Abstract
Cells communicate with each other to form organized structures by cell–cell adhesion and cell–cell repulsion, but it remains to be clarified how cell–cell contact information is converted into intracellular signals. Here, we show that cells in contact with neighbouring cells generate local transient intracellular Ca2+ signals (Ca2+ lightning). Ca2+ lightning was observed near cell–cell contact regions and was not observed in the central regions of cells or in solitary cells that were not in contact with other cells. We also show that Ca2+ lightning is able to regulate cell–cell repulsion by means of PYK2, a Ca2+-activated protein tyrosine kinase, which induces focal adhesion disassembly in a Ca2+-dependent manner. These results show that cell–cell contact information might be transmitted by Ca2+ lightning to regulate intracellular events.
Keywords: calcium signal, cell–cell repulsion, focal adhesion, imaging, PYK2
Introduction
Cells communicate with each other to maintain organized tissue structures by cell–cell adhesion and cell–cell repulsion (Takeichi, 1993; Fagotto & Gumbiner, 1996; Tessier-Lavigne & Goodman, 1996; Cavallaro & Christofori, 2004). The following sequence of events occurs during cell–cell repulsion: cell–cell contact, retractive cell movement, and cell separation (Hattori et al, 2000; Marston et al, 2003; Wilkinson, 2003; Zimmer et al, 2003). However, it remains to be clarified how cell–cell contact information is converted into intracellular signals and what induces the subsequent cellular retractive movement. Focal adhesions physically link the cytoskeleton and extracellular matrix (ECM), and focal-adhesion turnover mediates cell migration (Wehrle-Haller & Imhof, 2002; Ridley et al, 2003). As cell–cell repulsion involves retractive cell movement, the regulatory machinery of focal adhesion turnover should be important in cell–cell repulsion. Although recent studies have shown that cell–cell contact regulates focal adhesion turnover (Schaller, 2004; Yano et al, 2004), it remains to be clarified what signal conveys cell–cell contact information inside cells.
PYK2 (proline-rich tyrosine kinase 2, also known as RAFTK, CADTK or CAKβ), the second member of the focal adhesion kinase family, regulates focal adhesion turnover (Avraham et al, 2000; Gelman, 2003) and cell motility (Sieg et al, 1998; Guinamard et al, 2000; Ivankovic-Dikic et al, 2000; Okigaki et al, 2003); therefore, we studied the role of PYK2 in cell–cell repulsion. We found that PYK2 localizes at focal adhesions and promotes cell–cell repulsion. However, localization of PYK2 was not sufficient to induce retractive cell movements. The present study shows a novel form of spontaneous local Ca2+ transient (Ca2+ lightning) that is necessary to induce PYK2-dependent cell movements. Ca2+ lightning is observed near cell–cell borders and might convey cell–cell contact information inside cells.
Results And Discussion
PYK2-dependent cell–cell repulsion
Cells expressing green fluorescent protein (GFP)-tagged PYK2 (hereafter referred to as PYK2–GFP cells) and control cells expressing GFP (GFP cells) were examined on day 4 of culture after seeding at the same density on day 1 (Fig 1A). GFP cells spread well on the ECM and formed clusters. By contrast, PYK2–GFP cells were slender and evenly dispersed, with significant gaps between neighbouring cells. The average edge-to-edge distance between PYK2–GFP cells was significantly greater than between GFP cells (Fig 1B), even though the density of PYK2–GFP cells was higher than that of GFP cells (Fig 1C). The carboxy-terminal region of PYK2 is necessary for its localization at focal adhesions (Litvak et al, 2000). Cells expressing a mutant PYK2 (PYK2ΔC–GFP), which lacked the C-terminal region (supplementary Fig 1A online), grew in a similar manner to GFP cells (Fig 1A–C). Thus, PYK2 seems to promote cell–cell repulsion through its localization at focal adhesions.
Figure 1.

PYK2-induced cell–cell repulsion. (A) Fluorescence images of control (GFP), PYK2 (PYK2–GFF) and PYK2ΔC (PYK2ΔC–GFP) cells on days 1 and 4 of culture. Scale bar, 100 μm. (B) Average edge-to-edge distance between cells. Fluorescence images were used for the determination of nuclear position. Edge-to-edge distance was measured using simultaneously obtained surface reflection interference contrast microscopy images, which unambiguously defined cell borders. Values represent mean±s.e.m. (n>50). (C) Cell growth curves. Values represent mean±s.e.m. (n=3). GFP, green fluorescent protein.
To study the cellular processes underlying cell–cell repulsion, we observed the dynamic changes of focal adhesions around the cell–cell contact interface at a higher magnification by using surface reflection interference contrast (SRIC) microscopy (Izzard & Lochner, 1976). SRIC images are generated by the interference of light beams reflected at a glass–medium interface and a medium–cell interface. Therefore, focal adhesions and cell borders are seen as dark spots and lines, respectively. At thicker regions of cells, there could be higher order interference owing to reflections from other structures, such as reflection at the upper surface of cells. However, we can identify focal adhesions in the vicinity of cell borders where the cytoplasm is very thin and where there is little contribution from higher order interference. Fig 2A (left panels) shows the borders of three GFP cells, each of which is coloured differently for clarity. Although the lamellipodia of the cells made cyclic contacts with neighbouring cells, and sometimes invaded the cell–ECM interface of adjacent cells, the cells showed no appreciable global migration in 60 min of observation (supplementary Movie 1 online).
Figure 2.
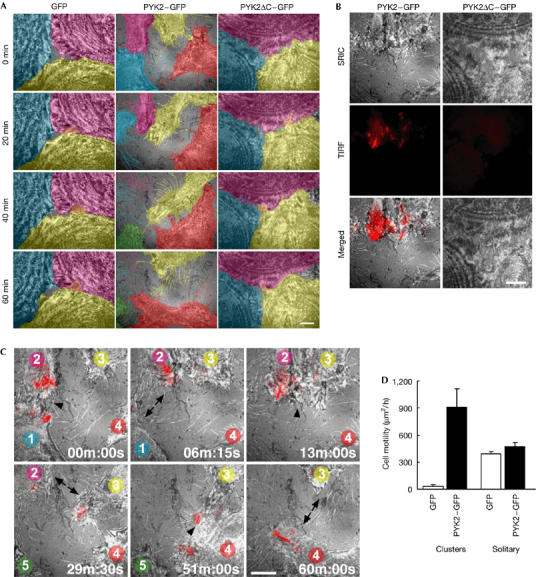
Dynamics of cell–cell repulsion regulated by PYK2 localized at focal adhesions in migrating cells. (A) Surface reflection interference contrast (SRIC) microscopy images of control cells (GFP; left), PYK2 cells (PYK2–GFP; middle) and PYK2ΔC cells (PYK2ΔC–GFP; right). Cells were distinguished using different colours for clarity. Note the instability of the cell–cell adhesion of PYK2 cells. GFP, green fluorescent protein. (B) PYK2–GFP localizes at focal adhesions by means of its carboxy-terminal region. SRIC microscopy images of focal adhesions and cell borders (top), total internal reflection fluorescence (TIRF) microscopy images of PYK2 (middle) and merged images (bottom) of PYK2–GFP cells and PYK2ΔC–GFP cells. The fringes of the SRIC image of PYK2ΔC–GFP cells are due to higher order interference showing the contours. (C) Merged images of SRIC (monochrome) and TIRF (red) microscopy images of PYK2–GFP cells. The cells are numbered from 1 to 4 (see text). The cells are the same as those in (A). Transient cell–cell adhesion (arrowhead) and repulsion (arrow) are indicated. Scale bars, 10 μm (A–C). (D) Motility of cells forming clusters and solitary cells, in the presence and absence of PYK-2 expression. The magnitude of cell motility was quantified as described in the Methods. Values represent mean±s.e.m. (n⩾4).
By contrast, PYK2–GFP cells showed vigorous lamellipodial movements at cell–cell borders (Fig 2A, middle panels). PYK2–GFP fluorescence at the cell–ECM border was simultaneously imaged using total internal reflection fluorescence (TIRF) microscopy (Fig 2B, left panels; Fig 2C, red signals). Because TIRF microscopy highlights fluorescent molecules only in the vicinity (within ∼100 nm) of the cover glass surface, we can effectively image PYK2–GFP in the vicinity of focal adhesions that attach to the cover glass by means of the ECM. Although the PYK2–GFP cells formed transient contacts with adjacent cells, they failed to maintain stable cell–cell contact. Fig 2C, and supplementary Movie 2 online, show four representative cells undergoing a sequence of such events. Although the dynamic cell–cell interaction can be fully appreciated in the movie, some of the notable features are described below. At 00 min:00 s, cells 1 and 2 were in contact (arrowhead), and at the same time PYK2 was localized at focal adhesions at the leading edges of the lamellipodia. Six minutes later (06 min:15 s), the focal adhesions disassembled, inducing the detachment of the lamellipodia from the ECM, lamellipodial retraction and cell–cell separation (arrows). During the separation, cell borders left fine processes owing to focal attachments to the ECM. At 13 min:00 s, cells 2 and 3 formed a transient contact (arrowhead), concomitant with PYK2 accumulation at focal adhesions. Subsequently (29 min:30 s), they detached from each other (arrows). Later (51 min:00 s to 60 min:00 s), cells 3 and 4 underwent a similar transient cell–cell adhesion and subsequent repulsion. Within the 60 min of observation, there was a net movement of each cell and the landscape changed markedly (Fig 2A, middle panels). The same dynamic changes in cell borders were observed in cells expressing non-GFP-tagged PYK2 (data not shown). Conversely, the TIRF image of PYK2ΔC–GFP showed faint and blurred images owing to excitation at the upper border of the evanescent field (Fig 2B, right panels). Therefore, PYK2ΔC–GFP did not localize at focal adhesions, and formed stable cell–cell adhesions similar to control cells (supplementary Movie 3 online).
These results show that PYK2 inhibits the formation of stable cell–cell adhesion and promotes cell–cell repulsion in cell clusters. The basal, dynamic cell-border movements were larger in solitary GFP cells, which formed no contact with neighbouring cells, than in GFP cells, which formed contacts with adjacent cells (Fig 2D; supplementary Movie 4 online). Interestingly, PYK2 had very little enhancing effect on cell border movement in solitary cells, although PYK2–GFP was constitutively localized at focal adhesions (Fig 2D; supplementary Movie 5 online). Thus, the PYK2-dependent cell–cell repulsion was confined to cell borders where cells formed contacts with adjacent cells, meaning that there must be another signal required for cell–cell repulsion in addition to PYK2 localization at focal adhesions. What can the signalling mechanism be?
Ca2+ dependence of PYK2-dependent cell movements
Because PYK2 is activated by agonist stimulation, which increases the intracellular Ca2+ concentration ([Ca2+]i; Avraham et al, 2000; Gelman, 2003), Ca2+ signals might be the additional signalling mechanism required for cell–cell repulsion. We first studied the Ca2+ dependence of PYK2 function, by observing the morphological changes of cells expressing PYK2–GFP under a confocal laser-scanning microscope (supplementary information online). In the resting state, PYK2–GFP was distributed diffusely in the cytoplasm, and more strongly at punctate structures on the periphery of the cell, which probably corresponds to focal adhesions (supplementary Fig 1C online). As a result of an increase in [Ca2+]i after thapsigargin treatment, most of the punctate structures disassembled, inducing the retraction of cell borders and decreasing cell area (supplementary Fig 1C,D online). The retractive movement of cell borders was also observed in the fluorescence images of cells loaded with Fura-red, a fluorescent molecule (data not shown). To identify the molecular domain required for the Ca2+- and PYK2-dependent cellular retraction, deletion mutants of PYK2 were generated (supplementary Fig 1A,B online). PYK2ΔC–GFP, which does not localize at focal adhesions (Fig 2B), failed to induce the retraction of cell borders after an increase in [Ca2+]i (supplementary Fig 1C,D online). The GFP-tagged C-terminal region of PYK2 (GFP–PR-FAT) was localized at focal adhesions but did not lead to cell retraction (supplementary Fig 1C,D online). PYK2 is cleaved by Ca2+-activated protease (Raja et al, 1997), increasing the possibility that only the C-terminal fragment of PYK2 was localized at focal adhesions. Thus, we generated GFP–PYK2, in which GFP was tagged at the amino terminus of PYK2. However, GFP–PYK2 also localized at focal adhesions and induced lamellipodial retraction (supplementary Fig 1C,D online), indicating that the full-length PYK2 is localized at focal adhesions. These results indicate that the coincidence of an increase in [Ca2+]i and PYK2 localization at focal adhesions, by means of its C-terminal region, induces cellular retractive movements by focal adhesions disassembly.
Ca2+ lightning
The above results indicate that a Ca2+ signal is the potential link between cell–cell contact and cell–cell repulsion. Previous studies suggest that Ca2+ signals might be involved in the intracellular signalling of information between neighbouring cells artificially brought into direct contact (Ko et al, 2001), and that Ca2+ signals promote focal adhesion disassembly (Ridley et al, 2003; Giannone et al, 2004). Therefore, we searched for intracellular Ca2+ signals in the vicinity of the cell–ECM interface by using TIRF microscopy of cells loaded with a Ca2+ indicator, Fluo-4. Interestingly, we observed spontaneous local transient increases in [Ca2+]i, each of which lasted for several seconds (1–10 s; Fig 3A–D; supplementary Movie 6 online). When cells expressing GFP (without Fluo-4 loading) were imaged by the same TIRF microscopy, no such changes in fluorescence intensity were observed. This confirmed that fluctuations in cell–ECM distance did not contribute to the changes in fluorescence intensity of the Ca2+ indicator. Although the time resolution of the imaging (1 frame/s) did not allow us to locate precisely the initial point of increase, these Ca2+ transients were generated near cell–cell borders. Ca2+ signals might cross the cell borders through the gap junction (Dunlap et al, 1987). The present Ca2+ signals are usually observed unilaterally, although they are rarely observed simultaneously in neighbouring cells. This type of Ca2+ signal in the absence of agonist stimulation has not been previously reported, and the Ca2+ transient was dubbed ‘Ca2+ lightning' because it appeared like lightning seen through a thundercloud.
Figure 3.

Spontaneous transient Ca2+ signals (Ca2+ lightning) near cell–cell borders. Images near cell borders obtained by surface reflection interference contrast microscopy (A) and total internal reflection fluorescence microscopy of Fluo-4 (B). (C) TIRF images (F/F0). Cell–cell borders are indicated by dotted lines. (D) Fluorescence intensity change of Fluo-4 in boxes 1–5 shown in (C). (E) Number of Ca2+ lightning events observed at various regions of cells (wild-type cells and cells expressing cyan fluorescent protein (CFP)-tagged PYK2) within 5 min. Values represent mean±s.e.m. (n=4). Green fluorescent protein (GFP) tag was replaced with CFP tag because GFP fluorescence interferes with fluo-4 Ca2+ imaging. (F) Number of Ca2+ lightning events at cell borders in the absence and presence of 20 μM Cd2+. Values represent mean±s.e.m. (n=8). Scale bar, 10 μm (A–C). SRIC, surface reflection interference contrast; TIRF, total internal reflection fluorescence.
We then analysed the subcellular location of Ca2+ lightning. Ca2+ lightning was observed around cell–cell borders, but was rarely observed in central regions away from cell borders (Fig 3E; supplementary Fig 2 online). Furthermore, Ca2+ lightning was not observed at the outer borders of clusters of cells or in solitary cells that formed no contact with other cells (Fig 3E). These features were also observed in cells expressing PYK2–cyan fluorescent protein (Fig 3E). Thus, the locations of Ca2+ lightning were essentially confined to the vicinity of cell–cell contacts and corresponded to those of PYK2-dependent cell retraction. Although we examined the Ca2+ source of Ca2+ lightning, it was not possible to test the effects of removal of extracellular Ca2+ or depletion of the intracellular Ca2+ store by thapsigargin, because such treatments compromised cell viability. Neither nicardipine (10 μM), a voltage-dependent Ca2+ channel inhibitor, nor 2-aminoethoxydiphenyl borate (30 μM), which inhibits both inositol 1,4,5-trisphosphate receptor and store-operated Ca2+ channels (Bootman et al, 2002), inhibited Ca2+ lightning. However, 20 μM Cd2+, a nonselective plasma membrane Ca2+ channel inhibitor, completely blocked Ca2+ lightning (Fig 3F). These results indicate that Ca2+ lightning depends on Ca2+ influx through Ca2+ channels in the plasma membrane that is in contact with neighbouring cells.
Role of Ca2+ lightning in cell–cell repulsion
The colocalization of Ca2+ lightning and PYK2-dependent cell retraction suggests that these events are causally related. We further tested the possible role of Ca2+ lightning in PYK2-dependent cell–cell repulsion, by inhibiting Ca2+ lightning using Cd2+. Although PYK2–GFP cells in the standard medium formed contacts with neighbouring cells only through narrow protrusions, they formed stable and broad contacts with the neighbouring cells through lamellipodia in the presence of Cd2+ (Fig 4A,B; supplementary Movie 7 online). To test whether the cell–cell contact induced by the inhibition of Ca2+ lightning is a type of conventional adhesion mediated by molecules such as cadherins, the cellular localization of cadherins was examined. In PYK2 cells, cadherins showed a diffuse distribution, indicating the absence of cell–cell adhesion (Fig 4C). However, cadherins became localized at the cell–cell borders of PYK2 cells in the presence of Cd2+ (Fig 4C,D). These results indicate that Ca2+ lightning promotes PYK2-dependent cell–cell repulsion and suppresses cell–cell adhesion.
Figure 4.

Cell–cell repulsion regulated by Ca2+ lightning and PYK2. (A) Confocal images of PYK2–green fluorescent protein (GFP) cells before and 3 h after addition of 20 μM Cd2+ (right panels). The left panels show control experiment without Cd2+ application. Note that PYK2–GFP cells formed broad contacts with the neighbouring cells in the presence of Cd2+. (B) Number of cell–cell contacts through narrow (⩽10 μm) protrusions or broad (>10 μm) lamellipodia in PYK2 cells before and after application of Cd2+ (20 μM, 3 h). (C) Localization of PYK2–GFP (green) and N-cadherin (red) in the presence and absence of Cd2+. (D) Quantitative analysis of cadherin localization (see Methods). Scale bars: 50 μm (A); 20 μm (C). Representative results of three replicate experiments are shown.
Role of endogenous PYK2 in cell–cell repulsion
We examined whether endogenous PYK2 is involved in cell–cell repulsion. Although wild-type cells are rather quiescent on day 4 or more of culture (Fig 2A), they make more pronounced movements on day 2 in subconfluent cultures. Therefore, we examined the effect of PYK2 short interfering RNA (siRNA) when cell–cell repulsion is enhanced in subconfluent cells. Wild-type cells were transfected with siRNA that had been successfully used previously (Yano et al, 2004). The cells were re-plated and cell–cell contact duration was observed by using SRIC microscopy on day 2 of culture. The siRNA-treated cells made contact with adjacent cells for a significantly longer duration than cells transfected with control RNA, whereas the frequency of Ca2+ lightning was not altered (supplementary Fig 3 online). Thus, endogenous PYK2 appears to have a role in cell–cell repulsion during cell growth.
We then studied the relationship between Ca2+ lightning and cell–cell repulsion in wild-type subconfluent cells. When cells were loaded with 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), an intracellular Ca2+ buffer, Ca2+ lightning was abolished (in ten out of ten cells) and cell border movements were inhibited (0.05±0.78 μm/10 min, n=6). We then analysed the temporal correlation between Ca2+ lightning and cell retraction. Cells generating Ca2+ lightning within a 10-min observation period made significantly greater cell retraction than cells not generating Ca2+ lightning (supplementary Fig 4 online).
PYK2 is tyrosine phosphorylated during activation. Tyrosine 580 is phosphorylated in a Ca2+-dependent manner (Wu et al, 2006). We therefore studied the effect of Cd2+-mediated inhibition of Ca2+ lightning on the level of phosphorylation of PYK2 in wild-type cells using anti-phosphotyrosine. As shown in the supplementary Fig 5 online, Cd2+ treatment reduced PYK2 phosphorylation at tyrosine 580.
The present results collectively show that a novel signalling pathway comprising cell–cell contact, Ca2+ lightning, focal adhesion disassembly and cell retraction regulates cell–cell repulsion. Focal adhesion disassembly mediates cell migration at the rear part of the cell (Geiger et al, 2001; Ridley et al, 2003). The present results provide a novel function of focal adhesion disassembly in cell–cell repulsion. The PYK2-dependent cell–cell repulsion is most pronounced during cell growth and becomes less obvious after cells become confluent. Ca2+ lightning is generated near cell–cell contact regions and requires Ca2+ influx. Therefore, it is possible that the cell–cell contact-dependent mechanical deflection of the plasma membrane or the subsequent changes in the cytoskeleton regulates the opening of Ca2+ channels in the plasma membrane. The identification of the molecular mechanism of Ca2+ lightning will facilitate the understanding of the generation of cell–cell repulsion signals.
Methods
Cell culture and transgene expression. HeLa cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (JRH, Lenexa, KS, USA) at 37°C and 5% CO2. For imaging, cells were cultured in a glass-bottom dish (Mattek, Ashland, MA, USA) coated with 0.1% polyethyleneimine (Sigma, St Louis, MO, USA) and 0.001% collagen (IFP, Yamagata, Japan). PYK2 complementary DNA was obtained from the rat brain cDNA library (Marathon-Ready cDNA, Clontech, Palo Alto, CA, USA) by PCR and confirmed by sequencing. PYK2 constructs (full-length, 1–3,030 bp; ΔC, 1–2,583 bp; PR-FAT, 2,038–3,030 bp) fused to the GFP gene of pEGFP-1 (Clontech) were introduced to the retrovirus system as described previously (Miyakawa et al, 2001).
Immunocytochemistry. Cells were fixed with 3.7% formaldehyde in PBS for 30 min and permeabilized with 0.1% Triton X-100 in a blocking solution, containing 1% BSA in PBS, for 30 min at room temperature (24°C). Samples were incubated with a primary N-cadherin antibody (BD Transduction, San Jose, CA, USA) and then with a secondary anti-mouse IgG antibody conjugated to Alexa Fluor 546 (Molecular Probes, Eugene, OR, USA) for 1 h at room temperature (24°C) in the blocking solution and observed under a confocal laser-scanning microscope (IX70, FV300, Olympus, Tokyo, Japan).
SRIC and TIRF microscopies. Imaging was carried out using an inverted microscope (TE2000S, Nikon, Tokyo, Japan) equipped with a cooled charge-coupled device camera (ORCA-ER, Hamamatsu Photonics, Hamamatsu, Japan), a light source (Polychrome IV, TILL Photonics, Planegg, Germany), a TIRF laser (Spectra-Physics, Mountain View, CA, USA) and an objective lens (PlanApo NA 1.40 100 × /IX-NPS, Olympus, or CFI Plan Apo TIRF 60 × H NA 1.45, Nikon). Cells were maintained under cell culture conditions (37°C, 5% CO2/95% air) during imaging using a stage chamber and a stage-and-objective-lens heating system (MI-IBC, Olympus). The microscope houses two dichroic mirrors in tandem, BP520-550/DM400 and DM505/BA520 (Nikon) in the upper and lower layers, respectively. For the imaging of Ca2+ lightning, cells were loaded with 5 μM Fluo-4AM (Molecular Probes) in DMEM containing 2.5 mM probenecid (Sigma) for 45 min at 37°C/5% CO2 with or without 20 μM Cd2+, and imaged at 37°C/5% CO2 at 1 s intervals. Transient increases in the fluorescence intensity of Fluo-4 with the peak size exceeding 0.1F0 were considered Ca2+ events. The time-averaged fluorescence intensity is defined as F0 at each pixel.
Image analyses. Obtained images were quantitatively analysed using IPLab software (Scanalytics, Fairfax, VA, USA) or NIH ImageJ (http://rsb.info.nih.gov/ij/). The magnitude of cell motility (Fig 2D) was estimated on the basis of the cumulative area of the trajectory of the cell edge. This was determined as follows. Contour lines of a cell were drawn at the beginning and end of 60-min SRIC observation. Then, the area surrounded by these lines was determined. Cell-area was estimated from the binarized fluorescence images of cells. Cadherin localization was assessed using confocal immunocytochemical images, and the cadherin signal was considered present when it was observed >10 μm along cell–cell borders.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Information and Figures
Supplementary Movie 1
Supplementary Movie 2
Supplementary Movie 3
Supplementary Movie 4
Supplementary Movie 5
Supplementary Movie 6
Supplementary Movie 7
Acknowledgments
This work was supported by Grants in Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- Avraham H, Park SY, Schinkmann K, Avraham S (2000) RAFTK/Pyk2-mediated cellular signalling. Cell Signal 12: 123–133 [DOI] [PubMed] [Google Scholar]
- Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM (2002) 2-Aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J 16: 1145–1150 [DOI] [PubMed] [Google Scholar]
- Cavallaro U, Christofori G (2004) Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer 4: 118–132 [DOI] [PubMed] [Google Scholar]
- Dunlap K, Takeda K, Brehm P (1987) Activation of a calcium-dependent photoprotein by chemical signalling through gap junctions. Nature 325: 60–62 [DOI] [PubMed] [Google Scholar]
- Fagotto F, Gumbiner BM (1996) Cell contact-dependent signaling. Dev Biol 180: 445–454 [DOI] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A, Pankov R, Yamada KM (2001) Transmembrane crosstalk between the extracellular matrix–cytoskeleton crosstalk. Nat Rev Mol Cell Biol 2: 793–805 [DOI] [PubMed] [Google Scholar]
- Gelman IH (2003) Pyk 2 FAKs, any two FAKs. Cell Biol Int 27: 507–510 [DOI] [PubMed] [Google Scholar]
- Giannone G, Ronde P, Gaire M, Beaudouin J, Haiech J, Ellenberg J, Takeda K (2004) Calcium rises locally trigger focal adhesion disassembly and enhance residency of focal adhesion kinase at focal adhesions. J Biol Chem 279: 28715–28723 [DOI] [PubMed] [Google Scholar]
- Guinamard R, Okigaki M, Schlessinger J, Ravetch JV (2000) Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat Immunol 1: 31–36 [DOI] [PubMed] [Google Scholar]
- Hattori M, Osterfield M, Flanagan JG (2000) Regulated cleavage of a contact-mediated axon repellent. Science 289: 1360–1365 [DOI] [PubMed] [Google Scholar]
- Ivankovic-Dikic I, Gronroos E, Blaukat A, Barth BU, Dikic I (2000) Pyk2 and FAK regulate neurite outgrowth induced by growth factors and integrins. Nat Cell Biol 2: 574–581 [DOI] [PubMed] [Google Scholar]
- Izzard CS, Lochner LR (1976) Cell-to-substrate contacts in living fibroblasts: an interference reflexion study with an evaluation of the technique. J Cell Sci 21: 129–159 [DOI] [PubMed] [Google Scholar]
- Ko KS, Arora PD, Bhide V, Chen A, McCulloch CA (2001) Cell–cell adhesion in human fibroblasts requires calcium signaling. J Cell Sci 114: 1155–1167 [DOI] [PubMed] [Google Scholar]
- Litvak V, Tian D, Shaul YD, Lev S (2000) Targeting of PYK2 to focal adhesions as a cellular mechanism for convergence between integrins and G protein-coupled receptor signaling cascades. J Biol Chem 275: 32736–32746 [DOI] [PubMed] [Google Scholar]
- Marston DJ, Dickinson S, Nobes CD (2003) Rac-dependent trans-endocytosis of ephrinBs regulates Eph–ephrin contact repulsion. Nat Cell Biol 5: 879–888 [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Mizushima A, Hirose K, Yamazawa T, Bezprozvanny I, Kurosaki T, Iino M (2001) Ca2+-sensor region of IP3 receptor controls intracellular Ca2+ signaling. EMBO J 20: 1674–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okigaki M, Davis C, Falasca M, Harroch S, Felsenfeld DP, Sheetz MP, Schlessinger J (2003) Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proc Natl Acad Sci USA 100: 10740–10745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja S, Avraham S, Avraham H (1997) Tyrosine phosphorylation of the novel protein-tyrosine kinase RAFTK during an early phase of platelet activation by an integrin glycoprotein IIb–IIIa-independent mechanism. J Biol Chem 272: 10941–10947 [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR (2003) Cell migration: integrating signals from front to back. Science 302: 1704–1709 [DOI] [PubMed] [Google Scholar]
- Schaller MD (2004) FAK and paxillin: regulators of N-cadherin adhesion and inhibitors of cell migration? J Cell Biol 166: 157–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieg DJ, Ilic D, Jones KC, Damsky CH, Hunter T, Schlaepfer DD (1998) Pyk2 and Src-family protein-tyrosine kinases compensate for the loss of FAK in fibronectin-stimulated signaling events but Pyk2 does not fully function to enhance FAK−cell migration. EMBO J 17: 5933–5947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M (1993) Cadherins in cancer: implications for invasion and metastasis. Curr Opin Cell Biol 5: 806–811 [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS (1996) The molecular biology of axon guidance. Science 274: 1123–1133 [DOI] [PubMed] [Google Scholar]
- Wehrle-Haller B, Imhof B (2002) The inner lives of focal adhesions. Trends Cell Biol 12: 382–389 [DOI] [PubMed] [Google Scholar]
- Wilkinson DG (2003) How attraction turns to repulsion. Nat Cell Biol 5: 851–853 [DOI] [PubMed] [Google Scholar]
- Wu SS, Jacamo RO, Vong SK, Rozengurt E (2006) Differential regulation of Pyk2 phosphorylation at Tyr-402 and Tyr-580 in intestinal epithelial cells: roles of calcium, Src, Rho kinase, and the cytoskeleton. Cell Signal, (in press) [DOI] [PubMed] [Google Scholar]
- Yano H, Mazaki Y, Kurokawa K, Hanks SK, Matsuda M, Sabe H (2004) Roles played by a subset of integrin signaling molecules in cadherin-based cell–cell adhesion. J Cell Biol 166: 283–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer M, Palmer A, Kohler J, Klein R (2003) EphB–ephrinB bi-directional endocytosis terminates adhesion allowing contact mediated repulsion. Nat Cell Biol 5: 869–878 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information and Figures
Supplementary Movie 1
Supplementary Movie 2
Supplementary Movie 3
Supplementary Movie 4
Supplementary Movie 5
Supplementary Movie 6
Supplementary Movie 7


