Abstract
Trypanosomes use RNA editing to produce most functional mitochondrial messenger RNA. Precise insertion and deletion of hundreds of uridines is necessary to make full-length cytochrome c oxidase III (COXIII) mRNA. We show that COXIII mRNA can be alternatively edited by a mechanism using an alternative guide RNA to make a stable mRNA. This alternatively edited mRNA is translated to produce a unique protein that fractionates with mitochondrial membranes and colocalizes with mitochondrial proteins in situ. Alternative RNA editing represents a previously unknown mechanism generating protein diversity and, as such, represents an important function for RNA editing.
Keywords: RNA editing, kinetoplast, Trypanosoma brucei
Introduction
Within the mitochondria of trypanosomes, many messenger RNAs are edited post-transcription (Benne et al, 1986; Kable et al, 1996; reviewed by Stuart et al, 2005). The proteins that carry out RNA editing exist as large protein complexes and small, guide RNAs (gRNAs) direct the editing activities (Blum et al, 1990; Pollard et al, 1992). Edited mRNAs are translated to produce components of the respiratory complexes and the ATP synthase (Horvath et al, 2000a). Fully edited mRNAs encoding the predicted complete open reading frames for these mitochondrial proteins have been identified; however, incomplete editing of extensively edited mRNA has been often observed (Bhat et al, 1990; Koslowsky et al, 1990). In extreme cases, cytochrome c oxidase III (COXIII) mRNA was found to be predominately edited incompletely (Abraham et al, 1988; Decker & Sollner-Webb, 1990).
African trypanosomes regulate the assembly of the mitochondrial respiratory complexes during their life cycle. A complete electron transport chain and oxidative phosphorylation occurs only in the insect vector developmental stage of the parasite's life cycle. In the bloodstream of the mammalian host, energy production is restricted to glycolysis and many mitochondrial gene products—including components of the cytochrome c reductase and cytochrome c oxidase respiratory complexes—are undetectable. Consistent with this downregulation of mitochondrial oxidative phosphorylation, both the abundance and editing of several mitochondrial mRNAs, encoding components of these electron transport complexes, are reduced in the bloodstream developmental stage of Trypanosoma brucei brucei (Feagin et al, 1987, 1988). However, despite the lack of conventional mitochondrial activities in bloodstream trypanosomes, editing of several mitochondrial mRNAs occurs and RNA-editing enzymes are essential (Schnaufer et al, 2001). Surprisingly, COXIII mRNA is abundant and extensively edited in both life-cycle stages, although its only known function is oxidative phosphorylation (Abraham et al, 1988). The steady-state pool of COXIII mRNAs is highly diverse and the constitutive expression of these transcripts during the trypanosome life cycle raised the possibility that the alternatively edited mRNAs could encode proteins with non-conventional functions in trypanosome mitochondria (Abraham et al, 1988; Decker & Sollner-Webb, 1990).
In this study, we investigated the editing of COXIII mRNAs in the bloodstream developmental stage of T. b. brucei. Among the highly diverse COXIII mRNAs, we identified a partially edited transcript that contained a long open reading frame including both pre-edited and edited sequences. A gRNA that could direct the alternative editing of COXIII mRNA was also identified. The predicted protein encoded by the alternatively edited COXIII mRNA is composed of a unique, hydrophilic amino-terminal domain and five membrane-spanning domains of COXIII in the carboxyl terminus. Antibodies against the predicted alternatively edited COXIII of T. b. brucei react with a mitochondrial membrane protein that colocalizes with mitochondrial markers in situ. These results indicate that alternative mRNA editing generates protein diversity in trypanosome mitochondria.
Results And Discussion
Identification of an alternatively edited COXIII mRNA
To investigate whether the alternative editing of COXIII mRNA could generate diverse protein products, mRNA was isolated from the bloodstream developmental stage of T. b. brucei, complementary DNAs were cloned and 186 full-length sequences were analysed. The sequences were aligned according to their extent and pattern of editing (data not shown). Most of the COXIII mRNAs are incompletely edited, as has been shown previously for the insect developmental stage of T. b. brucei (Abraham et al, 1988; Feagin et al, 1988; Decker & Sollner-Webb, 1990; Sturm & Simpson, 1990). In our library, fewer than 2% of the COXIII sequences were fully edited and entirely pre-edited COXIII cDNA sequences were absent. Thus, most of the COXIII mRNAs were partially edited. Most of the partially edited COXIII cDNAs had termination codons in all reading frames; however, a partially edited complementary DNA (cDNA; clone L07) containing an extended open reading frame was discovered and chosen for further analysis (Fig 1A). The alternatively edited (L07) cDNA contained an open reading frame starting with the alternative initiation codon (UUG) in the pre-edited sequence and extended to the authentic termination codon (UAA) for the fully edited COXIII (Fig 1A). The predicted UUG initiation codon is located 42 nucleotides from the 5′ end of the mRNA, consistent with the expected length for mitochondrial untranslated regions (supplementary Fig S1 online). The protein encoded by this alternatively edited mRNA would have unusual characteristics; it would contain a highly hydrophobic C-terminal region, with five predicted transmembrane domains—similar to COXIII—and a novel, arginine-rich, hydrophilic N-terminal domain.
Figure 1.
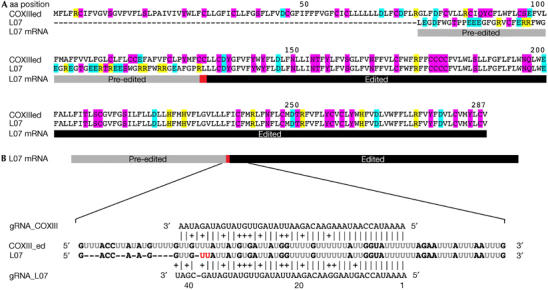
Alternatively edited cytochrome c oxidase III messenger RNA. (A) Predicted and aligned open reading frames for the fully (COXIIIed; COXIII, cytochrome c oxidase III) and alternatively edited (L07) complementary DNA sequences. The open reading frame for COXIII begins with an AUG (Met), whereas the alternative open reading frame starts with a UUG (Leu) alternative initiation codon. Amino acids are coloured according to their basic properties (white, non-polar; purple, polar; yellow, basic; turquoise, acidic). Under the predicted amino-acid sequences, the pre-edited (nucleotides 1–165, grey), the junction (nucleotides 166–168, red) and the fully edited (nucleotides 169–636, black) regions of the L07 cDNA are shown. (B) Alternatively edited junction region for L07 cDNA. Fully edited COXIII cDNA (COXIII_ed) and the alternatively edited COXIII cDNA (L07) are shown. In red are the uridyl residues that are inserted in the junction region joining the pre-edited and edited open reading frames. The guide RNA genes identified for the fully edited COXIII (gRNA_COXIII) and the alternatively edited COXIII (gRNA_L07) are shown above and below the cDNA sequences, respectively.
An examination of the L07 cDNA sequence explains how this partially edited mRNA could contain an open reading frame including both the 5′ pre-edited and 3′ edited sequence (Fig 1B). The junction between the pre-edited and edited sequence contains an editing site, where two uridines are inserted in the alternatively edited mRNA. By contrast, this editing site contains three uridines in the fully edited COXIII mRNA. This differential insertion of two uridines placed the pre-edited and the downstream edited sequence of the cDNA L07 in-frame.
The alternatively edited cDNA identified here (L07) might represent an intermediate in the editing of COXIII mRNA or a mis-edited COXIII mRNA destined for degradation. To address these possibilities, we searched for a gRNA that would direct editing of the alternative and incompletely edited COXIII mRNA, and which directly measured the stability of the alternatively edited mRNA.
Alternative editing of COXIII mRNA is gRNA directed
Our discovery that a partially edited COXIII mRNA contained an extended open reading frame raised the possibility that an alternative gRNA, specific for the junction of the pre-edited and edited mRNA, might be responsible for the creation of the open reading frame.
To identify gRNAs that could direct the editing pattern for the alternative COXIII mRNA, we initiated a minicircle sequencing project to expand the gRNA gene database for T. b. brucei. We purified minicircles from T. b. brucei, cloned and sequenced more than 500 minicircles (database and software tools will be published elsewhere). A gRNA gene (gRNA_L07) was identified with perfect complementarity, of more than 43 nucleotides, with the alternatively edited COXIII cDNA sequence (Fig 1B). Starting at the 5′ end, the gRNA_L07 is complementary for 39 bp with the bona fide COXIII mRNA and the alternative COXIII mRNA, inserting the same number of uridines at the same positions. The cytosine at position 40 requires binding to a guanosine residue in the edited mRNA and thereby restricts the number of uridine insertions at this site to two. In the bona fide COXIII mRNA, this site contains three uridines. We also identified the gRNA gene for the authentic editing of COXIII mRNA at this site (gRNA_COXIII; Fig 1B). The gRNA_COXIII differs from the gRNA_L07 at only two sites from nucleotides 1 to 39. Both changes are adenosines in the gRNA_COXIII and guanines in gRNA_L07 and maintain the correct guiding for uridine insertions at these sites to generate COXIII mRNA. On the basis of these results, we conclude that the alternative editing of COXIII mRNA is directed by a specific gRNA.
Alternatively edited COXIII mRNA is stable
To test directly whether the alternatively edited COXIII mRNA is stable, we inhibited trypanosome transcription by treatment with actinomycin D and then determined the abundance of pre-edited, alternatively edited and fully edited COXIII mRNAs at specific editing sites using a poisoned primer extension assay (Fig 2A–F). On the basis of our analysis of COXIII cDNA clones, we predict a rapid and complete editing of sites near the 3′ end of COXIII mRNA. Using a primer that binds immediately 3′ to the first editing site in COXIII, we detected pre-edited (+3 extension), partially edited (+5 product) and fully edited (+6 product) products at the time of actinomycin D addition (Fig 2B). The pre-edited extension product chased rapidly on inhibition of transcription (Fig 2C). As most of the COXIII transcripts were edited at this site when the drug was added, the increase in the fully edited product was slight but reproducible. We used a similar primer extension assay to investigate the stability of the alternatively edited COXIII mRNA (Fig 2D–F). We were able to detect the fully edited product (+4) and also the alternatively edited (+3) extension product, which corresponded to the cDNA L07. The products for both the fully edited and the alternatively edited COXIII are not only stable but also increase over time. Controls for both mitochondrial (9S ribosomal RNA) and nuclear genes (spliced leader RNA) show that actinomycin D ablates transcription (Fig 2I,J). These results indicate that the alternatively edited COXIII mRNA (L07) is a stable mRNA and is not an intermediate in the editing process.
Figure 2.
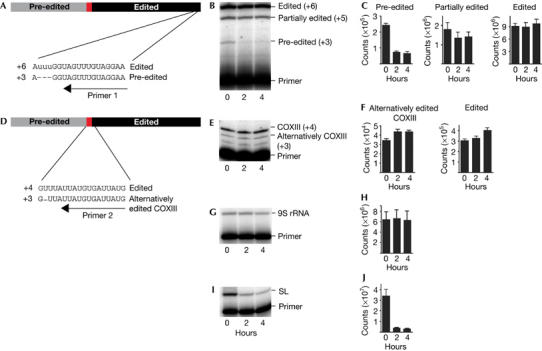
Analysis of the stability of edited, alternatively edited and pre-edited cytochrome c oxidase III transcripts. (A,D) Schematic representation of a cytochrome c oxidase III (COXIII) transcript and a poisoned primer extension assay. (A) Primer hybridizes 3′ of the first editing site in the 3′ untranslated region of COXIII. Extension occurs across the editing site and is terminated by the incorporation of ddTTP 5′ of that site. (B,E,G,I) Extension products from cells that are treated with actinomycin D for 0, 2 and 4 h are resolved on 20% (w/v) acrylamide and 8 M urea gels. (B) The (+6) extension product corresponds to the fully edited COXIII messenger RNA; (+5) extension product corresponds to a partially edited COXIII containing two uridines at this editing site; and (+3) extension product corresponds to the pre-edited COXIII mRNA. (C) After 2 h of actinomycin D treatment, more than 70% (±7%; n=7 biological replicates) was converted to edited/partially edited product. (D) Primer hybridizes 3′ of an alternatively edited site in the junction region of COXIII. Extension occurs across the editing site and is terminated by the incorporation of ddCTP 5′ of that site. (E) Fully and alternatively edited RNAs result in a +4 and +3 extension product, respectively. (F) After 2 h of actinomycin D treatment, the amount of alternatively edited product increased by 27% (±12%; n=3 biological replicates) and did not increase significantly thereafter. The fully edited product increased significantly by 32% after 4 h of actinomycin D treatment (±13%; n=3 biological replicates). The +2 extension product corresponds to a second complementary DNA discovered during this study and further analysis will be published elsewhere (data not shown). (G,H) Control poisoned primer extension experiments using an oligonucleotide complementary to 9S mitochondrial ribosomal RNA (9S rRNA) show the overall stability of the mitochondrial RNA preparations during the time-course analysed. (I,J) The rapid turnover of the spliced leader (SL) RNA from cytosolic RNA samples of the same cells verifies transcriptional arrest using actinomycin D.
Alternatively edited COXIII mRNA encodes a protein
To determine whether the alternatively edited COXIII mRNA was translated, we raised antisera against two unique synthetic peptides for the N-terminal domain, which were encoded by the pre-edited portion of alternative COXIII mRNA (supplementary Fig S2 online). Affinity-purified antibodies against the alternatively edited protein (AEP)-1 and AEP-1b were used to probe western blots of total cell protein, cytosolic protein, purified mitochondria, mitochondrial membrane, and matrix fractions. In the total cell extracts, both reacted with a single protein migrating at 49 kDa (Fig 3A). AEP-1 purified with mitochondria from bloodstream trypanosomes and mitochondrial fractionation localized AEP-1 exclusively to the mitochondrial membrane fraction relative to the cytosolic heat-shock protein-70 (HSP-70), the mitochondrial matrix heat-shock protein-70 (MTP-70) and the mitochondrial membrane trypanosome alternative terminal oxidase (TAO; Chaudhuri et al, 1995; Fig 3B). The relative mobility of the major band detected with anti-AEP-1 and anti-AEP-1b does not coincide with the predicted size of the protein, which would be around 27 kDa. Unusual gel mobility has also been shown for other trypanosome mitochondrial membrane proteins (Horvath et al, 2000a, 2000b). To determine whether AEP-1 was part of a larger protein complex in the mitochondrial membrane, detergent-extracted trypanosome mitochondrial membranes were fractionated on native gels (Fig 3C). The AEP-1 antibody reacted with a complex of approximately 550 kDa, suggesting assembly into high-molecular-weight membrane complexes. Under these conditions, we also detected other mitochondrial membrane complexes, the ATP synthase (>600 kDa; Williams & Frank, 1990) and the non-complexed monomeric and dimeric forms of TAO (Chaudhuri et al, 1995). These results indicate that AEP-1, encoded by an alternatively edited COXIII mRNA, is synthesized and assembled into a protein complex in trypanosome mitochondrial membranes.
Figure 3.
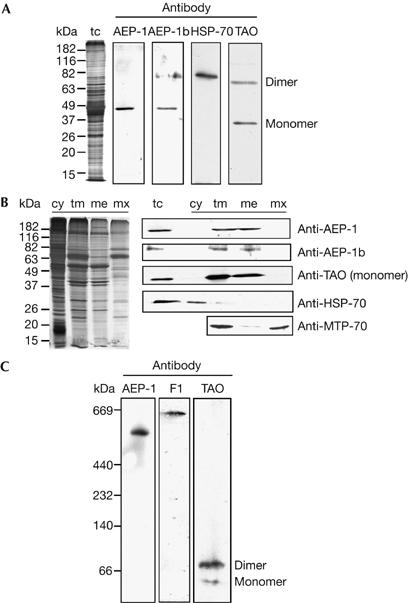
Expression and localization of alternatively edited protein-1. (A) Total cell (tc) protein lysate from Trypanosoma brucei bloodstream-form cells (2 × 105) fractionated on SDS–polyacrylamide gel electrophoresis, silver stained and analysed by western blotting with alternatively edited protein-1 (AEP-1) peptide antibodies (anti-AEP-1 and anti-AEP-1b), cytosolic heat-shock protein-70 (HSP-70) and mitochondrial alternative terminal oxidase (TAO) antibodies. Dimer and monomer refer to the respective forms of TAO. (B) Cytosolic (cy), total mitochondrial (tm), mitochondrial membrane (me) and mitochondrial matrix (mx) fractions from 2 × 105 bloodstream-form cells were separated by SDS–PAGE, silver stained and analysed by western blotting using the antibodies described in (A). MTP-70, mitochondrial matrix protein. (C) Western blot analysis of mitochondrial membrane protein complexes, fractionated by native gel electrophoresis using antibodies against AEP-1, the mitochondrial ATPase subunit F1 and mitochondrial TAO. The molecular weight standards were as follows: thyroglobulin, 669 kDa; ferritin, 440 kDa; catalase, 232 kDa; lactate dehydrogenase, 140 kDa; albumin, 66 kDa.
We used immunofluorescence microscopy to verify the mitochondrial localization of AEP-1 (Fig 4). Trypanosomes have a single mitochondrion running the length of the cell with a specialized region adjacent to the base of the flagellum containing the kinetoplast DNA (kDNA). Fixed and permeabilized cells were incubated with an AEP-1 antibody and a monoclonal antibody specific for the TAO; AEP-1 colocalized with TAO throughout the length of the mitochondrion (Fig 4B,C).
Figure 4.
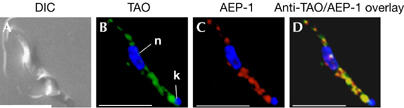
Localization of alternatively edited protein-1 in bloodstream-form Trypanosoma brucei using immunofluorescence microscopy. (A) Bloodstream-form T. brucei using differential interference contrast (DIC). (B) Distribution of the mitochondrial alternative terminal oxidase (TAO). Nucleus (n) and kinetoplast (k) were visualized by 4,6-diamidino-2-phenylindole staining. (C) Cellular distribution of alternatively edited protein-1 (AEP-1). (D) Overlaid images of cells stained with anti-AEP-1 and anti-TAO. Scale bars, 10 μm.
We have shown that alternative editing of the COXIII mRNA produces a stable product that contains the 5′ pre-edited and 3′ edited sequences. A minicircle gene encoding the gRNA specific for the formation of the AEP-1 mRNA was identified, suggesting that alternative mRNA editing is gRNA mediated. The fully edited COXIII mRNA encodes an essential core component of the cytochrome c oxidase complex, whereas AEP-1 is a novel mitochondrial protein with a highly hydrophobic C-terminal domain that is identical to COXIII and a novel hydrophilic N-terminal domain. Thus, RNA editing does more than simply repair mutations in mRNA to allow translation of conventional mitochondrial proteins. Alternative patterns of RNA editing can generate diverse open reading frames and proteins with potential novel functions in trypanosomes.
Methods
Trypanosomes, mitochondria and RNA isolation. Bloodstream-form trypanosomes (TREU667) were grown either in culture or in rats. Cells were lysed and mitochondria were purified as described by Harris et al (1990). Total cellular and mitochondrial RNA was extracted using the TriPure Isolation Reagent (Boehringer Mannheim, Mannheim, Germany) according to the manufacturer's instructions.
Cloning, sequencing and sequence analysis. cDNA libraries from bloodstream T. brucei mitochondrial mRNAs were made using the Creator® SMART™ cDNA library construction kit (Clontech, Palo Alto, CA, USA), according to the manufacturer's recommendations. Genemachines Revprep Orbit automated platform was used for template preparation and an Applied Biosystems (Foster City, CA, USA) 3730XL capillary sequencer for sequencing.
kDNA from T. brucei was isolated by sedimentation through a 20% (w/v) sucrose cushion at 65,000g for 80 min. After phenol:chloroform extraction and ethanol precipitation of the kDNA, the pellet was resuspended in water. Minicircles were released from the kDNA network with topoisomerase II (20 U; USB, Cleveland, OH, USA) and subsequently linearized using TaqI (20 U; NEB, Ipswich, MA, USA). After ligation and transformation into pCR Blunt II-TOPO (Invitrogen, Carlsbad, CA, USA), sequencing was carried out as described above.
Poisoned primer extension. Actinomycin D was added to trypanosome cultures at a final concentration of 5 μg/ml. Cells were either collected immediately or after incubation for 2 or 4 h at 37°C. Total RNA was isolated using the TriPure Isolation Reagent (Boehringer Mannheim). For each poisoned primer extension, 15 μg of total RNA was incubated with 20 U avian myeloblastosis virus reverse transcriptase, 40 U RNasin®, 0.5 mM ddNTP and 0.25 mM dNTPs in 50 mM Tris–HCl (pH 8.3), 50 mM KCl, 10 mM MgCl2, 0.5 mM spermidine, 10 mM dithiothreitol and 2 pmol of the corresponding 32P-labelled and gel-purified DNA oligonucleotide (primer 1: AACTTCCTACAAACTA; primer 2: CAAAACCATAATCACATAAT; 9S primer: TATTTGCATATACCTAATGG; SL: GTACAGAAACTGTTCTAATAATAG) at 48 or 54°C, for 45 min. Reaction products were separated on 20% (w/v) polyacrylamide sequencing gels containing 8 M urea, and quantified by PhosphorImager.
Bioinformatics. All bioinformatic analysis was carried out using the EMBOSS software package (Rice et al, 2000). A bioinformatic tool to analyse and graphically display cDNA and minicircle data will be published elsewhere. For the prediction of potential gRNAs, we used WUBLAST with a modified matrix to allow for G:U pairings. The prediction of open reading frames was made using the program GETORF from the EMBOSS program package using the protozoan mitochondrial genetic code EGC.4 from NCBI (Aldritt et al, 1989). All multiple alignments were constructed using EMMA, an EMBOSS interface to the Clustal W distribution (Thompson et al, 1994).
Generation of peptide antibodies. From the N terminus of AEP-1, two antigenic peptides were predicted and polyclonal rabbit antisera raised by Alpha Diagnostic Intl Inc. (San Antonio, TX, USA). Antibodies to AEP-1 (anti-AEP-1 and anti-AEP-1b) were purified using the peptide antigens. After elution from the peptide column, the antibodies were dialysed against phosphate buffered saline EDTA (pH 7.4) at 4°C for 12 h.
Purification of mitochondrial membrane fractions. To separate mitochondrial membrane from matrix proteins, mitochondria from 1 × 109 cells were lysed in 200 μl of 0.5% (v/v) Triton X-100, 20 mM Hepes–NaOH (pH 7.6), 1 mM phenylmethylsulphonyl fluoride and 1 × Complete® protease inhibitor cocktail (Roche, Indianapolis, IN, USA) for 45 min on ice. The membrane fraction was collected by centrifugation (12,000g, 10 min, 4°C) and resuspended in 3% (w/v) n-dodecyl-β-D-maltoside, 300 mM KCl, 50 mM BisTris–HCl (pH 7), 1 mM phenylmethylsulphonyl fluoride and 1 × Complete® protease inhibitor cocktail (Roche) and incubated for 45 min on ice. The membrane protein fraction was collected from the supernatant after centrifugation (12,000g, 10 min, 4°C). Membrane and matrix fractions were denatured by boiling in SDS and β-mercaptoethanol, and fractionated by SDS–polyacrylamide gel electrophoresis or on blue native gels without denaturation. Blue native gel electrophoresis was carried out as described previously (Schagger & von Jagow, 1991). For western blot analysis, proteins were transferred to nitrocellulose membranes, blocked overnight with 5% (w/v) milk in TBST (10 mM Tris–HCl, pH 8, 150 mM NaCl, 0.05% (v/v) Tween 20). The following primary antibodies were used: (i) polyclonal rabbit anti-AEP-1 (1:3,000), (ii) polyclonal rabbit anti-AEP-1b (1:500), (iii) monoclonal mouse anti-TAO (1:400; a kind gift from M. Chaudhuri, Nashville, TN, USA), (iv) polyclonal rabbit anti-HSP-70, and (v) monoclonal mouse anti-mtp-70 (1:1,000 and 1:500; a kind gift from J. Bangs, Madison, WI, USA). Blots were washed three times and then incubated with appropriate HRP-coupled secondary antibody at a 1:3,000 dilution.
Immunofluorescence microscopy. Bloodstream trypanosomes were washed in ice-cold PBS with 1% (w/v) glucose, fixed with 1% (v/v) paraformaldehyde in PBS for 60 min at 4°C and quenched with 0.1 M glycine/0.1 M Na2PO4 (pH 7.4) for 15 min at 24°C. Permeabilization was carried out with 0.1% (v/v) Triton X-100 in PBS for 10 min on ice. Cells were subsequently washed once and then blocked in 2% (v/v) native goat serum in PBS and incubated with the primary antibody. The following antibodies were used: (i) polyclonal rabbit AEP-1 (1:3,000), (ii) monoclonal mouse anti-TAO (1:400), and (iii) monoclonal mouse BBA4 (1:10; a kind gift from K. Gull, Oxford, UK). Primary antibodies were incubated with cells for 45 min, followed by two washes in 2% (v/v) native goat serum in PBS and incubated with the appropriate secondary antibody for 45 min at a 1:2,000 dilution. After incubation with the secondary antibody, cells were washed twice with PBS and then mounted on a microscopic slide using the 4,6-diamidino-2-phenylindole-containing antifade reagent ProlongGold® (Molecular Probes, Carlsbad, CA, USA). All centrifugation steps were carried out at 1,310g. Images were acquired using a motorized Zeiss Axioplan 2 and an Axiocam MRm camera, which were controlled by the Axiovisons 4.4 software, or by confocal microscopy using Zeiss LSM 510 NLO.
Supplementary information is available at EMBO reports online (http://www.emboreports.org)
Supplementary Material
Supplementary information Fig S1
Acknowledgments
We thank members of the Global Infectious Disease Laboratory at the Marine Biological Laboratory for stimulating discussions and comments on the manuscript. This work is supported by the National Institutes of Health. Computational resources were provided by the W.M. Keck Foundation and the G. Unger Vetlesen Foundation.
References
- Abraham JM, Feagin JE, Stuart K (1988) Characterization of cytochrome c oxidase III transcripts that are edited only in the 3′ region. Cell 55: 267–272 [DOI] [PubMed] [Google Scholar]
- Aldritt SM, Joseph JT, Wirth DF (1989) Sequence identification of cytochrome b in Plasmodium gallinaceum. Mol Cell Biol 9: 3614–3620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benne R, Van Den Burg J, Brakenhoff JPJ, Sloff P, Van Boom JH, Tromp MC (1986) Major transcript of the frameshifted coxII gene from trypanosome mitochondria contains four nucleotides that are not encoded in the DNA. Cell 46: 819–826 [DOI] [PubMed] [Google Scholar]
- Bhat GJ, Koslowsky DJ, Feagin JE, Smiley BL, Stuart K (1990) An extensively edited mitochondrial transcript in kinetoplastids encodes a protein homologous to ATPase subunit 6. Cell 61: 885–894 [DOI] [PubMed] [Google Scholar]
- Blum B, Bakalara N, Simpson L (1990) A model for RNA editing in kinetoplastid mitochondria: ‘guide' RNA molecules transcribed from maxicircle DNA provide the edited information. Cell 60: 189–198 [DOI] [PubMed] [Google Scholar]
- Chaudhuri M, Ajayi W, Temple S, Hill GC (1995) Identification and partial purification of a stage-specific 33 kDa mitochondrial protein as the alternative oxidase of the Trypanosoma brucei brucei bloodstream trypomastigotes. J Eukaryot Microbiol 42: 467–472 [DOI] [PubMed] [Google Scholar]
- Decker CJ, Sollner-Webb B (1990) RNA editing involves indiscriminate U changes throughout precisely defined editing domains. Cell 6: 1001–1011 [DOI] [PubMed] [Google Scholar]
- Feagin E, Jasmer DP, Stuart K (1987) Developmentally regulated addition of nucleotides within apocytochrome b transcripts in Trypanosoma brucei. Cell 49: 337–345 [DOI] [PubMed] [Google Scholar]
- Feagin JE, Abraham JM, Stuart K (1988) Extensive editing of the cytochrome c oxidase III transcript in Trypanosoma brucei. Cell 53: 413–422 [DOI] [PubMed] [Google Scholar]
- Harris ME, Moore DR, Hajduk SL (1990) Addition of uridines to edited RNAs in trypanosome mitochondria occurs independently of transcription. J Biol Chem 265: 11368–11376 [PubMed] [Google Scholar]
- Horvath A, Berry EA, Maslov DA (2000a) Translation of the edited mRNA for cytochrome b in trypanosome mitochondria. Science 287: 1639–1640 [DOI] [PubMed] [Google Scholar]
- Horvath A, Kingan TG, Maslov DA (2000b) Detection of the mitochondrially encoded cytochrome c oxidase subunit I in the trypanosomatid protozoan Leishmania tarentolae. Evidence for translation of unedited mRNA in the kinetoplast. J Biol Chem 275: 17160–17165 [DOI] [PubMed] [Google Scholar]
- Kable ML, Seiwert SD, Heidmann S, Stuart K (1996) RNA editing: a mechanism for gRNA-specified uridylate insertion into precursor mRNA. Science 273: 1189–1195 [DOI] [PubMed] [Google Scholar]
- Koslowsky DJ, Bhat GJ, Perrollaz AL, Feagin JE, Stuart K (1990) The MURF3 gene of T. brucei contains multiple domains of extensive editing and is homologous to a subunit of NADH dehydrogenase. Cell 62: 901–911 [DOI] [PubMed] [Google Scholar]
- Pollard VW, Harris ME, Hajduk SL (1992) Native mRNA editing complexes from Trypanosoma brucei mitochondria. EMBO J 11: 4429–4438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P, Longden I, Bleasby A (2000) EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet 16: 276–277 [DOI] [PubMed] [Google Scholar]
- Schagger H, von Jagow G (1991) Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal Biochem 199: 223–231 [DOI] [PubMed] [Google Scholar]
- Schnaufer A et al. (2001) An RNA ligase essential for RNA editing and survival of the bloodstream form of Trypanosoma brucei. Science 292: 2159–2162 [DOI] [PubMed] [Google Scholar]
- Stuart K, Schnaufer A, Lewis-Ernst N, Panigrahi AK (2005) Complex management: RNA editing in trypanosomes. Trends Biochem Sci 30: 97–105 [DOI] [PubMed] [Google Scholar]
- Sturm NR, Simpson L (1990) Partially edited mRNAs for cytochrome b and subunit III of cytochrome oxidase from Leishmania tarentolae mitochondria: RNA editing intermediates. Cell 61: 871–878 [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix. Nucleic Acids Res 22: 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams N, Frank PH (1990) The mitochondrial ATP synthase of Trypanosoma brucei: isolation and characterization of the intact F1 moiety. Mol Biochem Parasitol 43: 125–132 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information Fig S1


