Abstract
The nervous system receives a large amount of information about the environment through elaborate sensory routes. Processing and integration of these wide-ranging inputs often results in long-term behavioural alterations as a result of past experiences. These relatively permanent changes in behaviour are manifestations of the capacity of the nervous system for learning and memory. At the cellular level, synaptic plasticity is one of the mechanisms underlying this process. Repeated neural activity generates physiological changes in the nervous system that ultimately modulate neuronal communication through synaptic transmission. Recent studies implicate both presynaptic and postsynaptic ion channels in the process of synapse strength modulation. Here, we review the role of synaptic ion channels in learning and memory, and discuss the implications and significance of these findings towards deciphering the molecular biology of learning and memory.
Keywords: acetylcholine, GABA, long-term potentiation, neurotransmitter, NMDA, synaptic transmission
Introduction
Information storage and retrieval by the nervous system involves physical alterations in the neuronal substrate that modulate neuron activity and communication. These alterations can take many forms; for example, structural modifications include the re-wiring of neuronal networks, which can involve synapses being formed between previously unconnected neurons and existing connections being strengthened by the addition of new synapses. Moreover, information processing induces elaborate changes in the function of individual neurons, such as the adjustment of neuronal excitability and the regulation of synaptic strength (Nicoll & Schmitz, 2005; Zucker & Regehr, 2002).
Synaptic strength, or synaptic weight, is a measure of synapse efficacy in relaying a signal from the presynaptic to the postsynaptic neuron. More than 50 years ago, Donald Hebb postulated that modulation of synaptic weight underlies learning and memory phenomena (reviewed in Turrigiano & Nelson, 2000). His model, also known as the ‘Hebb synapse', suggests that synapses between neurons are strengthened by concerted pre- and postsynaptic neuronal activity. Conversely, synapses are weakened by non-coincidental neuronal firing. An integral component of this model is synaptic plasticity, which is the capacity of a synapse to adapt to overall neuronal activity. Indeed, a wealth of experimental data supports the idea that synaptic transmission can be potentiated or depressed, depending on neuronal stimulation. Long-term potentiation and depression (LTP and LTD, respectively) have been associated with learning and memory models in many organisms (Bear & Malenka, 1994; Siegelbaum & Kandel, 1991; Xu & Kang, 2005). But how is synapse reinforcement or attenuation achieved? Many molecular mechanisms have been implicated in the regulation of synaptic transmission (Debanne et al, 2003; Frick & Johnston, 2005; Magee & Johnston, 2005; Malenka & Bear, 2004). Several recent, elegant studies have shown that specific ion channels, localized at synapses, facilitate synaptic plasticity phenomena (Table 1). In this review, we aim to provide an entry point for further exploration of the vast literature on the mechanisms underlying learning and memory, focusing on the involvement of synaptic ion channels in shaping synaptic communication and plasticity.
Table 1.
Synaptic ion channels modulating learning and memory. Channel properties that are relevant to synaptic plasticity phenomena are listed
| Ion channel | Ligand, activator | Conducted ion | Relevant expression | Synaptic localization | Reference |
|---|---|---|---|---|---|
| NMDA receptor | L-Glutamate, NMDA | Na+,Ca2+ | Hippocampus | Presynaptic, postsynaptic | Daw et al, 1993; Humeau et al, 2003 |
| AMPA receptor | L-Glutamate, AMPA | Na+,K+ | Hippocampus, cerebellum | Presynaptic, postsynaptic | Lee et al, 2003; Lu et al, 2001 |
| Kainate receptor | L-Glutamate, kainate | Na+,Ca2+,K+ | Hippocampus, thalamocortical system | Presynaptic, postsynaptic | Lauri et al, 2003 |
| nAch receptor | Acetylcholine, nicotine | Na+,K+,Ca2+ | Hippocampus | Presynaptic, postsynaptic | Ji et al, 2001 |
| GABA receptor | γ-aminobutyric acid | Cl− | Hippocampus | Postsynaptic | Collinson et al, 2002 |
| L-type VGCC | Membrane potential | Ca2+ | Amygdala, hippocampus | Presynaptic, postsynaptic | Shinnick-Gallagher et al, 2003 |
| P/Q-type VGCC | Membrane potential | Ca2+ | Hippocampus, Purkinje cells | Presynaptic | Wu et al, 1999 |
| R-type VGCC | Membrane potential | Ca2+ | Hippocampus | Presynaptic | Wu et al, 1998 |
| BK channel | Ca2+ | K+ | Hippocampus, Purkinje cells | Presynaptic | Raffaelli et al, 2004; Sausbier et al, 2004 |
| SK channel | Ca2+ | K+ | Amygdala, hippocampus | Postsynaptic | Hammond et al, 2006 |
AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate; BK, large conductance calcium-gated potassium channel; GABA, γ-aminobutyric acid; LTP, long-term potentiation; nAChR, nicotinic acetylcholine receptor; NMDA, N-methyl-D-aspartate; SK, small conductance calcium-gated potassium channel; VGCC, voltage-gated calcium channel.
Neurotransmitter receptor ion channels
According to Hebb's conjecture, synaptic plasticity is manifested by experience-induced modifications in synaptic transmission (Cline, 2003). The Hebbian learning rule, formulated on the basis of this inference, dictates that such synaptic modifications depend on the excitation states of both the pre- and postsynaptic neuron. How is this type of learning implemented at the synapse? Numerous studies in both vertebrates and invertebrates have revealed that receptors for neurotransmitters have key roles in this process (Luscher et al, 2000). Neurotransmitter receptors can be classified into two categories: G-protein-coupled (metabotropic) receptors and ionotropic receptors. The binding of neurotransmitters to G-protein-coupled receptors triggers a series of signal transduction cascades at postsynaptic neurons. Ionotropic receptors are specialized synaptic ion channels, which are gated by the binding of specific neurotransmitters. The influx of ions that occurs on activation of ionotropic receptors alters the polarization state of the postsynaptic neuron, and action potentials are fired if a depolarization threshold is exceeded.
Ionotropic glutamate receptors. L-glutamate is the main excitatory neurotransmitter in the mammalian nervous system and is detected at postsynaptic terminals by both G-protein-coupled and ionotropic glutamate receptors (GluRs). GluRs are subdivided into three main types: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), N-methyl-D-aspartic acid (NMDA), and kainate. All three bind glutamate with high affinity and have varying preferences for other glutamate agonists, including AMPA, NMDA and kainate (Erreger et al, 2004). AMPA receptors are tetrameric ion channels that principally conduct sodium and potassium ions, although, depending on their subunit composition, they can also be permeable to calcium (Gouaux, 2004). NMDA receptors are tetrameric non-selective cation channels that, in addition to ligand gating, show voltage dependence (Dingledine et al, 1999). NMDA receptor opening requires both glutamate binding and the relief of a magnesium ion (Mg2+) block, which involves depolarization of the postsynaptic membrane. Kainate receptors are pentameric ion channels that are mostly permeable to sodium and potassium, with a low conductivity for calcium (Ferrer-Montiel & Montal, 1996).
An overwhelming number of studies implicate all three GluR types in learning and memory (Fig 1; Bliss & Collingridge, 1993). Furthermore, GluRs have been associated with various types of memory including long- and short-term memory, memory consolidation, spatial memory, episodic memory and contextual fear memory (Tsien et al, 1996; Zhao et al, 2005). AMPA and NMDA receptors synergize at postsynaptic terminals to facilitate various forms of synaptic plasticity. Sustained activation of AMPA receptors by a series of impulses arriving at presynaptic terminals initiates depolarization of the postsynaptic membrane and removes the Mg2+ ions that are obstructing NMDA receptors (Daw et al, 1993). Therefore, in agreement with the Hebb hypothesis, simultaneous excitation of pre- and postsynaptic neurons facilitates gating of NMDA channels and strengthens the synapse. An important feature of the NMDA channel, which is particularly relevant to synaptic plasticity, is its high permeability to calcium; in turn, calcium, which is a central messenger molecule, orchestrates a battery of signalling pathways and responses that collectively elicit synaptic modification.
Figure 1.

Glutamate receptors and synaptic plasticity. The arrival of a series of impulses at the presynaptic terminal triggers the release of glutamate, which binds to glutamate receptors at the postsynaptic membrane. On activation, AMPA and kainate receptors conduct sodium ions, which initiate postsynaptic depolarization. Membrane potential changes initiate the release of magnesium ions that block NMDA receptors. Calcium influx through NMDA channels sets off a chain of events that establish long-term potentiation. Kainate receptors at the presynaptic end also seem to facilitate synaptic transmission at specific synapses by augmenting neurotransmitter release. AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate; CaMKII, calcium/calmodulin-dependent kinase II; CREB, cAMP response element binding protein; MAPK, mitogen-activated protein kinase; NMDA, N-methyl-D-aspartate; PKA, protein kinase A; PKC, protein kinase C.
NMDA receptors participate in both the induction and the maintenance of LTP at postsynaptic hippocampal neurons. The induction of LTP involves opening NMDA channels to calcium through the removal of the Mg2+ block, whereas LTP maintenance is achieved by subsequent calcium-induced synapse alterations. One such modification engages AMPA receptor gating and transport. Signalling through the AMPA receptor is dynamically modulated by two principal mechanisms: direct phosphorylation of receptor subunits, and changes in the density of receptors at the postsynaptic membrane. During LTP in hippocampal pyramidal neurons, many kinases become activated including the calcium/calmodulin-dependent kinase II (CaMKII) and protein kinase A. These kinases respond to calcium transients and mediate the induction of enhanced synaptic transmission by phosphorylating specific AMPA receptor subunits (Lee et al, 2003). Phosphorylation increases the open probability of the receptor in LTP, whereas dephosphorylation is induced during LTD. AMPA receptor transport to and from the synapse is also important for synaptic plasticity. The concentration of AMPA receptors at the synapse increases after the induction of LTP, whereas it drops during LTD (Lu et al, 2001). Insertion of AMPA receptors at the postsynaptic membrane and their turnover is regulated through the phosphorylation of specific receptor subunits triggered by synaptic calcium fluctuations (Malenka, 2003; Malinow & Malenka, 2002).
This common theme has emerged by dissecting the involvement of NMDA receptors in synaptic plasticity, but in reality the situation is far more complex with many forms of LTP induced by diverse inputs in different neurons. One intriguing case is that of activity-dependent synaptic plasticity, stimulated by presynaptic NMDA channels. In the lateral nucleus of the amygdala, neuronal activity induces LTP, which requires NMDA receptors but is independent of postsynaptic activity (Humeau et al, 2003). These observations suggest that NMDA function, which is essential for learning and memory, is not limited to postsynaptic terminals.
Synaptic plasticity in the hippocampus does not always depend on NMDA receptors. The synapses between mossy fibres of the hippocampal dendate gyrus and CA3 neurons show a distinctive morphology with many sites for glutamate release, each of which has a corresponding postsynaptic bundle of glutamate receptors. At this synapse, LTP is NMDA-receptor-independent and requires calcium influx through presynaptic kainate receptors (Lauri et al, 2003). Although the mechanism responsible for this type of synaptic plasticity is not clear, it is likely that presynaptic kainate receptors amplify neurotransmitter release from presynaptic terminals through a positive feedback loop.
Nicotinic acetylcholine receptors. Neuronal nicotinic acetylcholine receptors (nAChRs) are pentameric ion channels gated by the neurotransmitter and the alkaloid acetylcholine agonist nicotine. nAChRs are mainly permeable to sodium and potassium, with much less conductance to calcium, and are located on hippocampal pyramidal neurons as well as interneurons (Hogg et al, 2003). Several studies have shown that nAChRs are important for learning and memory in humans and animal models. Blockage of nAChRs in the hippocampus of rats results in significant memory deficits, whereas nAChR agonists including nicotine improve certain types of memory, such as short-term and working memory, in humans (Ji et al, 2001; Levin et al, 2002; Seeger et al, 2004).
The molecular mechanisms underlying the effects of nAChR on learning and memory are not fully understood. nAChR currents are likely to take part in postsynaptic calcium signalling either directly through their calcium component or indirectly by contributing to postsynaptic depolarization. Notably, unlike NMDA receptors, nAChRs are not blocked by Mg2+ at negative resting potentials; therefore, postsynaptic nAChR activity might assist the removal of the Mg2+ block from NMDA receptors and facilitate LTP (Ji et al, 2001). The function of nAChRs at presynaptic terminals also seems to be important for synaptic plasticity by enhancing neurotransmitter release (Vizi & Lendvai, 1999). Activation of nAChRs by nicotine on presynaptic terminals in the ventral tegmental area enhances glutamatergic inputs to dopaminergic neurons. Thus, LTP of the excitatory synapses is induced by activation of NMDA receptors. Both the short- and long-term effects of nicotine require the activation of presynaptic nAChRs (Mansvelder & McGehee, 2000). Therefore, nicotine addiction might involve altered synaptic function in brain reward areas through mechanisms that are associated with learning and memory.
GABA-A receptors. Hippocampal synapses, which use the neurotransmitter γ-aminobutyric acid (GABA), are plastic (Lamsa et al, 2005). GABA is a general inhibitory neurotransmitter, which is sensed at GABAergic synapses by ionotropic (GABA-A) and metabotropic (GABA-B) receptors. GABA-A ion channels consist of five subunits forming a central pore that is permeable to negatively charged chloride ions (Cl−; Baumann et al, 2001). Cl− influx through GABA-A receptors hyperpolarizes mature postsynaptic neurons expressing appropriate Cl− transporters and inhibits synaptic transmission. These inhibitory postsynaptic currents are subject to both LTP and LTD in rat cerebellar neurons (Ouardouz & Sastry, 2006). Interestingly, a specific subtype of GABA-A receptors containing the α5-subunit localizes primarily in the CA1–CA3 fields of the hippocampus (Collinson et al, 2002). Mice lacking this subunit show increased spatial learning capacity together with attenuated inhibitory postsynaptic currents, suggesting that GABA-A activity in the hippocampus modulates synaptic plasticity.
Repeated exposure of rats to cocaine reduces the amplitude of GABA-A-mediated synaptic currents and increases the probability of spike initiation in dopaminergic neurons of the ventral tegmental area (Liu et al, 2005). In turn, dopamine neurons of the ventral tegmental area become highly susceptible to the induction of LTP by correlated pre- and postsynaptic activity. This cocaine-induced enhancement of synaptic plasticity might be important for the formation of drug-associated memories. In addition, GABA-A-mediated inhibition of dorsolateral prefrontal cortex neurons in monkeys seems to be important for the processes underlying spatial working memory (Rao et al, 2000).
Voltage-gated calcium channels
Voltage-gated calcium channels (VGCCs) are a diverse group of heteromultimeric ion channels that respond to changes in membrane potential. VGCCs are further classified into six classes—L-, N-, P-, Q-, R- and T-type—according to their sensitivity to pharmacological blocks, single-channel conductance kinetics and voltage dependence (Reuter, 1996). T-type channels have a low-voltage threshold for activation, whereas L-, N-, P-, Q- and R-type are high-voltage threshold-activated channels. VGCCs have key roles in signal transduction between neurons and several studies implicate specific types in various forms of synaptic plasticity (Fig 2).
Figure 2.
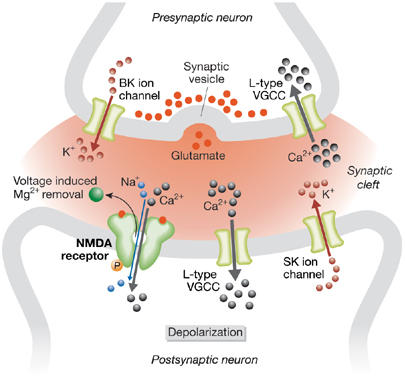
Contribution of voltage-gated calcium channels and potassium channels to synaptic plasticity. Voltage-gated calcium channels may function at both pre- and postsynaptic terminals to allow calcium influx that either augments neurotransmitter release at the presynaptic terminal or contributes to postsynaptic neuron depolarization. Excessive calcium influx at postsynaptic termini might impair long-term potentiation. Large-conductance calcium-activated potassium channels at presynaptic terminals and small-conductance calcium-activated potassium channels at postsynaptic terminals quench membrane depolarization by allowing potassium efflux, which facilitates neuron repolarization. BK, large-conductance calcium-activated potassium channel; NMDA, N-methyl D-aspartate; SK, small-conductance calcium-activated potassium channel; VGCC, voltage-gated calcium channel.
L-type ion channels are required for LTP at synapses between mossy fibres and CA3 pyramidal neurons. This type of LTP does not depend on NMDA receptors and involves postsynaptic calcium influx carried by L-type VGCCs (Kapur et al, 1998). Studies of synaptic transmission between the basolateral amygdala and the hippocampal dentate gyrus have established a similar role for L-type calcium channels in the induction of LTP (Niikura et al, 2004). Consistent with these observations, the administration of nimodipine—an L-type calcium channel antagonist—abolishes LTP and impairs fear conditioning in mice (Shinnick-Gallagher et al, 2003). Interestingly, calcium currents through L-type VGCCs in CA1 neurons of the hippocampus increase during ageing in rats, which is accompanied by a marked decline in learning and memory faculties. Chronic nimodipine treatment ameliorates memory loss, suggesting that excessive calcium influx through L-type calcium channels impairs learning and memory (Veng et al, 2003). It is intriguing that patients with Alzheimer's disease show higher L-type VGCC expression in the hippocampus compared with healthy individuals (Coon et al, 1999). This link suggests that aberrant L-type calcium channel levels might contribute to memory shortcomings in Alzheimer's patients.
Other VGCCs implicated in synaptic plasticity include N-, P-, Q- and R-type calcium channels (Wu et al, 1999). These channels influence neurotransmitter release in the central nervous system. P- and Q-type calcium channels are more effective than N- or R- type channels at triggering neurotransmitter release, probably because the latter are localized further from release sites (Wu et al, 1999). Therefore, modulation of P- and Q-type calcium channel conductance provides a mechanism for fine-tuning synaptic transmitter release. Nevertheless, R-type channels seem to carry almost one-third of the total calcium current at presynaptic terminals during a presynaptic action potential (Wu et al, 1998). Such an influx of calcium is an important component of certain forms of synaptic plasticity; for example, the induction of LTP at synapses between mossy fibres and CA3 hippocampal neurons requires a rise in presynaptic calcium but is independent of postsynaptic calcium currents. R-type calcium channel activity is involved in the induction of LTP in these synapses but is not required for fast neurotransmitter release induced by single action potentials (Dietrich et al, 2003).
Potassium channels
Potassium channels are the most diverse group of ion channels. Specific potassium channels, gated by intracellular calcium elevation, have been associated with synaptic plasticity (Fig 2).
Small-conductance calcium-activated potassium channels (SKs) are widely distributed in the nervous system and are involved in shaping neuronal responses to synaptic stimulation (Bond et al, 2005). In hippocampal CA1 neurons, SKs contribute to the after hyperpolarization and modulate neuronal excitability. By allowing potassium efflux after their activation, SKs have the capacity to quench postsynaptic potentials (Faber et al, 2005). In turn, repolarization of the postsynaptic membrane favours NMDA receptor obstruction by Mg2+ ions, which limits further calcium influx. Thus, SKs are part of a negative feedback loop that attenuates synaptic transmission (Ngo-Anh et al, 2005). Indeed, blockage of SKs enhances LTP in the hippocampus and the lateral amygdala, whereas SK overexpression diminishes LTP and impairs learning behaviours such as spatial learning and fear conditioning (Hammond et al, 2006).
Large-conductance, calcium-activated potassium channels (BKs) also influence synaptic plasticity. These channels are regulated by both calcium and voltage and are localized at presynaptic terminals throughout the nervous system (Hu et al, 2001). Inactivation of BKs increases the probability of neurotransmitter release at synapses between CA3 neurons of the hippocampus (Raffaelli et al, 2004). Similar observations suggest that BKs control synaptic transmission by shunting presynaptic potentials generated by calcium influx, which is required for neurotransmitter release. Mice lacking BKs are abnormal in their conditioned eye-blink reflex and in their motor coordination during locomotion, which indicate cerebellar learning deficiency (Sausbier et al, 2004). Cerebellar Purkinje neurons from these animals show reduced spontaneous discharge activity owing to depolarization-induced inactivation of the action-potential mechanism. Similar effects on learning behaviours have been observed in Drosophila, in which mutations in BKs slow habituation (Engel & Wu, 1998). Thus, BKs might provide negative feedback that moderates signalling through the synapse at the presynaptic side, under conditions of excessive depolarization and accumulation of intracellular calcium.
Concluding remarks and outlook
Considerable progress towards deciphering the molecular mechanisms of neuronal plasticity has been accomplished in recent years. The modulation of synaptic communication has emerged as a major mechanism underlying information storage and retrieval by the nervous system. Synaptic ion channels have a fundamental role in this process by facilitating and fine-tuning synaptic transmission. However, in spite of these exciting advances, many challenges still remain. We do not fully understand how synaptic ion channels are regulated to influence neuronal communication. Although many signalling pathways that control synaptic ion channel conductance are known, it is not clear how information processing by clusters of neurons engages these pathways to accomplish memory storage and retrieval. Even less clear are the mechanisms that support the maintenance of intricate memory traces in neuronal networks. The processes responsible for the time-dependent decay of memory traces are also poorly understood.
Non-synaptic forms of neuronal plasticity impose an additional layer of complexity. For example, in spontaneously firing neurons in the medial vestibular nucleus, CaMKII positively regulates calcium-activated potassium channels to maintain low levels of intrinsic excitability (Nelson et al, 2005). These neurons have a key role in motor learning in the vestibulo-ocular reflex and have capacity for firing rate potentiation (FRP), a form of plasticity in which synaptic inhibition triggers long-lasting increases in intrinsic excitability. In this system, CaMKII acts as a molecular switch, converting brief changes in synaptic activity or calcium influx into long-term changes in non-synaptic ion channel activity (Lisman et al, 2002). A decrease in CaMKII activity is both necessary and sufficient to induce FRP (Nelson et al, 2005).
Specific, non-synaptic voltage-gated potassium (Kv) channels are important for controlling neuron membrane electrical excitability and are localized to axons, somata and dendrites. During the early phase of LTP at postsynaptic terminals of CA1 hippocampal neurons, calcium entering through AMPA and NMDA receptors activates CaMKII, which phosphorylates Kv channels and increases neuronal excitability (Sweatt, 2001). Similarly, specific mitogen-activated protein kinases and protein kinase A are stimulated by elevated levels of cAMP as a result of calcium entry and subsequent activation of adenylyl cyclase-1, and also seem to phosphorylate Kv channels (Morozov et al, 2003). Deletion of the auxiliary Kvβ1.1 subunit in CA1 hippocampal neurons results in increased neuronal excitability and improved LTP induction in aged mice. These mice consistently performed better than wild-type mice in learning and memory tests (Murphy et al, 2004).
Hyperpolarization-activated, cyclic nucleotide-gated (HCN, Ih) ion channels are voltage-dependent, nonselective, cation channels that are widely expressed in the nervous system and localize to diverse pre- and postsynaptic terminals (Pape, 1996; Robinson & Siegelbaum, 2003). Neuronal HCN channels contribute to fundamental physiological processes such as the regulation of membrane potential, the integration of synaptic input to neurons and the rhythmicity of various brain regions (Robinson & Siegelbaum, 2003). HCN channels are modulated by both voltage and cAMP. In the absence of cAMP, HCN channels open when the intracellular potential becomes more negative (hyperpolarization). cAMP binding enhances the rate of activation of lh and promotes a larger current. HCN channels are ideally suited for modulating neurotransmitter release and effects because of their synaptic localization. Although they seem to have diverse functions in neuronal regulation, their contribution to synaptic transmission and plasticity is not fully understood. HCN channels in distal dendrites of pyramidal cells in the hippocampus are likely to dampen postsynaptic potentials that might otherwise trigger synaptic plasticity. Indeed, deletion of HCN1, which encodes an HCN ion channel subunit that contributes to Ih currents in hippocampal CA1 neurons, enhances LTP and hippocampal-dependent learning and memory (Nolan et al, 2004).
Uncovering the mechanisms that modulate neuronal communication through synaptic transmission to support information storage and retrieval by clusters of neurons is a central challenge for the future. The availability of new tools, such as non-invasive optical recording methods that allow real-time monitoring of neuronal activity in vivo, will facilitate the further molecular characterization of neuronal plasticity. The ultimate goal is to visualize synaptic activity and the formation of memory traces. Novel, fluorescent probes and microscopy techniques hold promise for significant advances towards this goal in the near future (Filippidis et al, 2005; Helmchen et al, 2001; Okumoto et al, 2005; Peleg et al, 1999). Indeed, modern genetically encoded fluorescent protein reporters can be targeted to specific neurons, allowing imaging of neuronal activity at single-synapse resolution (Bozza et al, 2004; Yuste et al, 2000). Combining this technology with genetic analyses in model organisms will provide a powerful platform for the comprehensive dissection of synaptic ion channel contributions to learning and memory. Such holistic approaches will ultimately bring together all pieces of the puzzle, exposing the elaborate links between genes, neurons and behaviour.

Nektarios Tavernarakis

Giannis Voglis
Acknowledgments
We acknowledge the contributions of numerous investigators that we could not include in this review owing to space limitations. Work in the authors' laboratory is funded by grants from the European Molecular Biology Organization, the European Union 6th Framework Programme and the Institute of Molecular Biology and Biotechnology (IMBB). N.T. is an EMBO Young Investigator.
References
- Baumann SW, Baur R, Sigel E (2001) Subunit arrangement of γ-aminobutyric acid type A receptors. J Biol Chem 276: 36275–36280 [DOI] [PubMed] [Google Scholar]
- Bear MF, Malenka RC (1994) Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol 4: 389–399 [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361: 31–39 [DOI] [PubMed] [Google Scholar]
- Bond CT, Maylie J, Adelman JP (2005) SK channels in excitability, pacemaking and synaptic integration. Curr Opin Neurobiol 15: 305–311 [DOI] [PubMed] [Google Scholar]
- Bozza T, McGann JP, Mombaerts P, Wachowiak M (2004) In vivo imaging of neuronal activity by targeted expression of a genetically encoded probe in the mouse. Neuron 42: 9–21 [DOI] [PubMed] [Google Scholar]
- Cline H (2003) Sperry and Hebb: oil and vinegar? Trends Neurosci 26: 655–661 [DOI] [PubMed] [Google Scholar]
- Collinson N et al. (2002) Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the α5 subunit of the GABAA receptor. J Neurosci 22: 5572–5580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coon AL, Wallace DR, Mactutus CF, Booze RM (1999) L-type calcium channels in the hippocampus and cerebellum of Alzheimer's disease brain tissue. Neurobiol Aging 20: 597–603 [DOI] [PubMed] [Google Scholar]
- Daw NW, Stein PS, Fox K (1993) The role of NMDA receptors in information processing. Annu Rev Neurosci 16: 207–222 [DOI] [PubMed] [Google Scholar]
- Debanne D, Daoudal G, Sourdet V, Russier M (2003) Brain plasticity and ion channels. J Physiol Paris 97: 403–414 [DOI] [PubMed] [Google Scholar]
- Dietrich D, Kirschstein T, Kukley M, Pereverzev A, von der Brelie C, Schneider T, Beck H (2003) Functional specialization of presynaptic Cav2.3 Ca2+ channels. Neuron 39: 483–496 [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF (1999) The glutamate receptor ion channels. Pharmacol Rev 51: 7–61 [PubMed] [Google Scholar]
- Engel JE, Wu CF (1998) Genetic dissection of functional contributions of specific potassium channel subunits in habituation of an escape circuit in Drosophila. J Neurosci 18: 2254–2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erreger K, Chen PE, Wyllie DJ, Traynelis SF (2004) Glutamate receptor gating. Crit Rev Neurobiol 16: 187–224 [DOI] [PubMed] [Google Scholar]
- Faber ES, Delaney AJ, Sah P (2005) SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nat Neurosci 8: 635–641 [DOI] [PubMed] [Google Scholar]
- Ferrer-Montiel AV, Montal M (1996) Pentameric subunit stoichiometry of a neuronal glutamate receptor. Proc Natl Acad Sci USA 93: 2741–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippidis G, Kouloumentas C, Voglis G, Zacharopoulou F, Papazoglou TG, Tavernarakis N (2005) Imaging of Caenorhabditis elegans neurons by second-harmonic generation and two-photon excitation fluorescence. J Biomed Opt 10: 024015. [DOI] [PubMed] [Google Scholar]
- Frick A, Johnston D (2005) Plasticity of dendritic excitability. J Neurobiol 64: 100–115 [DOI] [PubMed] [Google Scholar]
- Gouaux E (2004) Structure and function of AMPA receptors. J Physiol 554: 249–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond RS, Bond CT, Strassmaier T, Ngo-Anh TJ, Adelman JP, Maylie J, Stackman RW (2006) Small-conductance Ca2+-activated K+ channel type 2 (SK2) modulates hippocampal learning, memory, and synaptic plasticity. J Neurosci 26: 1844–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmchen F, Fee MS, Tank DW, Denk W (2001) A miniature head-mounted two-photon microscope. High-resolution brain imaging in freely moving animals. Neuron 31: 903–912 [DOI] [PubMed] [Google Scholar]
- Hogg RC, Raggenbass M, Bertrand D (2003) Nicotinic acetylcholine receptors: from structure to brain function. Rev Physiol Biochem Pharmacol 147: 1–46 [DOI] [PubMed] [Google Scholar]
- Hu H et al. (2001) Presynaptic Ca2+-activated K+ channels in glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. J Neurosci 21: 9585–9597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humeau Y, Shaban H, Bissiere S, Luthi A (2003) Presynaptic induction of heterosynaptic associative plasticity in the mammalian brain. Nature 426: 841–845 [DOI] [PubMed] [Google Scholar]
- Ji D, Lape R, Dani JA (2001) Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron 31: 131–141 [DOI] [PubMed] [Google Scholar]
- Kapur A, Yeckel MF, Gray R, Johnston D (1998) L-type calcium channels are required for one form of hippocampal mossy fiber LTP. J Neurophysiol 79: 2181–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamsa K, Heeroma JH, Kullmann DM (2005) Hebbian LTP in feed-forward inhibitory interneurons and the temporal fidelity of input discrimination. Nat Neurosci 8: 916–924 [DOI] [PubMed] [Google Scholar]
- Lauri SE, Bortolotto ZA, Nistico R, Bleakman D, Ornstein PL, Lodge D, Isaac JT, Collingridge GL (2003) A role for Ca2+ stores in kainate receptor-dependent synaptic facilitation and LTP at mossy fiber synapses in the hippocampus. Neuron 39: 327–341 [DOI] [PubMed] [Google Scholar]
- Lee HK et al. (2003) Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell 112: 631–643 [DOI] [PubMed] [Google Scholar]
- Levin ED, Bradley A, Addy N, Sigurani N (2002) Hippocampal α7 and α4-β2 nicotinic receptors and working memory. Neuroscience 109: 757–765 [DOI] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H (2002) The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci 3: 175–190 [DOI] [PubMed] [Google Scholar]
- Liu QS, Pu L, Poo MM (2005) Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature 437: 1027–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT (2001) Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron 29: 243–254 [DOI] [PubMed] [Google Scholar]
- Luscher C, Nicoll RA, Malenka RC, Muller D (2000) Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat Neurosci 3: 545–550 [DOI] [PubMed] [Google Scholar]
- Magee JC, Johnston D (2005) Plasticity of dendritic function. Curr Opin Neurobiol 15: 334–342 [DOI] [PubMed] [Google Scholar]
- Malenka RC (2003) Synaptic plasticity and AMPA receptor trafficking. Ann NY Acad Sci 1003: 1–11 [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF (2004) LTP and LTD: an embarrassment of riches. Neuron 44: 5–21 [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC (2002) AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 25: 103–126 [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS (2000) Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron 27: 349–357 [DOI] [PubMed] [Google Scholar]
- Morozov A, Muzzio IA, Bourtchouladze R, Van-Strien N, Lapidus K, Yin D, Winder DG, Adams JP, Sweatt JD, Kandel ER (2003) Rap1 couples cAMP signaling to a distinct pool of p42/44MAPK regulating excitability, synaptic plasticity, learning, and memory. Neuron 39: 309–325 [DOI] [PubMed] [Google Scholar]
- Murphy GG, Fedorov NB, Giese KP, Ohno M, Friedman E, Chen R, Silva AJ (2004) Increased neuronal excitability, synaptic plasticity, and learning in aged Kvβ1.1 knockout mice. Curr Biol 14: 1907–1915 [DOI] [PubMed] [Google Scholar]
- Nelson AB, Gittis AH, du Lac S (2005) Decreases in CaMKII activity trigger persistent potentiation of intrinsic excitability in spontaneously firing vestibular nucleus neurons. Neuron 46: 623–631 [DOI] [PubMed] [Google Scholar]
- Ngo-Anh TJ, Bloodgood BL, Lin M, Sabatini BL, Maylie J, Adelman JP (2005) SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat Neurosci 8: 642–649 [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Schmitz D (2005) Synaptic plasticity at hippocampal mossy fibre synapses. Nat Rev Neurosci 6: 863–876 [DOI] [PubMed] [Google Scholar]
- Niikura Y, Abe K, Misawa M (2004) Involvement of L-type Ca2+ channels in the induction of long-term potentiation in the basolateral amygdala-dentate gyrus pathway of anesthetized rats. Brain Res 1017: 218–221 [DOI] [PubMed] [Google Scholar]
- Nolan MF et al. (2004) A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell 119: 719–732 [DOI] [PubMed] [Google Scholar]
- Okumoto S, Looger LL, Micheva KD, Reimer RJ, Smith SJ, Frommer WB (2005) Detection of glutamate release from neurons by genetically encoded surface-displayed FRET nanosensors. Proc Natl Acad Sci USA 102: 8740–8745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouardouz M, Sastry BR (2006) Change in diazepam sensitivity of GABAA currents after LTP induction in neurons of deep cerebellar nuclei. Neurosci Lett 393: 147–149 [DOI] [PubMed] [Google Scholar]
- Pape HC (1996) Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu Rev Physiol 58: 299–327 [DOI] [PubMed] [Google Scholar]
- Peleg G, Lewis A, Linial M, Loew LM (1999) Nonlinear optical measurement of membrane potential around single molecules at selected cellular sites. Proc Natl Acad Sci USA 96: 6700–6704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaelli G, Saviane C, Mohajerani MH, Pedarzani P, Cherubini E (2004) BK potassium channels control transmitter release at CA3-CA3 synapses in the rat hippocampus. J Physiol 557: 147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS (2000) Destruction and creation of spatial tuning by disinhibition: GABA(A) blockade of prefrontal cortical neurons engaged by working memory. J Neurosci 20: 485–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H (1996) Diversity and function of presynaptic calcium channels in the brain. Curr Opin Neurobiol 6: 331–337 [DOI] [PubMed] [Google Scholar]
- Robinson RB, Siegelbaum SA (2003) Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol 65: 453–480 [DOI] [PubMed] [Google Scholar]
- Sausbier M et al. (2004) Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proc Natl Acad Sci USA 101: 9474–9478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger T, Fedorova I, Zheng F, Miyakawa T, Koustova E, Gomeza J, Basile AS, Alzheimer C, Wess J (2004) M2 muscarinic acetylcholine receptor knock-out mice show deficits in behavioral flexibility, working memory, and hippocampal plasticity. J Neurosci 24: 10117–10127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick-Gallagher P, McKernan MG, Xie J, Zinebi F (2003) L-type voltage-gated calcium channels are involved in the in vivo and in vitro expression of fear conditioning. Ann NY Acad Sci 985: 135–149 [DOI] [PubMed] [Google Scholar]
- Siegelbaum SA, Kandel ER (1991) Learning-related synaptic plasticity: LTP and LTD. Curr Opin Neurobiol 1: 113–120 [DOI] [PubMed] [Google Scholar]
- Sweatt JD (2001) The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem 76: 1–10 [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S (1996) The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell 87: 1327–1338 [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB (2000) Hebb and homeostasis in neuronal plasticity. Curr Opin Neurobiol 10: 358–364 [DOI] [PubMed] [Google Scholar]
- Veng LM, Mesches MH, Browning MD (2003) Age-related working memory impairment is correlated with increases in the L-type calcium channel protein α1D (Cav1.3) in area CA1 of the hippocampus and both are ameliorated by chronic nimodipine treatment. Brain Res Mol Brain Res 110: 193–202 [DOI] [PubMed] [Google Scholar]
- Vizi ES, Lendvai B (1999) Modulatory role of presynaptic nicotinic receptors in synaptic and non-synaptic chemical communication in the central nervous system. Brain Res Brain Res Rev 30: 219–235 [DOI] [PubMed] [Google Scholar]
- Wu LG, Borst JG, Sakmann B (1998) R-type Ca2+ currents evoke transmitter release at a rat central synapse. Proc Natl Acad Sci USA 95: 4720–4725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG, Westenbroek RE, Borst JG, Catterall WA, Sakmann B (1999) Calcium channel types with distinct presynaptic localization couple differentially to transmitter release in single calyx-type synapses. J Neurosci 19: 726–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kang J (2005) The mechanisms and functions of activity-dependent long-term potentiation of intrinsic excitability. Rev Neurosci 16: 311–323 [DOI] [PubMed] [Google Scholar]
- Yuste R, Miller RB, Holthoff K, Zhang S, Miesenbock G (2000) Synapto-pHluorins: chimeras between pH-sensitive mutants of green fluorescent protein and synaptic vesicle membrane proteins as reporters of neurotransmitter release. Methods Enzymol 327: 522–546 [DOI] [PubMed] [Google Scholar]
- Zhao MG et al. (2005) Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron 47: 859–872 [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG (2002) Short-term synaptic plasticity. Annu Rev Physiol 64: 355–405 [DOI] [PubMed] [Google Scholar]


