Abstract
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease characterized by motor neuron loss and muscle wasting. In muscles of ALS patients, Nogo-A—a protein known to inhibit axon regeneration—is ectopically expressed at levels that correlate with the severity of the clinical symptoms. We now show that the genetic ablation of Nogo-A extends survival and reduces muscle denervation in a mouse model of ALS. In turn, overexpression of Nogo-A in wild-type muscle fibres leads to shrinkage of the postsynapse and retraction of the presynaptic motor ending. This suggests that the expression of Nogo-A occurring early in ALS skeletal muscle could cause repulsion and destabilization of the motor nerve terminals, and subsequent dying back of the axons and motor neurons.
Keywords: amyotrophic lateral sclerosis, G86R mouse, neuromuscular junction, Nogo-A, skeletal muscle
Introduction
Amyotrophic lateral sclerosis (ALS) is the most common adult human motor neuron disease and causes motor neuron degeneration, progressive skeletal muscle atrophy, paralysis and death within 1–5 years of onset. Although most of the cases occur sporadically, approximately 10% of patients show an autosomal dominant pattern of inheritance and 10–20% of these are associated with missense mutations in the gene encoding Cu/Zn-superoxide dismutase 1 (SOD1), one of the main free radical scavenging enzymes that protects cells against oxidative stress (Bruijn et al, 2004). The nature of the selective degeneration of motor neurons in ALS still remains elusive, but several studies postulate that the disease might begin at the distal axon and induce pathological changes earlier in the muscle than in the spinal cord (Frey et al, 2000; Fischer et al, 2004; Pun et al, 2006); however, the contribution of skeletal muscle fibres to initiating such a ‘dying back' process has been largely undervalued.
Nogo-A was first identified as a high-molecular-weight membrane protein of spinal cord myelin, in part responsible for the non-permissive properties of the white matter in the central nervous system (Caroni & Schwab, 1988). At the molecular level, three distinct regions of Nogo-A have been shown to trigger neurite growth inhibition, growth cone collapse or inhibition of fibroblast spreading, which makes Nogo-A a key factor in restricting regeneration and repair of injured axons (Prinjha et al, 2000; GrandPre et al, 2002; Oertle et al, 2003). In vivo inactivation of Nogo-A by neutralizing antibodies or blockade of some of the components of its cognate signalling pathway leads to enhanced regeneration and improved functional recovery after spinal cord lesion (Li et al, 2004; Liebscher et al, 2005).
Previously, we have shown that the skeletal muscles of ALS-linked mutant Sod1 mice and patients with sporadic ALS express high amounts of Nogo-A (Dupuis et al, 2002). In addition, the levels of muscle Nogo-A in ALS patients are higher in atrophied slow-twitch fibres and correlate with the severity of motor impairment (Jokic et al, 2005). To extend our understanding of these findings, we investigated the effects of knocking out Nogo-A in ALS mice and evaluated the morphological changes at the neuromuscular junction (NMJ) after overexpressing Nogo-A in non-pathological muscle.
Results
Genetic ablation of Nogo-A delays disease in ALS mice
We first determined Nogo-A expression during ALS pathology in Sod1(G86R) mice, a transgenic model that recapitulates many of the characteristics of the human disease (Ripps et al, 1995). In the soleus muscle, which in mice consists of slow-twitch fibres, Nogo-A upregulation occurred early in presymptomatic animals and persisted after overt hindlimb paralysis (supplementary Fig 1A–C online). This expression accompanied the progressive decrease in muscle fibre size, also noticeable before the onset of apparent motor problems (supplementary Fig 1D online). To analyse the consequences of Nogo-A upregulation, we crossbred Sod1(G86R) mice with Nogo-A-knockout animals (Simonen et al, 2003). We checked that the soleus muscle of G86R/Nogo-A−/− mice did not contain Nogo-A and assessed that Nogo-A ablation did not affect the expression of mutant Sod1 at both the messenger RNA and protein levels (supplementary Fig 2 online). We then recorded the survival of double transgenic mice and analysed several parameters that are characteristic of ALS pathology in symptomatic animals at comparable disease stages. This allowed us to recognize true protection effects rather than the age-related delay of symptoms. The mean survival times were 167±11.2 days for G86R/Nogo-A−/− mice (n=16) and 142±6.4 days for the control littermates (n=17; P=0.0456, Mann–Whitney test), which accounts for a moderate but significant increase in lifespan as illustrated by the Kaplan–Meier curve representation (Fig 1A). Although the average levels of mutant Sod1 protein were almost identical in G86R/Nogo-A+/+ and G86R/Nogo-A−/− mice (supplementary Fig 2G online), some interindividual variability could be observed (supplementary Fig 2F online). To rule out the possibility that Nogo-A ablation might promote lower mutant Sod1 expression and hence better survival, we analysed the influence of mutant Sod1 protein level variability on survival rates (supplementary Fig 4 online). Correlation analyses between these two parameters showed no significant differences, as determined by Spearman's correlation test (P=0.4348, n=11, G86R/Nogo-A+/+ mice; P=0.1689, n=12, G86R/Nogo-A−/− mice). In addition, analysis of covariance was carried out in the G86R/Nogo-A-knockout group of animals, using survival as the dependent variable and mutant Sod1 protein levels as the explanatory variable. The probability corresponding to the F value (calculated by Fisher's F test) was 0.234 (n=12 mice), which means that the interindividual differences in the expression of mutant Sod1 have no significant influence on the survival of the animal.
Figure 1.

Genetic ablation of Nogo-A in Sod1(G86R) mice. (A) Cumulative probability of survival of G86R/Nogo-A+/+ and G86R/Nogo-A−/− mice (P=0.04, n=16–17 mice, log-rank test). (B) Cell counts in the ventral horns of the lumbar spinal cord of G86R/Nogo-A+/+ and G86R/Nogo-A−/− mice. Toluidine-blue-stained cells were divided into two groups of 300–600μm2 and >600 μm2, the latter clearly representing motor neurons. No differences were observed in the number of cells between non-Cu/Zn-superoxide dismutase 1 (Sod1)(G86R) Nogo-A-knockout and wild-type mice (data not shown; *P<0.05 versus corresponding +/+, n=3 mice, Student's t-test). (C) Ubiquitin immunoreactivity in large motor neurons (cross-sectional area >600 μm2; arrowheads) of the lumbar spinal cord from G86R/Nogo-A+/+ and G86R/Nogo-A−/− mice. Scale bar, 30 μm. (D) Messenger RNA levels of acetylcholine receptor α-subunit (AChRα) and muscle-specific receptor tyrosine kinase (MuSK) in the soleus muscle of wild-type (WT; open bars) and Sod1(G86R) mice (filled bars) containing (+/+) or not containing (−/−) Nogo-A (***P<0.001 versus wild type, #P<0.05 versus +/+, n=4–5 mice, analysis of variance followed by Tukey's test). (E) Levels of MuSK protein as determined by western blot of immunoprecipitated MuSK from muscle extracts of Sod1(G86R) mice containing (+/+) or not containing (−/−) Nogo-A. MW, molecular weight markers. In all cases, symptomatic animals reached the end point of the disease. Sod1, Cu/Zn-superoxide dismutase 1.
To determine the number of large motor neurons in the lumbar spinal cord, we stained sections with toluidine blue and classified cells according to their cross-sectional area. In earlier studies (supplementary Fig 3 online), we found that cells with a cross-sectional area greater than 600 μm2 correspond to cells positive for the specific motor neuronal marker choline acetyltransferase, which corroborates our toluidine-blue-based estimates. In diseased Sod1(G86R) mice, we found a higher number of motor neurons in Nogo-A−/− mice than in Nogo-A+/+ mice (Fig 1B). Furthermore, ubiquitin inclusions in the motor neuron cell bodies, which is a marker of cell stress typically detected in diseased animals, could not be seen in the double transgenic mice (Fig 1C). At the NMJ level, the increased expression of the acetylcholine receptor α-subunit mRNA—a typical marker of denervation in neuromuscular diseases (Duclert & Changeux, 1995)—was prevented in part in G86R/Nogo-A−/− mice (Fig 1D). A similar attenuation was observed for the increase of mRNA encoding the muscle-specific receptor tyrosine kinase (MuSK; Fig 1D), which is a receptor kinase involved in acetylcholine receptor clustering (Bezakova & Ruegg, 2003) and the expression of which is coordinated with that of the acetylcholine receptor on denervation (Valenzuela et al, 1995). We also carried out immunoprecipitation experiments with whole hindlimb muscles to detect MuSK protein levels, and found that the immunoreactivity specific for MuSK protein observed in the immunoprecipitates from G86R/Nogo-A+/+ mice had disappeared in G86R/Nogo-A−/− mice (Fig 1E). These data clearly show that Nogo-A ablation in ALS mice not only prevented the increase in musk gene expression, characteristically observed in these animals, but also reduced the levels of MuSK protein, thereby confirming the attenuated denervation of muscles. Therefore, although the overall effect of Nogo-A deletion on the survival of G86R mice was moderate, these findings show a protective action for motor neurons at both the cell body and the endplate levels.
Nogo-A overexpression in muscle affects NMJ
The neuroprotective effects of Nogo-A ablation and the increase of Nogo-A in diseased ALS muscle indicate that high levels of ectopic Nogo-A in adult skeletal muscle might influence the NMJ and thereby induce retrograde axon pathology. We tested this hypothesis by analysing the morphology of NMJs in vivo in the soleus muscle of wild-type animals on transfection of muscle fibres by electroporation with an expression plasmid encoding Nogo-A fused to a Myc tag. To allow independent identification of Nogo-A-transfected muscle fibres, a plasmid encoding green fluorescent protein (GFP) fused to a nuclear localization signal (NLS–GFP) was co-transfected. First, we examined the expression of Nogo-A in the transfected muscle fibres by staining the soleus muscle with antibodies directed against the Myc tag. As shown in Fig 2A,B, Nogo-A was detected 2 weeks after electroporation in muscle fibres that were also positive for NLS–GFP. This confirms that our experimental design is well suited for the overexpression of Nogo-A in muscle. To examine the influence of Nogo-A overexpression on the morphology of the NMJs, we used Thy-1 (T-cell antigen)/yellow fluorescent protein mice (Feng et al, 2000), which allow direct identification of presynaptic nerve terminals. Postsynaptic acetylcholine receptors were visualized with rhodamine-conjugated α-bungarotoxin. NMJs were examined only 6 weeks after electroporation because we had already observed that changes in morphology on perturbation of MuSK, which is required for the formation of the NMJ (DeChiara et al, 1996), require 4–6 weeks (Kong et al, 2004). In all experiments, NMJs located on transfected (that is, NLS–GFP-positive) muscle fibres were analysed. As shown in Fig 2C, NMJs on muscle fibres transfected with empty vector (control) looked normal. By contrast, NMJs on muscle fibres expressing Nogo-A were often smaller (Fig 2D), postsynaptic structures were fragmented and the presynaptic nerve terminal only partly occupied the postsynaptic region (Fig 2E). In a few cases, the presynaptic nerve terminal had retracted entirely from the fragmented postsynaptic region (Fig 2F). The effect of Nogo-A on both the pre- and postsynaptic sides was quantified using the groups described above (Table 1). Although most NMJs looked normal in control muscle, approximately 90% of the NMJs located on Nogo-A-expressing muscle fibres had an aberrant structure.
Figure 2.
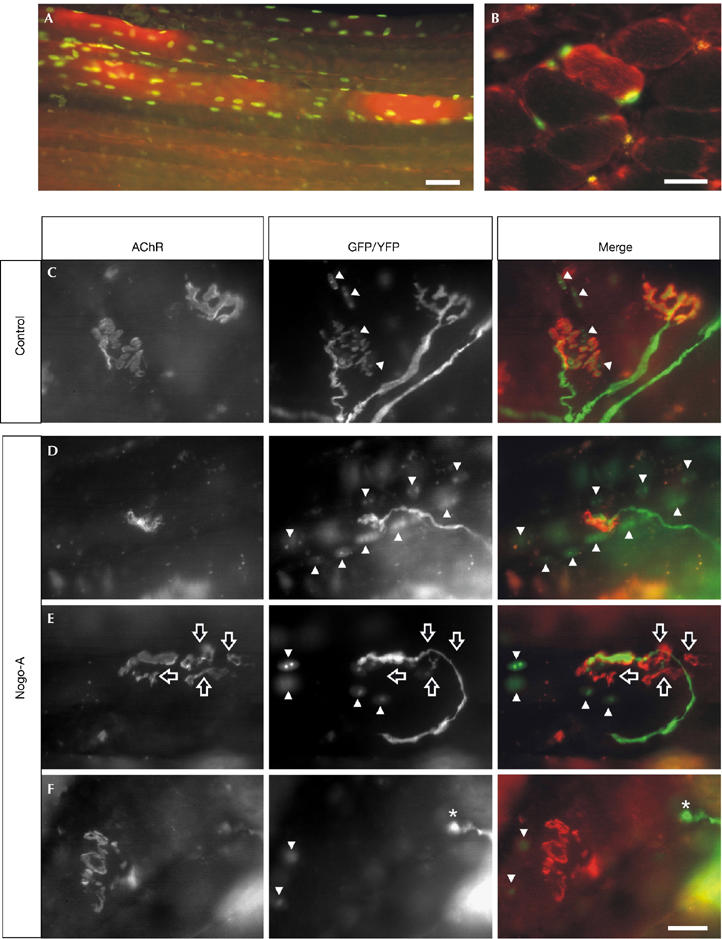
Overexpression of Nogo-A in muscle causes the disassembly of the neuromuscular junction. Electroporation of the soleus muscle with expression constructs for Nogo-A and nuclear-localized green fluorescent protein (GFP) results in muscle fibres that express high levels of Nogo-A (red) and GFP (green) after 2 weeks. (A) Whole-mount and (B) cross-section of the soleus muscle. Representative pictures of neuromuscular junctions (NMJs), 6 weeks after electroporation of empty vector (control; C) or Nogo-A expression constructs (D–F). The postsynaptic apparatus was visualized by rhodamine-labelled α-bungarotoxin (acetylcholine receptor; red in merge). Presynaptic nerve terminals were labelled by yellow fluorescent protein (YFP; green in merge) and transfected muscle fibres by nuclear localization signal–GFP (GFP, green in merge). For clarification, GFP-positive myonuclei are indicated by arrowheads. In control transfections, both pre- and postsynaptic structures look normal (C). By contrast, overexpression of Nogo-A often results in smaller NMJs (D) and fragmentation of the postsynaptic structure including partial (E; hollow arrows) or total (F; asterisks) retraction of the presynaptic nerve terminal. Scale bar, 25 μm.
Table 1.
Quantification of morphological changes at the neuromuscular junction after overexpression of Nogo-A in the soleus muscle
| Experiment | Side of NMJ | Total number of NMJs* | Normal‡ |
Abnormal‡ |
||
|---|---|---|---|---|---|---|
| Smaller | Fragmented | Lost | ||||
| Control |
Postsynaptic |
20 (3) |
18 (90) |
1 (5) |
1 (5) |
0 (0) |
| |
Presynaptic |
8 (3) |
7 (87.5) |
1 (12.5) |
0 (0) |
0 (0) |
| Nogo-A |
Postsynaptic |
47 (6) |
5 (11) |
25 (53) |
12 (25) |
5 (11) |
| Presynaptic | 45 (4) | 7 (17) | 19 (45) | 12 (29) | 4 (9) | |
The different classes for pre- and postsynaptic structures are as described in Fig 2.
NMJ, neuromuscular junction.
*The total number of NMJs is shown, with the number of mice in parentheses.
‡The data represent the total number of NMJs with a particular phenotype and the relative percentages are in parentheses. Note that in cases in which the phenotype was intermediate between different classes, the less severe class was chosen.
We also quantified the effect of Nogo-A on the size of the postsynaptic apparatus, by measuring the area stained with α-bungarotoxin in flat-mounted muscle preparations. Postsynapses on neighbouring, non-transfected muscle fibres acted as controls. Postsynapses on muscle fibres transfected with empty vector (control) occupied an area of 400±60 μm2 (n=4 mice; a total of 11 NMJs examined), whereas those located on non-transfected muscle fibres in the same muscle were 469±66 μm2 (n=3 mice; six NMJs). Thus, there is no significant difference between transfected and non-transfected muscle fibres in control muscles. By contrast, postsynapses in muscle fibres transfected with the expression construct for Nogo-A were only 241±15 μm2 (n=6 mice; 47 NMJs). Similar to control mice, postsynapses on non-transfected muscle fibres in the same muscles occupied an average area of 452±35 μm2 (n=4 mice; 11 NMJs). Thus, the postsynaptic regions of the muscle fibres that overexpress Nogo-A are significantly (P=0.005) smaller than those in control muscle fibres or neighbouring, non-transfected muscle fibres. Our data are consistent with the idea that increased levels of Nogo-A destabilize the NMJ and eventually lead to retraction of the nerve terminal.
Discussion
Several studies postulate that motor neuron death in ALS is preceded by pathological changes and degeneration of the motor axons and their nerve terminals (Ferri et al, 2003; Kieran et al, 2005). On the basis of our present findings, we propose that the high expression of Nogo-A in ALS muscle might affect the integrity of the NMJ, leading to its destabilization and retraction of the nerve terminal. This process could trigger a progressive ‘dying back' mechanism that ultimately leads to motor neuron degeneration. As muscle Nogo-A upregulation is observed in both mutant Sod1 mice and patients with sporadic ALS (Dupuis et al, 2002; Jokic et al, 2005), the deleterious effect of muscle Nogo-A on the NMJ might represent a common pathological event and a potential therapeutic target for the maintenance of the neuromuscular connections. Blocking the ability of Nogo-A to inhibit neurite outgrowth promotes axonal sprouting and functional recovery after spinal cord lesion (Li et al, 2004; Liebscher et al, 2005), which suggests that such an approach could be applicable to alleviate motor impairment in ALS.
Our findings show that specific transcriptional changes in skeletal muscle fibres, that is, increased Nogo-A expression, might hamper the dialogue between motor neurons and muscles. It is now accepted that motor neurons are not the only sites at which mutant Sod1 toxicity acts primarily to trigger ALS (Lino et al, 2002; Clement et al, 2003). Rather, the disease results from toxicity to the whole neuromuscular unit, as supported by recent studies (Wang et al, 2005). Thus, neurogenic injury and also other mechanisms of muscle origin, as discussed here, work together to cause the characteristic phenotype of the diseased NMJ in ALS (Fischer et al, 2004). Far from having a passive role in the establishment of neuromuscular connections, muscle-intrinsic signals can initiate the differentiation of neuromuscular synapses independently of neurogenic stimulation (Lin et al, 2001) and regulate motor neuron excitability (Nick & Ribera, 2000; Nakanishi et al, 2005). In ALS mice, the protective action for motor neurons offered by the intramuscular delivery of retrogradely transported active molecules might also imply benefit to muscle fibres (Kaspar et al, 2003; Azzouz et al, 2004; Miller et al, 2005). Indeed, the expression of a locally acting isoform of insulin-like growth factor 1 in only muscles is sufficient to maintain NMJ integrity, delay motor neuron death and prolong the lifespan of mutant Sod1 mice (Dobrowolny et al, 2005). Finally, we recently reported that the skeletal muscles of ALS mice show an unexpectedly augmented metabolic rate before overt motor symptoms appear (Dupuis et al, 2004), which further supports the involvement of non-neuronal mechanisms during the course of the disease. Compensating for such a hypermetabolic trait with a highly energetic diet reduces muscle denervation, offers neuroprotection and increases survival (Dupuis et al, 2004).
In summary, we provide new evidence for the active role of skeletal muscle in the maintenance of motor neuron function and suggest a role of Nogo-A in synapse integrity and stabilization. The suppression of Nogo-A increased the number of healthy motor neurons in end-stage ALS mice and moderately prolonged their lives. Much effort must now be concentrated on deciphering the molecular mechanisms underlying these effects.
Methods
Western blot, immunoprecipitation, immunohistochemistry, morphometry, real-time reverse transcription–PCR and analysis of transgene copy number are described in the supplementary information online.
Mice. Transgenic FVB/N males with the G86R murine Sod1 mutation were obtained from our animal facility. Sod1(G86R) mice were crossed with C57Bl/6 wild-type or Nogo-A-deficient mice. The resulting F1 generation animals were crossed again with new C57Bl/6 wild-type and Nogo-A-knockout mice; mice that were homozygous or wild type for the Nogo-A deficiency and heterozygous for the Sod1 mutation were obtained. Genotyping was carried out as described by Ripps et al (1995) and Simonen et al (2003). Details are given in the supplementary information online.
In vivo muscle transfection. Ectopic expression of Nogo-A in the soleus muscle was obtained by in vivo transfection of a pcDNA3/Myc-tagged/Nogo-A plasmid, previously described by Prinjha et al (2000). Identification of transfected fibres was achieved by co-transfection of a pGFP-S6 construct encoding a GFP that is targeted to the nucleus (Jones et al, 1999). The procedure for transfection has been described previously (Kong et al, 2004; for details, see supplementary information online).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
supplementary Fig 1
supplementary Fig 2
supplementary Fig 3
supplementary Fig 4
supplementary Methods online
Acknowledgments
We thank Mrs A. Picchinenna and Miss M.J. Ruivo for excellent technical assistance. This work was supported by grants from the French organizations Association Française contre les Myopathies (AFM), Biovalley, Association pour la Recherche sur la Sclérose Latérale Amyotrophique (ARS) and Association pour la Recherche contre les Maladies Neurodégéneratives (AREMANE) to J.P.-L.; the Swiss National Science Foundation (SNSF) and the National Center of Competence in Research (NCCR) on Neural Plasticity and Repair to M.E.S.; and the SNSF and the Swiss Foundation for Research on Muscle Diseases to M.A.R. N.J. was the recipient of a grant from Association Française contre les Myopathies (AFM). A.F. is the recipient of a grant from Région Alsace and Association pour L'Etude de la Culture d'Embryons et des Thérapeutiques des Maladies du Système Nerveux (ACE; France).
References
- Azzouz M, Ralph GS, Storkebaum E, Walmsley LE, Mitrophanous KA, Kingsman SM, Carmeliet P, Mazarakis ND (2004) VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature 429: 413–417 [DOI] [PubMed] [Google Scholar]
- Bezakova G, Ruegg MA (2003) New insights into the roles of agrin. Nat Rev Mol Cell Biol 4: 295–308 [DOI] [PubMed] [Google Scholar]
- Bruijn LI, Miller TM, Cleveland DW (2004) Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci 27: 723–749 [DOI] [PubMed] [Google Scholar]
- Caroni P, Schwab ME (1988) Antibody against myelin-associated inhibitor of neurite growth neutralizes nonpermissive substrate properties of CNS white matter. Neuron 1: 85–96 [DOI] [PubMed] [Google Scholar]
- Clement AM et al. (2003) Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science 302: 113–117 [DOI] [PubMed] [Google Scholar]
- DeChiara TM et al. (1996) The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell 85: 501–512 [DOI] [PubMed] [Google Scholar]
- Dobrowolny G, Giacinti C, Pelosi L, Nicoletti C, Winn N, Barberi L, Molinaro M, Rosenthal N, Musaro A (2005) Muscle expression of a local Igf-1 isoform protects motor neurons in an ALS mouse model. J Cell Biol 168: 193–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclert A, Changeux JP (1995) Acetylcholine receptor gene expression at the developing neuromuscular junction. Physiol Rev 75: 339–368 [DOI] [PubMed] [Google Scholar]
- Dupuis L et al. (2002) Nogo provides a molecular marker for diagnosis of amyotrophic lateral sclerosis. Neurobiol Dis 10: 358–365 [DOI] [PubMed] [Google Scholar]
- Dupuis L, Oudart H, Rene F, Gonzalez de Aguilar JL, Loeffler JP (2004) Evidence for defective energy homeostasis in amyotrophic lateral sclerosis: benefit of a high-energy diet in a transgenic mouse model. Proc Natl Acad Sci USA 101: 11159–11164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR (2000) Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28: 41–51 [DOI] [PubMed] [Google Scholar]
- Ferri A, Sanes JR, Coleman MP, Cunningham JM, Kato AC (2003) Inhibiting axon degeneration and synapse loss attenuates apoptosis and disease progression in a mouse model of motoneuron disease. Curr Biol 13: 669–673 [DOI] [PubMed] [Google Scholar]
- Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, Khan J, Polak MA, Glass JD (2004) Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol 185: 232–240 [DOI] [PubMed] [Google Scholar]
- Frey D, Schneider C, Xu L, Borg J, Spooren W, Caroni P (2000) Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motoneuron diseases. J Neurosci 20: 2534–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GrandPre T, Li S, Strittmatter SM (2002) Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature 417: 547–551 [DOI] [PubMed] [Google Scholar]
- Jokic N et al. (2005) Nogo expression in muscle correlates with amyotrophic lateral sclerosis severity. Ann Neurol 57: 553–556 [DOI] [PubMed] [Google Scholar]
- Jones G, Moore C, Hashemolhosseini S, Brenner HR (1999) Constitutively active MuSK is clustered in the absence of agrin and induces ectopic postsynaptic-like membranes in skeletal muscle fibers. J Neurosci 19: 3376–3383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar BK, Llado J, Sherkat N, Rothstein JD, Gage FH (2003) Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science 301: 839–842 [DOI] [PubMed] [Google Scholar]
- Kieran D, Hafezparast M, Bohnert S, Dick JR, Martin J, Schiavo G, Fisher EM, Greensmith L (2005) A mutation in dynein rescues axonal transport defects and extends the life span of ALS mice. J Cell Biol 169: 561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong XC, Barzaghi P, Ruegg MA (2004) Inhibition of synapse assembly in mammalian muscle in vivo by RNA interference. EMBO Rep 5: 183–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S et al. (2004) Blockade of Nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble Nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J Neurosci 24: 10511–10520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebscher T et al. (2005) Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann Neurol 58: 706–719 [DOI] [PubMed] [Google Scholar]
- Lin W, Burgess RW, Dominguez B, Pfaff SL, Sanes JR, Lee KF (2001) Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature 410: 1057–1064 [DOI] [PubMed] [Google Scholar]
- Lino MM, Schneider C, Caroni P (2002) Accumulation of SOD1 mutants in postnatal motoneurons does not cause motoneuron pathology or motoneuron disease. J Neurosci 22: 4825–4832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TM, Kaspar BK, Kops GJ, Yamanaka K, Christian LJ, Gage FH, Cleveland DW (2005) Virus-delivered small RNA silencing sustains strength in amyotrophic lateral sclerosis. Ann Neurol 57: 773–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi ST, Cope YC, Rich MM, Carrasco DI, Pinter MJ (2005) Regulation of motoneuron excitability via motor endplate acetylcholine receptor activation. J Neurosci 25: 2226–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nick TA, Ribera AB (2000) Synaptic activity modulates presynaptic excitability. Nat Neurosci 3: 142–149 [DOI] [PubMed] [Google Scholar]
- Oertle T et al. (2003) Nogo-A inhibits neurite outgrowth and cell spreading with three discrete regions. J Neurosci 23: 5393–5406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinjha R, Moore SE, Vinson M, Blake S, Morrow R, Christie G, Michalovich D, Simmons DL, Walsh FS (2000) Inhibitor of neurite outgrowth in humans. Nature 403: 383–384 [DOI] [PubMed] [Google Scholar]
- Pun S, Santos AF, Saxena S, Xu L, Caroni P (2006) Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat Neurosci 9: 408–419 [DOI] [PubMed] [Google Scholar]
- Ripps ME, Huntley GW, Hof PR, Morrison JH, Gordon JW (1995) Transgenic mice expressing an altered murine superoxide dismutase gene provide an animal model of amyotrophic lateral sclerosis. Proc Natl Acad Sci USA 92: 689–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonen M, Pedersen V, Weinmann O, Schnell L, Buss A, Ledermann B, Christ F, Sansig G, van der Putten H, Schwab ME (2003) Systemic deletion of the myelin-associated outgrowth inhibitor Nogo-A improves regenerative and plastic responses after spinal cord injury. Neuron 38: 201–211 [DOI] [PubMed] [Google Scholar]
- Valenzuela DM et al. (1995) Receptor tyrosine kinase specific for the skeletal muscle lineage: expression in embryonic muscle, at the neuromuscular junction, and after injury. Neuron 15: 573–584 [DOI] [PubMed] [Google Scholar]
- Wang J, Xu G, Slunt HH, Gonzales V, Coonfield M, Fromholt D, Copeland NG, Jenkins NA, Borchelt DR (2005) Coincident thresholds of mutant protein for paralytic disease and protein aggregation caused by restrictively expressed superoxide dismutase cDNA. Neurobiol Dis 20: 943–952 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary Fig 1
supplementary Fig 2
supplementary Fig 3
supplementary Fig 4
supplementary Methods online


