Abstract
Dengue virus (DV) is an important re-emerging arthropod-borne virus of global significance. The defining characteristic of DV infection-associated pathology is haemorrhagic fever, which often leads to a fatal shock-like syndrome (DHF/DSS) owing to an increase in vascular endothelial permeability. Here, we show, in a viral dose-dependent manner, that DV-infected immature dendritic cells overproduce soluble gelatinolytic matrix metalloproteinase (MMP)-9—and to a lesser extent MMP-2—which enhances endothelial permeability, but which are reduced by specific inhibitors and a neutralizing anti-MMP-9 antibody. This permeability was associated with a loss of expression of the platelet endothelial adhesion molecule 1 (PECAM-1) and vascular endothelium (VE)-cadherin cell adhesion molecules and redistribution of F-actin fibres. These in vitro observations were confirmed in an in vivo vascular-leakage mouse model. These results provide a molecular basis for DHF/DSS that could be a basis for a general model of haemorrhagic fever-inducing viruses, and identify a new therapeutic approach for the treatment of viral-induced vascular leakage by specifically targeting gelatinolytic metalloproteases.
Keywords: endothelial cells, haemorrhagic fever viruses, matrix metalloprotease inhibitors, plasma leakage, SB-3CT
Introduction
Dengue virus (DV serotypes 1–4) is a re-emerging arthropod-borne virus, from the Flaviviridae family, that infects humans. The World Health Organization classifies infection with DV as a relatively benign, self-limiting fever, and as a severe and sometimes fatal haemorrhagic disease referred to, respectively, as dengue fever and dengue haemorrhagic fever with a dengue shock syndrome (DHF/DSS; Mackenzie et al, 2004). Dengue physiopathology is correlated with activation of T lymphocytes and monocytes, as well as with overproduction of cytokines and soluble mediators leading to increased vascular permeability, serum leakage and hypovolemia (Lei et al, 2001). Less commonly, DV infection causes hepatic failure, encephalitis and haemorrhage (Gubler, 1998). The two main hypotheses to explain DHF/DSS in patients on the basis of epidemiological data are that DHF/DSS (i) depends on a second heterotypic antibody-dependent enhancement of DV infection (Halstead, 1970), and (ii) does not depend on a secondary infection, but rather a combination of viral load, strain virulence and host immune response (Rosen, 1986).
Although DV can infect different cell types, cells from the monocytic lineage, such as Langerhans cells in the skin and interstitial dendritic cells (DCs), are the primary viral targets. In these cells, expression of DC-specific intercellular adhesion molecule 3 (ICAM3)-grabbing non-integrin (DC-SIGN) is essential for productive DV infection (Navarro-Sanchez et al, 2003; Tassaneetrithep et al, 2003). It is noteworthy that immature, monocyte-derived DCs (iDCs) are permissive for infection with DV. This infection results in productive viral replication, cellular activation and maturation, and also increased production of cytokines (Ho et al, 2001), such as tumour necrosis factor-α (TNF-α; Marovich et al, 2001) and interleukin-8 (Juffrie et al, 2000), both of which are strongly correlated with severe DHF/DSS and death. Trans-endothelial migration of DCs occurring during normal physiological or inflammatory processes requires extracellular matrix remodelling, which involves changes in VE permeability regulated by the production of matrix metalloproteases (MMPs). In excess, these MMPs have deleterious effects on endothelial cell integrity (Asahi et al, 2001).
Understanding DHF/DSS pathogenesis is important because of the absence of a vaccine or effective treatment against DV. However, the molecular mechanisms governing virus-mediated enhancement of vascular permeability, leading to vascular damage, are not yet fully understood. In view of the important viro-immunological role of iDCs during DV infection, and in the absence of an in vivo DHF/DSS model, we have examined—both in an in vitro and in an in vivo vascular permeability model—whether DV-infected DCs might be involved in the pathogenesis of DHF/DSS through the production of MMP.
Results And Discussion
In vitro-generated iDCs expressing CD1a (Fig 1A) and DC-SIGN (Fig 1B) were incubated with DV. The results obtained by chemiluminescence protein arrays, show that virus-free supernatants of DV-infected iDCs (Sup iDC-DV; see the Methods section) have significantly higher levels of secreted pro-inflammatory cytokines interleukin-8, TNF-α, MMP-9 (92 kDa gelatinase) and MMP-13 (collagenase 3) as compared with uninfected iDC supernatants (Fig 1C,G). This is in agreement with the observation that MMP-13 activates MMP-9 (Knauper et al, 1997), and to a lesser extent MMP-2 (72 kDa gelatinase). After 3 h of infection by DV, iDCs only slightly increased gelatinase gene expression compared with uninfected iDCs (supplementary Fig S1 online). Moreover, MMP-9 activity was shown to be dependent on viral load iDC exposure (Fig 1H). DV infection also induced the production of tissue inhibitors of metalloproteinases (TIMP)-1 and TIMP-2 (Fig 2A,B), indicating that the enhanced production of these natural inhibitors of MMP-9 and MMP-2, respectively (Goldberg et al, 1989), was not able to restore the physiological balance between the MMPs and TIMPs or to reduce DV-induced vascular permeability. It has been reported that soluble gelatinolytic MMP secretion can be induced at different levels by the activation of pro-MMPs and by an increase in MMP gene transcription restricted to transcription factors induced or repressed by a large variety of soluble factors, including cytokines, growth factors and hormones, or by cellular contacts acting through specific signalling pathways (Van den Steen et al, 2002). Furthermore, viral double-stranded RNA (dsRNA) activation of the intracellular Toll-like receptor 3, present in iDCs (Matsumoto et al, 2003), might lead to secretion of pro-inflammatory cytokines and MMPs (Ritter et al, 2005). Interaction of purified glycoprotein 120—the surface envelope glycoprotein of human immunodeficiency virus-1—with its cell-surface receptor induces the secretion of active MMP-9 in a MAP-kinase-dependent manner and in the absence of dsRNA (Misse et al, 2001). Taken together with other reports, the present data indicate that gelatinolytic MMP secretion and regulation, initiated by DV infection of iDCs, could result from several levels of non-exclusive interactions between virus and surface or internal iDC ligands that would activate different pathways. These could include the pro-inflammatory pathways and upregulation of MMP-9 in a MAP-kinase-dependent manner (supplementary Fig S2 online).
Figure 1.

Dengue virus infection of immature dendritic cells triggers the overproduction of matrix metalloproteinases and inflammatory cytokines. Cell-surface expression of CD1a (A) and DC-SIGN (B) by myeloid immature dendritic cells (iDCs) was determined by flow cytometry. Cells were stained with an isotype-matched control antibody (a), an anti-CD1a antibody or an anti-DC-SIGN antibody (b). Supernatants from dengue-virus (DV)-infected iDCs (DV+) and uninfected iDCs (DV−) were collected at 3 h after infection to assess the amount of IL-8 (C), TNF-α (D), MMP-2 (E), MMP-9 (F) and MMP-13 (G) by Searchlight Proteome array technology. Statistical significance (****P<0.0001; Student's t-test) was determined from four independent experiments. After DV infection of iDCs with increasing viral loads, MMP-9 activity was analysed by gelatin zymography (H). DC-SIGN, DC-specific ICAM3-grabbing non-integrin; IL-8, interleukin-8; MMP, matrix metalloproteinase; MOI, multiplicity of infection; Sup iDC, supernatants of iDCs; Sup iDC-DV, supernatants of DV-infected iDCs; TNF-α, tumour necrosis factor-α.
Figure 2.
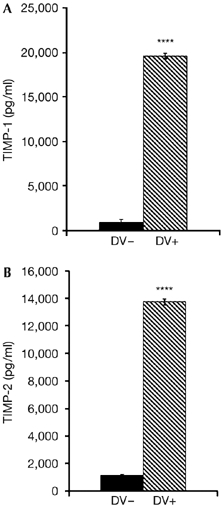
Levels of tissue inhibitors of metalloproteinases secreted by dengue-virus-infected cells. Supernatants of immature dendritic cells (iDC) and iDC/DV were collected to assess the amount of TIMP-1 (A) and TIMP-2 (B), measured using the sensitive Searchlight Proteome array detection method. Statistical significance (****P<0.0001; Student's t-test) was determined from four independent experiments. DV, dengue virus; TIMP, tissue inhibitors of metalloproteinases.
As MMP-9 was overexpressed in Sup iDC-DV, we investigated whether these supernatants could modify the permeability of endothelial cells by using primary human umbilical vascular endothelial cell (HUVEC) monolayers (Bonner & O'Sullivan, 1998). The HUVEC monolayers were incubated for 24 h with culture supernatants from uninfected or DV-infected iDCs. Sup iDC-DV enhanced the permeability of HUVECs by about twofold, as compared with those from uninfected iDCs (Fig 3A). To establish whether the endothelium-permeabilizing activity of the virus-free Sup iDC-DV was mediated by MMPs, the experiments were repeated in the presence of SB-3CT (3-(4-phenoxyphenylsulphonyl)-propylthiirane), a small and highly selective molecule that, at nanomolar concentrations, inhibits MMP-9 and MMP-2 gelatinase activities by binding to their catalytic zinc ion (Solomon et al, 2004). As shown in Fig 3A, SB-3CT restored the endothelial permeability of HUVECs to basal levels, similar to those observed on incubation with uninfected iDC supernatants. Similar effects were observed with GM-6001, a broad MMP inhibitor, as with a neutralizing antibody specific for MMP-9 (Fig 3B). These results highlight the important role of the secreted MMP gelatinases present in Sup iDC-DV in increasing endothelial permeability. In addition, Sup iDC-DV-induced permeability was markedly reduced, after MMP-9 depletion by a specific neutralizing antibody, whereas the consequence of MMP-13 removal was modest (supplementary Fig S3 online).
Figure 3.
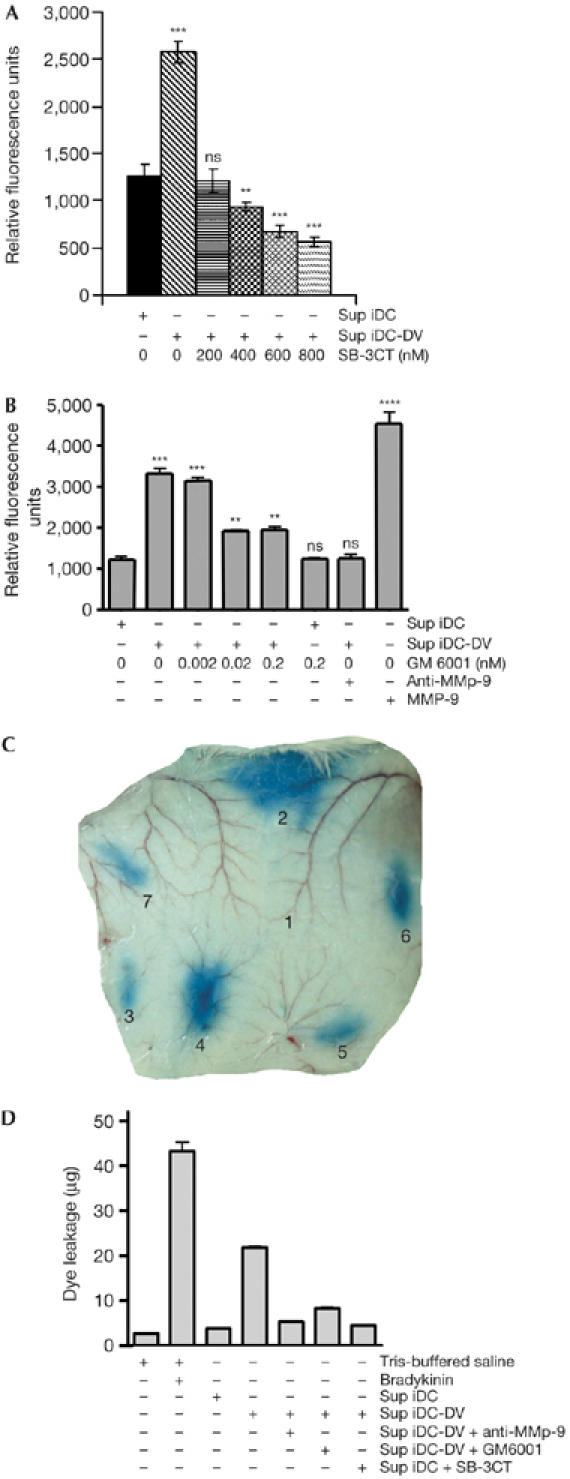
Supernatants from dengue-virus-infected immature dendritic cells increase endothelial cell permeability in a matrix metalloproteinase-dependent manner. Sup iDC or Sup iDC-DV were treated for 1 h at 37°C with SB-3CT (A), GM-6001 or neutralizing anti-MMP-9 polyclonal antibody (B) and subsequently added to confluent human umbilical vascular endothelial cells (HUVECs) grown on 24-well transwell polyethylene membranes for a period of 24 h. After the addition of fluorescein isothiocyanate–dextran to the HUVEC monolayer, the extent of permeability was determined by measuring fluorescence in the lower transwell chamber by spectrofluorometry. Data (mean±s.d.; n=4) are representative of four independent experiments (***P<0.001, **P<0.01, NS=P>0.5 versus controls). Vascular leakage of Evans blue was visualized after treatment of mouse skin with (1) 10 mM Tris-buffered saline, (2) 10 nM bradykinin, (3) Sup iDC, (4) Sup iDC-DV, or Sup iDC-DV preincubated with (5) 5 μg/ml neutralizing anti-MMP-9, (6) 20 nM GM-6001 or (7) 600 nM SB-3CT (C). Dye leakage was quantified in equal-size punch-biopsies as described in the Methods (D). Data (mean±s.d.; n=4) are representative of three independent experiments. MMP-9, matrix metalloproteinase-9; Sup iDC, supernatants of immature dendritic cells; Sup iDC-DV, supernatants of dengue-virus-infected immature dendritic cells.
In the absence of a DHF/DSS animal model (Bente & Rico-Hesse, 2006), we used a mouse vascular permeability enhancement model to investigate DV-induced endothelial disruption, and the effect of gelatinolytic MMP inhibitors in vivo. Sup iDC-DV induced substantial vascular leakage, which was reduced by both the gelatinolytic MMP inhibitors and a neutralizing anti-MMP-9 antibody (Fig 3C,D). Similar results were observed when TNF-α was used instead of Sup iDC-DV (supplementary Fig S4 online). These data confirm and extend the in vitro results, showing that the increased HUVEC permeability induced by TNF-α was reduced to basal levels by SB-3CT. These data support the TNF-α-mediated induction of MMPs (Lehmann et al, 2005) and consequent vascular permeability.
Increased serum levels of soluble factors, including the pro-inflammatory cytokines interleukin-6 (Nguyen et al, 2004), TNF-α (Fernandez-Mestre et al, 2004) , interleukin-8 (Juffrie et al, 2000), transforming growth factor-β (Agarwal et al, 1999), vascular endothelial growth factor (Tseng et al, 2005) and soluble vascular cell adhesion molecule 1 (sVCAM-1) (Murgue et al, 2001), are correlated with the vascular damage characteristics for DHF/DSS. Most of these factors activate the production of gelatinolytic MMP (Luca et al, 1997; Behzadian et al, 2001; Li et al, 2003), whereas factors such as sVCAM-1 could result from cell-surface shedding owing to the action of both gelatinolytic MMPs and TNF-α-converting enzyme (Hummel et al, 2001; Garton et al, 2003). In addition, most of the factors involved in the vascular permeability enhancement, such as bradykinin and anaphylatoxins, are located upstream of active gelatinolytic MMP secretion (Hsieh et al, 2004; DiScipio et al, 2006). Thus, our results unify data from the literature by suggesting that most of the vascular damage correlates could constitute, at least in part, a network of pathways triggering gelatinolytic MMP overproduction and, consequently, the vascular damage observed in DHF/DSS.
Endothelial cells express adhesion molecules such as cadherins and PECAM-1, which are concentrated at areas of cell–cell contact, and contain abundant F-actin fibres that are involved in changes of cell shape and in endothelial cell junction activities (Hordijk et al, 1999). To visualize modifications of endothelial junction integrity triggered by DV-infected iDC supernatants, we analysed the distribution of PECAM-1 and VE-cadherin, and the organization of the actin cytoskeleton in confluent HUVEC monolayers incubated with virus-free culture supernatants from uninfected or infected iDCs (Fig 4). Results from these experiments show that the expression of PECAM-1, and also that of VE-cadherin, was strongly decreased in HUVEC monolayers cultured in the presence of iDC-DV-infected supernatant and resulted in significantly reduced cell–cell adhesion (Fig 4B,E). In addition, MMP activity on HUVEC monolayer integrity was also reflected by a decrease and redistribution of F-actin staining (Fig 4H). The observed disruption of cell–cell adhesion induced by DV-infected iDC supernatants was found to be strictly dependent on the activity of MMP gelatinases as the addition of SB-3CT prevented the disruption of endothelial cell–cell adhesion molecules (Fig 4C,F) while maintaining proper organization of the endothelial cytoskeleton (Fig 4I). The results from western blot analysis confirmed the immunofluorescence data by showing a decrease in the expression of both VE-cadherin and PECAM-1 in HUVECs treated with iDC-DV supernatants (Fig 4J,K).
Figure 4.

Supernatants of dengue-virus-infected immature dendritic cells induce matrix metalloproteinase-mediated disruption of endothelial cell–cell adhesion. Double immunofluorescence microscopy was carried out on confluent human umbilical vascular endothelial cell monolayers after exposure for 24 h with either Sup iDC or Sup iDC-DV. Where indicated, Sup iDC-DV was pretreated for 1 h with 200 nM SB-3CT before incubation with cells. The cells were stained with either anti-PECAM-1 (A–C) or anti-VE-cadherin (D–F) (green) antibody, followed by fluorescein isothiocyanate-conjugated anti-mouse IgG. Rhodamine–phalloidin (red) was used to visualize F-actin fibres (G–I). Nuclei were stained with 4,6-diamidino-2-phenylindole (blue). Similar results were obtained from three separate experiments. The expression of VE-cadherin (J) and PECAM-1 (K) proteins was determined by western blot analysis. Data are representative of two independent experiments. PECAM-1, platelet endothelial adhesion molecule 1; Sup iDC, supernatants of immature dendritic cells; Sup iDC-DV, supernatants of dengue-virus-infected immature dendritic cells.
Conclusions And Speculation
Our experimental in vitro and in vivo results highlight the major role of secreted gelatinolytic MMP in increased vascular permeability on infection of iDCs by DV in an antibody-independent manner. Although the relevance of these results needs to be ascertained in humans, our results on DHF/DSS and the current literature allow us to propose the basis for a general model for other viral haemorrhagic fevers (VHF), as most of the soluble factors triggered by VHF-infected myeloid cells (Geisbert & Jahrling, 2004) can act as upstream activators of MMP-9 secretion. Finally, as neither vaccine nor therapeutic is available against DV infection and on the basis of the results of the present study, it is important to stress that the development of therapeutic approaches specifically targeting gelatinolytic MMP might be beneficial in controlling endothelial vascular leakage induced in DHF/DSS.
Methods
Cells and dengue virus. Myeloid iDCs were generated from peripheral blood mononuclear cells according to a modified method of Wong et al (2001). Immature DC CD14−, CD1a+, CDC86+ and DC-SIGN+ (>97% purity) were cultured and used after 5 days. Primary HUVECs were cultured according to a slightly modified method of Carr et al (2003). The DV strain 16681 from DV2 subtype was propagated in LLC-MK2 cells, and the virus titres expressed as plaque-forming units (PFU) were determined by plaque assay (Halstead et al, 1984).
Dengue virus infection of immature dendritic cells and cell stimulation conditions. Five hundred thousand iDCs were exposed to DV for 2 h at a multiplicity of infection of 1 PFU/cell, extensively washed to remove excess virus, and further starved at 37°C and 5% CO2 in RPMI-1640. All experiments were performed using virus-free (negative reverse transcription–PCR; Deubel et al, 1990) supernatants from DV-infected iDCs that contained the soluble factors produced in 1 h after the viral washing out, that is, 3 h after infection.
Protein arrays. Protein levels in cell supernatants were analysed by using the highly sensitive Searchlight Proteome array technology (Pierce Endogen, Boston, MA, USA), on the basis of chemiluminescence protein detection.
In vitro permeability assay. Permeability of the HUVEC monolayer cultured on collagen-coated semipermeable membranes was assessed using a commercialized in vitro vascular permeability assay (Chemicon International, Temecula, CA, USA), according to the manufacturer's instructions. The amount of fluorescein isothiocyanate–dextran that permeated the HUVEC monolayer into the plate well was determined by measuring fluorescence at an excitation wavelength of 485 nm and emission at 530 nm in a spectrofluorometer (GENios-TECAN, Trappes, France). Several gelatinolytic MMP inhibitors were tested.
In vivo vascular permeability enhancement assay. This assay was carried out according to the method of Imamura et al (2005) with some modifications. Briefly, adult BALB/c mice were anaesthetized by using a continuous inhalation flow of 3% isoflurane (AErrane, Baxter, Maurepas, France). Evans blue (30 mg/kg body weight) was injected into the tail vein. A 50 μl sample (iDC supernatants and/or products able to induce or inhibit vascular damage) was injected intradermally in the dorsal skin of mice. When necessary, the products were dissolved in 10 mM Tris–HCl and 150 mM NaCl buffer. After 1 h, the mice were killed by inhalation of a lethal dose of CO2. The blue, bleeding tissues were biopsied and incubated in 3 ml of formamide (Sigma, St Louis, MO, USA) at 60°C for 36 h. Vascular leakage was determined by measuring the amount of skin-extracted Evans blue by spectrophotometry at 620 nm. Several gelatinolytic MMP inhibitors were tested.
Immunofluorescence microscopy. HUVEC monolayers were incubated for 24 h at 37°C with virus-free Sup iDC-DV. After this incubation, HUVECs were treated and incubated with the appropriate fluorescent staining system to observe the localization of VE-cadherin, PECAM-1, F-actin or nuclei under a fluorescence microscope (Carl Zeiss, Gottingen, Germany). For immunoblotting, pretreated cells were lysed and VE-cadherin and PECAM-1 proteins were analysed by western blotting, as described previously (Misse et al, 2001).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
supplementary Fig S1
Acknowledgments
We thank C. Oblet, J. Pène, F. Brumas and Ch. Jaquet for their technical help and Dr D. Mathieu for HUVECs. We also thank Dr S.L. Salhi for pre-submission editorial assistance. This work was supported by an Institut de Recherche pour le Développement special grant, France. N.L. was a PhD student supported by the Royal Golden Jubilee, grant #4.A.MU/43/A.1, Bangkok, Thailand.
References
- Agarwal R, Elbishbishi EA, Chaturvedi UC, Nagar R, Mustafa AS (1999) Profile of transforming growth factor-β1 in patients with dengue haemorrhagic fever. Int J Exp Pathol 80: 143–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH (2001) Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood–brain barrier and white matter components after cerebral ischemia. J Neurosci 21: 7724–7732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadian MA, Wang XL, Windsor LJ, Ghaly N, Caldwell RB (2001) TGF-β increases retinal endothelial cell permeability by increasing MMP-9: possible role of glial cells in endothelial barrier function. Invest Ophthalmol Vis Sci 42: 853–859 [PubMed] [Google Scholar]
- Bente DA, Rico-Hesse R (2006) Models of dengue virus infection. Drug Discov Today Dis Models 3: 97–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner SM, O'Sullivan MA (1998) Endothelial cell monolayers as a model system to investigate dengue shock syndrome. J Virol Methods 71: 159–167 [DOI] [PubMed] [Google Scholar]
- Carr JM, Hocking H, Bunting K, Wright PJ, Davidson A, Gamble J, Burrell CJ, Li P (2003) Supernatants from dengue virus type-2 infected macrophages induce permeability changes in endothelial cell monolayers. J Med Virol 69: 521–528 [DOI] [PubMed] [Google Scholar]
- Deubel V, Laille M, Hugnot JP, Chungue E, Guesdon JL, Drouet MT, Bassot S, Chevrier D (1990) Identification of dengue sequences by genomic amplification: rapid diagnosis of dengue virus serotypes in peripheral blood. J Virol Methods 30: 41–54 [DOI] [PubMed] [Google Scholar]
- DiScipio RG, Schraufstatter IU, Sikora L, Zuraw BL, Sriramarao P (2006) C5a mediates secretion and activation of matrix metalloproteinase 9 from human eosinophils and neutrophils. Int Immunopharmacol 6: 1109–1118 [DOI] [PubMed] [Google Scholar]
- Fernandez-Mestre MT, Gendzekhadze K, Rivas-Vetencourt P, Layrisse Z (2004) TNF-α-308A allele, a possible severity risk factor of hemorrhagic manifestation in dengue fever patients. Tissue Antigens 64: 469–472 [DOI] [PubMed] [Google Scholar]
- Garton KJ, Gough PJ, Philalay J, Wille PT, Blobel CP, Whitehead RH, Dempsey PJ, Raines EW (2003) Stimulated shedding of vascular cell adhesion molecule 1 (VCAM-1) is mediated by tumor necrosis factor-α-converting enzyme (ADAM 17). J Biol Chem 278: 37459–37464 [DOI] [PubMed] [Google Scholar]
- Geisbert TW, Jahrling PB (2004) Exotic emerging viral diseases: progress and challenges. Nat Med 10: S110–S121 [DOI] [PubMed] [Google Scholar]
- Goldberg GI, Marmer BL, Grant GA, Eisen AZ, Wilhelm S, He CS (1989) Human 72-kilodalton type IV collagenase forms a complex with a tissue inhibitor of metalloproteases designated TIMP-2. Proc Natl Acad Sci USA 86: 8207–8211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler DJ (1998) Dengue and dengue hemorrhagic fever. Clin Microbiol Rev 11: 480–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead SB (1970) Observations related to pathogenesis of dengue hemorrhagic fever. VI. Hypotheses and discussion. Yale J Biol Med 42: 350–362 [PMC free article] [PubMed] [Google Scholar]
- Halstead SB, Venkateshan CN, Gentry MK, Larsen LK (1984) Heterogeneity of infection enhancement of dengue 2 strains by monoclonal antibodies. J Immunol 132: 1529–1532 [PubMed] [Google Scholar]
- Ho LJ, Wang JJ, Shaio MF, Kao CL, Chang DM, Han SW, Lai JH (2001) Infection of human dendritic cells by dengue virus causes cell maturation and cytokine production. J Immunol 166: 1499–1506 [DOI] [PubMed] [Google Scholar]
- Hordijk PL, Anthony E, Mul FP, Rientsma R, Oomen LC, Roos D (1999) Vascular-endothelial-cadherin modulates endothelial monolayer permeability. J Cell Sci 112: 1915–1923 [DOI] [PubMed] [Google Scholar]
- Hsieh HL, Yen MH, Jou MJ, Yang CM (2004) Intracellular signalings underlying bradykinin-induced matrix metalloproteinase-9 expression in rat brain astrocyte-1. Cell Signal 16: 1163–1176 [DOI] [PubMed] [Google Scholar]
- Hummel V, Kallmann BA, Wagner S, Fuller T, Bayas A, Tonn JC, Benveniste EN, Toyka KV, Rieckmann P (2001) Production of MMPs in human cerebral endothelial cells and their role in shedding adhesion molecules. J Neuropathol Exp Neurol 60: 320–327 [DOI] [PubMed] [Google Scholar]
- Imamura T, Tanase S, Szmyd G, Kozik A, Travis J, Potempa J (2005) Induction of vascular leakage through release of bradykinin and a novel kinin by cysteine proteinases from Staphylococcus aureus. J Exp Med 201: 1669–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juffrie M, van Der Meer GM, Hack CE, Haasnoot K, Sutaryo J, Veerman AJ, Thijs LG (2000) Inflammatory mediators in dengue virus infection in children: interleukin-8 and its relationship to neutrophil degranulation. Infect Immun 68: 702–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauper V, Smith B, Lopez-Otin C, Murphy G (1997) Activation of progelatinase B (proMMP-9) by active collagenase-3 (MMP-13). Eur J Biochem 248: 369–373 [DOI] [PubMed] [Google Scholar]
- Lehmann W, Edgar CM, Wang K, Cho TJ, Barnes GL, Kakar S, Graves DT, Rueger JM, Gerstenfeld LC, Einhorn TA (2005) Tumor necrosis factor α (TNF-α) coordinately regulates the expression of specific matrix metalloproteinases (MMPS) and angiogenic factors during fracture healing. Bone 36: 300–310 [DOI] [PubMed] [Google Scholar]
- Lei HY, Yeh TM, Liu HS, Lin YS, Chen SH, Liu CC (2001) Immunopathogenesis of dengue virus infection. J Biomed Sci 8: 377–388 [DOI] [PubMed] [Google Scholar]
- Li A, Dubey S, Varney ML, Dave BJ, Singh RK (2003) IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol 170: 3369–3376 [DOI] [PubMed] [Google Scholar]
- Luca M, Huang S, Gershenwald JE, Singh RK, Reich R, Bar-Eli M (1997) Expression of interleukin-8 by human melanoma cells up-regulates MMP-2 activity and increases tumor growth and metastasis. Am J Pathol 151: 1105–1113 [PMC free article] [PubMed] [Google Scholar]
- Mackenzie JS, Gubler DJ, Petersen LR (2004) Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med 10: S98–S109 [DOI] [PubMed] [Google Scholar]
- Marovich M et al. (2001) Human dendritic cells as targets of dengue virus infection. J Invest Dermatol Symp Proc 6: 219–224 [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Funami K, Tanabe M, Oshiumi H, Shingai M, Seto Y, Yamamoto A, Seya T (2003) Subcellular localization of Toll-like receptor 3 in human dendritic cells. J Immunol 171: 3154–3162 [DOI] [PubMed] [Google Scholar]
- Misse D, Esteve PO, Renneboog B, Vidal M, Cerutti M, St Pierre Y, Yssel H, Parmentier M, Veas F (2001) HIV-1 glycoprotein 120 induces the MMP-9 cytopathogenic factor production that is abolished by inhibition of the p38 mitogen-activated protein kinase signaling pathway. Blood 98: 541–547 [DOI] [PubMed] [Google Scholar]
- Murgue B, Cassar O, Deparis X (2001) Plasma concentrations of sVCAM-1 and severity of dengue infections. J Med Virol 65: 97–104 [PubMed] [Google Scholar]
- Navarro-Sanchez E, Altmeyer R, Amara A, Schwartz O, Fieschi F, Virelizier JL, Arenzana-Seisdedos F, Despres P (2003) Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep 4: 723–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TH et al. (2004) Dengue hemorrhagic fever in infants: a study of clinical and cytokine profiles. J Infect Dis 189: 221–232 [DOI] [PubMed] [Google Scholar]
- Ritter M, Mennerich D, Weith A, Seither P (2005) Characterization of Toll-like receptors in primary lung epithelial cells: strong impact of the TLR3 ligand poly(I:C) on the regulation of Toll-like receptors, adaptor proteins and inflammatory response. J Inflamm (Lond) 2: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen L (1986) Dengue in Greece in 1927 and 1928 and the pathogenesis of dengue hemorrhagic fever: new data and a different conclusion. Am J Trop Med Hyg 35: 642–653 [DOI] [PubMed] [Google Scholar]
- Solomon A, Rosenblum G, Gonzales PE, Leonard JD, Mobashery S, Milla ME, Sagi I (2004) Pronounced diversity in electronic and chemical properties between the catalytic zinc sites of tumor necrosis factor-α-converting enzyme and matrix metalloproteinases despite their high structural similarity. J Biol Chem 279: 31646–31654 [DOI] [PubMed] [Google Scholar]
- Tassaneetrithep B et al. (2003) DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J Exp Med 197: 823–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng CS, Lo HW, Teng HC, Lo WC, Ker CG (2005) Elevated levels of plasma VEGF in patients with dengue hemorrhagic fever. FEMS Immunol Med Microbiol 43: 99–102 [DOI] [PubMed] [Google Scholar]
- Van den Steen PE, Dubois B, Nelissen I, Rudd PM, Dwek RA, Opdenakker G (2002) Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9). Crit Rev Biochem Mol Biol 37: 375–536 [DOI] [PubMed] [Google Scholar]
- Wong EC, Maher VE, Hines K, Lee J, Carter CS, Goletz T, Kopp W, Mackall CL, Berzofsky J, Read EJ (2001) Development of a clinical-scale method for generation of dendritic cells from PBMC for use in cancer immunotherapy. Cytotherapy 3: 19–29 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary Fig S1


