. S., Zhou X. W., Andressen J. C., Meng T. C., Johansson S. L., West W. W., Taylor R. J., Anderson J. R., Lin F. F. Decreased expression of cellular prostatic acid phosphatase increases tumorigenicity of human prostate cancer cells. J. Urol. 2001;166:1943–1950. [PubMed] [Google Scholar]
- Loewenstein W. R. Junctional intercellular communication: the cell-to-cell membrane channel. Physiol. Rev. 1981;61:829–913. doi: 10.1152/physrev.1981.61.4.829. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. The cell-to-cell channel of gap junctions. Cell. 1987;48:725–726. doi: 10.1016/0092-8674(87)90067-5. [DOI] [PubMed] [Google Scholar]
- McCracken A. A., Brodsky J. L. Evolving questions and paradigm shifts in endoplasmic-reticulum-associated degradation (ERAD) Bio Essays. 2003;25:868–877. doi: 10.1002/bies.10320. [DOI] [PubMed] [Google Scholar]
- Meda P. Gap junction involvement in secretion: the pancreas experience. Clin. Exp. Pharm. Physiol. 1996;23:1053–1057. doi: 10.1111/j.1440-1681.1996.tb01168.x. [DOI] [PubMed] [Google Scholar]
- Mehdi S. Cell-penetrating inhibitors of calpains. Trends Biochem. Sci. 1991;16:150–153. doi: 10.1016/0968-0004(91)90058-4. [DOI] [PubMed] [Google Scholar]
- Mehta P. P., Bertram J. S., Loewenstein W. R. Growth inhibition of transformed cells correlates with their junctional communication with normal cells. Cell. 1986;44:187–196. doi: 10.1016/0092-8674(86)90497-6. [DOI] [PubMed] [Google Scholar]
- Mehta P. P., Hotz-Wagenblatt A., Rose B., Shalloway D., Loewenstein W. R. Incorporation of the gene for a cell-to-cell channel proteins into transformed cells leads to normalization of growth. J. Membr. Biol. 1991;124:207–225. doi: 10.1007/BF01994355. [DOI] [PubMed] [Google Scholar]
- Mehta P. P., Lokeshwar B. L., Schiller P. C., Bendix M. V., Ostenson R. C., Howard G. A., Roos B. A. Gap-junctional communication in normal and neoplastic prostate epithelial cells and its regulation by cAMP. Mol. Carcinogen. 1996;15:18–32. doi: 10.1002/(SICI)1098-2744(199601)15:1<18::AID-MC4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Mehta P. P., Perez-Stable C., Nadji M., Mian M., Asotra K., Roos B. A. Suppression of human prostate cancer cell growth by forced expression of connexin genes. Dev. Genet. 1999;24:91–110. doi: 10.1002/(SICI)1520-6408(1999)24:1/2<91::AID-DVG10>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Mehta P. P., Yamamoto M., Rose B. Transcription of the gene for the gap junctional protein connexin43 and expression of functional cell-to-cell channels are regulated by cAMP. Mol. Biol. Cell. 1992;3:839–850. doi: 10.1091/mbc.3.8.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meusser B., Hirsch C., Jarosch E., Sommer T. ERAD: the long road to destruction. Nat. Cell Biol. 2005;7:766–772. doi: 10.1038/ncb0805-766. [DOI] [PubMed] [Google Scholar]
- Munari-Silem Y., Audebet C., Rousset B. Hormonal control of cell to cell communication: regulation by thyrotropin of the gap junction-mediated dye transfer between cells. Endocrinology. 1991;128:3299–3309. doi: 10.1210/endo-128-6-3299. [DOI] [PubMed] [Google Scholar]
- Munari-Silem Y., Rousset B. Gap junction-mediated cell-to-cell communication in endocrine glands—molecular and functional aspects: a review. Eur. J. Endocrinol. 1996;135:251–264. doi: 10.1530/eje.0.1350251. [DOI] [PubMed] [Google Scholar]
- Musil L. S., Cunningham B. A., Edelman G. M., Goodenough D. A. Differential phosphorylation of the gap junction protein connexin43 in junctional communication-competent and -deficient cell lines. J. Cell Biol. 1990;111:2077–2088. doi: 10.1083/jcb.111.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musil L. S., Goodenough D. A. Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly of gap junctional plaques. J. Cell Biol. 1991;115:1357–1374. doi: 10.1083/jcb.115.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musil L. S., Le A. C., VanSlyke J. K., Roberts L. M. Regulation of connexin degradation as a mechanism to increase gap junction assembly and function. J. Biol. Chem. 2000;275:25207–25215. doi: 10.1074/jbc.275.33.25207. [DOI] [PubMed] [Google Scholar]
- Rose B., Mehta P. P., Loewenstein W. R. Gap-junction protein gene suppresses tumorigenicity. Carcinogenesis. 1993;14:1073–1075. doi: 10.1093/carcin/14.5.1073. [DOI] [PubMed] [Google Scholar]
- Ruch R. J. The role of gap junctional intercellular communication in neoplasia. Ann. Clin. Lab. Sci. 1994;24:216–231. [PubMed] [Google Scholar]
- Saez J. C., Berthoud V. M., Branes M. C., Martinez A. D., Beyer E. C. Plasma membrane channels formed by connexins: their regulation and functions. Physiol. Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- Segretain D., Falk M. M. Regulation of connexin biosynthesis, assembly, gap junction formation, and removal. Biochim. Biophys. Acta. 2004;1662:3–21. doi: 10.1016/j.bbamem.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Shen H. C., Coetzee G. A. The androgen receptor: unlocking the secrets of its unique transactivation domain. Vitam. Horm. 2005;71:301–319. doi: 10.1016/S0083-6729(05)71010-4. [DOI] [PubMed] [Google Scholar]
- Solan J. L., Lampe P. D. Connexin phosphorylation as a regulatory event linked to gap junction channel assembly. Biochim. Biophys. Acta. 2005;1711:154–163. doi: 10.1016/j.bbamem.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Sosinsky G. E., Nicholson B. J. Structural organization of gap junction channels. Biochim. Biophys. Acta. 2005;1711:99–125. doi: 10.1016/j.bbamem.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Stock A., Sies H., Stahl W. Enhancement of gap junctional communication and connexin43 expression by thyroid hormones. Biochem. Pharmacol. 1998;55:475–479. doi: 10.1016/s0006-2952(97)00473-5. [DOI] [PubMed] [Google Scholar]
- Stutenkemper R., Geisse S., Schwarz H. J., Look J., Traub O., Nicholson B. J., Wilecke K. The hepatocyte-specific phenotype of murine liver cells correlates with high expression of connexin32 and connexin26 but very low expression of connexin43. Exp. Cell Res. 1992;201:43–54. doi: 10.1016/0014-4827(92)90346-a. [DOI] [PubMed] [Google Scholar]
- Tartakoff A. M. Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell. 1983;32:1026–1028. doi: 10.1016/0092-8674(83)90286-6. [DOI] [PubMed] [Google Scholar]
- Temme A., Buchmann A., Gabriel H. D., Nelles E., Schwarz M., Willecke K. High incidence of spontaneous and chemically induced liver tumors in mice deficient for connexin32. Curr. Biol. 1997;7:713–716. doi: 10.1016/s0960-9822(06)00302-2. [DOI] [PubMed] [Google Scholar]
- Trosko J. E., Ruch R. J. Cell-cell communication in carcinogenesis. Front. Biosci. 1998;3:208–236. doi: 10.2741/a275. [DOI] [PubMed] [Google Scholar]
- VanSlyke J. K., Deschenes S. M., Musil L. S. Intracellular transport, assembly, and degradation of wild-type and disease-linked mutant gap junction proteins. Mol. Biol. Cell. 2001;11:1933–1946. doi: 10.1091/mbc.11.6.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanSlyke J. K., Musil L. S. Analysis of connexin intracellular transport and assembly. Methods. 2000;20:156–164. doi: 10.1006/meth.1999.0933. [DOI] [PubMed] [Google Scholar]
- VanSlyke J. K., Musil L. S. Dislocation and degradation from the ER are regulated by cytosolic stress. J. Cell Biol. 2002;157:381–394. doi: 10.1083/jcb.200111045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanSlyke J. K., Musil L. S. Cytosolic stress reduces degradation of connexin43 internalized from the cell surface and enhances gap junction formation and function. Mol. Biol. Cell. 2005;16:5247–5257. doi: 10.1091/mbc.E05-05-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber M. M., Bello D., Quader S. Immortalized and tumorigenic adult human prostatic epithelial cell lines: characteristics and applications. Part 1. Cell markers and immortalized nontumorigenic cell lines. Prostate. 1996;29:386–394. doi: 10.1002/(SICI)1097-0045(199612)29:6<386::AID-PROS7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Wei J. C., Xu X., Lo C. W. Connexins and cell signaling in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:811–838. doi: 10.1146/annurev.cellbio.19.111301.144309. [DOI] [PubMed] [Google Scholar]
- Wheelock M. J., Johnson K. R. Cadherins as modulators of cellular phenotype. Annu. Rev. Cell Dev. Biol. 2003;19:207–235. doi: 10.1146/annurev.cellbio.19.011102.111135. [DOI] [PubMed] [Google Scholar]
- Wright M. E., Tsai M. J., Aebersold R. Androgen receptor represses the neuroendocrine transdifferentiation process in prostate cancer cells. Mol. Endocrinol. 2003;17:1726–1737. doi: 10.1210/me.2003-0031. [DOI] [PubMed] [Google Scholar]
- Zhu D., Caveney S., Kidder G. M., Naus C.C.G. Transfection of C6 glioma cells with connexin43 cDNA: Analysis of expression, intercellular coupling, and cell proliferation. Proc. Natl. Acad. Sci. USA. 1991;88:1883–1887. doi: 10.1073/pnas.88.5.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]