Abstract
Ubiquitin-like (UBL)-ubiquitin-associated (UBA) proteins such as Rad23 and Dsk2 mediate the delivery of polyubiquitinated proteins to the proteasome in the ubiquitin–proteasome pathway. We show here that budding yeast peptidyl-tRNA hydrolase 2 (Pth2), which was previously recognized as a peptidyl-tRNA hydrolase, is a UBL domain-binding protein that participates in the ubiquitin–proteasome pathway. Pth2 bound to the UBL domain of both Rad23 and Dsk2. Pth2 also interacted with polyubiquitinated proteins through the UBA domains of Rad23 and Dsk2. Pth2 overexpression caused an accumulation of polyubiquitinated proteins and inhibited the growth of yeast. Ubiquitin-dependent degradation was accelerated in the pth2Δ mutant and was retarded by overexpression of Pth2. Pth2 inhibited the interaction of Rad23 and Dsk2 with the polyubiquitin receptors Rpn1 and Rpn10 on the proteasome. Furthermore, Pth2 function involving UBL-UBA proteins was independent of its peptidyl-tRNA hydrolase activity. These results suggest that Pth2 negatively regulates the UBL-UBA protein-mediated shuttling pathway in the ubiquitin–proteasome system.
Keywords: Dsk2, Pth2, proteasome, Rad23, UBL domain
Introduction
Recruitment of ubiquitinated proteins to the proteasome is a crucial step in the ubiquitin–proteasome pathway. The integrity of this pathway is ensured by a sequential mode of action: capture of ubiquitin conjugates, delivery to the proteasome and release from the proteasome ubiquitin receptors. Ubiquitinated proteins can be recognized directly by the 19S proteasomal subunit Rpn10 (van Nocker et al, 1996; Saeki et al, 2002; Elsasser et al, 2004; Verma et al, 2004). In addition to this pathway, UBL-UBA proteins help to deliver proteins to be degraded to the proteasome. UBL-UBA proteins belong to a family of proteins that contain a UBL domain at the N terminus and a UBA domain at the C terminus. Through the ability of the UBL domain to interact with polyubiquitin receptors on the 19S proteasome regulatory particle and the UBA domain to bind to polyubiquitin, UBL-UBA proteins such Rad23 and Dsk2 in Saccharomyces cerevisiae serve as shuttle factors delivering ubiquitinated substrates to the proteasome (Schauber et al, 1998; Lambertson et al, 1999; Wilkinson et al, 2001; Chen and Madura, 2002; Funakoshi et al, 2002; Rao and Sastry, 2002). These shuttle factors interact with the 19S proteasomal subunit Rpn1, which acts as a scaffold on the proteasome (Elsasser et al, 2002). A recently developed cell-free system has demonstrated that UBL-UBA shuttle proteins promote protein degradation by the proteasome (Verma et al, 2004). These accumulated findings have advanced our understanding of the molecular basis of recognition of polyubiquitinated proteins by the proteasome (reviewed by Hartmann-Petersen et al, 2003; Madura, 2004; Elsasser and Finley, 2005). However, how the shuttle proteins regulate the flux of polyubiquitinated proteins to the proteasome receptors is not well understood.
To investigate the regulation of UBL-UBA proteins in shuttling pathways, we searched for interacting factors of UBL-UBA proteins in a two-hybrid screen, and identified peptidyl-tRNA hydrolase 2 (Pth2) from S. cerevisiae as an interacting protein of Rad23 and Dsk2. Pth is an enzyme that cleaves peptidyl-tRNA to tRNA and peptides during translation elongation (Menninger, 1976) and is essential for growth in bacteria. In archaea, a second Pth enzyme named Pth2 has been identified, whose amino-acid sequence is completely different from bacterial Pth (Rosas-Sandoval et al, 2002). Eukaryotic cells encode both Pth and Pth2, and curiously, the two eukaryotic enzymes are dispensable for yeast viability (Menez et al, 2002; Rosas-Sandoval et al, 2002; Fromant et al, 2003). Pth2 has two domains: a conserved C-terminal half is the catalytic domain (de Pereda et al, 2004), but no function has been ascribed to the highly divergent N-terminal half, suggesting that this region may confer species specificity to Pth2. As we show in this report, budding yeast Pth2 is a UBL domain-binding protein that inhibits ubiquitin-mediated protein degradation. In addition, the C-terminal domain of Pth2 is required for the interaction with Rad23 and Dsk2, but this Pth2 function is independent of its C-terminal peptidyl-tRNA hydrolase activity. Based on our findings, we discuss a regulatory role of Pth2 in substrate delivery to the proteasome via an interaction with the UBL domain of UBL-UBA proteins. Budding yeast Pth2 may have a role in the ubiquitin–proteasome pathway and as a peptidyl-tRNA hydrolase.
Results
Yeast Pth2 interacts with Dsk2 and Rad23 in vivo and in vitro
We screened for proteins that interact with a UBL-UBA protein in a two-hybrid screen using Dsk2 as bait. Among 2 × 105 transformants from an S. cerevisiae cDNA library, three clones interacted with Dsk2 strongly, and each of these clones was identified as PTH2 (Figure 1A), which encodes a full-length peptidyl-tRNA hydrolase. First, we tested for a direct interaction in vitro between Pth2 and the UBL-UBA proteins (Figure 1B). His6-T7-tagged Pth2 bound directly to GST-Dsk2 (lane 5) and GST-Rad23 (lane 6), but not to the DNA damage-inducible UBL-UBA protein, Ddi1 (lane 7). Next, we coexpressed the T7-Pth2 with GST-Dsk2 or GST-Rad23 in yeast, and in vivo binding was tested using a GST pull-down assay followed by immunoblotting with anti-T7 (Figure 1C). T7-Pth2 co-precipitated with GST-Dsk2 (lane 10) and GST-Rad23 (lane 11) and, to a lesser extent, with GST-Ddi1 (lane 12). The weak binding to Ddi1 was abolished in the dsk2Δrad23Δ mutant (lane 16), suggesting that the interaction of Pth2 is meditated by Dsk2 and Rad23. Therefore, Pth2 binds selectively to the UBL-UBA proteins Rad23 and Dsk2 in vivo. Next, we confirmed in vivo interaction of Pth2 with UBL-UBA protein at endogenous expression level (Figure 1D). FLAG-tagged Pth2 was expressed from PTH2 own promoter in pth2Δ cells (middle panel, lane 4) and the extracts were immunoprecipitated with anti-FLAG beads. FLAG-Pth2 expressing at endogenous levels or even less in pth2Δ co-precipitated with endogenous Dsk2 (bottom panel, lane 4).
Figure 1.
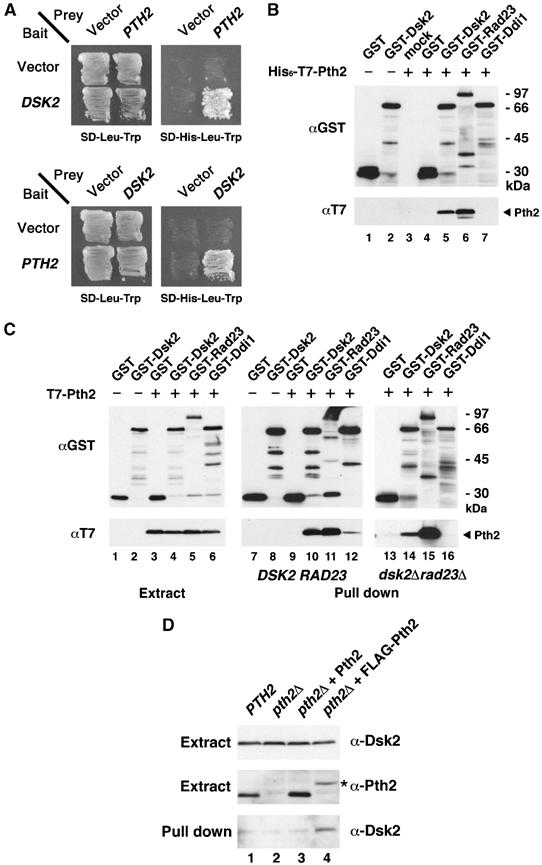
Pth2 binds to Dsk2 and Rad23 but not to Ddi1. (A) Identification of PTH2 in a two-hybrid assay. Pth2 was obtained as a Dsk2-interacting clone. Interaction of Pth2 with Dsk2 was tested by histidine-prototrophic growth, using DSK2 as bait and PTH2 as prey (upper panel) or vice versa (lower panel). (B) Binding of Pth2 to Dsk2 and Rad23 in vitro. His6-T7-Pth2 (1 μg) was mixed with 1 μg of GST-Dsk2, GST-Rad23, GST-Ddi1 or GST alone in lysis buffer and incubated with 20 μl glutathione-Sepharose (GSH beads) for 1 h. Material bound to GSH beads was immunoblotted with the indicated antibodies. (C) Binding of Pth2 to Dsk2 and Rad23 in vivo. T7-Pth2 was coexpressed with GST-Dsk2, GST-Rad23, GST-Ddi1 or GST alone in wild-type yeast or in dsk2Δrad23Δ. The extracts (lanes 1–6) and the GST precipitates from wild-type (YPH499) (lanes 7–12) and dsk2Δrad23Δ (lanes 13–16) were immunoblotted as indicated. (D) Interaction of Pth2 with Dsk2 at endogenous expression level in yeast. FLAG-Pth2 was expressed from PTH2 own promoter in pth2Δ, and was immunoprecipitated with anti-FLAG M2 beads. The extracts (top and middle panels) and the FLAG-precipitates from the extracts (bottom panel) were immunoblotted with anti-Dsk2 or anti-Pth2 antibodies as indicated. An asterisk indicates FLAG-Pth2. Because the sensitivity of our anti-Rad23 is lower than that of anti-Dsk2 (ca. 1/10, see Materials and methods), we could not show a Rad23 signal under this condition. Also, we could not test co-immunoprecipitation of Dsk2/Rad23 with anti-Pth2 antibody, probably because our Pth2 antibody was not effective for immunoprecipitations.
Pth2 interacts with polyubiquitinated proteins indirectly via Dsk2 and Rad23 in vivo
To further investigate the interaction of Pth2 with UBL-UBA proteins, yeast cell extracts were fractionated by gel filtration, and each fraction was immunoblotted (Figure 2A). Pth2 reproducibly eluted in fractions containing high molecular weight proteins (bottom panel) but not at the expected monomer size (∼25 kDa). The Pth2-containing fractions contained Dsk2, Rad23 and polyubiquitinated proteins in cells. Also, GST-Pth2 expressed in yeast co-precipitated with polyubiquitinated proteins (Figure 2B, lane 7) together with Dsk2 and Rad23 in wild-type cells but did not co-precipitate with polyubiquitinated proteins in dsk2Δrad23Δ (lane 10). Like the wild-type strain, GST-Pth2 co-precipitated polyubiquitin in either dsk2Δ (lane 8) or rad23Δ cells (lane 9). It thus seems that the Pth2–polyubiquitin interaction seen in wild-type cells depends on Dsk2 and Rad23. Pth2 alone did not bind directly to polyubiquitin in vitro (Figure 2C).
Figure 2.
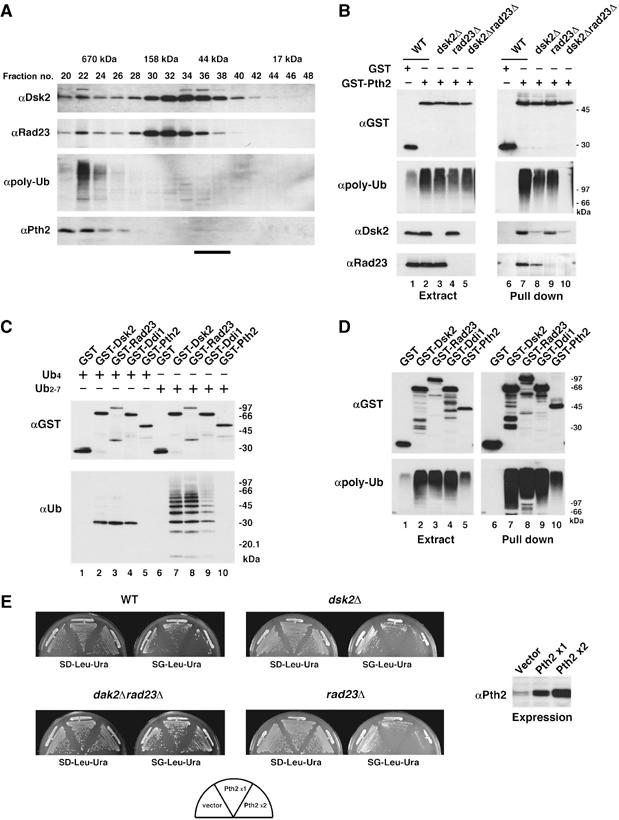
Pth2 associates with polyubiquitinated proteins via Dsk2 and Rad23. (A) Gel filtration of yeast extracts on a Superdex 200 column. Each fraction was immunoblotted with the indicated antibodies. The bar at the bottom indicates the position of monomeric Pth2 (∼25 kDa) (Rosas-Sandoval et al, 2002). (B) Binding of Pth2, Dsk2, Rad23 and polyubiquitin conjugates. GST-Pth2 was expressed in wild-type (YPH499), dsk2Δ, rad23Δ or dsk2Δrad23Δ strains. The GSH beads precipitates were immunoblotted as indicated. (C) In vitro GST pull-down assays with Pth2 and polyubiquitin. In total, 1 μg of tetraubiquitin (lanes 1–5) or polyubiquitin (lanes 6–10) was incubated with GST-Pth2, GST-Dsk2, GST-Rad23, GST-Ddi1 or GST alone (1 μg each). The samples were precipitated with GSH beads and immunoblotted as indicated. (D) In vivo GST pull-down assays with Pth2 and polyubiquitin. GST-Pth2, GST-Dsk2, GST-Rad23, GST-Ddi1 or GST alone was expressed in wild-type cells. Cell extracts were precipitated with GSH beads and immunoblotted as indicated. (E) Growth of yeast overexpressing Pth2. A single copy of PTH2 was expressed from a galactose-inducible pGAL1-YEplac181 ( × 1), or two copies of PTH2 were expressed from both pGAL1-YEplac181 and pGAL1-YEplac195 ( × 2) in wild-type, dsk2Δ, rad23Δ or dsk2Δrad23Δ strains. Cells were incubated for 2–5 days at 30°C, the permissive-temperature for growth of dsk2Δrad23Δ (Biggins et al, 1996). Expression levels were determined by Pth2 immunoblotting (on the right).
Overexpression of Pth2 caused accumulation of polyubiquitinated proteins (Figure 2D, lane 5; and B, lane 2); therefore, we also tested the growth of cells overexpressing Pth2 (Figure 2E). Growth was inhibited in wild-type cells overexpressing Pth2 (top left panel), whereas Pth2-induced growth inhibition was not observed in the double-deletion mutant dsk2Δrad23Δ (bottom left panel). However, growth of the single-deletion mutants, dsk2Δ and rad23Δ, was inhibited when PTH2 was overexpressed (right top and bottom panels). These genetic analyses suggest that the Pth2-induced growth inhibition we observed is also mediated by Dsk2 and Rad23.
Pth2 inhibits ubiquitin-mediated degradation
We next investigated whether Pth2 affects ubiquitin-mediated protein degradation. Degradation of the N-end rule substrate Leu-β-gal was accelerated in pth2Δ (Figure 3A) compared with wild-type cells. As a control, degradation of Ala-β-gal was not affected by PTH2 deletion. The in vivo substrate Sic1, a cyclin-dependent kinase inhibitor, was also examined (Figure 3B; Supplementary Figure 1). Sic1-HA was expressed in cells arrested by α-factor and released synchronously into the cell cycle. Degradation of Sic1-HA was slightly accelerated in pth2Δ (Figure 3B, left panels). To confirm this Pth2 effect, we also tested Sic1 degradation in cells overexpressing PTH2. Supporting the acceleration by the PTH2 deletion, overexpression of PTH2 retarded degradation of Sic1-HA (right panels). Consistent with these effects of Pth2 on degradation, PTH2 disruption suppressed the growth defect of the temperature-sensitive proteasome mutant pre9Δ (Figure 3C). Furthermore, the cells' response to amino-acid analogs was affected by PTH2 disruption (Figure 3D); that is, pth2Δ became resistant to the amino-acid analogs azetidine and canavanine. Contrary to our expectations, however, the triple mutant dsk2Δrad23Δpth2Δ was more sensitive to azetidine than dsk2Δrad23Δ, suggesting that Pth2 functions in a distinct pathway in the ubiquitin–proteasome system (see Discussion). In contrast to the drug response results, pth2Δ did not affect survival after UV irradiation (Figure 3D). Collectively, these data indicate that Pth2 is involved negatively in the ubiquitin-mediated degradation pathway.
Figure 3.
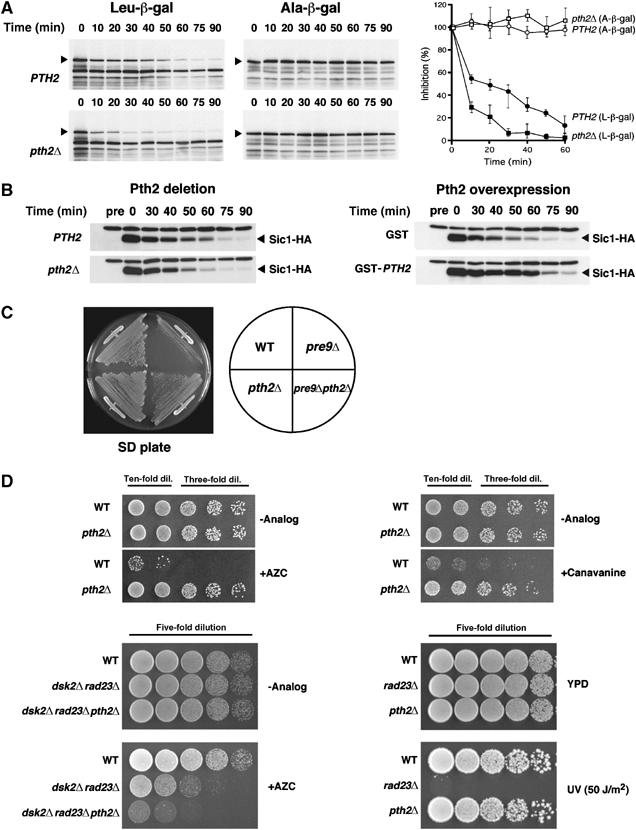
Pth2 inhibits the ubiquitin–proteasome pathway. (A) Degradation of the N-end rule substrate was accelerated in pth2Δ. Leu-β-gal and Ala-β-gal (indicated by arrowheads) were induced with galactose in wild-type (YPH499) or pth2Δ cells. Cell samples were collected at the indicated times after the induction was terminated, and degradation was assessed by immunoblotting with anti-β-gal. The quantification of the experiments is shown on the right. Note that, despite our expectations, Pth2 overexpression barely inhibited degradation of Leu-β-gal (data not shown), which might be indicative that Pth2 is involved in substrate specificity for degradation (cf Figure 3B). (B) Degradation of Sic1 in pth2Δ and PTH2-overexpressing cells. Wild-type (YPH499) and pth2Δ cells were arrested by α-factor treatment and then Sic1-HA was expressed for 1 h by galactose induction. For overexpression, Sic1-HA and GST-Pth2 were coexpressed in synchronized wild-type cells for 2 h by galactose induction. After release into the cell cycle, cell samples were collected at the indicated times and Sic1-HA protein was followed by immunoblotting with anti-HA. (C) Rescue of the proteasome defect of pre9Δ by PTH2 deletion. Growth was tested for 2–3 days at 30°C, at which temperature the pre9Δ mutant shows impaired growth. (D) Effects of PTH2 deletion on sensitivity to amino-acid analogs and UV irradiation. Wild-type (YPH499) and pth2Δ were spotted onto SD plates containing 0.5 mM AZC or 3 μg/ml canavanine in serial dilutions (upper panels). Wild-type and the mutants (dsk2Δrad23Δ and dsk2Δrad23Δpth2Δ) were spotted onto YPD plates containing 5 mM AZC in serial dilutions (bottom left panel). Cells were also spotted on YPD plates and exposed to 50 J/m2 UV irradiation (bottom right panel). The plates were incubated at 30°C for 2–4 days.
Pth2 inhibits interactions of Rad23/Dsk2 with polyubiquitin receptors on the proteasome
To further delineate Pth2's role in delivery of ubiquitinated proteins to the proteasome, we examined the effects of Pth2 on the interaction between the proteasome ubiquitin receptors Rpn1 and Rpn10 and UBL-UBA proteins Rad23 and Dsk2. First, we confirmed that Rpn1 interacts with Rad23 (Figure 4A, lane 6) but not with Dsk2 (lane 5) in vitro (cf; Elsasser et al, 2002). Then, to test whether Pth2 alters the Rad23–Rpn1 interaction, GST pull-down experiments were carried out using purified GST-Rpn1 and His6-T7-Rad23 in the presence of His6-T7-Pth2. Figure 4B shows that increasing amounts of Pth2 decreased binding of Rad23 to Rpn1 in vitro (lanes 4–6). Similarly, we confirmed that Rpn10 interacts with Dsk2 (Figure 4A, lane 12) but not with Rad23 (lane 13) in vitro. Again, increasing amounts of Pth2 competed with Dsk2 for Rpn10 binding (Figure 4C).
Figure 4.
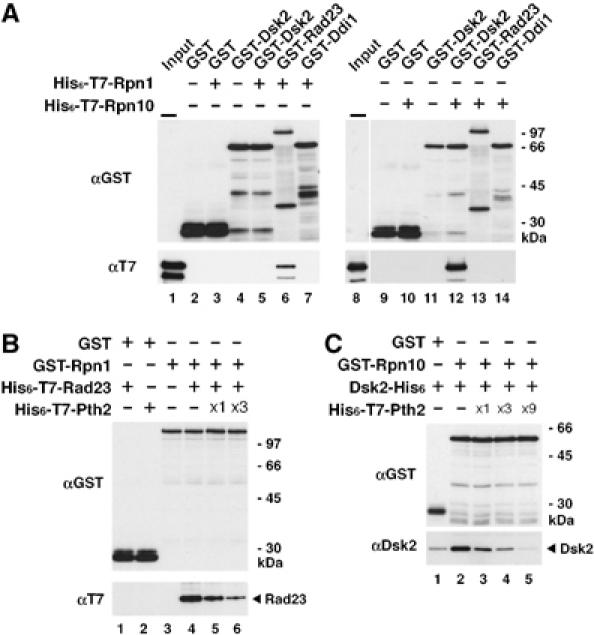
Pth2 inhibits the interaction between UBL-UBA proteins and ubiquitin receptors. (A) Rpn1–Rad23 and Rpn10–Dsk2 interactions in vitro. GST-Dsk2, GST-Rad23, GST-Ddi1 or GST alone (1 μg each) was incubated with 1 μg of either His6-T7-Rpn1 (lanes 1–7) or His6-T7-Rpn10 (lanes 8–14) for 1 h, followed by precipitation with GSH beads and immunoblotting as indicated. Inputs represent 5% of the amounts used for the assay. (B) Effect of Pth2 on the Rad23–Rpn1 interaction in vitro. GST-Rpn1 (1 μg) was incubated with His6-T7-Rad23 (1 μg) in the presence of increasing amounts of His6-T7-Pth2 ( × 1=1 μg) for 1 h, followed by precipitation with GSH beads and immunoblotting as indicated. (C) Effect of Pth2 on the Dsk2–Rpn10 interaction in vitro. GST-Rpn10 (1 μg) was incubated with Dsk2-His6 (1 μg) in the presence of increasing amounts of His6-T7-Pth2 ( × 1=1 μg) for 1 h, followed by precipitation with GSH beads and immunoblotting as indicated.
Next, we tested the effect of Pth2 on the interaction between the proteasome and UBL-UBA proteins (Figure 5A). To address this issue, an intact proteasome 19S regulatory particle containing FLAG-Rpt1 and polyubiquitin receptors Rpn1 and Rpn10 (data not shown, Elsasser et al, 2002) was immunoprecipitated from yeast cells with anti-FLAG beads and then incubated with the UBL domain fragment (1–77) of Rad23-GST or Dsk2-GST in the presence of His6-T7-Pth2. We used the 19S particle instead of the 26S proteasome because the FLAG-Pre1-tagged 26S proteasome was prone to dissociate in our buffer conditions in the GST pull-down assay (data not shown). Figure 5A shows that increasing amounts of Pth2 decreased the binding of 19S regulatory particle to Dsk2 (lanes 3–5) and to Rad23 (lanes 6–8), indicating that Pth2 inhibits the interaction of Dsk2 and Rad23 with the 19S particle in vitro. In the case of Rad23, a higher dose of Pth2 was required to compete with the proteasome–Rad23 interaction in vitro (lanes 6–8) (Supplementary Figure 2).
Figure 5.
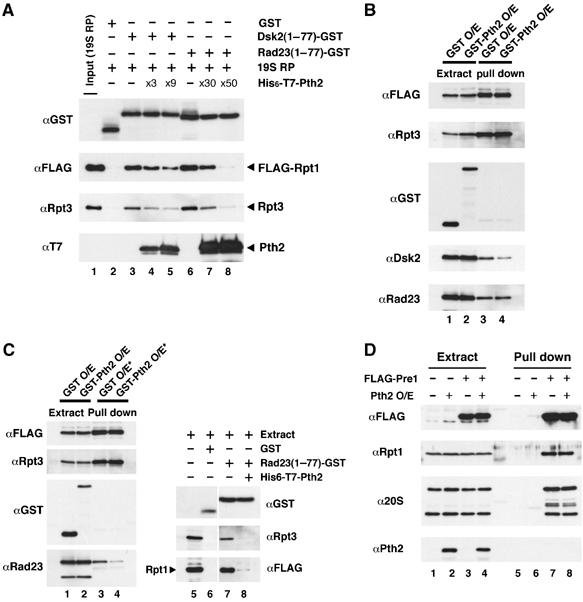
Pth2 inhibits the interaction between UBL-UBA proteins and the proteasome. (A) Effect of Pth2 on the interaction between the proteasome and Dsk2/Rad23 in vitro. 19S regulatory particle was affinity-purified with FLAG-Rpt1 from yeast cells in which endogenous RPT1 had been replaced with FLAG-RPT1. Rad23-GST UBL domain (1–77) protein or Dsk2-GST UBL domain (1–77) protein (1 μg) was mixed with the 19S proteasome (19S RP) and incubated with GSH beads for 1 h in the presence of increasing amounts of His6-T7-Pth2 ( × 1=1 μg) followed by immunoblotting of the precipitates with anti-Rpt3, anti-FLAG, anti-T7 and anti-GST. (B) Effect of Pth2 on the interaction between the proteasome and Dsk2/Rad23 in yeast. GST or GST-Pth2 was overexpressed (O/E) by the galactose-inducible pGAL1-YEplac112-GST or pGAL1-YEplac112-GST-PTH2 for 4 h in yeast in which endogenous RPT1 was replaced with FLAG-His6-tagged RPT1. Extracts were precipitated with anti-FLAG M2 beads, followed by immunoblotting with anti-Rpt3, anti-GST, anti-FLAG, anti-Dsk2 or anti-Rad23. Extracts were immunoblotted as controls (lanes 1–2). (C) Effect of Pth2 on the interaction between the proteasome and Rad23 in yeast extracts. Lanes 1–4: GST or GST-Pth2 was overexpressed in yeast cells as in (B). Cell extracts were precipitated in the presence of purified GST-Pth2 (lane 4, 15-fold in molar excess; asterisk) with anti-FLAG M2 beads, followed by immunoblotting with anti-Rpt3, anti-GST, anti-FLAG and anti-Rad23 (lanes 1–4). Extracts were immunoblotted as a control (lanes 1–2). Lanes 5–8: experimental conditions were the same as (A), except that cell extracts were used instead of a purified 19S proteasome. Purified His6-T7-Pth2 (30-fold excess) was added to the cell extract (lane 8). (D) The proteasome does not contain Pth2. Endogenous PRE1 was replaced with FLAG-His6-tagged PRE1. Pth2 was overexpressed either in wild-type (YPH499) or the FLAG-His6-tagged PRE1-integrated strain (lanes 2 and 4, respectively). Extracts were precipitated with anti-FLAG M2 beads, followed by immunoblotting with anti-Rpt1, anti-20S, anti-FLAG and anti-Pth2 (lanes 5–8). Extracts were immunoblotted as a control (lanes 1–4).
We also examined whether Pth2 prevents the interaction of Rad23 and Dsk2 with the proteasome in vivo. As shown in Figure 5B, overexpression of Pth2 in yeast decreased the amount of Dsk2 associated with the proteasome (lanes 3 and 4). Under the same conditions, we could not detect a decreased amount of Rad23 associated with the proteasome, consistent with the results in Figure 5A (lanes 6–8). Therefore, we examined the effect of greater amounts of Pth2 on the proteasome–Rad23 interaction. Figure 5C shows that a higher dose of Pth2 inhibited the interaction of Rad23 with the proteasome in the cell extracts (lanes 3 and 4). This result was confirmed by a GST pull-down assay of the proteasome from extracts with Rad23-GST, followed by immunoblotting for the proteasomal subunits, FLAG-tagged Rpt1 and Rpt3 (lanes 7 and 8). Pth2 itself did not associate with the proteasome; no Pth2 signal was detected in the 26S proteasome-precipitated fraction, even in cells overexpressing Pth2 (Figure 5D). Taking these results together, it appears that Pth2 competitively inhibits the interaction between UBL-UBA proteins and polyubiquitin receptors on the proteasome.
Pth2 binds directly to the UBL domains of Rad23 and Dsk2
We tested which domain of UBL-UBA proteins is required for binding to full-length Pth2. A series of deletion mutants of Rad23 and Dsk2 were constructed (Figure 6A), and their binding to Pth2 was tested in vitro and in vivo. Figure 6B shows that the N-terminal UBL domain of Rad23 (lane 5) bound to Pth2 in vitro, but the N-terminal Rad23 truncation (lane 6) did not. The requirement of the UBL domain of Dsk2 for Pth2 binding was also shown by deletion analysis of Dsk2 in vitro (Figure 6B, lanes 12–16). Thus, Pth2 binds directly to the UBL domains of Rad23 and Dsk2.
Figure 6.
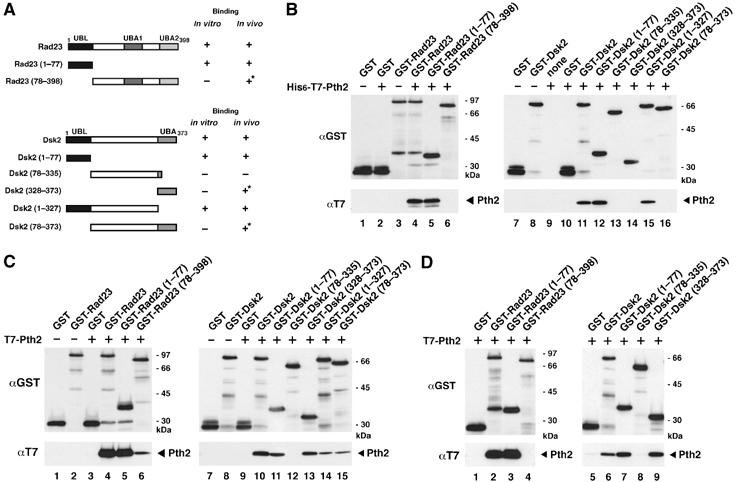
Pth2 binds to the UBL domains of Rad23 and Dsk2. (A) A diagram of Rad23 and Dsk2 constructs used for in vitro and in vivo binding assays. Summary of Pth2 binding to the mutants are shown on the right. Asterisks indicate an indirect interaction detected in cells (see text). (B) Binding of Pth2 to Rad23 and Dsk2 mutants in vitro. His6-T7-Pth2 (1 μg) was incubated with various GST-Rad23 mutants (lanes 1–6) or Dsk2 mutants (lanes 7–16) for 1 h, precipitated with GSH beads, and immunoblotted with the indicated antibody. Because Dsk2 contains only a single C-terminal UBA domain, we constructed and used a series of Dsk2 mutants for domain analysis. (C) Binding of Pth2 to Rad23 and Dsk2 mutants in vivo. T7-Pth2 was coexpressed with GST-Rad23 (lanes 1–6) or GST-Dsk2 (lanes 7–15) mutants in wild-type (YPH499) cells, and the cell extracts were precipitated with GSH beads followed by immunoblotting with the indicated antibodies. (D) Binding of Pth2 to Rad23 and Dsk2 mutants in vivo in dsk2Δrad23Δ. The in vivo assay was carried out as in (C), using cell extracts of dsk2Δrad23Δ.
Pth2 bound in vivo to the UBL domain of Rad23 (Figure 6C, lane 5) and Dsk2 (lane 11); Pth2 also bound weakly to the N-terminal truncation mutants of Rad23 and Dsk2 (lanes 6, 13, 15). However, when a double deletion dsk2Δrad23Δ mutant was tested in the in vivo binding assay (Figure 6D), the Rad23 mutant fragment (78–398) did not interact with Pth2 (lane 4), indicating that the weak signal is due to an indirect interaction that polyubiquitin chains bridge between the GST-UBA domain fusion proteins and endogenous Rad23 or Dsk2, which then binds to Pth2.
Notably, Pth2 also interacted with the Dsk2 UBA domain in vivo but not with Rad23 UBA domain (Figure 6C and D). Pth2 appeared to bind both the Dsk2 UBL and the UBA domains at equivalent levels (Figure 6C, lanes 11 and 13). Even in dsk2Δrad23Δ (Figure 6D), Pth2 co-precipitated with the UBA domain alone (lane 9) in vivo. By contrast, a Dsk2 mutant fragment (78–335) that lacks both the UBL and UBA domains did not bind to Pth2 (lane 8). Therefore, in addition to the UBL domain, Pth2 interacts with the Dsk2 UBA domain in vivo. However, this Pth2–UBA interaction seems to be mediated by an additional cellular factor(s) in yeast cells because Pth2 did not bind the UBA domain in vitro (Figure 6B) (see Discussion).
Pth2 function in the ubiquitin–proteasome pathway is independent of its C-terminal peptidyl-tRNA hydrolase activity
The Pth2 C-terminal domain, which contains the peptidyl-tRNA hydrolase activity, is conserved across species; however, the N-terminal domain is not conserved, and its function is unknown (Rosas-Sandoval et al, 2002). We investigated whether the peptidyl-tRNA hydrolase activity of Pth2 is required for its binding to UBL-UBA proteins. Guided by a structural analysis of the catalytic domain (de Pereda et al, 2004), we produced a series of Pth2 peptidyl-tRNA hydrolase mutants by site-directed mutagenesis of the putative active center (Figure 7A). The peptidyl-tRNA hydrolase activity of these mutants was tested by complementation of the Escherichia coli mutant, pthts (Rosas-Sandoval et al, 2002). The yeast Pth2 mutants D171A, D171N, DRTQ171AAAA, DRTQ171NLVL and RTQ174LVL failed to rescue the temperature-sensitive growth of pthts (summarized in Figure 7A), indicating a lack of peptidyl-tRNA hydrolase activity in the Pth2 mutants. When we examined the ability of these Pth2 mutants to bind to Rad23 (Figure 7B, lanes 1–9) and Dsk2 (lanes 10–18), we found that these Pth2 mutants could bind to Rad23 (lanes 5–9) and to Dsk2 (lanes 14–18). Overexpression of mutant Pth2 (D171A) inhibited growth to a similar degree as wild-type Pth2 (Figure 7C). We also confirmed this result by complementation test for cell sensitivity to amino-acid analog: pth2Δ expressing Pth2 mutant (D171A) became sensitive to azetidine compared with pth2Δ alone (Figure 7D, see also Figure 3D). These data, therefore, demonstrated that Pth2 function in the ubiquitin-mediated pathway is independent of its peptidyl-tRNA hydrolase activity. In addition, this complementation assay also shows that FLAG-tagged Pth2 expressing from PTH2's own promoter in pth2Δ made more sensitive to azetidine than pth2Δ, supporting that the tagged Pth2 is functional in yeast cells (Figure 7D). Like FLAG-Pth2, GST-Pth2 expressing in pth2Δ at endogenous level rescued the azetidine-resistant phenotype of pth2Δ (data not shown). As shown in Figure 7E, the C-terminal half (83–208) of Pth2 bound to Rad23 (lane 12) and Dsk2 (lane 24) in vivo; however, the N-terminal domain (1–82) did not bind these proteins (lanes 11 and 23). Furthermore, overexpression of the N-terminal domain (1–82), which lacks the Rad23/Dsk2-binding site, did not inhibit the growth of yeast, unlike wild-type Pth2 (Supplementary Figure 3). Therefore, the C-terminal domain of Pth2 is necessary for binding to Rad23 and Dsk2.
Figure 7.
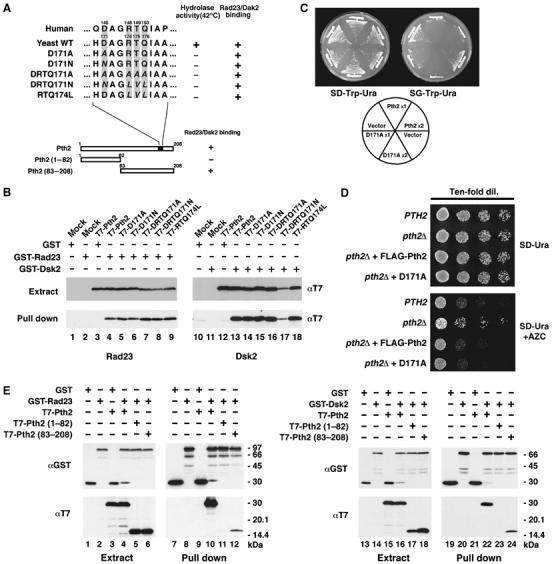
The function of Pth2 in the ubiquitin–proteasome pathway is independent of its peptidyl-tRNA hydrolase activity. (A) Pth2 mutants and the sequences of the peptidyl-tRNA hydrolase active site. Conserved residues of the active site (D171, R174, T175 and Q176) were changed by site-directed mutagenesis as indicated in italics. A summary of the hydrolase activity and Rad23/Dsk2 binding of these mutants is shown on the right. (B) Binding of Pth2 hydrolase mutants to Rad23 and Dsk2. Each T7-tagged Pth2 mutant was coexpressed with GST-Rad23 (lanes 1–9) or with GST-Dsk2 (lanes 10–18) in yeast, and cell extracts were precipitated with GSH beads and immunoblotted with anti-GST or anti-T7. Note that the expression of the mutant protein DRTQ171N (lane 17) is rather low when it was coexpressed with GST-Dsk2 but not with GST-Rad23. (C) Overexpression of Pth2 hydrolase mutants in yeast. Pth2 hydrolase mutant (D171A) or wild-type Pth2 was overexpressed in YPH499 cells harboring a single ( × 1) pGAL1-YEplac112 or both ( × 2) pGAL1-YEplac112 and pGAL1-YEplac195 at 30°C for 2–4 days. (D) Complementation test for drug sensitivity of Pth2 hydrolase mutant. pth2Δ expressing D171A from PTH2 promoter was spotted onto SD plate (minus uracil) containing 0.5 mM AZC in serial dilutions (lower panel). pth2Δ expressing FLAG-Pth2 from PTH2 promoter were spotted in parallel. The plates were incubated at 30°C for 2 days. (E) Requirement of the Pth2 C-terminal domain for binding to Rad23 and Dsk2 in vivo. GST-fused Rad23 (lanes 1–6) or GST-Dsk2 (lanes 7–12) was coexpressed in yeast with T7-tagged full-length, N-terminal (1–82) or C-terminal (83–208) domains of Pth2. The extracts were precipitated with GSH beads and immunoblotted with anti-GST and anti-T7.
Discussion
We have found a novel role for yeast Pth2 in the ubiquitin-mediated protein degradation pathway through its interaction with UBL domains of Rad23 and Dsk2. This Pth2 function is independent of its peptidyl-tRNA hydrolase activity.
Yeast Pth2 negatively regulates substrate delivery via its interaction with shuttle proteins
Polyubiquitinated proteins are targeted to the 19S proteasome regulatory particle via either the proteasome subunit Rpn10 or the shuttle proteins Rad23 and Dsk2. The Rad23/Dsk2-mediated shuttling pathway thus is redundant with Rpn10, and it remains controversial as to which is the primary pathway to the proteasome (Elsasser et al, 2004; Verma et al, 2004). We present evidence that Pth2 inhibits the binding of proteasome receptors to UBL-UBA proteins Rad23 and Dsk2 (Figures 4 and 5) and that Pth2 inhibits degradation of the authentic in vivo substrate Sic1 and the N-end rule substrate Leu-β-gal (Figure 3), suggesting that Pth2 suppresses ubiquitin-mediated degradation. The interaction of Pth2 with UBL-UBA protein exists in yeast cells (Figures 1 and 2). Interactions between Pth2 and the UBL domain of UBL-UBA proteins (Figure 6) may ultimately prevent delivery of polyubiquitinated substrates to the proteasome receptor. Such a negative cascade might be important in ensuring a proper rate of substrate degradation by preventing a high flux of substrates from reaching the proteasome.
A proper rate of delivery of polyubiquitinated substrates to the proteasome presumably requires an optimal concentration of UBL-UBA proteins. Although UBL-UBA proteins are positive factors in proteasomal degradation, at higher concentrations, they inhibit protein degradation (Raasi and Pickart, 2003). Such inhibitory effects could be explained by an overflow of polyubiquitinated substrates onto the proteasome due to an excess of UBL-UBA proteins. In this regard, we suspect that Pth2 binding may decrease the affinity of the UBL domain for the proteasomal receptor. This interpretation is supported by our data that Pth2 itself did not bind to the proteasome (Figure 5D) and that the majority of Pth2 was associated with Rad23 and Dsk2 that were bound to polyubiquitinated substrates in cells rather than to free Rad23 or Dsk2 (Figure 2A). Moreover, overexpression of Pth2 accumulated polyubiquitinated substrates in cells (Figures 2B and D), although the accumulation is less than cells overexpressing Rad23, Dsk2 or Ddi1 (cf lanes 2–5), implying that Pth2 functions indirectly in the ubiquitin–proteasome pathway through the UBL-UBA proteins. Therefore, we propose that Pth2 plays a role in regulating the flow of polyubiquitinated substrates to the proteasome by interfering with the interaction between UBL-UBA proteins and proteasome receptors. An efficient substrate flux to the proteasome also requires a transient interaction of UBL-UBA proteins with proteasomal receptors, so as not to occupy the receptor site permanently. Therefore, Pth2 may be involved in the release of UBL-UBA proteins on the proteasome because overexpression of Pth2 decreases the amounts of Rad23 and Dsk2 associated with the proteasome (see Figure 5B). However, it is unlikely that Pth2 itself occupies the receptor site, because Pth2 associated with the proteasome was not detected even when PTH2 was overexpressed (Figure 5D).
Interaction of Pth2 with the UBL domain in the shuttling pathway
To date, several UBL domain-associated proteins have been reported to modulate substrate delivery: ataxin-3 (Wang et al, 2000; Doss-Pepe et al, 2003), Ufd2 (E3/E4) (Koegl et al, 1999; Kim et al, 2004) and 19S subunit Rpn1 (Elsasser et al, 2002; Saeki et al, 2002). Similarly, Pth2 binding may induce a structural change in the UBL domain and hinder its subsequent docking to proteasome receptors. Indeed, Pth2 overexpression prevented the association of the proteasome with Rad23 and Dsk2 in vitro and Dsk2 in vivo (Figures 4 and 5). Although we could not observe a competitive inhibition by Pth2 in cells in the case of Rad23 in vivo (Figure 5B), it is conceivable that Rad23 is insensitive to Pth2 overexpression because Rad23 is much more abundant than Dsk2 (Figure 5, Elsasser et al, 2002).
In addition to the direct interaction of Pth2 with the UBL domain, we observed that Pth2 interacts with polyubiquitinated proteins. This Pth2–polyubiquitin interaction seen in vivo is indirectly mediated via the UBA domains of Rad23 and Dsk2 (Figure 6). We suspect that polyubiquitin chains bridge an interaction between the GST-UBA domain fusion proteins and endogenous Rad23 or Dsk2, which then binds to Pth2. Interestingly, we observed that Pth2 is also capable of interacting with the Dsk2 UBA domain in vivo (Figure 6C and D). By contrast, Pth2 appears not to interact with the Rad23 UBA domain (compare lanes 4 and 9 in Figure 6D). Because Pth2 itself does not bind the UBA domain in vitro (Figure 6B), it thus appears that the interaction of Pth2 with the Dsk2 UBA domain in cells is mediated by some additional factors. Such putative Pth2-associated factors could bridge the UBA domain and the UBL domain, which may give Dsk2 a distinct function in the shuttling pathway.
The structure of the UBL domain has been determined (Mueller and Feigon, 2003; Lowe et al, 2006), and the N-terminal UBL domain can interact intramolecularly with the C-terminal UBA domain (Ryu et al, 2003; Walters et al, 2003; Lowe et al, 2006). The UBL-UBA protein hHR23a undergoes a conformational change between ‘closed' and ‘open' states defined by an intramolecular interaction of the UBL and UBA domains (Walters et al, 2003). On the contrary to the ‘open' conformation predicted for the S5a receptor, it is plausible that Pth2 binding alters the conformation of Rad23 so as to form the ‘closed' states, causing reduced substrate flow to the proteasome. In the case of Dsk2, the interaction of the UBA domain with Pth2 might cause Dsk2 to adopt more ‘closed' conformation.
Pth2 function and redundancy of proteasomal ubiquitin receptors
Three different proteasomal ubiquitin receptors, the Rad23 and Dsk2 shuttle proteins and the Rpn10 regulatory particle subunit, have been analyzed extensively, and it has been shown that they have overlapping functions in the ubiquitin–proteasome pathway. According to the alternative receptor model (Elsasser et al, 2004; Verma et al, 2004), Rad23 or Dsk2 acts as a shuttle receptor pathway via the Rpn1 scaffolding protein, whereas Rpn10 seems to prefer the intrinsic proteasomal gateway via its direct binding to polyubiquitin chains. Moreover, another ubiquitin receptor(s) seems to exist in cells, because double deletion mutants and the triple deletion mutant of these receptors are still viable under certain growth conditions (Lambertson et al, 1999; Saeki et al, 2002; Elsasser et al, 2004). The proteasomal ATPase subunit Rpt5 (Lam et al, 2002) and elongation factor 1A (eEF1A) (Chuang et al, 2005) are candidates for additional ubiquitin receptors. However, it seems that Pth2 is not involved in an Rpt5 or eEF1A pathway, but rather interacts with Rad23 and Dsk2. Pth2-induced growth inhibition is mediated mainly by Rad23 and Dsk2. In the shuttling pathway, Rpn1 seems to be the major receptor pathway for Rad23 and Dsk2. We cannot exclude the direct involvement of Dsk2 in the Rpn10-mediated pathway in cells; however, it appears that Dsk2 interacts with the large scaffolding protein Rpn1 in vivo, whose association may be facilitated by its interaction with Rpn10. In light of these aspects, it is conceivable that Pth2 mainly affects Rad23 and Dsk2 through an Rpn1-mediated pathway.
Recent studies suggest that the Cdc48 receptor is involved in ER-associated degradation (ERAD) pathway for misfolded proteins (reviewed by Elsasser and Finley, 2005), where unfolded substrates are recruited to Cdc48 and polyubiquitinated by the E4 enzyme Ufd2, and this complex is subsequently delivered to the proteasome (Koegl et al, 1999; Kim et al, 2004; Medicherla et al, 2004; Richly et al, 2005). Dsk2 and Rad23 also mediate delivery in the ERAD pathway (Medicherla et al, 2004); therefore, our expectation is that Pth2 may also participate in the degradation of ERAD substrates. More recently, the shuttling pathway involving elongation factor 1A was shown to participate in the ubiquitin-mediated degradation of nascent damaged proteins (Chuang et al, 2005). A link between ubiquitin-mediated degradation and protein synthesis has been reported (Sato et al, 1998; Schubert et al, 2000; Turner and Varshavsky, 2000). During translational elongation, a significant proportion of nascent peptidyl-tRNA dissociates from the ribosome prematurely, and the prematurely dissociated polypeptides are degraded by proteasome-mediated proteolysis. Considering these points, we hypothesize that yeast Pth2 may play bifunctional roles by switching between hydrolysis of peptidyl-tRNA and an interaction with UBL-UBA proteins in protein degradation.
Possible role of the C-terminal and N-terminal domains of yeast Pth2 in the ubiquitin–proteasome pathway
Pth2 homologs have been found in archaea and eukaryotes but not in bacteria (Rosas-Sandoval et al, 2002). Although the C-terminal peptidyl-tRNA hydrolase catalytic site is not necessary for Rad23 or Dsk2 binding, another region in the C-terminal domain is required for binding (Figure 7). In addition to the C-terminal domain, the Pth2 N-terminal half may also contribute to full binding of Rad23 and Dsk2 (see Figure 7E). The N-terminal domain has phylogenetically diverged in length and sequence: a protein database search indicated that the N-terminal domains of Pth2 from different eukaryotes, such as yeast, nematode, fly and mammal, are distinct, implying that this domain could define species-specific functions of Pth2. Indeed, mammalian Pth2 has an N-terminal signal sequence for mitochondrial localization mediating apoptosis in humans (Jan et al, 2004), while archaeal Pth2 lacks the N-terminal domain entirely (Rosas-Sandoval et al, 2002). Caenorhabditis elegans Pth2 (C24G6.8) contains the UBA domain in its N-terminal half and its sequence shows high homology to the deubiquitinating enzyme Ubp14. In yeast, it is possible that the N-terminal domain also plays a part in ubiquitin-mediated degradation, together with the C-terminal Rad23/Dsk2-binding domain.
Materials and methods
Strains and media
The yeast strains used in this study were in the S. cerevisiae YPH499 (MATa ade2-101 leu2-Δ1 trp1-Δ63 ura3-52 lys2-801 his3-Δ200) background (Sikorsky and Hieter, 1989). Yeast strains were grown in YPD or selective medium. The yeast strain deleted for the PTH2 gene was generated by PCR-mediated gene disruption (Adams et al, 1997) and verified by PCR and immunoblot analysis. For overexpression experiments, yeast cells transformed with a galactose-inducible plasmid were cultured in minimal medium containing 2% raffinose at 30°C to A600=0.8. Galactose (2%) then was added to the medium and the cells were incubated at 30°C for 4 h. The E. coli DH5α strain was used for DNA manipulation, and E. coli BL21 (DE3) was used for the expression of recombinant protein. The yeast strain Y190 (MATa ade2-101 his3-Δ200 leu2-3,112 trp1-901 ura3-52 gal4Δ gal80Δ cyhr2 URA3::Gal-lacZ LYS2∷GAL-HIS3) and PJ69-4A (MATa trp1-901 leu2-3,112 ura3-52 his3Δ200 gal4Δ gal80Δ LYS2∷GAL1-HIS3 GAL2-ADE2 met2∷GAL7-lacZ) were used for the two-hybrid screening. DSK2 lacking the N terminus (72–373) was subcloned into pAS404 and used as bait in the two-hybrid screen using a yeast Gal4AD-cDNA library (Funakoshi et al, 2002). Transformants that grew on the SD-His-Trp-Leu +25 mM 3-aminotriazole plate were selected and the library clones were obtained and sequenced.
Plasmids and mutants
For the binding assays, the PCR-amplified PTH2 ORF was subcloned into a BamHI–HindIII site in the galactose-inducible vector pGAL1-YEplac112-T7. For overexpression, PTH2 DNA was subcloned into a BamHI–HindIII site in the galactose-inducible vectors, pGAL1-YEplac112, pGAL1-YEplac181 and pGAL1-YEplac195. For the expression of GST-fused Pth2, the PCR-amplified PTH2 ORF was subcloned into a XbaI–HindIII site in the galactose-inducible vector pEG(KG) and expression vector pGEX(KG). For the expression of His6-T7 tagged Pth2, PTH2 DNA was subcloned into a BamHI–HindIII site in the expression vector pET-28a(+). PTH2 deletion mutants were constructed by the PCR method and subcloned into pGAL1-YEplac112-T7. For the complementation test, the PCR-amplified PTH2 ORF was subcloned into an EcoRI–HindIII site in the expression vector pKQV4. PTH2 gene (1.1 kb) that has 5′UTR containing its promoter, ORF, 3′UTR, and terminator was generated by PCR amplification from yeast genome DNA and subcloned into a BamHI–HindIII site in pRS316 vector. FLAG-PTH2 in pRS316 was made by the whole-plasmid PCR amplification method (Parikh and Guengerich, 1998). PTH2 hydrolase mutants were generated by the whole-plasmid PCR amplification method similarly, and subcloned into either pKQV4 and pGAL1-YEplac112-T7 or pRS316.
The PCR-amplified RAD23 and DDI1 ORFs were subcloned into a BamHI–SalI site in pEG(KG) and pGEX(KG) to construct GST-tagged Rad23 and GST-Ddi1, and RAD23 deletion mutants were constructed by the PCR method and subcloned into a XbaI–XhoI site in pEG(KG) and pGEX(KG). The RPN1 ORF was generated by the PCR method and subcloned into a BamHI–XhoI site in pGEX(KG) and pET-28a(+). The PCR-amplified RPN10 ORF was subcloned into an XbaI–HindIII site in pGEX(KG), and into an SacI–HindIII site in pET-28a(+). For the expression of 3′-His6-tagged Dsk2, the PCR-amplified DSK2 ORF was subcloned into a NdeI–HindIII site in the expression vector pET-21a, and the other plasmids carrying DSK2 were described previously (Funakoshi et al, 2002). Plasmids carrying Ub-Leu-β-galactosidase (β-gal) and Ub-Ala-β-gal were originally provided by Dr A Varshavsky.
Pull-down assays and immunoblotting
Cell extracts were prepared using glass beads in lysis buffer: 20 mM HEPES pH 7.5, 100 mM NaCl, 0.5% Nonidet P-40, 1 mM EDTA, 1 mM p-amidinophenylmethanesulfonyl fluoride and 1 μg/ml each of leupeptin, pepstatin and chymostatin. For the GST pull-down assay, cell extracts (5.0 A600 cells equivalent) or recombinant proteins (1 μg) were incubated with 20 μl glutathione-Sepharose 4B (Pharmacia) in 500 μl lysis buffer for 1 h at 4°C. The beads were washed three times with lysis buffer, followed by SDS–PAGE of the retained proteins and immunoblotting with anti-GST. For FLAG pull-down, cell extracts (5.0 A600) were immunoprecipitated with anti-FLAG M2 beads (Sigma). Cell extracts equivalent to 0.2 A600 were used for immunoblotting of cell extracts, except for anti-Dsk2 antibody (0.02 A600). Recombinant GST-fused proteins and His6-tagged proteins were prepared from E. coli BL21 (DE3) that was transformed with expression vectors. Proteasomes were affinity-purified from the cell lysates prepared by manually ground powder methods with pestle and mortar (Verma et al, 2000).
Gel filtration
Gel filtration chromatography was carried out using an AKTA FPLC system equipped with a Superdex 200 HR 10/30 column (Pharmacia). Yeast cell extract was applied to the column and eluted with a buffer (20 mM HEPES pH 7.5, 100 mM NaCl, 1 mM EDTA) at a flow rate of 0.4 ml/min. Then, each fraction was analyzed by immunoblotting. The Gel Filtration Standard (Bio-Rad) containing thyroglobulin (670 kDa), gamma globulin (158 kDa), ovalbumin (44 kDa), myoglobin (17 kDa) and vitamin B-12 (1.35 kDa), and bovine serum albumin (67 kDa) was used for molecular size markers.
Degradation assay of N-end rule substrate and Sic1
The plasmid carrying the Ub-lacZ fusion gene (Bachmair et al, 1986) was transformed into strain YPH499 and pth2Δ. Ub-Leu-β-gal and Ub-Ala-β-gal (Varshavsky, 1996) were expressed in yeast under the control of the GAL1 promoter. Cells were collected by centrifugation and transferred to galactose medium lacking uracil. The incubation was continued for 2 h to induce Ub-Leu-β-gal or Ub-Ala-β-gal, and then dextrose and cycloheximide were added to a final concentration of 2% and 0.5 mg/ml, respectively. Cell extracts were prepared (Runder et al, 2000), followed by immunoblotting with anti-β-gal. The plasmid carrying HA-tagged Sic1, pMF920, was transformed into YPH499 and pth2Δ. The transformants were pregrown at 30°C overnight in raffinose medium lacking tryptophan. Cells were transferred to fresh raffinose medium and incubated 2 h at 26°C; succinate and α-factor were then added to the culture at a concentration of 0.5% and 10 μg/ml, respectively. After incubating another 4 h at 26°C, galactose (2%) was added to the medium and the incubation was continued for 1 h to induce Sic1-HA protein. Cells were washed twice with sterile water, transferred to glucose medium, and incubated for cell sampling at the time intervals. For overexpression of GST-Pth2 in Sic1-expressing cells, both pMF920 and pEG(KG)-PTH2 were transformed into YPH499 cells. After synchronization with α-factor, the cells were incubated in galactose medium for 2 h to induce Sic1-HA and GST-Pth2.
Growth sensitivity to amino-acid analogs and UV irradiation
Yeast strains were cultured in synthetic complete medium (for the canavanine assay, lacking arginine) at 30°C for 14–16 h and their cell densities were adjusted to OD600=1.0. Ten- and three-fold serial dilutions were prepared in sterile water, and 10 μl was spotted on duplicate synthetic complete medium plates (SD) and synthetic complete medium plates supplemented with either 0.5 mM azetidine-2-carboxylic acid (AZC) or 3 μg/ml canavanine. In case of YPD medium, cells were spotted on YPD plates supplemented with 5 mM AZC. Yeast cells on YPD plates were exposed to UV irradiation (50 J/m2). The sensitivity to amino-acid analogs and UV irradiation was determined after 2–4 days of growth at 30°C.
Complementation of E. coli strain C600 pthts
Competent E. coli C600 pthts cells (Rosas-Sandoval et al, 2002) were prepared and transformed with the pKQV4 vector (Strauch et al, 1989) containing wild-type PTH2 and its mutants. Expression in each construct was controlled by the IPTG-inducible tac promoter. The transformants were plated on LB agar containing 0.1 mg/ml ampicillin and 1 μM IPTG, and duplicate plates were incubated at 32 and 42°C.
Antibodies and ubiquitins
Anti-Pth2 polyclonal antiserum was raised in rabbits using purified Pth2 prepared from thrombin-digested recombinant GST-Pth2. Other reagents included anti-Dsk2 (Funakoshi et al, 2002), anti-Ub, anti-GST, anti-Rad23 and anti-Cdc28 from Santa Cruz Biotechnology (Santa Cruz, CA), anti-T7 and anti-β-gal (Promega), anti-polyubiquitin (FK1, Nippon Bio-Test Laboratories, Tokyo), anti-20S proteasome ‘core', anti-Rpt1, anti-Rpt3, tetra-Ub and poly-Ub chains from Affiniti (Exeter, UK), anti-FLAG (M2, Sigma) and anti-HA (Babco).
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Acknowledgments
We thank Dr Dieter Soll for providing the E. coli C600 pthts strain and pKQV4, Dr Ray Deshaies for FLAG-RPT1 and FLAG-PRE1, and Dr Akio Toh-e for pMF920. This work was supported by grants from the Ministry of Education, Science, Sports and Technology of Japan and by CREST, JST Japan.
References
- Adams A, Gottschling DE, Kaiser CA, Stearns T (1997) Gene replacement. In Methods in Yeast Genetics, pp 59–70. New York: CSHL Press [Google Scholar]
- Bachmair A, Finley D, Varshavsky A (1986) In vivo half-life of a protein is a function of its amino-terminal residue. Science 234: 179–186 [DOI] [PubMed] [Google Scholar]
- Biggins S, Ivanovska I, Rose MD (1996) Yeast ubiquitin-like genes are involved in duplication of the microtubule organizing center. J Cell Biol 133: 1331–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Madura K (2002) Rad23 promotes the targeting of proteolytic substrates to the proteasome. Mol Cell Biol 22: 4902–4913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang S-M, Chen L, Lambertson D, Anand M, Kinzy TG, Madura K (2005) Proteasome-mediated degradation of cotranslationally damaged proteins involves translation elongation factor 1A. Mol Cell Biol 25: 403–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pereda JM, Waas WF, Jan Y, Ruoslahti E, Schimmel P, Pascual J (2004) Crystal structure of a human peptidyl-tRNA hydrolase reveals a new fold and suggests basis for a bifunctional activity. J Biol Chem 279: 8111–8115 [DOI] [PubMed] [Google Scholar]
- Doss-Pepe EW, Stenroos ES, Johnson WG, Madura K (2003) Ataxin-3 interactions with Rad23 and Valosin-containing protein and its associations with ubiquitin chains and the proteasome are consistent with a role in ubiquitin-mediated proteolysis. Mol Cell Biol 23: 6469–6483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsasser S, Chandler-Militello D, Muller B, Hanna J, Finley D (2004) Rad23 and Rpn10 serve as alternative ubiquitin receptors for the proteasome. J Biol Chem 279: 26817–26822 [DOI] [PubMed] [Google Scholar]
- Elsasser S, Finley D (2005) Delivery of ubiquitinated substrates to protein-unfolding machines. Nat Cell Biol 7: 742–749 [DOI] [PubMed] [Google Scholar]
- Elsasser S, Gali RR, Schwickart M, Larsen CN, Leggett DS, Muller B, Feng MT, Tubing F, Dittmar GAG, Finley D (2002) Proteasome subunit Rpn1 binds ubiquitin-like protein domains. Nat Cell Biol 4: 725–730 [DOI] [PubMed] [Google Scholar]
- Fromant M, Ferri-Fioni M-L, Plateau P, Blanquet S (2003) Peptidyl-tRNA hydrolase from Sulfolobus solfataricus. Nucleic Acids Res 31: 3227–3235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi M, Sasaki T, Nishimoto T, Kobayashi H (2002) Budding yeast Dsk2p is polyubiquitin-binding protein that can interact with the proteasome. Proc Natl Acad Sci USA 99: 745–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann-Petersen R, Seeger M, Gordon C (2003) Transferring substrates to the 26S proteasome. Trends Biochem Sci 28: 26–31 [DOI] [PubMed] [Google Scholar]
- Jan Y, Matter M, Pai JT, Chen Y-L, Pilch J, Komatsu M, Ong E, Fukuda M, Ruoslahti E (2004) A mitochondrial protein, Bit1, mediates apoptosis regulated by integrins and groucho/TLE corepressors. Cell 116: 751–762 [DOI] [PubMed] [Google Scholar]
- Kim I, Mi K, Rao H (2004) Multiple interactions of Rad23 suggest a mechanism for ubiquitylated substrate delivery important in proteolysis. Mol Biol Cell 15: 3357–3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegl M, Hoppe T, Schlenker S, Ulrich HD, Mayer TU, Jentsch S (1999) A novel ubiquitination factor, E4, is involved in multiubiquitin chain assembly. Cell 96: 635–644 [DOI] [PubMed] [Google Scholar]
- Lam YA, Lawson TG, Velayutham M, Zweler JL, Pickart CM (2002) A proteasomal ATPase subunit recognizes the polyubiquitin degradation signal. Nature 416: 763–767 [DOI] [PubMed] [Google Scholar]
- Lambertson D, Chen L, Madura K (1999) Pleiotropic defects caused by loss of the proteasome-interacting factors Rad23 and Rpn10 of Saccharomyces cerevisiae. Genetics 153: 69–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe ED, Hasan N, Trempe JF, Fonso L, Noble ME, Endicott JA, Johnson LN, Brown NR (2006) Structures of the Dsk2 UBL and UBA domains and their complex. Acta Cryst D 62: 177–188 [DOI] [PubMed] [Google Scholar]
- Madura K (2004) Rad23 and Rpn10: perennial wallflowers join the melee. Trends Biochem Sci 29: 637–640 [DOI] [PubMed] [Google Scholar]
- Medicherla B, Kostova MZ, Schaefer A, Wolf DH (2004) A genomic screen identifies Dsk2p and Rad23p as essential components of ER-associated degradation. EMBO Rep 5: 692–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menez J, Buckingham RH, de Zamaroczy M, Campelli CK (2002) Peptidyl-tRNA hydrolase in Bacillus substilis, encoded by spoVC, is essential to vegetative growth, whereas the homologous enzyme in Saccharomyces cerevisiae is dispensable. Mol Microbiol 45: 123–129 [DOI] [PubMed] [Google Scholar]
- Menninger JR (1976) Peptidyl transfer RNA dissociates during protein synthesis from ribosomes of Escherichia coli. J Biol Chem 251: 3392–3398 [PubMed] [Google Scholar]
- Mueller TD, Feigon J (2003) Structural determinants for the binding of ubiquitin-like domains to the proteasome. EMBO J 22: 4634–4645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh A, Guengerich FP (1998) Random mutagenesis by whole-plasmid PCR amplification. Biotechniques 24: 428–431 [DOI] [PubMed] [Google Scholar]
- Raasi S, Pickart CM (2003) Rad23 ubiquitin-associated domains (UBA) inhibit 26S proteasome-catalyzed proteolysis by sequestering lysine 48-linked polyubiquitin chains. J Biol Chem 278: 8951–8959 [DOI] [PubMed] [Google Scholar]
- Rao H, Sastry A (2002) Recognition of specific ubiquitin conjugates is important for the proteolytic functions of the ubiquitin-associated domain proteins Dsk2 and Rad23. J Biol Chem 277: 11691–11695 [DOI] [PubMed] [Google Scholar]
- Richly H, Rape M, Braun S, Rumpf S, Hoege C, Jentsch S (2005) A series of ubiquitin binding factors connects CDC48/p97 to substrate multiubiquitylation and proteasomal targeting. Cell 120: 73–84 [DOI] [PubMed] [Google Scholar]
- Rosas-Sandoval G, Ambrogelly A, Rinehart J, Wei D, Cruz-Vera LR, Graham DE, Stetter KO, Guarneros G, Soll D (2002) Orthologs of a novel archaeal and of the bacterial peptidyl-tRNA hydrolase are nonessential in yeast. Proc Natl Acad Sci USA 99: 16707–16712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runder AD, Hardwick KG, Murray A (2000) Cdc28 activates exit from mitosis in budding yeast. J Cell Biol 149: 1361–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu KS, Lee KJ, Bae SH, Kim BK, Kim KA, Choi BS (2003) Binding surface mapping of intra- and interdomain interactions among hHR23B, ubiquitin, and polyubiquitin binding site 2 of S5a. J Biol Chem 278: 36621–36627 [DOI] [PubMed] [Google Scholar]
- Saeki Y, Saitoh A, Toh-e A, Yokosawa H (2002) Ubiquitin-like proteins and Rpn10 play cooperative roles in ubiquitin-dependent proteolysis. Biochem Biophys Res Commun 293: 986–992 [DOI] [PubMed] [Google Scholar]
- Sato S, Ward CL, Kopito RR (1998) Cotranslational ubiquitination of cystic fibrosis transmembrane conductance regulator in vitro. J Biol Chem 273: 7189–7192 [DOI] [PubMed] [Google Scholar]
- Schauber C, Chen L, Tongaonkar P, Vega I, Lambertson D, Potts W, Madura K (1998) Rad23 links DNA repair to the ubiquitin/proteasome pathway. Nature 391: 715–718 [DOI] [PubMed] [Google Scholar]
- Schubert U, Anton LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR (2000) Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature 404: 770–774 [DOI] [PubMed] [Google Scholar]
- Sikorsky RS, Hieter P (1989) A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122: 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch MA, Spiegelman GB, Perego M, Johnson WC, Burbulys D, Hoch JA (1989) The transition state transcription regulator abrB of Bacillus subtilis is a DNA binding protein. EMBO J 8: 1615–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner GC, Varshavsky A (2000) Detecting and measuring cotranslational protein degradation in vivo. Science 289: 2117–2120 [DOI] [PubMed] [Google Scholar]
- van Nocker S, Sadis S, Rubin DM, Glickman M, Fu H, Coux O, Wefes I, Finley D, Vierstra RD (1996) The multiubiquitin-chain-binding protein Mcb1 is a component of the 26S proteasome in Saccharomyces cerevisiae and plays a non-essential, substrate-specific role in protein turnover. Mol Cell Biol 16: 6020–6028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A (1996) The N-end rule: functions, mysteries, uses. Proc Natl Acad Sci USA 93: 12142–12149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R, Chen S, Feldman R, Schieltz D, Yates J, Dohmen J, Deshaies RJ (2000) Proteasome proteomics: identification of nucleotide-sensitive proteasome-interacting proteins, by mass spectrometric analysis of affinity-purified proteasomes. Mol Biol Cell 11: 3425–3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R, Oania R, Graumann J, Deshaies RJ (2004) Multiubiquitin chain receptors define a layer of substrate selectivity in the ubiquitin–proteasome system. Cell 118: 99–110 [DOI] [PubMed] [Google Scholar]
- Walters KJ, Lech PJ, Goh AM, Wang Q, Howley PM (2003) DNA-repair protein hHR23a alters its protein structure upon binding proteasomal subunit S5a. Proc Natl Acad Sci USA 100: 12694–12699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Sawai N, Kotliarova S, Kanazawa I, Nukina N (2000) Ataxin-3, the MJD1 gene product, interacts with the two human homologs of yeast DNA repair protein RAD23, HHR23A and HHR23B. Hum Mol Genet 9: 1795–1803 [DOI] [PubMed] [Google Scholar]
- Wilkinson CRM, Seeger M, Hartmann-Petersen R, Stone M, Wallace M, Semple C, Gordon C (2001) Proteins containing the UBA domain are able to bind to multi-ubiquitin chains. Nat Cell Biol 3: 939–943 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3


