Abstract
During embryonic development, the kidney epithelium originates from cells that undergo a mesenchymal to epithelial transition (MET). The reverse process, epithelium to mesenchyme transition (EMT), has been implicated in epithelial tumor progression and in the fibrosis that leads to end-stage kidney failure. Snail transcription factors induce both natural and pathological EMT, but their implication in renal development and disease is still unclear. We show that Snail genes are downregulated during the MET that occurs during renal development and that this is correlated with Cadherin-16 expression. Snail suppresses Cadherin-16 via the direct repression of the kidney differentiation factor HNF-1β, a novel route by which Snail disrupts epithelial homeostasis. Indeed, Snail activation is sufficient to induce EMT and kidney fibrosis in adult transgenic mice. Significantly, Snail is also activated in patients with renal fibrosis. Thus, Snail expression is suppressed during renal development and it must remain silent in the mature kidney where its aberrant activation leads to fibrosis.
Keywords: Cadherin-16, epithelial–mesenchymal transition, HNF-1β, renal fibrosis, Snail transcription factors
Introduction
During embryonic development, epithelial and mesenchymal phenotypic transitions are necessary for the correct formation of different tissues. However, these processes are not only involved in development but they are also thought to be implicated in pathological conditions such as cancer and fibrosis (Nieto, 2002; Kalluri and Neilson, 2003; Liu, 2004; Thiery and Sleeman, 2006). One striking example is in kidney development and disease. During development, mesenchymal cells are initially formed by epithelium to mesenchyme transition (EMT) and subsequently, some of these cells undergo mesenchymal to epithelial transition (MET) to form the epithelia of the pronephros, mesonephros and metanephros (Dressler, 2002). In the adult, the reverse process (EMT) is implicated in the progress of carcinomas and in organ fibrosis. Fibrosis is regarded as the main event leading to end-stage kidney failure in most progressive renal diseases (Liu, 2004). Thus, while kidney formation involves reciprocal transformations, suppression of this plasticity in the adult is critical to maintain normal tissue architecture and homeostasis.
The Snail gene family of transcription factors is best known for its ability to trigger EMT, converting epithelial cells into mesenchymal cells with migratory properties. Snail genes influence both tissue formation during embryonic development and the acquisition of invasive properties in epithelial tumors (Barrallo-Gimeno and Nieto, 2005). Of the three vertebrate Snail family members, Snail and Slug (recently been renamed as Snail1 and Snail2, respectively; Barrallo-Gimeno and Nieto, 2005) are functionally equivalent (del Barrio and Nieto, 2002; Bolós et al, 2003).
Prompted by our observation that Snail1 is a strong suppressor of kidney-specific cadherin (Cadherin-16) expression in cell culture, we have studied the role of Snail genes in the kidney. We found that Snail genes are repressed during kidney differentiation and identified that they regulate Cadherin-16 expression by repressing HNF-1β. To determine to what extent plasticity may be retained in the adult, we developed a system allowing us to reactivate Snail expression. We show that Snail activation induces renal fibrosis in transgenic mice and report the presence of pathological Snail expression in human fibrotic kidneys. Hence, the activation of Snail in the adult has profound effects on epithelial homeostasis in the kidney, which can be considered as a process of reverse embryogenesis likely to be pivotal in the development of renal fibrosis.
Results
To gain insight into the mechanisms used by Snail to repress the epithelial phenotype, we established an in vitro model of Snail1-induced EMT in the mouse mammary gland-derived epithelial cell line NMuMG. These cells typically display a cobblestone-like phenotype in culture (Figure 1A), yet they acquire a mesenchymal phenotype when stably transfected with Snail1 (Figure 1B). This morphological change is accompanied by the loss of E-Cadherin and a reorganization of the actin cytoskeleton (Supplementary Figure 1). Likewise, Snail1 transfectants become motile and acquire invasive properties (Supplementary Figure 1). Indeed, the transcription of many epithelial genes was strongly repressed in Snail1 transfectants, including that of E-Cadherin (Figure 1C) and other known Snail1 targets such as mucin-1, claudins and occludins (data not shown and Barrallo-Gimeno and Nieto, 2005; Huber et al, 2005). Thus, this cell-culture system provides a potent tool to investigate EMT driven by Snail.
Figure 1.
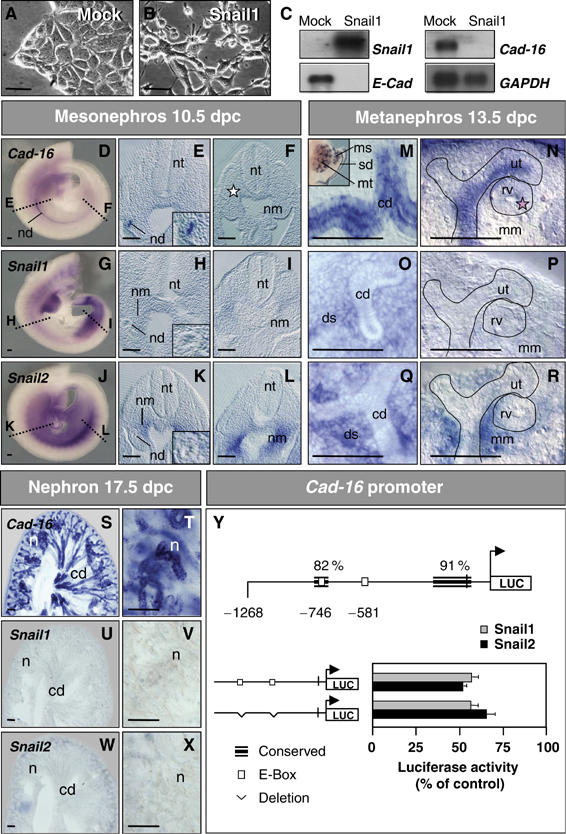
Snail1 represses the kidney epithelial Cadherin-16 both in cell culture and in the embryo. (A, B) Phase-contrast images and (C) Snail1, E-Cadherin and Cadherin-16 expression in stable mock- and Snail1-transfected cells. Snail1 expression represses E-cadherin and Cadherin-16 transcription. GAPDH levels are shown as a control. (D–X) ISH for Cadherin-16, Snail1 and Snail2 in whole-mount mouse embryos and transverse sections taken at the mid (E, H, K) and posterior (F, I, L) trunk levels. Cadherin-16 is expressed in the newly formed nephric duct epithelium (nd, E) that no longer expresses Snail genes (H, K insets). Snail transcripts are observed in the undifferentiated anterior (H, K) and posterior (I, L) nephrogenic mesenchyme (nm). Dissected urogenital system (see M, inset) or gelatin sections (M–R) hybridized with Cadherin-16 and Snail probes. Cadherin-16 is expressed in the collecting duct epithelia (M) and their ureteric tips (ut, N) of the developing metanephros but it is absent from the newly forming renal vesicle (pink star, rv; N). Expression is also detected in the sexual ducts and in the tubules of the transient mesonephros (sd and ms; M, inset). Snail1 and Snail2 expression is restricted to the metanephric mesenchyme and to the deep stroma (mm and ds, O–R). Nephrons (n) appear after the complete epithelialization of the metanephric mesenchyme. The nephron epithelia and the collecting ducts (cd) strongly express Cadherin-16 (S, T), whereas Snail1 and Snail2 expression disappears when the mesenchyme transforms into epithelia (U–X). nt, neural tube. Scale bar, 100 μm. (Y) Snail proteins repress the Cadherin-16 promoter. Schematic representation of the mouse Cadherin-16 promoter showing the regions of high similarity between mouse and human, and the location of the consensus Snail-binding sequences (white boxes). Luciferase reporter constructs carrying the wild-type mouse Cadherin-16 promoter (−1268) or deletions in the two E-boxes were assayed in the NMuMG cells together with either the mouse Snail1 or Snail2 expression vectors or an empty vector as a control (pcDNA3). Luciferase activity was measured 24 h after transfection and the activity is expressed relative to that of the wild-type construct. The results are the mean values±S.E. of duplicates from four independent experiments. Deletions of the Snail-binding sites do not relieve the repression of the Cadherin-16 promoter activity.
Mock-transfected NMuMG cells were found to contain a high level of Cadherin-16 transcripts, and its expression was completely downregulated in stable Snail1 transfectants (Figure 1C). Cadherin-16 is a kidney-specific cadherin expressed in renal tubular epithelial cells and collecting ducts of the differentiating and mature kidney (Thomson et al, 1995). Hence, Cadherin-16 expression was unexpected in a mammary gland-derived cell line and indeed, it was not expressed in the mouse mammary gland or in human mammary gland-derived cell lines (Supplementary Figure 2), and we concluded that Cadherin-16 expression was merely a peculiarity of NMuMG cells. Given the importance of MET and EMT in renal development, this result prompted us to determine whether Snail genes regulate Cadherin-16 expression in the kidney.
Inverse correlation between Cadherin-16 and Snail gene expression during kidney development
To determine whether Snail1 could repress Cadherin-16, we first examined the expression of each gene at different stages during murine kidney development. As Snail2 also represses E-Cadherin and it is considered functionally equivalent to Snail1 (del Barrio and Nieto, 2002; Bolós et al, 2003), we also analyzed its expression in the kidney. Cadherin-16 expression commences during the differentiation of the developing kidney, concomitant with the MET that gives rise to the different renal epithelial cell populations (Wertz and Herrmann, 1999). The first renal epithelium to form is the nephric duct, derived from the intermediate mesoderm in the caudal part of the trunk region. Although the early mesoderm expresses Snail1 in mouse embryos, the expression of the Snail1 and 2 genes becomes more complex as the mesoderm segregates into distinct populations. As such, when Cadherin-16 is not yet expressed in the embryo (not shown), Snail2 expression is upregulated in the intermediate and lateral mesoderms (Sefton et al, 1998). Once the nephric duct became epithelialized, Snail genes expression was downregulated and Cadherin-16 transcripts could be detected (Figure 1E, H and K). The mesenchyme adjacent to the nephric duct (nm, nephrogenic mesenchyme) expresses both Snail genes (Figure 1H and K) and later epithelializes to form the transient mesonephros. The resulting mesonephric tubules express high levels of Cadherin-16 (Figure 1M, inset).
In the posterior embryo, Snail transcripts are expressed in the nephrogenic mesenchyme where the metanephros will form (Figure 1I and L). Strong Cadherin-16 expression is observed in the collecting ducts of the metanephros that arise from the branching of the nephric duct, whereas Snail genes expression is maintained in the deep stroma (Figure 1M, O and Q). The ureteric tips of the collecting ducts also express Cadherin-16 unlike the condensing mesenchyme that gives rise to the renal vesicle and is still devoid of transcripts (Figure 1N). In the metanephric mesenchyme that surrounds the renal vesicle and that gives rise to the nephrons, Snail2 and weaker Snail1 expression was detected (Figure 1P and R). Again, the epithelialization of this tissue was correlated with Snail downregulation (Figure 1M–R).
At 17.5 days post-coitum (dpc), the nephron displayed robust Cadherin-16 expression (Figure 1S and T), having downregulated Snail genes by this stage (Figure 1U–X). This situation was maintained throughout adulthood (Supplementary Figure 3). Thus, the expression of the Snail and Cadherin-16 genes is complementary during kidney ontogenesis and tissues that express Cadherin-16 are derived from Snail1- and Snail2-expressing mesenchyme. These results are compatible with Snail genes acting as repressors of Cadherin-16 in vivo.
Direct repression of HNF-1β transcription by Snail genes prevents Cadherin-16 expression
Given the precedent of E-Cadherin (Batlle et al, 2000; Cano et al, 2000), we examined whether Snail proteins could directly repress Cadherin-16 transcription. Two consensus E-boxes for Snail binding were identified at −581 and −746 bp in the mouse Cadherin-16 promoter (Figure 1Y). A construct containing these two boxes within sequences able to recapitulate the physiological expression of Cadherin-16 in the developing kidney (Whyte et al, 1999; Shao et al, 2002) was cotransfected with either Snail1 or Snail2 constructs. Promoter activity decreased to 61 and 56%, respectively, indicating that Snail proteins do indeed repress Cadherin-16 transcription. However, the modification or deletion of the individual E-boxes (Supplementary Figure 4) or simultaneous deletion of both boxes did not impair this repression (Figure 1Y), indicating that Snail1 and 2 do not directly downregulate Cadherin-16 expression.
As Snail proteins act as transcriptional repressors (Nieto, 2002), we speculated that they might downregulate an activator of Cadherin-16. The HNF-1β transcription factor promotes the epithelial phenotype in the kidney by strongly activating Cadherin-16 expression (Bai et al, 2002). Thus, we compared HNF-1β expression with that of Cadherin-16 during kidney development. HNF-1β was expressed in the differentiated epithelia of the anterior nephric duct (Figure 2B), where Snail genes had been downregulated and Cadherin-16 transcripts were found at 10.5 dpc (Figure 1E, H and K, insets). At this stage, HNF-1β transcripts were also detected in the differentiating posterior nephric duct (dnd, white star; Figure 2C), where Cadherin-16 was not yet expressed (white star; Figure 1F). In the cortex of the metanephros at 13.5 dpc, HNF-1β expression was observed in the ureteric tips of the collecting ducts and in the forming renal vesicles that were still devoid of Cadherin-16 transcripts (Figures 1N and 2D and G). However, Cadherin-16 was expressed strongly in the previously differentiated collecting ducts (Figure 2E), while the tips of the collecting ducts and the renal vesicles are surrounded by Snail2-expressing cells yet to condense (Figure 2F). Accordingly, HNF-1β transcripts were detected in the areas of epithelializing mesenchyme (Figure 2D and G), some of which do not yet express Cadherin-16 (compare Figures 1F with 2C, white stars, and Figures 1N with 2G, pink stars). The differentiated nephrons that give rise to functional epithelial structures of the definitive kidney express both HNF-1β (Figure 2H and Cadherin-16 (Figure 1S and T). Thus, the expression of HNF-1β is compatible with it acting as an activator of Cadherin-16.
Figure 2.
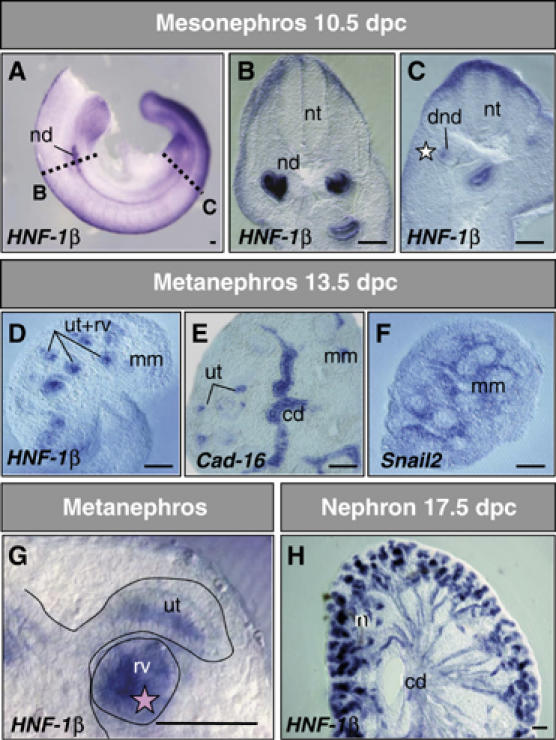
HNF-1β expression precedes that of Cadherin-16 in the developing kidney epithelia. (A–H) ISH of embryos or dissected kidneys. HNF-1β expression was observed in the epithelia at all stages of kidney development. At 10.5 dpc, HNF-1β transcripts are seen in the nephric duct (nd, B) and in the condensing mesenchyme of the differentiating posterior nephric duct (dnd, C), which is still devoid of Cadherin-16 transcripts (white star in Figure 1F). At 13.5 dpc, in addition to the collecting duct (cd), the ureteric tips of the ducts (ut) and the renal vesicle (rv) contain HNF-1β transcripts (D, G), some of which remain negative for Cadherin-16 (E and pink star in Figure 1N). Snail2 is expressed in the metanephric mesenchyme (mm) but not in the areas expressing HNF-1β. (H) At 17.5 dpc, the epithelia of the nephrons (n) and the collecting ducts express high levels of HNF-1β and both Snail genes have been downregulated (Figure 1U–X). nt, neural tube; scale bars, 100 μm.
We detected HNF-1β transcripts in NMuMG cells, explaining the high levels of Cadherin-16 expression, but both genes were completely downregulated in stable Snail1 transfectants (not shown and Figure 1C). To determine if activating Snail was sufficient to repress the expression of these genes, we used a chimeric construct in which Snail1 could be activated by tamoxifen (Snail1-ER; see Materials and methods). Snail activation in stable transfectants triggered EMT within 24 h (Figure 3A) analyzed by the loss of E-cadherin, reorganization of the actin cytoskeleton and the acquisition of motile and invasive properties (not shown, but see Supplementary Figure 1). These phenotypic changes were concomitant with the downregulation of HNF-1β and Cadherin-16 (Figure 3C). Interestingly, within 6 h of Snail1 activation HNF-1β levels fell by 20%, whereas Cadherin-16 transcripts remained unaffected (not shown), indicative of the sequential downregulation of HNF-1β and Cadherin-16 and suggesting that Snail1 inhibits Cadherin-16 expression by repressing HNF-1β. Indeed, the two consensus sequences for Snail binding in the HNF-1β promoter indicate that Snail proteins could directly repress HNF-1β transcription. The most proximal of these was conserved and lies in a highly conserved region in mouse, rat and human (Figure 3D). Accordingly, both Snail proteins repressed HNF-1β promoter activity when assayed using a reporter construct that included both these boxes (Figure 3D). Moreover, while deletion of the distal nonconserved E-box did not affect repression, deleting the conserved box abrogated inhibition (Figure 3D).
Figure 3.
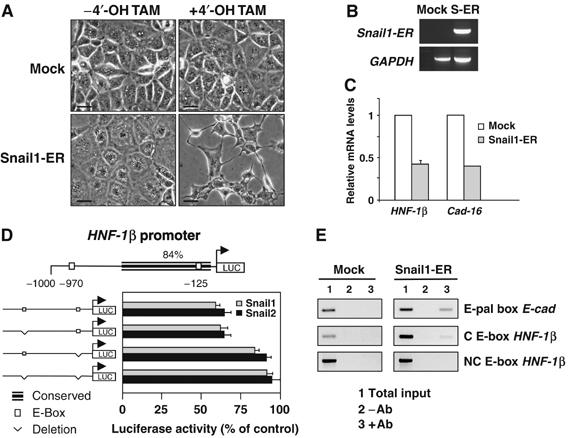
Snail genes directly repress HNF-1β transcription, which in turn impairs Cadherin-16 expression. (A) NMuMG cells stably transfected with an inducible Snail1 construct (Snail1-ER) or with the empty vector (Mock) were analyzed 24 h after induction. Note the phenotypic change of the Snail1-transfected cells upon 4′-OH-tamoxifen (4′-OH-TAM) administration. Scale bar, 25 μm. (B) Transgene expression visualized by RT–PCR in Mock and Snail1-ER transfectants (S-ER). (C) Quantitative RT–PCR for Cadherin-16 and HNF-1β 24 h after 4′-OH-tamoxifen administration. (D) Diagram of the 1 kb region upstream of the translational initiation site in the mouse HNF-1β gene showing regions highly conserved between mouse and human. Of the two consensus E-boxes for Snail binding, only one lies within the conserved region. Snail1 and Snail2 repressed the activity of the wild-type HNF-1β promoter (measured as described for the Cadherin-16 promoter), but they did not affect the promoter constructs in which the conserved E-box was deleted. (E) ChiP analyses show that Snail1 binds directly to the HNF-1β promoter. ChIP assays were carried out with anti-ER antibodies on Mock- and Snail1-ER cells 24 h after induction. As a positive control, the interaction of Snail with the E-pal element of the E-cadherin promoter is shown. Snail does not bind to the nonconserved (NC) E-box in the HNF-1β promoter. Amplifications of the indicated promoter regions in the input (1), nonimmunoprecipitated (2) and immunoprecipitated (3) fractions are shown. The data presented are representative of three independent experiments.
Chromatin immunoprecipitation (ChiP) further demonstrated that Snail1 binds to the HNF-1β promoter (Figure 3E), repressing its transcriptional activity and surely accounting for the subsequent downregulation of Cadherin-16 expression. This interaction is specific for the fragment containing the conserved E-box, as HNF-1β promoter sequences were not recovered when a nonconserved promoter region was used. Indeed, Snail1 bound in a similar manner to the HNF-1β promoter sequences as to E-cadherin promoter sequences (Figure 3E). As NMuMG cells are derived from the mammary gland, we examined whether this repression also occurred in a kidney cell line. Indeed, HNF-1β and Cadherin-16 transcription was completely downregulated by Snail1 in MDCK cells (Supplementary Figure 5).
Snail activation induces renal fibrosis in transgenic mice
As Snail genes repress HNF-1β expression in culture and consequently the epithelial phenotype, we assessed whether they behave similarly in vivo. More importantly, we assessed whether they could induce full EMT in the kidney and thus, the loss of the epithelial homeostasis. A transgenic mouse expressing the same tamoxifen-inducible Snail1-ER construct was generated identifying a line that specifically expressed significant levels of transgenic protein in the kidney (Supplementary Figure 6). This inducible system is appropriate to analyze transcription factor activity since despite its constitutive expression, the exogenous protein is only active after nuclear translocation upon tamoxifen administration (Figure 4A–H). In these animals, nuclear exogenous Snail1 protein was observed after two weeks of subcutaneal tamoxifen administration (Figure 4B, D, F and H). Both HNF-1β and Cadherin-16 were downregulated in the cortex and the medulla of kidneys from tamoxifen-treated mice (Figure 4I–P and U). Interestingly, we also observed the induction of Snail2 expression (Figure 4Q–T and U), the family member whose expression is prominent during kidney development. These data are in agreement with our previous finding that Snail1 induces Snail2 transcription during neural crest development in Xenopus embryos (Aybar et al, 2003).
Figure 4.
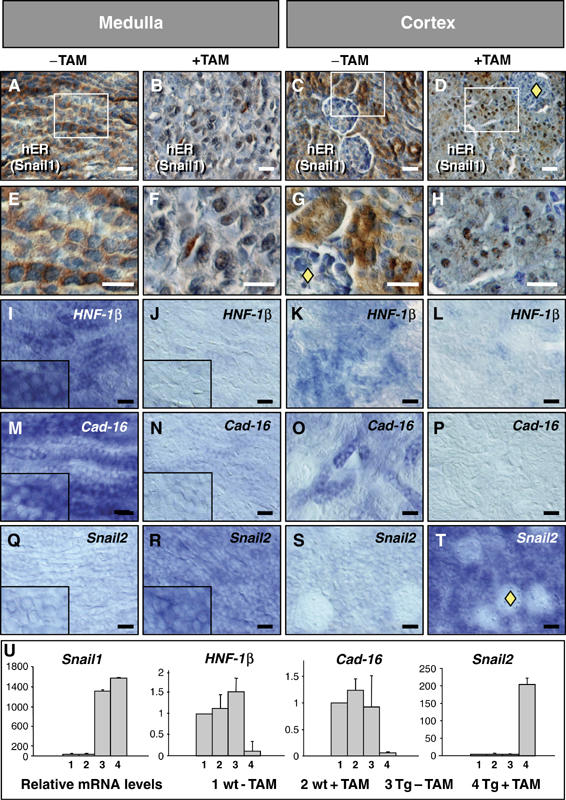
Snail expression induces the loss of the epithelial character in postnatal transgenic kidneys. (A–H) Exogenous Snail1 protein was translocated to the nucleus upon tamoxifen (TAM) administration, as seen with an anti-hER antibody in both the medulla and the cortex. Note the absence of the transgenic protein in the glomeruli (yellow diamonds). (I–P) HNF-1β (I–L) and Cadherin-16 expression (M–P) in sections from 2-week-old transgenic kidneys show the loss of the two epithelial and differentiation markers. (Q–T) Snail1 activation also induces Snail2 expression but not in the glomeruli (yellow diamond in T). (U) Quantitative RT–PCR analysis of Snail1, HNF-1β, Cadherin-16 and Snail2 expression in wild-type and transgenic kidneys in the absence or presence of TAM. Transcription is normalized to GAPDH mRNA expression and the error bars represent the standard error of the mean.
The downregulation of Cadherin-16 in vivo can be explained by Snail1 repressing HNF-1β transcription and the loss of both markers should be associated with the loss of epithelial characteristics. Indeed, upon Snail activation, collecting duct cells in the medulla seemed to acquire a fibroblast-like morphology (Figure 4F). The phenotype of the transgenic kidneys with active Snail1 was further analyzed and the collecting ducts in the medulla were clearly disorganized (Figure 5B). This disorganization correlated with the morphological changes characteristic of EMT (Figure 5B inset) and the repression of epithelial markers such as HNF-1β, Cadherin-16 (Figure 4) and also E-Cadherin (Figure 5C and D). In addition, cells expressed mesenchymal markers such as the intermediate filament vimentin (Figure 5E and F) and smooth muscle actin (SMA, Figure 5G and H). These histological changes are reminiscent of those observed after experimentally induced or pathological fibrosis (Li et al, 2003; Sato et al, 2003; Zeisberg et al, 2003; McMorrow et al, 2005; Slattery et al, 2005). The deposition of Collagen I is a hallmark of renal fibrosis (Alexakis et al, 2005) and focal deposition was observed after the activation of Collagen I transcription by Snail1 (Figure 5I–L). Epithelial disorganization was also observed in the cortex, where dilated tubules and disrupted basement membranes were readily apparent as well as Collagen I deposition (Figure 5M–T). The mice died after about 2 weeks of treatment, presumably of renal failure given the distorted morphology of the kidney and the high levels of urea in their serum (not shown).
Figure 5.
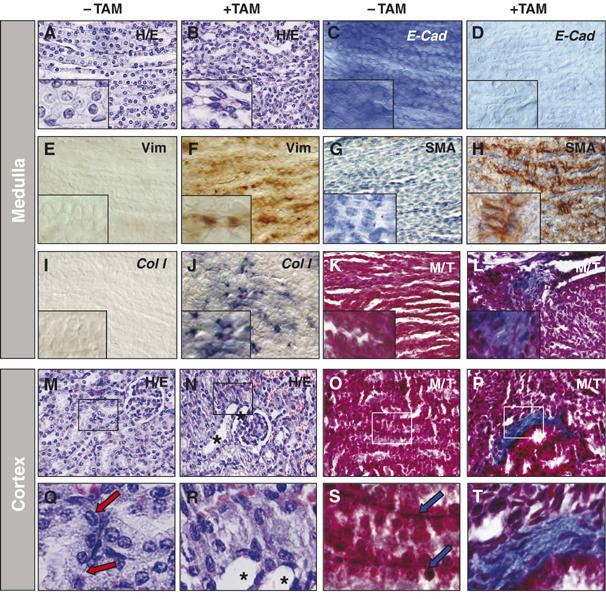
Snail expression induces EMT and features of fibrosis in postnatal transgenic mice. (A, B) Hematoxylin/eosin staining of sections from the kidneys shown in Figure 4. Note the depolarized and fibroblast morphology of the collecting duct cells in the medulla region of animals treated with tamoxifen (+TAM). (C, D) E-Cadherin expression is completely downregulated in the kidneys of transgenic mice upon Snail1 activation, confirming the loss of epithelial character. (E–H) Snail 1 activation also induces the expression of vimentin (E, F) and smooth muscle actin (SMA, G, H), both indicating the appearance of mesenchymal characteristics. (I–L) Collagen I transcripts (I, J) and Collagen fibrotic deposits (K, L) detected by Masson–Trichrome staining in the transgenic kidneys upon Snail1 activation. (M–T) Similar signs of epithelial disruption and fibrosis are also observed in the cortex of the transgenic kidneys from tamoxifen-treated mice. Hematoxylin/eosin staining (M, N) and collagen deposits (O, P). (Q–T) High-power images to better assess the dilation of renal tubules (asterisks) and the disruption of basement membranes upon Snail1 activation. Basement membranes that can be clearly observed in the untreated transgenic kidneys (arrows).
As the postnatal kidney may be considered immature or prone to revert to the embryonic phenotype, it was important to check whether Snail1 activation alone could induce fibrosis in the adult kidney. When tamoxifen was administered for 2 months commencing 2 months after birth (Figure 6), the collecting ducts and renal tubules became disorganized and dilated (Figure 6A–D), and Collagen I deposits were formed (Figure 6E–H). A feature frequently associated with renal fibrosis is the appearance of cysts, a phenomenon that was incipient in the postnatal transgenic kidneys following Snail activation. As the young mice died very early, we could not follow the progress of this putative polycystic kidney disease. Nevertheless, we observed the appearance of scattered cysts in the adult kidneys when Snail1 was activated over several weeks (Figure 6D).
Figure 6.
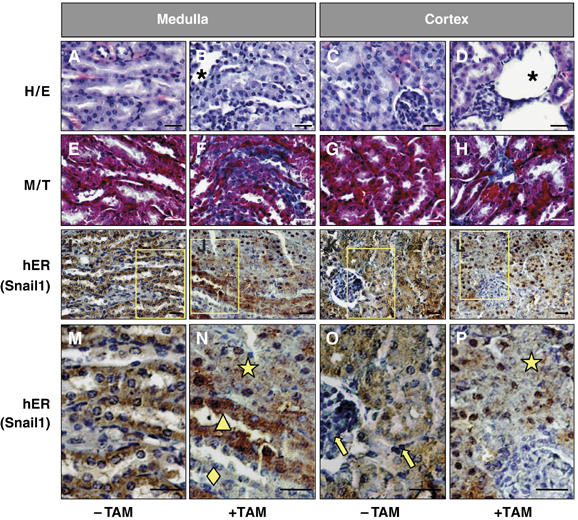
Snail activation is sufficient to induce renal fibrosis in adult transgenic mice. (A–D) Hematoxylin/eosin staining of sections from 4-month-old transgenic mice kidneys. Tamoxifen treatment in mice (+TAM) was initiated 2 months after birth. Note the defective overall morphology including the presence of dilated tubules. (E–H) Fibrotic deposits can be observed by Masson–Trichrome staining in tamoxifen-treated mice. Fibrosis is also manifested by the presence of cysts (D). (I–P) Detection of the transgenic Snail1 protein with an anti-hER antibody (brown) in paraffin sections counterstained with hematoxylin (blue). Note the absence of transgenic protein in the glomeruli and in the interstitial cells (yellow arrows in O) and in some ducts in the medulla (yellow diamond in N). The yellow triangle in (N) indicates ducts in which the Snail1 protein has not been efficiently translocated to the nucleus (note the blue nuclei and brown cytoplasms). The ducts and tubules with Snail1 nuclear expression lost the epithelial character and present a completely disorganized structure (yellow star in N and P). Scale bars, 25 μm.
The loss of epithelial features perfectly correlated with Snail activation since the transgenic protein was expressed in the renal tubules and the collecting ducts, but it was absent from the glomeruli and the interstitial cells (Figure 6O). Accordingly, Snail1 activation did not induce morphological changes or Snail2 expression in the glomeruli of postnatal animals (yellow diamond in Figure 4D, G and T) and adult mice (yellow arrow in Figure 6O). In the medulla of adult mice, the transgenic protein displayed a mosaic distribution. A tubular structure was maintained in ducts that did not express Snail1 or when the protein was not efficiently translocated to the nucleus upon tamoxifen administration (Figure 6N, yellow diamond and triangle, respectively). However, when Snail1 reached the nucleus, the tubules were disorganized and the epithelial structure was lost in both the medulla and the cortex (Figure 6N and P, yellow stars). The mosaic expression observed in the adult tubules explains why epithelial structures are less disorganized than in the young animals. These results provide an internal control of the activity of the Snail1 protein and highlight its ability to repress the epithelial phenotype in a cell autonomous manner. Indeed, the activation of Snail1 is sufficient to induce the features of renal fibrosis in both young and adult animals.
Snail genes are pathologically active during human renal fibrosis
We next analyzed normal and fibrotic human kidney tissue obtained from patients subjected to nephrectomy (see Materials and methods). Features of renal fibrosis were observed in the tissue from patients affected by renal failure, including dilation and tubule disorganization in the medulla and cortex (Figure 7B and H). Strong vimentin expression (Figure 7D and J) was accompanied by deposition of Collagen I in the sclerotic areas of the tubule and massive interstitial fibrosis in both the cortex and the medulla (Figure 7F and L). While no Snail1 or Snail2 expression was observed in the normal human tissue (Figure 7M; n=4, see also Supplementary Figure 3), high levels of Snail1 were detected in patients with renal fibrosis (Figure 7M). As in the experimental mouse model, Snail2 was also expressed in the fibrotic tissue. Interestingly, the presence of fibrotic and nonfibrotic tissue in the same kidney showed that Snail activation was only associated with fibrosis (Figure 7M). Thus, human fibrosis is accompanied by the aberrant activation of Snail expression.
Figure 7.
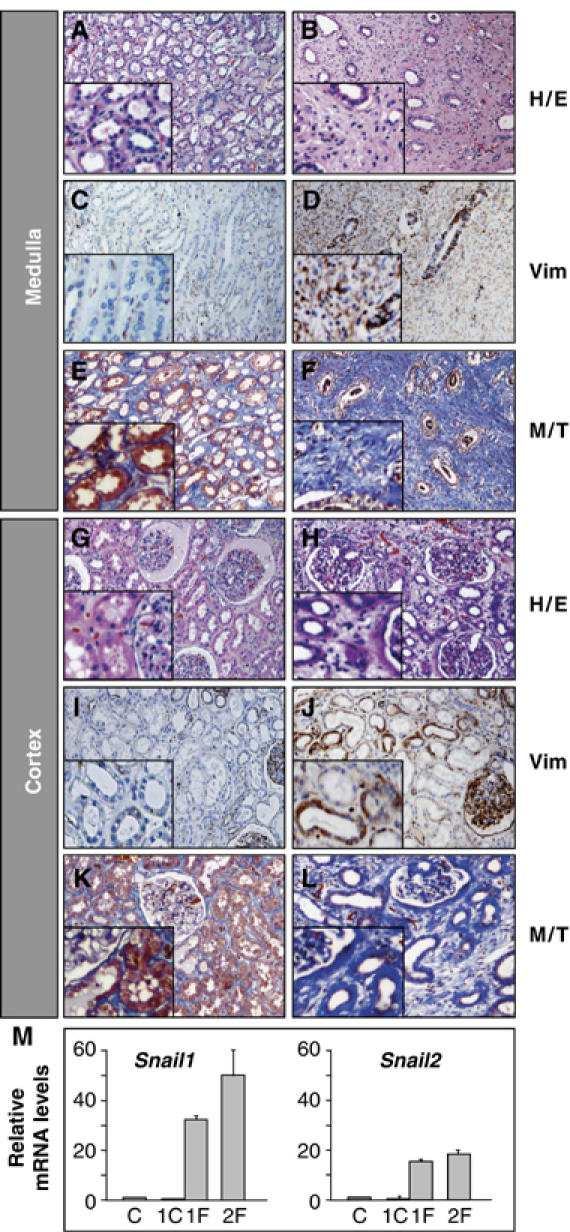
Fibrotic human kidneys show strong Snail expression. Sections from normal human kidney tissue (A, C, E, G, I, K) and from a patient subjected to nephrectomy due to urinary obstruction and kidney failure (B, D, F, H, J, L) showing hematoxylin/eosin staining (H/E), vimentin expression (Vim) and fibrotic deposits in blue following Masson–Trichome staining (M/T). (M) Quantitative RT–PCR analysis of Snail1 and Snail2 expression in normal human kidney tissue (C, n=4), nonfibrotic (1C) and fibrotic tissue from patient 1 (1F) and patient 2 (2F). Transcript levels are normalized to GAPDH mRNA expression and the error bars represent the standard error of the mean.
Discussion
The data presented here implies that Snail not only represses the prototypical epithelial cadherin, E-Cadherin, but also the kidney-specific Cadherin-16. This repression is indirect, via the well-characterized regulator of renal differentiation, HNF-1β, in contrast with Snail's direct transcriptional suppression of E-Cadherin through promoter binding. This defines a new pathway contributing to Snail's activity as a general epithelial repressor and in the disruption of tissue homeostasis, leading to the appearance of pathological features common to end-stage renal failure.
Genetic observations in mice have shown that HNF-1β is important for normal kidney function. Polycystic renal disease is seen in transgenic mice expressing either a mutant HNF-1β under the control of the Cadherin-16 promoter (Hiesberger et al, 2004) or carrying a kidney-specific deletion (Gresh et al, 2004). HNF-1β inhibits the autosomal-recessive polycystic kidney disease gene, Pkdh1 (Hiesberger et al, 2004) and other genes in which mutations are associated with cystic diseases (Gresh et al, 2004). Thus, Snail genes can now be added to this transcriptional network, as upstream effectors and the first known repressors of HNF-1β. Interestingly, mutations in HNF-1β cause abnormalities in human renal development (Edghill et al, 2006), including cysts, familial hypoplastic glomerulocystic disease, malformations and atypical familial hyperuricemic nephropathy. Our finding that Snail genes repress the expression of HNF-1β suggests that genetic or epigenetic alterations influencing Snail expression or activity can cause renal developmental abnormalities independently of HNF-1β mutations. Interestingly, our adult mice in which Snail gene expression was reactivated showed a milder cystic phenotype than that described when HNF-1β function is lost in mouse models (Gresh et al, 2004; Hiesberger et al, 2004). This is very likely due to the fact that in those models the inactivation of HNF-1β occurs at an early developmental stage, as the transgene is under the control of the Cadherin-16 promoter. However, in our model, inactivation was induced in the mature kidney that had developed normally but nevertheless, we show that Snail1 activation in the adult kidney was sufficient to drive epithelial to mesenchymal transition and to favor the deposit of Collagen. Thus, Snail is a good candidate to coordinate the fibrosis and cyst formation that occurs in human renal disease.
Kidney fibrosis and scarring is thought to be a key process linking progressive loss of renal function with a wide range of primary diseases such as glomerulonephritis (including IgA nephropathy), diabetes, toxic injury, congenital abnormalities, urinary tract obstruction and chronic rejection of transplanted kidneys (Kalluri and Neilson, 2003; Liu, 2004; Zeisberg and Kalluri, 2004; Vongwiwatana et al, 2005). Although renal fibrosis was believed to be associated with the activation of interstitial fibroblasts, myofibroblasts also appear to originate from renal tubular epithelial cells that undergo an EMT (Strutz et al, 1995; Iwano et al, 2002). There is evidence that tubular EMT occurs in the kidneys of patients with renal fibrosis (Jinde et al, 2001; Rastaldi et al, 2002) and in animal models where as much as 36% of the fibroblasts involved seem to be derived from local tubular epithelial cells (Kalluri and Neilson, 2003).
We show that Snail1 activation in the kidney promotes EMT of both tubular and collecting duct cells inducing fibrosis. Although studies in vitro suggest that medulla cells are resistant to EMT (Liu, 2004), it does not strike us as strange to find both the cortex and the medulla affected by Snail in vivo. Both epithelial cells from the renal collecting duct and the tubular epithelium are derived from Snail-expressing embryonic mesenchyme (the intermediate mesoderm and the metanephric mesenchyme, respectively). Hence, pathological activation of Snail in the adult could be regarded as a return to the embryonic phenotype, evidence of the reversibility of these processes and the restoration of a plastic state. Importantly, this also highlights the relevance and consequences of the reactivation of Snail in the adult, capable of inducing EMT and fibrosis in a cell-autonomous manner.
Although Snail genes play important roles during embryonic development, it seems critical that they are maintained silent in the adult. Epigenetic mechanisms are probably fundamental in this silencing, and are presumably reversed when Snail is activated in renal fibrosis and cancer. Indeed, we have found aberrant Snail expression in the fibrotic areas of human kidneys and higher levels of Snail expression due to promoter demethylation have been correlated with the increase in the invasive properties of carcinoma derived cell lines (Fraga et al, 2004). This supports the idea that the reversal of silencing or transcriptional regulation rather than irreversible genetic loss contributes to tumor progression (Cheng et al, 2001) and perhaps, to organ fibrosis. Interestingly, changes in expression related to epigenetic mechanisms have been associated with aging (Burzynski, 2005) and the aged kidney is much more susceptible to fibrosis (Melk, 2005).
It seems likely that the pathological activation of Snail may contribute to other fibrotic diseases. Thus, it would be interesting to analyze Snail's involvement in lung and cavernous fibrosis (Chilosi et al, 2003; Ryu et al, 2005) and even in the EMT of epithelial cells, pericytes and other progenitors from the circulatory pool during systemic sclerosis and related rheumatic diseases (Postlethwaite et al, 2004). In relation to this, Snail is upregulated during the EMT undergone by mesothelial cells in patients treated with peritoneal dialysis (Yañez-Mo et al, 2003), renal cells following unilateral ureteral obstruction in mice (Sato et al, 2003) and hepatocytes (Valdés et al, 2002). Interestingly, these pathological processes can be induced by TGF-β, a potent Snail activator (Barrallo-Gimeno and Nieto, 2005). As abnormal high levels of TGF-β have been associated with renal fibrosis (Liu, 2006) and activation of Snail is sufficient to trigger fibrosis in the adult, we propose that Snail may be the key transducer of TGF-β signalling in these pathologies. Importantly, the antagonism of TGF-β by BMP7 seems to be a promising strategy to treat renal fibrosis as BMP7 can reverse TGF-β-induced renal fibrosis in mice (Zeisberg et al, 2003; Zeisberg and Kalluri, 2004), and mice mutant for the BMP signaling activator kielin are more susceptible to the disease (Lin et al, 2005). Signalling molecules such as TGF-β and BMPs trigger complex transduction cascades. Thus, if Snail transduces the pathological TGF-β signal and its sole activation induces all the features of renal fibrosis, inhibiting Snail activity could provide a more specific way to prevent or reverse the disease. In summary, we show that the reactivation of otherwise silent developmental pathways leads to loss of adult tissue homeostasis, and may make a major contribution to the progression of pathological conditions, in particular those associated with aging and degenerative processes.
Materials and methods
Plasmids and antibodies
Expression plasmids
pcDNA3-Snail1 and pcDNA3-Snail2 contain the mouse Snail1 and Snail2 cDNAs, respectively. pcDNA3-Snail1-ER (CMV promoter) contains the Snail1-coding sequence fused to a mutated version of the ligand-binding domain of the human estrogen receptor that recognizes the synthetic ligand 4′-OH-Tamoxifen. The fragment corresponding to the binding site was obtained from pCre-ERT2 kindly provided by P Chambon (Feil et al, 1996). This construct was used to generate the stable Snail1-inducible NMuMG transfectants and for the generation of Snail1-inducible transgenic mice (see below). Reporter plasmids. The mouse Cadherin-16 promoter reporter construct pKsp(1268F)-Luc was a kind gift from Dr Peter Igarashi (U Texas Southwestern Medical Center, Dallas, TX). The mouse HNF-1β promoter sequence containing 1072 bp upstream of the ATG was amplified by PCR from NMuMG cells DNA using the primers: 5′-ggtaccATCTACACATTCACTACTAGA-3′ and 5′-acgcgtTTTCCAAGGACGGAAAAAGAA-3′ (GenBank™ sequence X55842 plus KpnI and MluI restriction sites, respectively). The purified PCR product was subcloned into the pGL3-basic vector (Promega). The Quickchange Site Directed Mutagenesis kit (Stratagene) was used to mutate the E-boxes in the mouse Cadherin-16 promoter. The core 5′-CA(G/C)(G/C)TG-3′ sequence was mutated to 5′-AA(G/C)(G/C)TA-3′. The sequences of the oligonucleotides used to delete the E-boxes on the Cadherin-16 and HNF-1β promoters are available upon request.
Antibodies
Anti-E-cadherin (1:200, Takara), anti-vimentin (1:200, Dako), anti-smooth muscle actin (1:200, Dako). The Snail1-ER fusion protein was detected by immunoblotting or immunohistochemistry using an anti-human estrogen receptor antibody (1:100, Santa Cruz). F-actin was detected using FITC-phalloidin (1:10, Sigma).
Cell culture and generation of stable Snail1- and Snail1-ER-transfected cells
For transfection studies, the NMuMG and MDCK lines were plated in six-well plates (5 × 104 cells per well) in a 1:1 mixture of Ham's F12 Medium and DMEM supplemented with 100 IU/ml penicillin, 0.1 mg/ml streptomycin, 2 mM glutamine, 2.5 μg/ml amphotericin B, 10% fetal bovine serum and 10 μg/ml insulin. DNA constructs for constitutive or inducible Snail1 expression or control vectors (500 ng) were added to the cells 24 h after seeding in the presence of LIPOFECTAMIN (Roche) and the transfected cells were selected for neomycin resistance 24 h later using G-418 (Calbiochem, 400 μg/ml). Two independent Snail1- and Snail1-ER transfectant clones were isolated in each case.
Migration and invasion assays
Cells were seeded in six-well culture dishes at a density of 3 × 105 cells/well. A wound was made in the center of the confluent culture 24 h later, and after washing and adding fresh medium, phase-contrast pictures were taken of the wounded area at different intervals. Invasion assays on collagen type IV gels were carried out using the two-compartment Boyden chambers (Cano et al, 2000). Having carefully removed the cells from the upper surface after 8 h, the fixed cells on the lower surface of the filters were stained with DAPI.
Analysis of gene transcripts
Poly(A)+ mRNA was isolated using the Microfast Track kit (Invitrogen). For Northern blots, 1.5 μg aliquots of Poly(A)+ oligo(dT)-cellulose-purified mRNA were transferred to nylon membranes and hybridized with [α-32P]dCTP-labelled probes (rediprime II, Amersham Biosciences) for mouse Snail1, E-Cadherin, Cadherin-16 and GAPDH. RT–PCR for Snail1 and GAPDH was carried out as described (Cano et al, 2000). Quantitative RT–PCR was carried using an ABI PRISM® 7000 sequence detection system and TaqMan® probes. The primers and probes were obtained from Applied Biosystems Assays-on-Demand as follows: Mm-99999915-g1 (GAPDH), Mm-00441533-g1 (Snail1), Mm 00441531-m1 (Snail2), Mm-00483196-m1 (Cadherin-16), Mm-00447452-m1 (HNF-1β), Hs 99999905-m1 (GAPDH), Hs 00193591-m1 (SNAI1), Hs 00161904-m1 (SNAI2), Hs 00187880-m1 (CADH16) and Hs 00172123-m1 (HNF1 β). RNA expression was calculated using the comparative Ct method normalized to GAPDH. Data are expressed relative to a calibrator (mock cells or wild-type mice) using the 2−(ΔΔCt)±SD formula. RNA was extracted and cDNA synthesized from cultured Snail1-ER and mock-transfected cells at different times after 4′-OH-Tamoxifen administration and from the kidneys of wild-type and transgenic mice after different periods of tamoxifen or vehicle administration.
In situ hybridization
Mouse embryos were obtained from Balb-C mice and their ages established in dpc, the day on which the vaginal plug was detected being designated 0.5 dpc. The urogenital systems or isolated kidneys were removed at stages 13.5 and 17.5 dpc, respectively, fixed in 4% paraformaldehyde and either processed directly for in situ hybridization (ISH), or gelatin embedded to obtain 50 μm vibratome sections. ISH was performed as described previously (Cano et al, 2000), using mouse Snail1, Snail2 and E-Cadherin probes (Sefton et al, 1998; Cano et al, 2000). The mouse Cadherin-16 and HNF-1β probes were obtained by RT–PCR from NMuMG cells cDNA using the primers (sense/antisense): Cadherin-16, 5′-ACTGTAGAGATCTCCATAGTA-3′/5′-CTCCACTAGTATAGTCACTGT-3′; HNF-1β, 5′GACACTCCTCCCATCCTCAA-3′/5′-TGTAGCGCACTCCTGACATC-3′. The human Cadherin-16 and Snail2 probes were obtained by RT–PCR from human kidney and lung cDNA obtained from BD Biosciences. Amplification was performed with the primers (sense/antisense): human CDH16, 5′-GCAGAGCTGTCTGTGGAAGTT-3′/5′-CTCCAGGTGATAGTGCACATC-3′; human SNAI2, 5′-CTGTAGGGACCGCCGTGT-3′/5′-GCTTCGGAGTGAAGAAATGC-3′. Human Snail1, Snail2 and E-Cadherin were analyzed in renal biopsies as described (Blanco et al, 2002). After hybridization, whole embryos or free-floating slices were processed as described (Cano et al, 2000).
Promoter analyses
Cadherin-16 and HNF-1β promoter activities were measured by cotransfecting NMuMG cells with 50 ng of pcDNA3-Snail1, pcDNA3-mSnail2 or empty pcDNA3 vector and with 300 ng of pKsp(1268F)-Luc or 400 ng of pmHNF-1β-Luc. A Renilla reniformis luciferase plasmid (phRL-CMV-Luc from Promega) was also cotransfected as a control of efficiency. At 24 h after transfection, firefly luciferase (Luc) and renilla luciferase activity was measured using the Dual Luciferase Reporter Assay System (Promega) according to the manufacturer's instructions. Luc activity was normalized to renilla luciferase activity. In all experiments, the total amount of DNA transfected was standardized by adding empty vector. The results are presented as the percentage of luciferase activity relative to controls (luciferase values in cells cotransfected with empty vector).
ChIP assays
Cells were crosslinked with formaldehyde before DNA sonication. The chromatin was immunoprecipitated with the anti-human ER antibody (see above) and sheared to an average length of 0.5–1 kb. A 252-bp fragment of the mouse HNF-1β promoter containing the conserved E-box was amplified with the primers (sense/antisense) 5′-ATCTTGCCGAAAGCTGAGCCC-3′/5′-TTTCCAAGGACGGAAAAAGAAG-3′ and a more distal 500-bp fragment of the promoter carrying the nonconserved E-box with 5′-CACATTCACTACTAGAAAGGA-3′/5′-CTCCAAATCTTCTCTACCGAT-3 to be used as a negative control. As a positive control, a 191-bp fragment from the E-cadherin promoter carrying the Snail1-binding E-pal element (Cano et al, 2000) was amplified with the primers: (sense/antisense) 5′-CATGCCACCAACTACAGACAG-3′/5′-AGTTCTTGGGAACTCAGTAGT-3′.
Transgenic mice
A tamoxifen-inducible Snail1 transgenic mouse was generated according to standard procedures (Hogan et al, 1994) with the pcDNA3-Snail1-ER (see above) and a line was selected that expressed significant levels of the transgenic protein in the kidney. The Snail1-ER protein is constitutively expressed, but it is only functional when translocated into the nucleus upon tamoxifen administration. The subcellular localization of the protein and the level of expression were assessed by immunohistochemistry using a human estrogen-receptor antibody also used to assay the amount of Snail1-ER protein in the tissues obtained from 2-month-old transgenic mice in Western blots. A solution of Tamoxifen 30 mg/ml was sonicated and 30 or 200 μg of tamoxifen/gram body weight was injected subcutaneously into neonatal or intraperitoneally into 2-month-old animals every 3 days over different periods. The animals were killed and their kidneys processed for mRNA extraction, ISH or immunohistochemistry, or paraffin sections were stained with hematoxylin–eosin or Masson–Trichrome.
Human tissue samples
Normal kidney tissue was obtained from the unaffected areas of tumor nephrectomies (four cases) and fibrotic tissue from patients affected by kidney failure caused by urinary obstruction. Specimens approximately 1 cm3 were fixed in 10% formaline or snap frozen in liquid nitrogen-cooled isopentane. The Research Ethics Committees of Sant Joan d' Alacant, Guy's and St Thomas's Hospitals approved the protocols to analyze the tissues, which were processed for real-time RT–PCR, ISH, immunohistochemistry and histology as described above.
Supplementary Material
Supplementary Figures 1 to 6
Acknowledgments
We thank P Igarashi for his generous gift of the mouse Cadherin-16 promoter construct, JM Flores for her help in the preliminary histological analysis of an independent transgenic line, M Tran for collecting human renal samples and M Sefton for editorial assistance. This work has been supported by the Spanish Ministry of Education and Science (BFU2004-02665, BFU2005-05772 and NAN2004-09230-C04-04) to MAN. CAF was also supported by BFU2004-02665 and NAN2004-09230-C04-04. AB was supported by a Marie Curie Fellowship and by the Spanish Ministry of Education and Science.
References
- Alexakis C, Maxwell P, Bou-Gharios G (2005) Organ-specific collagen expression: implications for renal disease. Nephron Exp Nephrol 102: e71–e75 [DOI] [PubMed] [Google Scholar]
- Aybar M, Nieto MA, Mayor R (2003) Snail precedes Slug in the genetic cascade required for the specification and migration of the Xenopus neural crest. Development 130: 483–494 [DOI] [PubMed] [Google Scholar]
- Bai Y, Pontoglio M, Hiesberger T, Sinclair AM, Igarashi P (2002) Regulation of kidney-specific Ksp-cadherin gene promoter by hepatocyte nuclear factor-1β. Am J Physiol Renal Physiol 283: F839–F851 [DOI] [PubMed] [Google Scholar]
- Barrallo-Gimeno A, Nieto MA (2005) The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development 132: 3151–3161 [DOI] [PubMed] [Google Scholar]
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A (2000) The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2: 84–89 [DOI] [PubMed] [Google Scholar]
- Blanco MJ, Moreno-Bueno G, Sarrio D, Locascio A, Cano A, Palacios J, Nieto MA (2002) Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene 21: 3241–3246 [DOI] [PubMed] [Google Scholar]
- Bolos V, Peinado H, Perez-Moreno MA, Fraga MF, Esteller M, Cano A (2003) The transcription factor Slug represses E-cadherin expression induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci 116: 499–511 [DOI] [PubMed] [Google Scholar]
- Burzynski SR (2005) Aging: gene silencing or gene activation? Med Hypotheses 64: 201–208 [DOI] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA (2000) The transcription factor snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2: 76–83 [DOI] [PubMed] [Google Scholar]
- Cheng CW, Wu PE, Yu JC, Huang CS, Yue CT, Wu CW, Shen CY (2001) Mechanisms of inactivation of E-cadherin in breast carcinoma: modification of the two-hit hypothesis of tumor suppressor gene. Oncogene 20: 3814–3823 [DOI] [PubMed] [Google Scholar]
- Chilosi M, Poletti V, Zamo A, Lestani M, Montagna L, Piccoli P, Pedron S, Bertaso M, Scarpa A, Murer B, Cancellieri A, Maestro R, Semenzato G, Doglioni C (2003) Aberrant Wnt/β-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol 162: 1495–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Barrio MG, Nieto MA (2002) Overexpression of Snail family members highlights their ability to promote chick neural crest formation. Development 129: 1583–1593 [DOI] [PubMed] [Google Scholar]
- Dressler GR (2002) Development of the excretory system. In Mouse Development, J Rossant and PPL Tam (eds) pp 395–420. Canada: Academic Press [Google Scholar]
- Edghill EL, Bingham C, Ellard S, Hattersley AT (2006) Mutations in hepatocyte nuclear factor-1-beta and their related phenotypes. J Med Genet 43: 84–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil R, Brocard J, Mascrez B, LeMeur M, Metzger D, Chambon P (1996) Ligand-activated site-specific recombination in mice. Proc Natl Acad Sci USA 93: 10887–10890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga MF, Herranz M, Espada J, Ballestar E, Paz MF, Ropero S, Erkek E, Bozdogan O, Peinado H, Niveleau A, Mao JH, Balmain A, Cano A, Esteller M (2004) A mouse skin multistage carcinogenesis model reflects the aberrant DNA methylation patterns of human tumors. Cancer Res 64: 5527–5534 [DOI] [PubMed] [Google Scholar]
- Gresh L, Fischer E, Reimann A, Tanguy M, Garbay S, Shao X, Hiesberger T, Fiette L, Igarashi P, Yaniv M, Pontoglio M (2004) A transcriptional network in polycystic kidney disease. EMBO J 23: 1657–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiesberger T, Shao X, Gourley E, Reimann A, Pontoglio M, Igarashi P (2004) Mutation of hepatocyte nuclear factor-1β inhibits Pkhd1 gene expression and produces renal cysts in mice. J Clin Invest 113: 814–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Constantini F, Lacy E (1994) Manipulating the Mouse Embryo. A Laboratory Manual. New York: Cold Spring Harbor Laboratory Press [Google Scholar]
- Huber MA, Kraut N, Beug H (2005) Molecular requirements for epithelial–mesenchymal transition during tumour progression. Curr Opin Cell Biol 17: 548–558 [DOI] [PubMed] [Google Scholar]
- Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG (2002) Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 110: 341–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinde K, Nikolic-Paterson DJ, Huang XR, Sakai H, Kurokawa K, Atkins RC, Lan HY (2001) Tubular phenotypic change in progressive tubulointerstitial fibrosis in human glomerulonephritis. Am J Kidney Dis 38: 761–769 [DOI] [PubMed] [Google Scholar]
- Kalluri R, Neilson EG (2003) Epithelial–mesenchymal transition and its implications for fibrosis. J Clin Invest 112: 1776–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Yang J, Dai C, Wu C, Liu Y (2003) Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J Clin Invest 112: 503–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Patel SR, Cheng X, Cho EA, Levitan I, Ullenbruch M, Phan SH, Park JM, Dressler GR (2005) Kielin/chordin-like protein, a novel enhancer of BMP signaling, attenuates renal fibrotic disease. Nat Med 11: 387–393 [DOI] [PubMed] [Google Scholar]
- Liu Y (2004) Epithelial to mesenchymal transition in renal fibrogenesis: pathological significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol 15: 1–12 [DOI] [PubMed] [Google Scholar]
- Liu Y (2006) Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int 69: 213–217 [DOI] [PubMed] [Google Scholar]
- McMorrow T, Gaffney MM, Slattery C, Campbell E, Ryan MP (2005) Cyclosporine A induced epithelial–mesenchymal transition in human renal proximal tubular epithelial cells. Nephrol Dial Transplant 20: 2215–2225 [DOI] [PubMed] [Google Scholar]
- Melk A (2005) Transcriptional analysis of the molecular basis of human kidney aging cDNA microarray profiling. Kidney Int 68: 2667–2679 [DOI] [PubMed] [Google Scholar]
- Nieto MA (2002) The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol 3: 155–166 [DOI] [PubMed] [Google Scholar]
- Postlethwaite AE, Shigemitsu H, Kanangat S (2004) Cellular origins of fibroblasts: possible implications for organ fibrosis in systemic sclerosis. Curr Opin Rheumatol 16: 733–738 [DOI] [PubMed] [Google Scholar]
- Rastaldi MP, Ferrario F, Giardino L, Dell'Antonio G, Grillo C, Grillo P, Strutz F, Muller GA, Colasanti G, D'Amico G (2002) Epithelial–mesenchymal transition of tubular epithelial cells in human renal biopsies. Kidney Int 62: 137–146 [DOI] [PubMed] [Google Scholar]
- Ryu JK, Song SU, Han JY, Chu YC, Lee M, Kim JS, Kim SJ, Suh JK 2005. Establishment of penile fibrosis model in a rat using mouse NIH 3T3 fibroblasts expressing transforming growth factor β1. Biol Reprod 72: 916–921 [DOI] [PubMed] [Google Scholar]
- Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A (2003) Targeted disruption of TGF-β1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest 112: 1486–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton M, Sanchez S, Nieto MA (1998) Conserved and divergent roles for members of the Snail family of transcription factors in the chick and mouse embryo. Development 125: 3111–3121 [DOI] [PubMed] [Google Scholar]
- Shao X, Johnson JE, Richardson JA, Hiesberger T, Igarashi P (2002) A minimal Ksp-cadherin promoter linked to a green fluorescent protein reporter gene exhibits tissue-specific expression in the developing kidney and genitourinary tract. J Am Soc Nephrol 13: 1824–1836 [DOI] [PubMed] [Google Scholar]
- Slattery C, Campbell E, McMorrow T, Ryan MP (2005) Cyclosporine A-induced renal fibrosis: a role for epithelial–mesenchymal transition. Am J Pathol 167: 395–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG (1995) Identification and characterization of a fibroblast marker: FSP1. J Cell Biol 130: 393–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery JP, Sleeman JP (2006) Complex networks orchestrate epithelial–mesenchymal transitions. Nat Rev Mol Cell Biol 7: 131–142 [DOI] [PubMed] [Google Scholar]
- Thomson RB, Igarashi P, Biemesderfer D, Kim R, Abu-Alfa A, Soleimani M, Aronson S (1995) Isolation and cDNA cloning of Ksp-cadherin, a novel kidney-specific member of the cadherin multigene family. J Biol Chem 270: 17594–17601 [DOI] [PubMed] [Google Scholar]
- Valdés F, Alvarez AM, Locascio A, Vega S, Herrera B, Fernandez M, Benito M, Nieto MA, Fabregat I (2002) The epithelial mesenchymal transition confers resistance to the apoptotic effects of transforming growth factor β in fetal rat hepatocytes. Mol Cancer Res 1: 68–78 [PubMed] [Google Scholar]
- Vongwiwatana A, Tasanarong A, Rayner DC, Melk A, Halloran PF (2005) Epithelial to mesenchymal transition during late deterioration of human kidney transplants: the role of tubular cells in fibrogenesis. Am J Transplant 5: 1367–1374 [DOI] [PubMed] [Google Scholar]
- Wertz K, Herrmann BG (1999) Kidney-specific cadherin (cdh16) is expressed in embryonic kidney, lung, and sex ducts. Mech Dev 84: 185–188 [DOI] [PubMed] [Google Scholar]
- Whyte DA, Li C, Thomson RB, Nix SL, Zanjani R, Karp SL, Aronson PS, Igarashi P (1999) Ksp-cadherin gene promoter I characterization and renal epithelial cell-specific activity. Am J Physiol 277: F587–F598 [DOI] [PubMed] [Google Scholar]
- Yañez-Mo M, Lara-Pezzi E, Selgas R, Ramirez-Huesca M, Dominguez-Jimenez C, Jimenez-Heffernan JA, Aguilera A, Sanchez-Tomero JA, Bajo MA, Alvarez V, Castro MA, del Peso G, Cirujeda A, Gamallo C, Sanchez-Madrid F, Lopez-Cabrera M (2003) Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N Engl J Med 348: 403–413 [DOI] [PubMed] [Google Scholar]
- Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R (2003) BMP-7 counteracts TGF-β1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med 9: 964–968 [DOI] [PubMed] [Google Scholar]
- Zeisberg M, Kalluri R (2004) The role of epithelial-to-mesenchymal transition in renal fibrosis. J Mol Med 82: 175–181 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1 to 6


