Abstract
In healthy individuals the immune system does not react aggressively toward host cells, a phenomenon defined as self tolerance. If self tolerance is broken autoimmune disease can develop, during which autoreactive lymphocytes are directed to a variety of autoantigenic epitopes. However, researchers have yet to determine whether immune responses to multiple autoantigens develop independently of each other or are the result of the response “spreading” from one autoantigen to another. In a study of NOD mice in this issue of the JCI, Krishnamurthy et al. show that the autoreactive T cell response to the autoantigen proinsulin lies upstream of that to islet-specific glucose-6-phosphatase catalytic subunit–related protein, suggesting that the pathogenic autoimmune response to proinsulin subsequently spreads to other antigens (see the related article beginning on page 3258). These data support the current view that this pancreatic β cell hormone is the first autoantigen targeted by the immune response in autoimmune diabetes.
Pathologic autoimmunity is characterized by an aberrant, self-perpetuating, immune-mediated, inflammatory response. It is the uncontrolled chronicity of this response that eventually leads to irreversible destruction of the target tissue. Among the major mechanisms underlying this chronicity is the diversification of the pathogenic autoimmune response, also termed epitope spreading.
The concept of epitope spreading was initially described by Eli Sercarz in the early 1990s in autoantigen-induced EAE, which is a model for multiple sclerosis (1). This term was used to describe how a self-directed immune response induced by a single peptide (or epitope) could spread to include other peptides (or epitopes) not only on the same autoantigen (i.e., intramolecular spreading), but also on other self molecules clustered in close vicinity within the target cell (i.e., intermolecular spreading). Thereafter, several studies confirmed the crucial role of epitope spreading in EAE (2–4) and also in demyelinating diseases of the central nervous system that follow some viral infections (e.g., Theiler’s murine encephalomyelitis; ref. 5) and IDDM, also known as type 1 diabetes (6, 7).
However, a central question remains as to whether an autoantigen triggers a primary insult, causing the release of other autoantigens from the damaged target cell in the context of a proinflammatory environment, and thus promotes the subsequent immune responses.
Epitope spreading in autoimmune diabetes: from proinsulin to other islet antigens
This crucial question is addressed by Krishnamurthy et al. in this issue of the JCI using a set of sophisticated in vivo experimental tools in a mouse model of autoimmune diabetes (8). The authors directly demonstrate that epitope spreading plays a role in a central loop that amplifies the autoimmune process, leading to disease chronicity. In addition, they provide strong and clear evidence in support of proinsulin being the primary autoantigen.
The analysis focused on 2 major autoantigens involved in type 1 diabetes: proinsulin 2 and islet-specific glucose-6-phosphatase catalytic subunit–related protein (IGRP). Pathogenic T cells specific for these 2 antigens are present in significant numbers in infiltrated islets of NOD mice (which develop spontaneous IDDM) and exhibit efficient diabetogenic properties, as demonstrated by their capacity to transfer disease to immunoincompetent syngeneic recipients (9–13). Two different transgenic animal models were analyzed: NOD-PI mice, which overexpress proinsulin 2 in their APCs (14), and NOD-IGRP mice, which overexpress IGRP in their APCs. While the 2 transgenic mouse lines were fully tolerant to the autoantigen they overexpressed, they exhibited quite a different response in terms of disease. NOD-PI mice were insulitis and diabetes free as well as completely deficient of IGRP-reactive T cells. In contrast, NOD-IGRP mice were not protected from disease in spite of being tolerant to IGRP, as shown by the total absence of IGRP-specific CD8+ cytotoxic T cells. In fact, these animals exhibited an anti-proinsulin–autoreactive response that was identical to that observed in conventional NOD mice (8).
These data support the conclusion that the immune responses to IGRP lie downstream of those to proinsulin and are tightly dependent on the generation of a primary anti-proinsulin response. Proinsulin has long represented an ideal “primary” candidate for triggering autoimmune diabetes based on its highly restricted expression in pancreatic β cells. However, until recently only indirect evidence had accumulated in support of such a conclusion. The recent data from Eisenbarth and colleagues (15) represented the first direct demonstration that, in NOD mice, part of the sequence of the B insulin chain is a primary target of the immune response. NOD mice lacking native insulin genes and carrying a mutated proinsulin transgene do not develop insulin autoantibodies, insulitis, or diabetes (15). In contrast, autoimmunity develops in mice carrying even a single copy of the native insulin gene (15). The study by Krishnamurthy et al. (8), via the use of a different experimental approach, provides additional proof for such a key role of proinsulin.
What cellular and molecular factors propagate the spread?
The study by Krishnamurthy et al. provides important clues regarding the initiation of epitope specificity and epitope dominance as well as the hierarchy of the immune responses to autoantigens in type 1 diabetes (8). However, important questions concerning the cellular and molecular events that initiate and perpetuate epitope spreading remain to be addressed.
At least 3 distinct factors may be involved: the nature of the antigenic determinant, the cytokines present in the milieu, and the type of APC involved. One important implication of the epitope spreading phenomenon is that, at least in the case of intramolecular spreading, subdominant or cryptic epitopes (i.e., not normally “seen” by the immune system) become “visible” and thus contribute to the autoimmune response. The type of cytokine present in the environment is also a key element. In particular, high levels of IFN-γ produced by pathogenic CD4+ Th1 cells enhance target cell immunogenicity by upregulating MHC and costimulatory molecules at the surface of APCs and somatic cells. In addition, a number of reports highlight the essential role of nonprofessional antigen presentation (i.e., mediated by cells other than dendritic cells, the professional APCs) in perpetuating autoimmune responses. In the peptide- or Theiler virus–induced EAE model, microglial cells resident in the CNS function as efficient APCs capable of activating T cells and contributing to epitope spreading (16, 17). Similarly, in a model of the autoimmune disease myasthenia gravis, presentation of an epitope of the acetylcholine receptor by myoblasts favors spreading of the immune response (18). Lastly, autoreactive B cells were shown to be strongly involved in the diversification of autoimmune T cell responses. Thus, during the course of autoimmune thyroiditis, autoantibodies to thyroglobulin could alter antigen processing, favoring the presentation of subdominant pathogenic epitopes (19). In type 1 diabetes, NOD mice deprived of B cells are disease and insulitis free (20). It has also been shown that B lymphocytes can process β cell autoantigens captured by surface immunoglobulins and present them to T cells contributing to the maintenance of the autoimmune response (21–23).
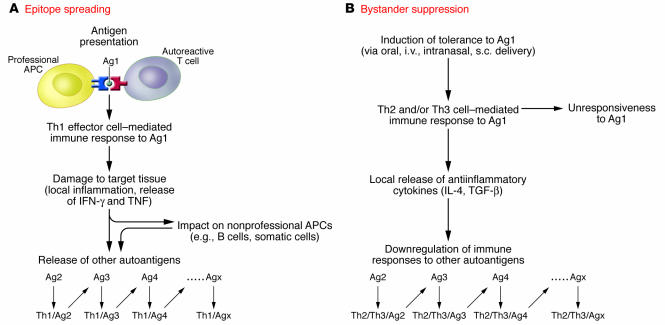
Therefore, professional APCs such as dendritic cells may be required to initiate the autoimmune reaction by adequately processing and presenting the primary autoantigen to naive autoreactive T cells. Once activated, these autoreactive effectors create a proinflammatory environment that in turn influences and modifies the behavior of other cell types, including immune or somatic cells, to acquire APC-like functional properties (Figure 1).
Figure 1. Epitope spreading versus bystander suppression.
(A) In the event that an autoimmune response is triggered by a primary autoantigen (Ag1), the cytokine-mediated proinflammatory environment favors first the development of a Th1 effector cell–mediated immune response to Ag1 (Th1/Ag1), then the release from the damaged target tissue of other autoantigens (Ag2, Ag3, etc.), which trigger specific responses. This spread of specificity of the autoimmune response is one major molecular basis for its chronicity. (B) Bystander suppression operates when self tolerance is induced to one of the candidate autoantigens. The antiinflammatory environment generated may in turn downregulate the autoimmune responses to the other autoantigens involved in the autoimmune response. Major cytokines participating in this antiinflammatory effect are IL-4 and TGF-β, which are produced by Th2 and Th3 regulatory cells, respectively.
The experimental model described by Krishnamurthy et al. (8) represents an interesting tool with which to obtain further insights into these issues, which are important from both fundamental and therapeutic points of view. In fact, the molecular processes that spread pathogenic responses are probably similar to those operating to spread protection in the case of bystander suppression (24, 25). It has been well established in different models of autoimmunity, including EAE (24) and type 1 diabetes, that protection from disease may be induced following delivery by various routes of any of the candidate autoantigens (in the case of autoimmune diabetes using proinsulin, insulin, heat shock protein 60, or glutamic acid decarboxylase). Results of mechanistic studies confirmed that the effective downregulation of the immune response specific to the therapeutic autoantigen rapidly extends to other candidate autoantigens (24, 25) (Figure 1). A better understanding of the underlying molecular mechanisms of these interactions will be essential to expedite the transfer of autoantigen therapy to the clinic.
Footnotes
Nonstandard abbreviations used: IGRP, islet-specific glucose-6-phosphatase catalytic subunit–related protein.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 116:3108–3110 (2006). doi:10.1172/JCI30760.
See the related article beginning on page 3258.
References
- 1.Lehmann P.V., Forsthuber T., Miller A., Sercarz E.E. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358:155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- 2.Yu M., Johnson J.M., Tuohy V.K. A predictable sequential determinant spreading cascade invariably accompanies progression of experimental autoimmune encephalomyelitis: a basis for peptide-specific therapy after onset of clinical disease. J. Exp. Med. 1996;183:1777–1788. doi: 10.1084/jem.183.4.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuohy V.K., Yu M., Weinstock-Guttman B., Kinkel R.P. Diversity and plasticity of self recognition during the development of multiple sclerosis. J. Clin. Invest. 1997;99:1682–1690. doi: 10.1172/JCI119331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuohy V.K., et al. The epitope spreading cascade during progression of experimental autoimmune encephalomyelitis and multiple sclerosis. Immunol. Rev. 1998;164:93–100. doi: 10.1111/j.1600-065x.1998.tb01211.x. [DOI] [PubMed] [Google Scholar]
- 5.Miller S.D., et al. Persistent infection with Theiler’s virus leads to CNS autoimmunity via epitope spreading. Nat. Med. 1997;3:1133–1136. doi: 10.1038/nm1097-1133. [DOI] [PubMed] [Google Scholar]
- 6.Tisch R., et al. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature. 1993;366:72–75. doi: 10.1038/366072a0. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman D.L., et al. Spontaneous loss of T-cell tolerance to glutamic acid decarboxylase in murine insulin-dependent diabetes. Nature. 1993;366:69–72. doi: 10.1038/366069a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krishnamurthy B., et al. Responses against islet antigens in NOD mice are prevented by tolerance to proinsulin but not IGRP. J. Clin. Invest. . 2006;116:3258–3265. doi: 10.1172/JCI29602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wegmann D.R., Norbury-glaser M., Daniel D. Insulin-specific T cells are a predominant component of islet infiltrates in pre-diabetic NOD mice. Eur. J. Immunol. 1994;24:1853–1857. doi: 10.1002/eji.1830240820. [DOI] [PubMed] [Google Scholar]
- 10.Daniel D., Gill R.G., Schloot N., Wegmann D. Epitope specificity, cytokine production profile and diabetogenic activity of insulin-specific T cell clones isolated from NOD mice. Eur. J. Immunol. 1995;25:1056–1062. doi: 10.1002/eji.1830250430. [DOI] [PubMed] [Google Scholar]
- 11.Verdaguer J., et al. Spontaneous autoimmune diabetes in monoclonal T cell nonobese diabetic mice. J. Exp. Med. 1997;186:1663–1676. doi: 10.1084/jem.186.10.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lieberman S.M., et al. Identification of the beta cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc. Natl. Acad. Sci. U. S. A. 2003;100:8384–8388. doi: 10.1073/pnas.0932778100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Utsugi T., et al. Major histocompatibility complex class I-restricted infiltration and destruction of pancreatic islets by NOD mouse-derived beta-cell cytotoxic CD8(+) T-cell clones in vivo. Diabetes. 1996;45:1121–1131. doi: 10.2337/diab.45.8.1121. [DOI] [PubMed] [Google Scholar]
- 14.French M.B., et al. Transgenic expression of mouse proinsulin II prevents diabetes in nonobese diabetic mice. Diabetes. 1997;46:34–39. doi: 10.2337/diab.46.1.34. [DOI] [PubMed] [Google Scholar]
- 15.Nakayama M., et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMahon E.J., Bailey S.L., Castenada C.V., Waldner H., Miller S.D. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat. Med. 2005;11:335–339. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- 17.Vanderlugt C.L., Miller S.D. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat. Rev. Immunol. 2002;2:85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]
- 18.Curnow J., Corlett L., Willcox N., Vincent A. Presentation by myoblasts of an epitope from endogenous acetylcholine receptor indicates a potential role in the spreading of the immune response. J. Neuroimmunol. 2001;115:127–134. doi: 10.1016/s0165-5728(01)00272-7. [DOI] [PubMed] [Google Scholar]
- 19.Dai Y., et al. Enhancing or suppressive effects of antibodies on processing of a pathogenic T cell epitope in thyroglobulin. J. Immunol. 1999;162:6987–6992. [PubMed] [Google Scholar]
- 20.Serreze D.V., et al. B lymphocytes are essential for the initiation of T cell- mediated autoimmune diabetes: analysis of a new ‘’speed congenic’’ stock of NOD.Ig mu(null) mice. J. Exp. Med. 1996;184:2049–2053. doi: 10.1084/jem.184.5.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serreze D.V., et al. B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. J. Immunol. 1998;161:3912–3918. [PubMed] [Google Scholar]
- 22.Reijonen H., Daniels T.L., Lernmark A., Nepom G.T. GAD65-specific autoantibodies enhance the presentation of an immunodominant T-cell epitope from GAD65. Diabetes. 2000;49:1621–1626. doi: 10.2337/diabetes.49.10.1621. [DOI] [PubMed] [Google Scholar]
- 23.Tian J., Zekzer D., Lu Y., Dang H., Kaufman D.L. B cells are crucial for determinant spreading of T cell autoimmunity among beta cell antigens in diabetes-prone nonobese diabetic mice. J. Immunol. 2006;176:2654–2661. doi: 10.4049/jimmunol.176.4.2654. [DOI] [PubMed] [Google Scholar]
- 24.Al Sabbagh A., Miller A., Santos L.M., Weiner H.L. Antigen-driven tissue-specific suppression following oral tolerance: orally administered myelin basic protein suppresses proteolipid protein-induced experimental autoimmune encephalomyelitis in the SJL mouse. Eur. J. Immunol. 1994;24:2104–2109. doi: 10.1002/eji.1830240926. [DOI] [PubMed] [Google Scholar]
- 25.Tian J., Lehmann P.V., Kaufman D.L. Determinant spreading of T helper cell 2 (Th2) responses to pancreatic islet autoantigens. J. Exp. Med. 1997;186:2039–2043. doi: 10.1084/jem.186.12.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]