Abstract
Dendritic cells (DCs) capture and process Ag in the periphery. Thus, traffic through lymphatic vessels is mandatory before DCs relocate to lymph nodes where they are dedicated to T-cell priming. Here, we show that the ubiquitous self-marker CD47 selectively regulates DC, but not T and B cell trafficking across lymphatic vessels and endothelial barriers in vivo. We find an altered skin DC migration and impaired T-cell priming in CD47-deficient mice at steady state and under inflammatory conditions. Competitive DC migration assays and active immunization with myeloid DCs demonstrate that CD47 expression is required on DCs but not on the endothelium for efficient DC trafficking and T-cell responses. This migratory defect correlates with the quasi-disappearance of splenic marginal zone DCs in nonmanipulated CD47-deficient mice. Nonetheless, CCR7 expression and CCL19-driven chemotaxis remain intact. Our data reveal that CD47 on DCs is a critical factor in controlling migration and efficient initiation of the immune response.
Keywords: CCR7, CD47, dendritic cell, migration, SIRP-α
Introduction
Dendritic cells (DCs), sentinels of the immune system, are unique in their capacity to coordinate innate and adaptive immune responses. Upon encounter with pathogens or danger signals, DCs adapt their chemokine receptors and migrate to the lymph nodes (LN) where they transfer their information to T cells and initiate T-cell differentiation and polarization (Palucka and Banchereau, 2002; Steinman et al, 2003). The trafficking of DCs through lymphatic vessels (LV) is an obligatory route for the accomplishment of their functions (Randolph et al, 2005a). In homeostatic and inflammatory conditions, DC mobilization is under the control of CCR7, although additional signals that include leukotrienes, prostaglandin E2 and CD38 usually found at sites of inflammation, appear to be required to sensitize CCR7 to its ligands (Randolph et al, 2005b). Activated DCs are first attracted to the connective tissue by CCL21 expressed by lymphatic endothelial cells until they enter the afferent lymph and produce CCL19, a chemokine that promotes their final maturation (Bachmann et al, 2006).
Yet, a large gap persists in our understanding of the regulation of DC interactions with the LV, their passage across the endothelial barrier and finally their exit and relocation to LN, where they encounter Ag-specific T cells. Another important issue relates to how DCs egress from the bloodstream to penetrate lymph nodes through high endothelial venules (HEV) (Randolph et al, 2005a). Adhesion (ICAM), junctional (JAMA-1) and extracellular matrix (SPARC) proteins expressed by the host environment or DCs themselves favor DC retention in the peripheral tissues (Xu et al, 2001; Cera et al, 2004; Sangaletti et al, 2005).
A link exists between CD47/signal regulatory proteins (SIRP)-α interactions and neutrophil transepithelial migration in vitro (Liu et al, 2002; Zen and Parkos, 2003). Neutrophil mobilization is delayed in vivo in CD47-deficient mice after intraperitoneal inoculation of Escherichia coli (Lindberg et al, 1996). The CD47 Ag (integrin-associated protein) is a highly hydrophobic and unusual pentaspanning transmembrane protein that is physically and functionally associated with αvβ3 integrin, the vitronectin receptor (Brown and Frazier, 2001). Ubiquitously expressed on hematopoietic and non-hematopoietic cells, CD47 serves both as a receptor for the extracellular matrix protein thrombospondin-1 (TSP) and as a ligand for the transmembrane SIRP-α and -γ (Gao et al, 1996; van Beek et al, 2005). CD47 is considered to be a marker of self in immune and nonimmune cells in vivo, as it delivers a negative signal to SIRP-α expressed on resident macrophages or DCs, thus inhibiting the clearance of intact hematopoietic cells (Oldenborg et al, 2000; Gardai et al, 2005). Interaction of CD47 with either SIRP-α or TSP molecules regulates several important biological phenomena, including cell–cell communication, macrophage multinucleation, neuronal survival, maintenance of lung homeostasis as well as DC maturation and their pro-inflammatory cytokine production (Cant and Ullrich, 2001; Latour et al, 2001).
Considering that interference with CD47/SIRP-α interactions by means of specific antibodies or respective fusion proteins favors Langerhans cell (LC) retention in the epidermis under inflammatory conditions (Fukunaga et al, 2004; Yu et al, 2006), we hypothesized that CD47 deficiency affects the mobilization of SIRP-α-expressing myeloid DCs of the immune system, that is, LC, dermal DCs and CD11b+CD11c+ cells, and thus regulates the T-cell priming that ensues (Edwards et al, 2003; Fukunaga et al, 2004). We investigated the impact of CD47 expressed in DCs or as part of the host environment on their trafficking in vivo. We report that the lack of CD47 curtails in vivo skin DC migration and the Ag-specific T-cell response under steady state and inflammatory conditions. We demonstrate that CD47 expression is dispensable on the endothelium but its expression on DCs themselves is essential for their passage across HEV and vascular sinusoids prior their relocation to the LN and the spleen, respectively. In vivo, the impaired DC migration is reflected by a profound defect in myeloid CD11b+CD4+ DCs of the splenic marginal zone. Thus, we propose CD47 molecule as a positive self-regulator on myeloid DCs for the control of their trafficking to secondary lymphoid organs.
Results
CD47 is required for DC entry into dermal lymphatic vessels
CD47 homophilic and/or CD47/SIRP-α interactions appear to be involved in leukocyte trafficking (Liu et al, 2004b; Rebres et al, 2005). Whether these interactions are also implicated in DC migration and which is the precise step of DC trafficking affected by CD47 deficiency remains to be elucidated. We thus examined the role of CD47 expression for LC emigration from the epidermis to the LN. We quantified the number MHC I-E+ cells in freshly isolated epidermal sheets and first noticed a slight reduction in LC density (10%) (P<0.01) in CD47−/− skin (Figure 1A, top). After in vitro culture of the skin, the epidermis was separated from the dermis to examine LC morphology and distribution (Figure 1A, middle and bottom panels). LCs acquired a round shape in the epidermis of both mice. Whereas CD47+/+ DCs accumulated along LV forming dermal cord structures, CD47−/− DCs were distributed evenly within the dermis. The absence of dermal cord formation in the CD47-deficient mice suggests that CD47 is required for DC entry into LV. We observed a significant decrease in the accumulation of skin-derived DCs in the CD47−/− LN (Figure 1B and C). As the CD11c+IEhigh subset may represent activated resident DCs, we examined CD40 expression and found a significant reduction in the proportion and absolute number of both dermal (CD11clowCD40+, I) and epidermal (CD11chighCD40+, II) skin-derived DCs. However, CCR7 expression on CD11c+IEhigh and CD11c+CD40high DCs was not significantly affected by the lack of CD47 expression. The proportion of resident CD8+DEC205+ DCs was comparable in both strains of mice, but the total DC number was significantly reduced in CD47-deficient mice. This reflected a decrease in CD11clowB220 DCs (Supplementary Figure S1).
Figure 1.
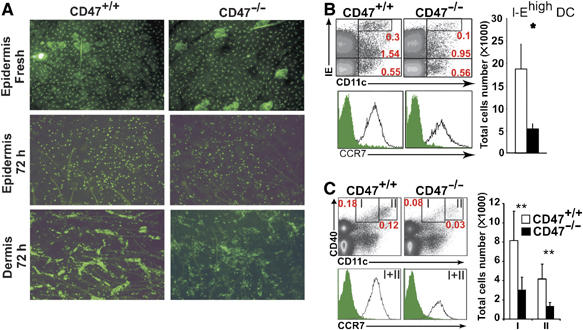
Effect of CD47 deficiency on the distribution and mobilization of LCs and skin DCs. (A) Epidermal sheets were prepared from untreated CD47−/− and CD47+/+ mice and stained with MHC I-E mAb (top panel). After 72 h culture, the epidermis (middle panel) was separated from the dermis (bottom panel) and stained with I-E mAb. The data represent one of three individually analyzed mice. (B, C) LN cell suspensions were analyzed for the expression of CD11c, I-E and CD40. The proportion of CD11c+I-Ehigh cells (B) and of each CD11c+CD40+ subset (I, dermal DCs and II, epidermal DCs) (C) is shown. CCR7 staining was examined for CD11c+ I-Ehigh (B, lower panel) and skin-derived DCs (I+II) (C, lower panel). Dot plots are representative of five individually analyzed LN from separate mice. Bars represent absolute number (mean±s.d., n=5 mice) of CD11c+IEhigh cells and CD11c+CD40+ DC subsets. *P<0.05, **P<0.01.
To further clarify the impairment of DC trafficking across the LV to the LN, we examined the impact of CD47 deficiency on the Ag-specific response of passively transferred DO11.10 TCR transgenic CD4+ T cells following subcutaneous (s.c.) immunization with OVA protein in the absence of adjuvant (Figure 2A). T-cell proliferation, measured by CFSE dilution on day 2, was impaired in the ipsilateral LN in CD47-deficient compared to BALB/c mice. No T-cell priming was detected in the contralateral LN owing to specificity of the response (data not shown). The decreased T-cell priming was observed at 72 h whether mice were immunized with 1 or 10 μg OVA, as demonstrated by the percent of undivided cells. Note that a similar proportion of transferred naïve T cells were recovered in both strains of unmanipulated mice, suggesting that the CD47-deficient environment did not hamper their migration to LN (Figure 2C).
Figure 2.
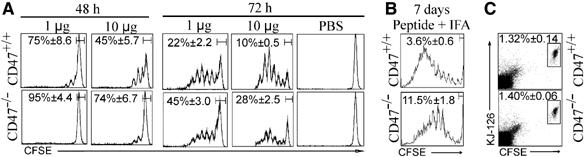
Impaired T-cell response in CD47−/− mice under homeostatic and inflammatory conditions. (A, B) One day after adoptive transfer of CFSE-labelled CD4+ DO11.10 T cells into CD47+/+ and CD47−/− mice, the recipients were immunized s.c. into the left footpad with 1 or 10 μg OVA protein (A) or 10 μg OVA peptide with IFA (B). PBS was injected into right footpad. Proliferation of CD4+ T cells was measured by CFSE dilution in the draining LN after 48 or 72 h (A) and 7 days (B). CFSE profile (% of undivided cells, mean±s.e.m.) is representative of five individually analyzed LN from separate mice. (C) Proportion of CFSE-labelled CD4+ DO11.10 T cells (KJ-126+ cells) recovered from naive CD47+/+ and CD47−/− mice after 24 h.
Altogether, the observed impairment of dermal cord formation, decreased accumulation of skin-derived DCs in the LNs and inefficient T-cell priming in CD47-deficient mice support the hypothesis of altered skin DC migration.
CD47/SIRP-α interactions are required for skin DC migration under inflammatory conditions
To examine how the absence of CD47 influences DC trafficking under inflammatory conditions, mice were immunized with OVA peptide in IFA 1 day after passive transfer of transgenic T cells and T-cell priming was examined at day 7 (Figure 2B). At this time point, T-cell proliferation continued to be decreased in CD47−/− hosts demonstrating that CD47 deficiency has a negative impact on the priming of T cells under inflammatory conditions. By using an OVA peptide, we also ascertained that impaired Ag processing did not account for the altered T-cell response.
Cutaneous sensitization with FITC is an inflammatory stimulus commonly used to induce a robust migration of skin DCs, along with other immune cells, into draining LN (Figure 3A). However, LN cellularity as well as T and B cell number remained unchanged in CD47-deficient mice after FITC painting (Figure 3A). The absence of immune cell recruitment to the LN is paralleled by a significant decrease in the proportion and accumulation of DCs carrying FITC (Figure 3B and C). The early (i.e. 24 h) and late (i.e. 72 h) defects in DC migration delineated a decrease in the accumulation of dermal DCs and LCs, respectively (Kissenpfennig et al, 2005). From 24 to 72 h after FITC painting, we observed a 3.1- and 5.3-fold increase in the recovery of CD47−/− and CD47+/+IE+FITC+ DCs, respectively, in the LN. These data suggest that the defect in migration is more pronounced for LCs than dermal CD47−/− DCs.
Figure 3.
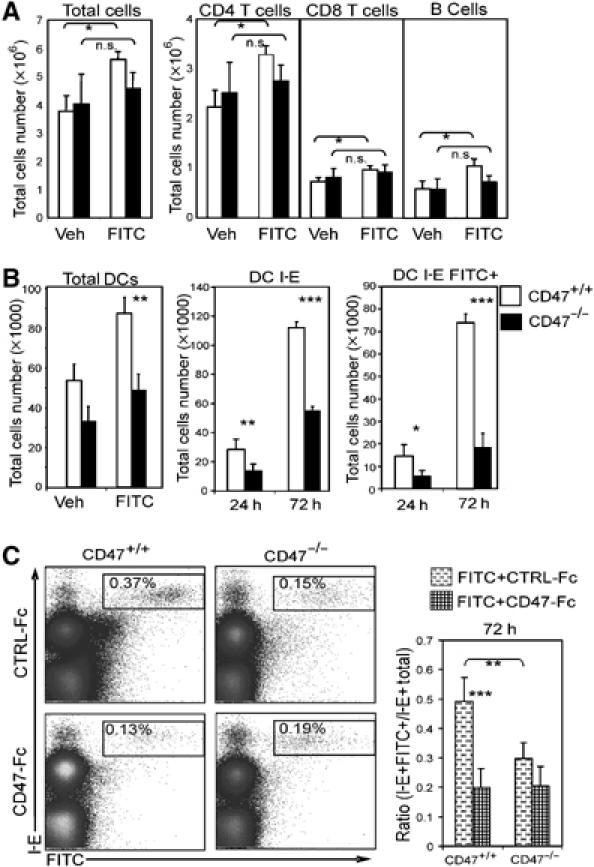
CD47/SIRP-α requirement for skin DC migration under inflammatory conditions. LN cell recovery 24 h after FITC painting is shown for various cell subsets (A). Relative number of total DCs (24 h), MHC-I-E+ and MHC-I-E+ carrying FITC (B). Proportion of MHC-I-E+ FITC+ DCs in LN (72 h) of mice injected intradermally with CD47-Fc or CTRL-Fc. Sixty minutes before FITC challenge (C). For each panel, data are representative of at least five individually analyzed LN from separate mice. *P<0.05, **P<0.01, ***P<0.001.
To examine the contribution of CD47 to SIRP-α interactions in BALB/c mice, intradermal injections of CD47-Fc or control fusion protein were performed 1 h before FITC application. CD47-Fc treatment significantly reduced the accumulation of epidermal (72 h) and dermal (24 h) IE+FITC+DCs in BALB/c mice (Figure 3C, data not shown). The results were expressed as a ratio of IE+FITC+DCs/Total IE+DCs in the LN to eliminate independent variability owing to differences in LN cellularity in the two strains of mice. In contrast to BALB/c mice, pretreatment with CD47-Fc did not further inhibit DC migration at any time point in CD47-deficient mice (Figure 3C). These data demonstrate that CD47-Fc probably disrupted CD47/SIRP-α interactions between DCs and their environment, rather than delivering a negative signal via SIRP-α ligation on DCs and/or the endothelium.
Decreased trafficking of CD47−/− DCs across lymphatic vessels
In vitro transepithelial migration of leukocytes requires CD47 to be expressed on the epithelium side and CD47 overexpression enhances this passage (Liu et al, 2004a; Rebres et al, 2005). We thus examined whether a similar requirement applied to in vivo transendothelial DC migration. To investigate the importance of CD47 as part of the host environment for DC trafficking, OVA peptide-pulsed bone-marrow-derived (BM) CD47+/+ DCs were administered s.c. to CD47+/+ and CD47−/− mice in which CFSE transgenic T cells had been adoptively transferred (Figure 4A). The T-cell response was comparable in the two types of mice and directly correlated with the number of injected DCs (Figure 4A, top and middle panels). Indeed, a five-fold reduction in the number of BM-CD47+/+ DCs (0.05 versus 0.25 × 106) resulted in an equivalent decrease in T-cell response. These results indicated that the absence of CD47 in the host did not influence the efficiency of CD47+/+ DC migration and the quality of the ensuing T-cell response. The next series of experiments were then exclusively performed in CD47-deficient hosts, a mandatory setting considering that injected CD47−/− cells were readily eliminated from BALB/c mice as a result of the lack of an inhibitory signal delivered through SIRP-α (Gardai et al, 2005). When compared to CD47+/+ BM-DCs, CD47−/− BM-DCs yielded a strong decrease in the magnitude of the T-cell response in CD47−/− mice. In fact, the number of undivided T cells was comparable for CD47−/− mice immunized with either 0.25 × 106 CD47−/− or 0.05 × 106 CD47+/+ BM-DCs. Therefore, inefficient T-cell priming in vivo is observed only when CD47 is absent on the DC and not in the endothelium or in the microenvironment.
Figure 4.
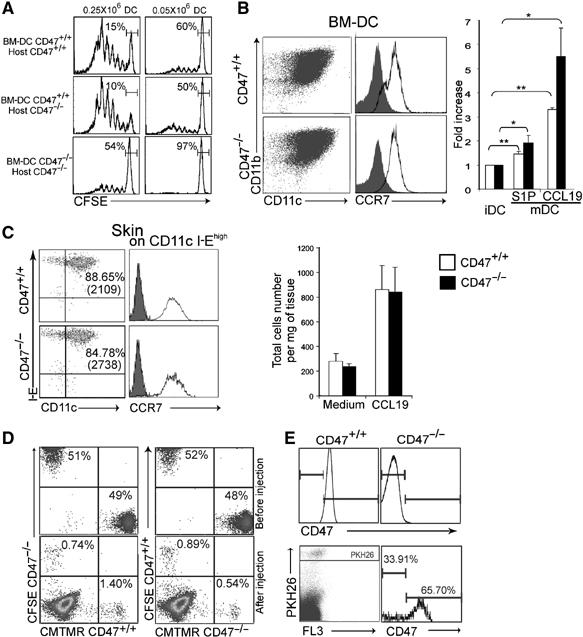
Decreased CD47−/− DC trafficking across lymphatic vessels. (A) 0.25 and 0.05 × 106 CD47+/+ and CD47−/− LPS-activated and OVA-pulsed BM-DCs were injected s.c. into the footpad 24 h after adoptive transfer of CFSE-labelled transgenic CD4+ T cells. Proliferation of CD4+ T cells in the draining LN was analyzed after 72 h. Data are representative of four individually analyzed mice. (B) Activated BM-DCs were analyzed for CD11b and CCR7 expression and their in vitro migration toward CCL19 and S1P in transwell chambers. Dot plots and histograms represent one of four BM-DC preparations. Bars represent fold increase DC migration compared to immature DCs (mean±s.d., n=5 mice). (C) Ears from four to ten mice were pooled to determine the proportion (MFI, in parenthesis) of I-E+ cells emigrated into the tissue culture medium after 72 h. CCR7 staining is shown for CD11c+I-Ehigh DCs. Bars represent the number of IE+ cells/mg of tissue cultured in the absence or presence of CCL19 (mean±s.d., n=3 pools of mice). (D) For the competitive migration assay, activated CD47+/+ and CD47−/− BM-DCs were stained with CFSE or CMTMR and vice versa, and mixed at a 1:1 ratio before s.c. injection into mice. Top panel; a mixture of cells before injection. Bottom panel; cells were isolated from LN on day 2 and retraced (E) CD47+/+ and CD47−/− activated BM-DC were stained with PKH-26 and injected s.c. in CD47−/− footpad, then traced by staining with anti-CD47 antibody after gating on PKH-26-positive cells. Upper panel shows negative and positive CD47 staining of a BALB/c LN. For panels D and E, data are representative of at least five individually analyzed mice. *P<0.05, **P<0.01.
As CD47+/+ DCs primed naive transgenic T cells in vitro as efficiently as CD47−/− DCs (Supplementary Figure S2), the altered T-cell priming observed in vivo could result from an impaired DC response to chemokines or transmigration through the endothelial barrier. It was thus essential to ascertain the in vitro migratory capacity of these two types of DCs to chemo-attractants. We found that CD47+/+ and CD47−/− DCs displayed equivalent amounts of CCR7 and chemotaxis to CCL19 (Figure 4B). Sphingosine-1-phosphate (S1P) also controls egress of lymphocytes from the LN and is a CCR7-independent mediator of mature DC migration (Lan et al, 2005). We found no difference in the in vitro migratory potential of the two types of DCs toward S1P. Similarly, CD47+/+ and CD47−/− DCs isolated from ex vivo skin explants migrated efficiently toward CCL19. CCR7 expression and cell recovery of CD11c+IEhigh cells were comparable in both mice (Figure 4C).
Taken together, these data strongly support the concept of an altered passage of CD47−/− DCs through LV rather than a defect in chemotaxis toward CCL19. The following experiments were designed to directly and concomitantly trace the in vivo migration of CD47+/+ and CD47−/− DCs in CD47-deficient hosts (Figure 4D). Each BM-DC population was labelled with CFSE or CMTMR, respectively, and vice versa before s.c. injection at a 1:1 ratio (top panel). On day 2, DCs were found in the draining LN in the proportion of one CD47−/− to two CD47+/+ DCs (bottom panel). As a complementary approach to visualize the differential DC migration, both types of DCs were labelled with the same red fluorescent vital dye (i.e. PKH-26), coinjected at 1:1 ratio and retraced in the LN by CD47 staining (Figure 4E). On day 2, the recovery of CD11c+CD47+cells gated on PKH-26 positive cells was 66%±4.1 (mean±s.d. of four experiments). These two competitive in vivo migration assays performed in a CD47-deficient host demonstrate that CD47 must be expressed on the DC to allow for efficient lymphatic transendothelial trafficking, but is dispensable on the endothelium itself.
The selective altered CD47−/− DC migration is correlated with a depletion of marginal zone DCs in the spleen
As CD47 expression on DCs was required to gain entry into the LV and prime T cells in the LN, we hypothesized that it was also critical for DCs to exit the bloodstream and travel across the endothelial barriers to enter the spleen. BM-DCs were administered intravenously (i.v.) into mice passively transferred with transgenic T cells 1 day before and the T-cell response was examined at day 3. As depicted in Figure 5A, CD47 expression was dispensable on the endothelium for migration into the spleen as CD47+/+ BM-DCs efficiently primed T cells in both strains of mice. In contrast, i.v. injection of CD47−/− DCs resulted in impaired T-cell priming in CD47−/−mice. Note that no T-cell response occurred when CD47−/− DCs were injected in BALB/c mice because of their elimination. In vivo competitive migration assays in CD47-deficient hosts further showed that the absence of CD47 on DCs led to a decrease in their recovery from the spleen. However, we demonstrated that the altered entry into the spleen was rather selective for CD47−/− DCs, as we observed a comparable recovery of CD47−/− and CD47+/+ T and B cells (Figure 5B).
Figure 5.
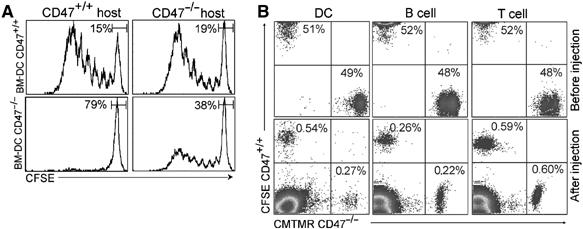
Selective altered CD47−/− DC transendothelial migration. (A) 0.5 × 106 CD47+/+ and CD47−/− LPS-activated and OVA-pulsed BM-DCs were injected i.v. 24 h after adoptive transfer of CFSE-labelled transgenic CD4+ T cells. Proliferation of CD4+ T cells in the spleen was analyzed after 72 h. (B) For the competitive migration assay, activated CD47+/+ and CD47−/− BM-DC, and unfractionated B and T cells were stained with CFSE or CMTMR and mixed at 1:1 ratio before i.v. injection into mice. Top panel: a mixture of the cells before injection. Bottom panel: cells were isolated from the spleen and retraced at day 1 postinjection. In each panel, data represent one of four individually analyzed spleens.
The faulty in vivo migration of the in vitro generated CD47−/− BM-DCs was taken as a strong argument in favor of their inefficient trafficking through vascular sinusoids in the spleen. In support of this concept, we next demonstrated in unmanipulated CD47-deficient mice, a drastic reduction in the proportion and the accumulation of splenic CD11bhighCD11chigh myeloid DCs that correlated with a significant increase of immediate DC precursors in the blood (Figure 6A). In fact, the absolute number of CD11chigh DCs was significantly diminished; more specifically, the CD4+CD11chigh subset was virtually absent in CD47-deficient mice, whereas the number of CD8+CD11chigh and plasmacytoid DCs (120G8 positive cells) remained unchanged as it was weighed down by a 30% decrease in the cellularity of the spleen (Figure 6B and C). By immunohistological techniques, we observed that the marginal zone was almost devoid of CD11c staining, the precise location of the CD4+CD11chigh subset. The number of DCs in T-cell areas, mostly comprised of CD8+CD11chigh was similar in both strains of mice (Figure 6D). Of interest, the CD4+ DC subset expressed the lowest quantity of CD47 in BALB/c mice suggesting that the regulation of CD47 expression may be critical for the localization of this DC subset (Supplementary Figure S3).
Figure 6.
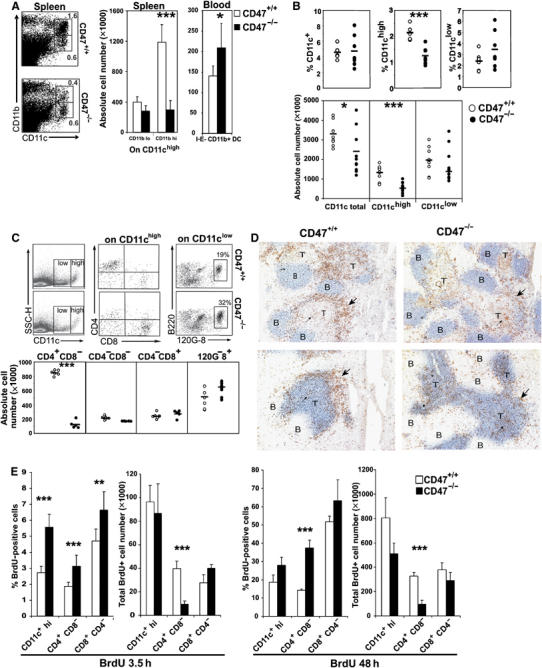
Depletion of myeloid CD4+CD8− CD11chigh DC subset in the spleen of CD47-deficient mice. (A) Proportion and absolute number of CD11c+CD11b+DCs in spleens (left bar graph; mean±s.d., n=4 mice) and immediate DC precursors (CD11c+CD11b+I-E− DCs) in blood (right bar graph; mean±s.d., n=5 mice). (B–C) Proportion and absolute number of total DCs, CD11chigh, CD11clow and the different DC subsets per spleen. (D) Serial sections from frozen spleens were stained with CD11c (brown) and B220 (blue, top) or Thy1 (blue, bottom). Large arrowhead points to marginal zone DCs whereas narrow arrowhead points to DCs in the T-cell area. Data are representative of three individually analyzed spleens. (E) Proportion and absolute number of BrDU-positive CD11chigh, CD4+ and CD8+ DCs in mice treated with BrdU for 3.5 and 48 h. Bars represent mean±s.d. of six (3.5 h) and four (48 h) individually analyzed mice. *P<0.05, **P<0.01, ***P<0.001.
At this point, one may speculate that there is a selective developmental defect of the CD4+ DC subset in CD47−/− mice. Because BM chimera experiments are not feasible in CD47−/− mice (Blazar et al, 2001), we relied on in vitro and in vivo DC differentiation experiments to disprove such a hypothesis. No defect was observed during BM-DC differentiation in vitro with GM-CSF (Figure 4 and data not shown) and the analysis of DC subsets after in vivo administration of Fms-like tyrosine kinase 3 ligand (Flt3-L), known to promote the differentiation and the expansion of both CD4+ DC and CD8+ DC subsets, showed that the number of progenitors and total DCs recovered in the BM and blood was comparable in Flt3-L-treated CD47+/+ and CD47−/− mice. However, the reduction in CD4+ DCs was maintained in the spleen of Flt3-L-treated CD47 mutants (Supplementary Figure S4).
Recent studies provided evidence that the pool of splenic myeloid DCs is maintained by a combination of continuous extravasation from the blood of DC precursors and the local proliferation of immediate DC precursors in the spleen (Kabashima et al, 2005). Here, we show a similar or increased proportion of BrdU+ cells among CD11chigh DCs, as well as the CD4+ and CD8+ subsets, in the two strains of mice 3.5 or 48 h after BrdU administration (Figure 6E and Supplementary Figure S5). However, the absolute number of proliferating DC precursors that had previously differentiated into the CD4+ subset was significantly reduced in the CD47−/− spleen mice. Thus, an additional mechanism underlying the quasi-disappearance in CD4+CD11chigh DCs in CD47-deficient mice may be through an altered migration into the spleen of the low-frequency immediate DC precursors that otherwise proliferated efficiently.
Collectively, the present results revealed that CD47 expression on DCs positively regulates myeloid DC trafficking and as such may control the pool of marginal zone DCs in the spleen.
Discussion
At peripheral sites, DCs require direct recognition of pathogens to appreciate the quality of the danger and initiate their migration toward the LN, where they are dedicated to T-cell priming. Yet, the integrated signals that lead to DC migration and the elicitation of a protective immune response versus tolerance are not completely identified.
Earlier in vitro studies indicated that through the interaction with its counter-receptor SIRP-α on neutrophils or monocytes, CD47 on the epithelium or endothelium controls leukocyte migration (Liu et al, 2002; de Vries et al, 2002; Zen and Parkos, 2003). Here, we hypothesized that CD47 expression governs epidermal and dermal skin transendothelial DC trafficking to the LN. In the absence of CD47, there was a decrease in the accumulation of skin-derived DCs in the draining LN under both homeostatic and inflammatory conditions. We have established that CD47 expression on DCs, rather than on the endothelium, is essential for DCs to gain entry into the afferent lymph and for their relocation to the LN, where stimulation of T cell occurs. We propose that after skin DCs have penetrated through the extracellular matrix network into the dermis, their entrance into LV is positively controlled by CD47.
Although it is not yet clear whether CD47/CD47 or CD47/SIRP-α interactions predominantly regulate LC retention in the epidermis, LC emigration is impaired in SIRP-α mutants lacking its intracytoplasmic domain (Fukunaga et al, 2004). In addition, phosphorylated SIRP-α expression on a melanoma cell line positively controls its migration (Motegi et al, 2003). In contrast, SIRP-α ligation by CD47-Fc fusion protein impairs skin DC migration in BALB/c mice and promotes SIRP-α tyrosine phosphorylation and SHP-1 recruitment under inflammatory conditions (Okazawa et al, 2005). Obviously, in CD47-deficient mice, SIRP-α is bound by CD47 neither in trans nor in cis and the predominant effect appeared to be impaired myeloid cell migration. Here, we report that the proportion of CD11c+ DCs carrying FITC in the LN of mutant mice was not further altered by treatment with CD47-Fc fusion protein. These data suggest that CD47-Fc interrupted a positive interaction between CD47/SIRP-α rather than delivering a negative signal to the DCs via SIRP-α. Alternatively, the SIRP-α signalling pathway may not be functional in the absence of CD47 coexpression.
Inflammatory stimuli delivered by contact elicitation or active immunization with OVA peptide in the presence of adjuvant induces less efficient skin DC migration in CD47-deficient mice when compared to BALB/c mice. The general defect in skin DC migration is reminiscent of the phenotype observed in CCR7-deficient mice (Ohl et al, 2004). The latter is much more severe, as there is a near complete absence of naïve T and B cells in the LN, precluding DC/T-cell interactions (Forster et al, 1999). In contrast, T- and B-cell migration were entirely normal in CD47-deficient mice. Moreover, we show that the absence of CD47 did neither modulate CCR7 expression nor the chemotaxis toward CCL19. During the revision of this paper, Hagnerud et al (2006) similarly reported on the in vivo altered DC migration in CD47−/−C57BL/6 mice with no modulation of CCR7 expression. In contrast to our data, these authors observed a defect in DC chemotaxis in vitro. As the function of CD47 is partly mediated through interactions with SIRP-α, this apparent controversy may be attributed to the polymorphism in SIRP-α in the two strains of mice (Sano et al, 1999).
We further showed that i.v. injected CD47−/− BM-DCs poorly primed T cells in the spleen, owing to their inefficient migration across endothelial barriers. Owing to the observed parallel between the cellular distribution of SIRP-α expression (i.e. neutrophils, LCs and dermal DCs) and the defect in cell migration of the corresponding cells in CD47−/− mice, we postulated that the absence of CD47 commonly affects the mobilization of SIRP-α-expressing cells. This prediction fits with our in vivo findings showing a selective depletion of the CD4+CD11b+CD11chigh marginal zone DC subset in the spleen. In support of our hypothesis, SIRP-α is not expressed on CD8+ resident DCs, and their numbers were unaltered in the CD47-deficient spleen and LN.
Other mice carrying mutations for various transcription factors display a deficiency in CD4+ DC numbers. Indeed, a reduction in the CD4+ DC subset was demonstrated in Rel-B, PU.1, TRAF-6, IRF-2 and IRF-4-deficient mice as a result of a developmental defect in their DCs (Wu et al, 1998; Guerriero et al, 2000; Kobayashi et al, 2003; Ichikawa et al, 2004; Suzuki et al, 2004). Specifically, PU.1, Rel-B and IRF-4-deficient mice all show defects in BM-DC differentiation in vitro, and in contrast to CD47 deficiency, they show no evidence of altered DC migration. Hence, the decrease in splenic CD4+ DCs observed in CD47−/− mice is most likely linked to a defect in cellular migration rather than differentiation.
Nonetheless, we cannot rule out a DC developmental problem in CD47−/− mice, although the following observations argued against such a defect. First, Flt3-L administration yielded a similar recovery of progenitors and total DCs in the BM and blood in CD47+/+ and CD47−/− mice. Second, the proportion of CD11b+I-E−CD11c+DC precursors was increased in the CD47−/− blood. Third, intrasplenic DC proliferation, a pathway to maintain DC homeostasis, was not decreased in the mutant mice. Yet, the absolute number of CD4+ DCs was significantly reduced after BrdU or Flt3-L administration. We thus propose that a lack of CD47 leads to a defective migration of immediate DC precursors into the spleen, which results in the quasi-disappearance of marginal zone DCs. Naik et al (2006) recently identified such a low frequency DC precursor (0.05% total spleen cells), that is, CD11cint, CD43int, CD4−CD8−, MHC classII− SIRP-αint DC, corroborating our hypothesis of a general defect in the mobilization of SIRP-α+ cells in the absence of CD47.
Taken together, we provide evidence that the CD47 molecule expressed on myeloid DCs controls their ability to efficiently traffic across lymphatic and endothelial vessels, seed in secondary lymphoid organs and participate in T-cell priming. Thus, regulating CD47 expression and as such DC migration may have an impact on undesired T-cell responses in autoimmune diseases and organ transplantation.
Materials and methods
Mice
A 16–18th-generation BALB/c backcross of CD47-deficient mice was a generous gift from Dr Oldenborg. BALB/c mice were purchased from Charles River Labs. Eight- to 12-week-old mice were used in all experimental protocols as approved by the Canadian Council on Animal Care and maintained under SPF conditions.
Preparation of epidermal sheets and skin explant cultures
The dorsal halves of ear explants were incubated in PBS with 20 mM EDTA for 2 h. The epidermal and dermal fractions were separated using fine forceps. The tissues were stained with FITC-anti-I-E mAb (14.4.4S3). In some experiments, ear explants were floated on tissue culture medium with or without 100 ng/ml of CCL19 (R&D System) for 72 h the before dermis was separated from the epidermis and stained. The images were acquired on a microscope equipped with a mercury lamp, monochrome filter, Kodak camera, and with the Metamorph software. Emigrated cells in the culture supernatant were counted by flow cytometry using latex beads for calibration (Beckman Coulter) and stained for CD11c, I-E and CCR7 expression. The data were acquired on a FACSCalibur and analyzed using Cellquest Software.
Preparation of BM-DCs
BM-DCs were prepared as described previously (Inaba et al, 1992), with slight modifications. BM cell suspensions were obtained from femurs and tibias and left to adhere in vitro for 6 h in serum-free RPMI at 5 × 106 cells/ml in six-well plates. GM-CSF 5 ng/ml (Peprotech) was added for 10–14 days to 1 × 107 nonadherent cells/3 ml to induce DC differentiation. The culture medium was renewed every 3 days. Cell purity was >98% CD11c+ cells. In some experiments, BM-DCs were activated overnight with 1 μg/ml of LPS (E. coli, Sigma) and 1 μg/ml OVA peptide (323–339, Peptide International).
In vitro migration assay
3 × 105 activated or nonactivated BM-DCs in RPMI were placed on the upper compartment of 5-μm pore size Transwell plates (ChemoTx, Neuro Probe). CCL19 measuring 100 ng/ml or 1 μM of S1P (R&D system) in RPMI was added to the lower chamber. After 3 h of incubation at 37°C, cells that had transmigrated to the bottom compartment were collected and counted by flow cytometry using latex beads for calibration (Beckman Coulter).
Passive transfer experiments
BALB/c and CD47-deficient mice were injected i.v. with 5 × 106 CFSE-labelled CD4+ T cells from DO11.10 mice. One day later, the mice were immunized s.c in the right footpad with 1 or 10 μg of OVA protein dissolved in 20 μl of PBS or 10 μg of OVA peptide emulsified in IFA. The left footpad received 20 μl of PBS. The draining LN were extracted 2, 3 or 7 days after the immunization. In some experiments, mice were immunized with activated OVA-pulsed BM-DCs s.c. or i.v. and the T-cell response was examined in the LN or the spleen after 72 h.
Competitive migration assays
2 × 106 (s.c.) or 4 × 106 (i.v.) activated CD47+/+ and CD47−/− BM-DCs were stained with two different colors, CFSE (Sigma) or CMTMR (Molecular Probes), respectively, and injected into CD47−/− hosts. Twenty-four hours later, DCs were retraced in draining LN or spleen by flow cytometry. For some experiments, activated CD47+/+ and CD47−/− BM-DCs were stained with PKH26 (Sigma), injected s.c. and retraced by CD47 staining.
Spleen and lymph node staining
Antibodies were purchased from BD, except for anti-I-E (14.4.4S3) and anti-CD11c (N418), purified in our laboratory, and an antibody that specifically recognizes pDCs, 120G-8 (Asselin-Paturel et al, 2003), which was kindly provided by Dr G Trinchieri. Spleens were injected with 1 ml liberase at 0.4 mg/ml and minced in 2 ml (Roche). Axillary, brachial and inguinal LN were minced in 3 ml liberase. The tissues were incubated at 37°C for 15 min and passed through a 70-μm nylon cell strainer. Spleen cells were then treated with NH4Cl for red blood cell lysis.
FITC sensitization
Mice were shaved and 100 μl of a 1% FITC (sigma) solution or vehicle alone (1:1 v/v acetone and dibutylphthalate) was painted on the abdomen. At 24 or 72 h after painting, the inguinal LN were stained for DCs, T and B cells, and analyzed by flow cytometry. In CD47-Fc experiments, mice received 50 μg of CD47-Fc or CTRL-Fc fusion proteins intradermally before FITC sensitization.
Spleen immunohistochemistry
Frozen spleen sections (8 μm) were fixed on slides in acetone for 20 min at −20°C, blocked with Universal Blocking reagent (DAKO, Mississauga, Ontario) and stained with biotin anti-CD11c and either FITC-anti-B220 or anti-Thy1. The biotin-coupled antibody was revealed using Vectastain ABC kit (Vector Labs, Burlingame, CA), followed by DAB, and the FITC-antibodies using alkaline phosphatase-coupled anti-FITC (Roche, Indianapolis, IN) followed by Vector Blue.
BrdU staining
BrdU (2 mg/mouse) was injected intraperitoneally and added to the drinking water (0.8 mg/ml). After 3.5 or 48 h, spleens were harvested and stained for different DC subsets. The cells were then fixed, permeabilized (BD Cytofix/cytoperm) and treated with DNase I (Sigma) for 1 h at 37°C before staining with anti-BrdU-FITC.
Statistical analysis
Student' t-test; ***P<0.001, **P<0.01, *P<0.05.
Supplementary Material
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4
Supplementary Figure S5
Acknowledgments
This work was supported by grants from the CIHR. S Lesage is a recipient of the Senior Research Fellowship Award (CIHR). P Gautier is a recipient of a COPSE award.
References
- Asselin-Paturel C, Brizard G, Pin JJ, Briere F, Trinchieri G (2003) Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J Immunol 171: 6466–6477 [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Kopf M, Marsland BJ (2006) Chemokines: more than just road signs. Nat Rev Immunol 6: 159–164 [DOI] [PubMed] [Google Scholar]
- Blazar BR, Lindberg FP, Ingulli E, Panoskaltsis-Mortari A, Oldenborg PA, Iizuka K, Yokoyama WM, Taylor PA (2001) CD47 (integrin-associated protein) engagement of dendritic cell and macrophage counterreceptors is required to prevent the clearance of donor lymphohematopoietic cells. J Exp Med 194: 541–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EJ, Frazier WA (2001) Integrin-associated protein (CD47) and its ligands. Trends Cell Biol 11: 130–135 [DOI] [PubMed] [Google Scholar]
- Cant CA, Ullrich A (2001) Signal regulation by family conspiracy. Cell Mol Life Sci 58: 117–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cera MR, Del Prete A, Vecchi A, Corada M, Martin-Padura I, Motoike T, Tonetti P, Bazzoni G, Vermi W, Gentili F, Bernasconi S, Sato TN, Mantovani A, Dejana E (2004) Increased DC trafficking to lymph nodes and contact hypersensitivity in junctional adhesion molecule-A-deficient mice. J Clin Invest 114: 729–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries HE, Hendriks JJ, Honing H, De Lavalette CR, van der Pol SM, Hooijberg E, Dijkstra CD, van den Berg TK (2002) Signal-regulatory protein alpha-CD47 interactions are required for the transmigration of monocytes across cerebral endothelium. J Immunol 168: 5832–5839 [DOI] [PubMed] [Google Scholar]
- Edwards AD, Chaussabel D, Tomlinson S, Schulz O, Sher A, Reis e Sousa C (2003) Relationships among murine CD11c(high) dendritic cell subsets as revealed by baseline gene expression patterns. J Immunol 171: 47–60 [DOI] [PubMed] [Google Scholar]
- Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M (1999) CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99: 23–33 [DOI] [PubMed] [Google Scholar]
- Fukunaga A, Nagai H, Noguchi T, Okazawa H, Matozaki T, Yu X, Lagenaur CF, Honma N, Ichihashi M, Kasuga M, Nishigori C, Horikawa T (2004) Src homology 2 domain-containing protein tyrosine phosphatase substrate 1 regulates the migration of Langerhans cells from the epidermis to draining lymph nodes. J Immunol 172: 4091–4099 [DOI] [PubMed] [Google Scholar]
- Gao AG, Lindberg FP, Finn MB, Blystone SD, Brown EJ, Frazier WA (1996) Integrin-associated protein is a receptor for the C-terminal domain of thrombospondin. J Biol Chem 271: 21–24 [DOI] [PubMed] [Google Scholar]
- Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, Henson PM (2005) Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 123: 321–334 [DOI] [PubMed] [Google Scholar]
- Guerriero A, Langmuir PB, Spain LM, Scott EW (2000) PU.1 is required for myeloid-derived but not lymphoid-derived dendritic cells. Blood 95: 879–885 [PubMed] [Google Scholar]
- Hagnerud S, Manna PP, Cella M, Stenberg A, Frazier WA, Colonna M, Oldenborg PA (2006) Deficit of CD47 results in a defect of marginal zone dendritic cells, blunted immune response to particulate antigen and impairment of skin dendritic cell migration. J Immunol 176: 5772–5778 [DOI] [PubMed] [Google Scholar]
- Ichikawa E, Hida S, Omatsu Y, Shimoyama S, Takahara K, Miyagawa S, Inaba K, Taki S (2004) Defective development of splenic and epidermal CD4+ dendritic cells in mice deficient for IFN regulatory factor-2. Proc Natl Acad Sci USA 101: 3909–3914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM (1992) Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med 176: 1693–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabashima K, Banks TA, Ansel KM, Lu TT, Ware CF, Cyster JG (2005) Intrinsic lymphotoxin-beta receptor requirement for homeostasis of lymphoid tissue dendritic cells. Immunity 22: 439–450 [DOI] [PubMed] [Google Scholar]
- Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, Tripp CH, Douillard P, Leserman L, Kaiserlian D, Saeland S, Davoust J, Malissen B (2005) Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity 22: 643–654 [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Walsh PT, Walsh MC, Speirs KM, Chiffoleau E, King CG, Hancock WW, Caamano JH, Hunter CA, Scott P, Turka LA, Choi Y (2003) TRAF6 is a critical factor for dendritic cell maturation and development. Immunity 19: 353–363 [DOI] [PubMed] [Google Scholar]
- Lan YY, De Creus A, Colvin BL, Abe M, Brinkmann V, Coates PT, Thomson AW (2005) The sphingosine-1-phosphate receptor agonist FTY720 modulates dendritic cell trafficking in vivo. Am J Transplant 5: 2649–2659 [DOI] [PubMed] [Google Scholar]
- Latour S, Tanaka H, Demeure C, Mateo V, Rubio M, Brown EJ, Maliszewski C, Lindberg FP, Oldenborg A, Ullrich A, Delespesse G, Sarfati M (2001) Bidirectional negative regulation of human T and dendritic cells by CD47 and its cognate receptor signal-regulator protein-alpha: down-regulation of IL-12 responsiveness and inhibition of dendritic cell activation. J Immunol 167: 2547–2554 [DOI] [PubMed] [Google Scholar]
- Lindberg FP, Bullard DC, Caver TE, Gresham HD, Beaudet AL, Brown EJ (1996) Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science 274: 795–798 [DOI] [PubMed] [Google Scholar]
- Liu Y, Buhring HJ, Zen K, Burst SL, Schnell FJ, Williams IR, Parkos CA (2002) Signal regulatory protein (SIRPalpha), a cellular ligand for CD47, regulates neutrophil transmigration. J Biol Chem 277: 10028–10036 [DOI] [PubMed] [Google Scholar]
- Liu Y, O'Connor MB, Mandell KJ, Zen K, Ullrich A, Buhring HJ, Parkos CA (2004a) Peptide-mediated inhibition of neutrophil transmigration by blocking CD47 interactions with signal regulatory protein alpha. J Immunol 172: 2578–2585 [DOI] [PubMed] [Google Scholar]
- Liu Y, Shaw SK, Ma S, Yang L, Luscinskas FW, Parkos CA (2004b) Regulation of leukocyte transmigration: cell surface interactions and signaling events. J Immunol 172: 7–13 [DOI] [PubMed] [Google Scholar]
- Motegi S, Okazawa H, Ohnishi H, Sato R, Kaneko Y, Kobayashi H, Tomizawa K, Ito T, Honma N, Buhring HJ, Ishikawa O, Matozaki T (2003) Role of the CD47-SHPS-1 system in regulation of cell migration. EMBO J 22: 2634–2644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik SH, Metcalf D, van Nieuwenhuijze A, Wicks I, Wu L, O'Keeffe M, Shortman K (2006) Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat Immunol 7: 663–671 [DOI] [PubMed] [Google Scholar]
- Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, Zwirner J, Blankenstein T, Henning G, Forster R (2004) CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity 21: 279–288 [DOI] [PubMed] [Google Scholar]
- Okazawa H, Motegi S, Ohyama N, Ohnishi H, Tomizawa T, Kaneko Y, Oldenborg PA, Ishikawa O, Matozaki T (2005) Negative regulation of phagocytosis in macrophages by the CD47-SHPS-1 system. J Immunol 174: 2004–2011 [DOI] [PubMed] [Google Scholar]
- Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP (2000) Role of CD47 as a marker of self on red blood cells. Science 288: 2051–2054 [DOI] [PubMed] [Google Scholar]
- Palucka K, Banchereau J (2002) How dendritic cells and microbes interact to elicit or subvert protective immune responses. Curr Opin Immunol 14: 420–431 [DOI] [PubMed] [Google Scholar]
- Randolph GJ, Angeli V, Swartz MA (2005a) Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol 5: 617–628 [DOI] [PubMed] [Google Scholar]
- Randolph GJ, Sanchez-Schmitz G, Angeli V (2005b) Factors and signals that govern the migration of dendritic cells via lymphatics: recent advances. Springer Semin Immunopathol 26: 273–287 [DOI] [PubMed] [Google Scholar]
- Rebres RA, Kajihara K, Brown EJ (2005) Novel CD47-dependent intercellular adhesion modulates cell migration. J Cell Physiol 205: 182–193 [DOI] [PubMed] [Google Scholar]
- Sangaletti S, Gioiosa L, Guiducci C, Rotta G, Rescigno M, Stoppacciaro A, Chiodoni C, Colombo MP (2005) Accelerated dendritic-cell migration and T-cell priming in SPARC-deficient mice. J Cell Sci 118: 3685–3694 [DOI] [PubMed] [Google Scholar]
- Sano S, Ohnishi H, Kubota M (1999) Gene structure of mouse BIT/SHPS-1. Biochem J 344 (Part 3): 667–675 [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Hawiger D, Nussenzweig MC (2003) Tolerogenic dendritic cells. Annu Rev Immunol 21: 685–711 [DOI] [PubMed] [Google Scholar]
- Suzuki S, Honma K, Matsuyama T, Suzuki K, Toriyama K, Akitoyo I, Yamamoto K, Suematsu T, Nakamura M, Yui K, Kumatori A (2004) Critical roles of interferon regulatory factor 4 in CD11bhighCD8alpha− dendritic cell development. Proc Natl Acad Sci USA 101: 8981–8986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beek EM, Cochrane F, Barclay AN, van den Berg TK (2005) Signal regulatory proteins in the immune system. J Immunol 175: 7781–7787 [DOI] [PubMed] [Google Scholar]
- Wu L, D'Amico A, Winkel KD, Suter M, Lo D, Shortman K (1998) RelB is essential for the development of myeloid-related CD8alpha− dendritic cells but not of lymphoid-related CD8alpha+ dendritic cells. Immunity 9: 839–847 [DOI] [PubMed] [Google Scholar]
- Xu H, Guan H, Zu G, Bullard D, Hanson J, Slater M, Elmets CA (2001) The role of ICAM-1 molecule in the migration of Langerhans cells in the skin and regional lymph node. Eur J Immunol 31: 3085–3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Fukunaga A, Nagai H, Oniki S, Honma N, Ichihashi M, Matozaki T, Nishigori C, Horikawa T (2006) Engagement of CD47 inhibits the contact hypersensitivity response via the suppression of motility and B7 expression by Langerhans cells. J Invest Dermatol 126: 797–807 [DOI] [PubMed] [Google Scholar]
- Zen K, Parkos CA (2003) Leukocyte–epithelial interactions. Curr Opin Cell Biol 15: 557–564 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4
Supplementary Figure S5


