Abstract
An increase in adipocyte number is a major contributor to the increase in adipose tissue mass that is characteristic of obesity. The identity and regulation of the adipocyte precursor cell (or preadipocyte) and the preadipocyte precursor cell (or progenitor cell) have been intensely studied for many years. In this issue of the JCI, Crossno et al. report that progenitor cells originating from outside the adipose tissue, in particular the bone marrow, can contribute to an increase in adipocyte number (see the related article beginning on page 3220). Their study in mice reveals that treatment with the thiazolidinedione rosiglitazone or exposure to a high-fat diet promotes the trafficking of circulating bone marrow–derived progenitor cells into adipose tissue, where they become multilocular adipocytes. This adds a new and unexpected dimension to this research arena.
Historical perspective
Early investigations noted that the full range of cells common to red bone marrow, including monocytes, reticular cells, lymphocytes, normoblasts, erythroblasts, and basophils, were observed in developing adipose tissue (1). In particular, a wide range of cell phenotypes common to red bone marrow, including endothelial cell precursors, pericytes, macrophages, immature macrophages, fibroblast-like perivascular reticular cells, and perivascular mesenchymal cells, were among those implicated as the adipocyte precursor cell (1). Adipose tissue and bone marrow–derived mesenchymal cells have a similar protein expression phenotype based on cell-surface markers (2, 3). Furthermore, adipogenic differentiation of both adipose tissue and bone marrow–derived mesenchymal cells are similarly characterized by increases in the expression of key adipocyte markers such as fatty acid–binding protein, lipoprotein lipase, PPARγ, and CCAAT/enhancer-binding protein α (C/EBPα) (4, 5). Bone marrow–derived adipocytes also secrete the adipocyte-specific factors leptin and adiponectin, indicating functional similarity between bone marrow–derived and adipose tissue–derived adipocytes (4). Interestingly, clusters of red bone marrow cell types have been observed in developing adipose tissue (6). The number of these cell types was associated temporally and spatially with the development of large clusters of multilocular (comprising many compartments) adipocytes representative of an immature stage of adipocyte development in white adipose tissue. These observations would seem to counter the long-held belief that new adipocytes arise solely from resident preadipocyte progenitors and hint that progenitor cells from tissues outside adipose depots could contribute to the formation of new adipocytes. In support of the latter concept, in this issue of the JCI Crossno et al. (7) provide evidence that bone marrow–derived progenitor cells do indeed contribute to new adipocytes in adipose tissue. The authors exposed GFP-labeled bone marrow–transplanted mice to 2 known inducers of new fat cell formation, either a high-fat diet or treatment with the thiazolidinedione (TZD) rosiglitazone. Subsequent examination demonstrated the presence of GFP+ multilocular adipocytes within adipose tissue that were significantly increased in number by both high-fat feeding and TZD treatment. Furthermore, the GFP+ multilocular adipocytes expressed many genes that are known markers of the adipocyte phenotype, providing additional evidence that the bone marrow–derived progenitor cells had trafficked to adipose tissue where they differentiated into multilocular adipocytes.
TZDs, adipogenesis, and endothelial cells
TZDs are PPARγ ligands currently used in the treatment of type 2 diabetes mellitus. In vitro, TZDs consistently induce adipogenesis, although in many instances the accompanying lipid accretion is minimal (8, 9). TZD treatment in vivo typically increases the number of small multilocular adipocytes and decreases the number of larger adipocytes (10). As a result, adipose tissue undergoes “remodeling,” since clusters of small multilocular adipocytes develop throughout the tissue (10). The morphological results of TZD treatment observed by Crossno et al. (7) are consistent with these previous findings. Similar clusters of multilocular adipocytes were also reported in adipose tissue of mice treated with β3-adrenergic receptor agonists that exert antidiabetic effects in rodents (11). Although transitory in nature, clusters of multilocular adipocytes in developing adipose tissue and those induced by TZDs (10) and β3-adrenergic receptor agonists (11) may share some biochemical characteristics. TZDs also promote mobilization and homing of bone marrow–derived circulatory endothelial progenitor cells to various tissues in the process of endothelial regeneration (12, 13). Do TZDs direct circulatory endothelial progenitor cells to adipose tissue as well? Or, since TZDs enhance migration of endothelial progenitor cells (12), could TZDs influence migration of endothelial progenitor cells in situ in addition to increasing the number of nonresident progenitor cells? The demonstration of preadipocyte trafficking from nonresident sources has stimulated these and many other intriguing questions, which will undoubtedly be addressed as mechanisms and regulatory influences on adipose tissue expansion are further defined.
High-fat feeding
Calorie-dense diets, including high-fat diets, increase adipocyte number and size in rodents. In mice fed high-fat diets from birth, increases in fat pad weight were associated with a greater fat cell size through 18 weeks of age, followed by an increase in fat cell number through 52 weeks of age (14). High-fat feeding–induced obesity in adult rodents is also characterized by an increase in fat cell size (7, 15), followed by an increase in fat cell number, which is a consequence of proliferation of resident preadipocyte progenitors, as demonstrated by increased rates of progenitor cell [3H] thymidine incorporation (16). Crossno et al. (7) have shown that trafficking of bone marrow–derived circulating progenitors to adipose tissue represents an additional and novel mechanism of expanding the number of preadipocyte progenitors in response to calorie-dense diets. Future studies will determine the role or contribution of nonresident preadipocyte progenitors in obesity caused by factors other than high-fat feeding.
Adipose tissue depot–dependent traits
A large number of characteristics distinguish adipose tissue locations or depots from each other, including adipocyte number and size, insulin signaling, glucose and lipid metabolism, lipolytic rates, and cytokine expression and secretion (17, 18). Depot-dependent blood flow and innervation density may be the most important depot-dependent traits, since they are associated with adipocyte growth patterns and adipocyte metabolism (17, 18). Subcutaneous adipose tissue and internal (mesenteric) adipose tissue represent extremes in blood flow and innervation density (17). In humans, internal or visceral adipose tissue is associated with the insulin resistance syndrome to a greater degree than is subcutaneous adipose tissue (18). The abundance of non-adipocyte cell types and their differentiation potential are very depot dependent in rat adipose tissue (19). These studies indicate that adipose tissue–derived stromal vascular cells (progenitor cells) give rise to osteoblasts, endothelial cells, and hematopoietic cells, depending on culture conditions and depot (19). In particular, the number of resident bone progenitor cells was highest in white adipose tissue and lowest in brown adipose tissue (19). Could there be a depot-dependent influence on nonresident progenitor cell pools as well? The results presented by Crossno et al. (7) further hint that this may be the case, as they demonstrated that bone marrow–derived adipocyte progenitors (nonresident) were distributed as large clusters in white adipose tissue (omental), but as single cells in brown (interscapular) adipose tissue.
Adipocyte development and angiogenesis
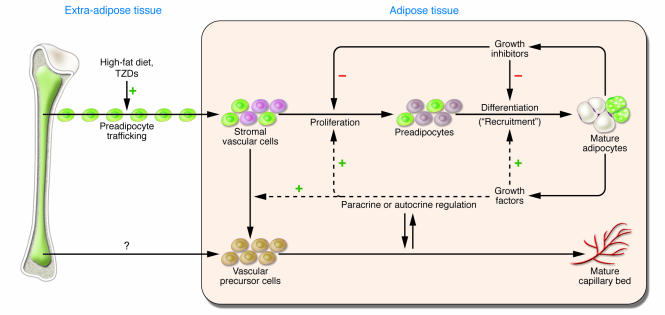
It is important to consider adipocyte development in relation to vasculogenesis and angiogenesis (Figure 1), since adipocyte development (adipogenesis) and vasculogenesis/angiogenesis are reciprocally regulated events (20). For instance, vasculogenesis and angiogenesis either precede or are coincidental with adipogenesis during fetal adipose tissue development (20). There are many other lines of evidence of developmental or autocrine/paracrine relationships between capillaries/endothelial cells and preadipocytes (Figure 1; ref. 20).
Figure 1. Schematic representation of the basic features of adipose tissue expansion.
The major processes at work within adipose tissue are the proliferation of a progenitor cell population within the stromal vascular cell pool and the differentiation of these cells into adipose or vascular cells. These processes are regulated by stimulatory and inhibitory endocrine, neural, and paracrine/autocrine controls (17). Monoclonal antibodies raised against adipocyte surface proteins identify preadipocyte progenitors and closely associated developing blood vessels in developing adipose tissues (24). These observations were substantiated by examination of the preadipocyte progenitor cells and endothelial cells by electron microscopy and studies of the major extracellular components (25, 26). The study by Crossno et al. (7) in this issue of the JCI indicates that progenitor cells may also be delivered to adipose tissue by trafficking from extra-adipose sources, such as bone marrow, and that this trafficking my be upregulated by high-fat diet and TZD administration. TZDs also promote mobilization and homing of bone marrow–derived circulatory endothelial progenitor cells to various tissues in the process of endothelial regeneration (12, 13). The green cells in this figure represent cells originating from bone marrow that differentiate within adipose tissue to form clusters of multilocular adipocytes.
Implanting murine preadipocytes in a dorsal skin–fold chamber has been shown to induce angiogenesis and formation of fat pads (21). The induced blood vessels subsequently developed and remodeled into a mature vasculature associated with differentiated adipocytes (21). The vasculature development and remodeling demonstrated by Fukumura et al. (21) were virtually identical to those observed during adipose tissue development in vivo (20). Implantation of murine preadipocytes transduced with a recombinant adenovirus encoding a PPARγ–dominant-negative mutant receptor in a dorsal skin–fold chamber markedly reduced adipocyte differentiation but also markedly reduced angiogenesis compared with the effects of implanting preadipocytes transduced with a mock adenovirus (21). Furthermore, blocking angiogenesis with an antibody to VEGFR-2 (a transducer of the major signals of angiogenesis via tyrosine kinase activity) reduced angiogenesis and inhibited adipocyte differentiation (21). Conversely, neovasculature induced with injections of Matrigel supplemented with basic FGF recruited and induced migration of preadipocyte progenitors from adjacent connective tissue, which ultimately resulted in the formation of a fat pad at the injection site (22).
Related studies have demonstrated that adipose tissue can be effectively regulated by its vasculature (23). For instance, treatment with angiogenesis inhibitors selectively inhibits adipose tissue growth and prevents high-fat feeding–induced and genetic obesity in mice (23). This and other studies indicate that vascular stability, i.e., vascular maturity, per se can dictate adipocyte development and adiposity.
Considering the reciprocal relationship between blood vessel and adipocyte development and the intriguing results presented by Crossno et al. (7) provokes many additional questions: What is the relationship, if any, between nonresident bone marrow–derived preadipocyte progenitors and vasculogenesis/angiogenesis? Do bone marrow–derived progenitors home in on newly formed adipose tissue vasculature? Or, conversely, can nonresident (bone marrow–derived) preadipocyte progenitors induce adipose tissue neovascularization? Does the maturity of adipose tissue blood vessels play a role in homing and incorporation of bone marrow–derived progenitor cells?
Finally, the study by Crossno et al. (7) has ushered in terminology distinguishing nonresident and resident preadipocyte progenitors and has clearly expanded the possibilities in the search for the preadipocyte progenitor cell.
Footnotes
Nonstandard abbreviations used: TZD, thiazolidinedione.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 116:3103–3106 (2006). doi:10.1172/JCI30666.
See the related article beginning on page 3220.
References
- 1.Hausman G.J., Campion D.R., Martin R.J. Search for the adipocyte precursor cell and factors that promote its differentiation. J. Lipid Res. 1980;21:657–670. [PubMed] [Google Scholar]
- 2.Gronthos S., et al. Surface protein characterization of human adipose tissue-derived stromal cells. J. Cell. Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 3.De Ugarte D.A., et al. Differential expression of stem cell mobilization-associated molecules on multi-lineage cells from adipose tissue and bone marrow. Immunol. Lett. 2003;89:267–270. doi: 10.1016/s0165-2478(03)00108-1. [DOI] [PubMed] [Google Scholar]
- 4.Dicker A., et al. Functional studies of mesenchymal stem cells derived from adult human adipose tissue. Exp. Cell Res. 2005;308:283–290. doi: 10.1016/j.yexcr.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 5.Sekiya I., Larson B.L., Vuoristo J.T., Cui J.G., Prockop D.J. Adipogenic differentiation of human adult stem cells from bone marrow stroma (MSCs). J. Bone Miner. Res. 2004;19:256–264. doi: 10.1359/JBMR.0301220. [DOI] [PubMed] [Google Scholar]
- 6.Hausman G.J., Martin R.J. The development of adipocytes located around hair follicles in the fetal pig. J. Anim. Sci. 1982;54:1286–1296. doi: 10.2527/jas1982.5461286x. [DOI] [PubMed] [Google Scholar]
- 7.Crossno J.T., Majka S.M., Grazia T., Gill R.G., Klemm D.J. Rosiglitazone promotes development of a novel adipocyte population from bone marrow–derived circulating progenitor cells. . J. Clin. Invest. 2006;116:3220–3228. doi: 10.1172/JCI28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poulos S.P., Hausman G.J. A comparison of thiazolidinedione-induced adipogenesis and myogenesis in stromal-vascular cells from subcutaneous adipose tissue or semitendinosus muscle of postnatal pigs. J. Anim. Sci. 2006;84:1076–1082. doi: 10.2527/2006.8451076x. [DOI] [PubMed] [Google Scholar]
- 9.Hutley L.J., et al. Effects of rosiglitazone and linoleic acid on human preadipocyte differentiation. Eur. J. Clin. Invest. 2003;33:574–581. doi: 10.1046/j.1365-2362.2003.01178.x. [DOI] [PubMed] [Google Scholar]
- 10.De Souza C.J., et al. Effects of pioglitazone on adipose tissue remodeling within the setting of obesity and insulin resistance. Diabetes. 2001;50:1863–1871. doi: 10.2337/diabetes.50.8.1863. [DOI] [PubMed] [Google Scholar]
- 11.Granneman J.G., Li P., Zhu Z., Lu Y. Metabolic and cellular plasticity in white adipose tissue I: effects of beta3-adrenergic receptor activation. Am. J. Physiol. Endocrinol. Metab. 2005;289:E608–E616. doi: 10.1152/ajpendo.00009.2005. [DOI] [PubMed] [Google Scholar]
- 12.Pistrosch F., et al. PPARgamma-agonist rosiglitazone increases number and migratory activity of cultured endothelial progenitor cells. Atherosclerosis. 2005;183:163–167. doi: 10.1016/j.atherosclerosis.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 13.Wang C.H., et al. Rosiglitazone facilitates angiogenic progenitor cell differentiation toward endothelial lineage: a new paradigm in glitazone pleiotropy. Circulation. . 2004;109:1392–1400. doi: 10.1161/01.CIR.0000123231.49594.21. [DOI] [PubMed] [Google Scholar]
- 14.Lemonnier D. Effect of age, sex, and sites on the cellularity of the adipose tissue in mice and rats rendered obese by a high-fat diet. J. Clin. Invest. 1972;51:2907–2915. doi: 10.1172/JCI107115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faust I.M., Johnson P.R., Stern J.S., Hirsch J. Diet-induced adipocyte number increase in adult rats: a new model of obesity. Am. J. Physiol. 1978;235:E279–E286. doi: 10.1152/ajpendo.1978.235.3.E279. [DOI] [PubMed] [Google Scholar]
- 16.Ellis J.R., McDonald R.B., Stern J.S. A diet high in fat stimulates adipocyte proliferation in older (22 month) rats. Exp. Gerontol. 1990;25:141–148. doi: 10.1016/0531-5565(90)90045-4. [DOI] [PubMed] [Google Scholar]
- 17.Hausman D.B., DiGirolamo M., Bartness T.J., Hausman G.J., Martin R.J. The biology of white adipocyte proliferation. Obes. Rev. 2001;2:239–254. doi: 10.1046/j.1467-789x.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- 18.Laviola L., Perrini S., Cignarelli A., Giorgino F. Insulin signalling in human adipose tissue. Arch. Physiol. Biochem. 2006;112:82–88. doi: 10.1080/13813450600736174. [DOI] [PubMed] [Google Scholar]
- 19.Prunet-Marcassus B., et al. From heterogeneity to plasticity in adipose tissues: site-specific differences. Exp. Cell Res. 2006;312:727–736. doi: 10.1016/j.yexcr.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 20.Hausman G.J., Richardson R.L. Adipose tissue angiogenesis. J. Anim. Sci. 2004;82:925–934. doi: 10.2527/2004.823925x. [DOI] [PubMed] [Google Scholar]
- 21.Fukumura D., et al. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ. Res. 2003;93:e88–e97. doi: 10.1161/01.RES.0000099243.20096.FA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawaguchi N., et al. De novo adipogenesis in mice at the site of injection of basement membrane and basic fibroblast growth factor. Proc. Natl. Acad. Sci. U. S. A. . 1998;95:1062–1066. doi: 10.1073/pnas.95.3.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brakenhielm E., et al. Angiogenesis inhibitor, TNP-470, prevents diet-induced and genetic obesity in mice. Circ. Res. 2004;94:1579–1588. doi: 10.1161/01.RES.0000132745.76882.70. [DOI] [PubMed] [Google Scholar]
- 24.Wright J.T., Hausman G.J. Adipose tissue development in the fetal pig examined using monoclonal antibodies. J. Anim. Sci. 1990;68:1170–1175. doi: 10.2527/1990.6841170x. [DOI] [PubMed] [Google Scholar]
- 25.Hausman G.J., Wright J.T., Thomas G.B. Vascular and cellular development in fetal adipose tissue: lectin binding studies and immunocytochemistry for laminin and type IV collagen. Microvasc. Res. 1991;41:111–125. doi: 10.1016/0026-2862(91)90012-z. [DOI] [PubMed] [Google Scholar]
- 26.Hausman G.J., Richardson L.R. Histochemical and ultrastructural analysis of developing adipocytes in the fetal pig. Acta Anat. (Basel). 1982;114:228–247. doi: 10.1159/000145593. [DOI] [PubMed] [Google Scholar]