Abstract
The generation of regular and irregular past tense verbs has been an important issue in cognitive science and has been used to advance different models of the organization of language in the brain. The dual-system view holds that the regular past tense forms are generated by a rule while irregular forms are retrieved from memory. The single-system view, on the other hand, holds that both forms are generated by a single integrated system and differ only in their reliance on factors such as phonology and semantics. We conducted an event-related fMRI study to examine the activation patterns associated with the generation and reading of regular and irregular past tense forms, in addition to the reading of their stems. Regular and irregular past tense generation activated similar brain regions compared to the reading of their respective stems. The areas activated more for irregular generation compared to regular generation included inferior frontal, precentral, and parietal regions bilaterally. This activation can be interpreted as reflecting the greater attentional and response selection demands of irregular generation. Compared to irregular generation, regular generation activated a small region in the left superior temporal gyrus when the regular and irregular past tense forms were mismatched on phonological complexity. No areas were more activated for regulars than irregulars when the past tense forms were matched on this variable. This suggests that the activation specific to regulars was related to the higher phonological complexity of their past tense forms rather than to their generation. A contrast of the reading of regular and irregular past tense forms was consistent with this hypothesis. These results support a single-system account of past tense generation.
INTRODUCTION
A particularly contentious issue in psycholinguistics involves the computation of inflectional morphology. The debate surrounding the English past tense has been used to advance broader, competing claims regarding the fundamental nature of cognitive processes including language. One view holds that language is modular, symbolic, and domain-specific and proposes a fundamental dichotomy between the lexicon and grammar. The other proposal suggests that cognitive processes are graded and domain-general, and various components such as the lexicon and grammar are intertwined and interactive. Issues related to the generation of English past tense have been extensively discussed elsewhere (see McClelland & Patterson, 2002; Pinker & Ullman, 2002 for a recent summary); here we briefly describe the competing hypotheses.
For the vast majority of English verbs, the past tense is formed by the addition of the affix -ed, without any change to the stem. In the final phonological form, this past tense marker is realized as [d], [t], or [Id], depending on the last phoneme of the stem (e.g., free/freed, push/pushed, want/wanted). These regular verbs account for approximately 86% of the 1000 most frequent verbs in English by type count (Plunkett & Nakisa, 1997). For the rest of the verbs, formation of the past tense usually involves a change in the vowel and/or final consonant in the stem (e.g., sit/sat, make/made, sleep/slept). A few involve no change (e.g., hit/hit). Two of the principal accounts of past tense formation can be termed the “Dual-system” (DS) and “Single-system” (SS) theories.
One version of the DS theory is the “Words and Rules” theory, developed by Pinker (1991, 1999), which holds that distinct systems or modules are responsible for the generation of regular and irregular past tense forms. Regular past tenses are generated by the productive, combinatorial system of grammar, by application of a rule without reference to the phonology or semantics of the stem. Irregular past tense forms, by contrast, are learned by rote, stored in the lexicon, and retrieved through an associative memory mechanism. When a verb is inflected, both the lexicon and grammar are initially accessed. The retrieval of a stored inflected form of an irregular verb blocks the application of the rule to that verb (e.g., sang pre-empts singed). This theory has been extended by Ullman (2004, in press) in the Declarative/Procedural hypothesis, which proposes distinct neural substrates of the lexicon and grammar. According to this hypothesis, lexical memory, used for irregular forms, is a subdivision of declarative memory. Medial-temporal and temporo-parietal regions are considered to be responsible for the consolidation and long-term retention of declarative memories. Other regions are involved in searching this memory for retrieval. By contrast, grammatical processing, and thus, regular inflection, depends on the procedural system, which is thought to be sub-served by the basal ganglia, Broca's area, and neighboring anterior regions.
The DS theory, then, would predict that damage to different regions in the brain is associated with differential impairment of either regular or irregular past tense processing. In fact, such behavioral double dissociations have been frequently described. On tests of past tense elicitation and reading, Ullman, Corkin, et al. (1997) reported preferential impairment of regular relative to irregular verb inflection in patients with nonfluent aphasia secondary to vascular lesions in anterior left hemisphere regions, although performance was not completely intact for irregulars. In a similar nonfluent aphasia sample, Tyler et al. (2002) reported reduced priming for regularly inflected past tense forms and their stems relative to irregular pairs. In contrast, greater impairments on irregular past tense processing have been reported in association with damage to temporal or temporo-parietal regions resulting from herpes simplex encephalitis (Tyler et al., 2002), semantic dementia (Patterson, Lambon Ralph, Hodges, & McClelland, 2001), and stroke (Ullman, Corkin, et al., 1997).
Such behavioral dissociations may also be explained, however, by an alternative account, originally proposed by Rumelhart and McClelland (1986). In this model, all past tense forms are generated through a single, interactive system, but component processes within the network such as phonology and semantics have distinct representations. The behavioral differences between regular and irregular forms are attributed to differential contributions of phonological and semantic components, not to categorically separate systems. Computing irregular forms depends on semantics more than computing regular forms, therefore irregular inflection may be more vulnerable to disruption from a semantic deficit. On the other hand, in English, regular past tense forms place a greater demand on the phonological system than irregular forms do (Lambon Ralph, Braber, McClelland, & Patterson, 2005; Bird, Lambon Ralph, Seidenberg, McClelland, & Patterson, 2003; Burzio, 2002). This is because, in English, the transformation of the present to the regular past tense always involves the addition of extra phonemes, which is rarely the case for irregulars. Consequently, phonological impairments may be more likely to disrupt regular inflection. This theory has received support from a connectionist simulation (Joanisse & Seidenberg, 1999) as well as a behavioral study in which the preferential impairment in regular past tense forms of patients with nonfluent aphasia was no longer evident when the regular and irregular past tense forms were matched on phonological complexity (Bird et al., 2003).
Here, we present an fMRI study of brain activation patterns associated with the generation of regular and irregular past tense in English, with specific attention to phonological complexity, which has not been addressed in imaging studies thus far. When the activation patterns for regular and irregular past tense generation are contrasted, the DS theory predicts that regular past tense generation will be associated with greater activation of frontal and striatal regions hypothesized to subserve the procedural grammar/rule system. Processing irregular forms should preferentially activate the declarative memory/lexical system in temporal and temporo-parietal regions, and possibly areas involved in searching this memory. The SS theory predicts greater activation of semantic regions for irregulars, greater activation of regions related to phonological complexity for regulars, and a considerable overlap between the two, to the extent that the cognitive demands of both types of morphological transformations are similar. If, however, phonological complexity is matched, there should be no area of greater activation for regular relative to irregular forms, thus providing only a single dissociation.
Next, we briefly review some of the prior functional imaging studies of past tense generation and compare these results with the predictions of the two theories above.
Previous Functional Imaging Studies
A summary of some of the previous imaging studies of past tense generation (Sach, Seitz, & Indefrey, 2004; Beretta et al., 2003; Dhond, Marinkovic, Dale, Witzel, & Halgren, 2003; Indefrey et al., 1997; Ullman, Bergida, et al., 1997; Jaeger et al., 1996) is presented in Table 1. With the exception of Sach et al., the results of all studies have been interpreted as supporting the DS theory, based on the fact that they find some differences in the activation produced by regulars and irregulars. However, a closer look at the results reveals that this conclusion is not obvious. As shown in Table 1, the areas activated more for regular generation compared to irregular generation vary considerably between studies. Due to differences in methodologies, it might be expected that the results of various experiments would differ somewhat in their precise activation patterns. However, if a rule module or grammatical system is being used to generate the regular past tense, it should be activated with at least some consistency. Furthermore, in only one of the studies, the MEG study performed by Dhond et al. (2003), was greater activation for regulars found in the left inferior frontal gyrus (IFG), as predicted by the DS theory. This finding could also be consistent with the SS theory, however, if the regulars and irregulars were not matched on phonological complexity. The PET and fMRI studies either found no difference in this area (Sach et al., 2004; Indefrey et al., 1997) or greater activation for the irregulars (Beretta et al., 2003; Ullman, Bergida, et al., 1997). The precise pattern of irregular > regular activation was also highly variable across studies.
Table 1.
A Summary of Some Imaging Studies of Past Tense Generation, Showing Areas Activated Differentially for Regular and Irregular Past Tense Generation in a Direct Contrast
| Study | Language | Technique | Design | NI | NS | Task | Regular > Irregular | Irregular > Regular |
|---|---|---|---|---|---|---|---|---|
| Jaeger et al. (1996) | English | PET | Blocked | 46 | 9 | Overt generation | ?a | ? |
| Indefrey et al. (1997) | German | PET | Blocked (mixed)b | NA | 12 | Overt generation | L BA 39; R BA 37 | L BA 46, 44/6, 10, 39/40, 5, 37; R BA 47, 46, 9/32, 18 |
| Ullman, Bergida, et al. (1997) | English | fMRI | Blocked | 160 | 5 | Silent generation | L temporal | Bilateral frontal, basal ganglia |
| Beretta et al. (2003) | German | fMRI | Event-related | 12 | 8 | Silent generation | ?c | ? |
| Dhond et al. (2003) | English | MEG | Random order | 80 | 12 | Silent generation, finger-raised | L inferior frontal gyrus | L fusiform gyrus; R prefrontal (BA 10) |
| Sach et al. (2004) | German | PET | Blocked (mixed) | 9 | 12 | Overt Generation | None | None |
NA = not available; NI = number of items in each comparison group; NS = number of subjects.
A direct contrast between regular and irregular past tense generation was not presented, so it is unclear whether any areas were significantly differentially activated. In comparison with a baseline of reading, irregulars produced much greater activation than regulars.
In this blocked design, past tense and past participle generation were mixed within a block in order to prevent the use of a simple strategy within a block.
Statistically thresholded group maps are not presented, so it is unclear which areas were activated significantly for regular generation compared to irregular generation. In subtraction maps, irregulars produced much more extensive activation compared to regulars.
This task required the subjects to raise a finger if the past tense of the given verb ended in -ed.
Importantly, although studies have shown that people require more time and are less accurate when generating low-frequency irregulars in comparison with regulars of matched frequency, the reliance on covert responses in some of these imaging studies precluded the collection of on-line RT and accuracy data (see Seidenberg & Arnoldussen, 2003). Consequently, the extent to which the regular and irregular conditions were or were not equated on processing difficulty is unknown. Thus, the large variation found in the results of these imaging studies suggests that experiment-specific factors, such as blocked presentation, small numbers of subjects or stimuli, or confounds such as phonological complexity or processing difficulty may be responsible for some of the differences in activation.
There are two main areas of agreement in these studies. First, there are large areas of common activation for regular and irregular past tense generation compared to a common baseline. Second, irregulars activate more areas than regulars (with the exception of the Sach et al. study). The conclusions that can be drawn from these studies regarding adjudication between DS and SS theories are, then, unclear at best. Additionally, there are a number of electrophysiological studies (e.g., Lavric, Pizzagalli, Forstmeier, & Rippon, 2001; Rodriguez-Fornells, Clahsen, Lleo, Zaake, & Munte, 2001; Munte, Say, Clahsen, Schiltz, & Kutas, 1999; Newman, Izvorski, Davis, Neville, & Ullman, 1999; Marslen-Wilson & Tyler, 1998; Penke et al., 1997), which also show variable results. Space limitations preclude a full review of these studies.
Current Study
Here we build on this previous research in several ways. In comparing the activation patterns of regular and irregular past tense inflection in English, we remove several possible confounds resulting from the fact that regulars and irregulars can differ in several dimensions, including frequency, letter and syllable length, friend–enemy ratio (FER),1 and especially phonological complexity. Following Bird et al. (2003), we operationalized phonological complexity in terms of CV structure. That is, a form such as crawled, with the structure CCVCC, is considered to be more complex than a form such as shook, with the structure CVC. We also used overt generation of the past tense as opposed to silent generation used in most prior studies in this area. Although silent generation is useful for avoiding movement artifacts, it does not permit the collection of reliable behavioral data. By using overt generation, we were able to examine the relative processing difficulty of the two conditions and the possible effects of this variable on the activation patterns. We also used a larger number of subjects and stimuli (within the constraints of multiple matching criteria and a limited number of irregular verbs), so that even subtle differences between conditions would be more detectable due to increased statistical power.
Verb stems were presented visually, and subjects said the past tenses of the verbs aloud during the generate (Gen) task. In the read (Read) task, present and past tense forms of verbs were presented and were read aloud by the subjects. A group of 50 regulars and 50 irregulars, matched on the log frequency of the past tense form, FER, letter length of the stem, and syllable length of the stem and past tense forms, was included in the Gen condition. To examine the effects of phonological complexity, a subgroup of 40 regulars and 40 irregulars was created that was matched pairwise, not only on the number of phonemes, but also on the precise CV structure of the past tense form. For a direct comparison to this phonologically matched contrast, a subgroup of 40 regulars and 40 irregulars, mismatched on the number of phonemes in the past tense form, was created. Similarly, subgroups matched and mismatched on the number of phonemes were also created in the past tense Read condition. The past tense forms were included in the Read task to dissociate activation associated with generation of the past tense from effects of phonological complexity on articulation of the past tense forms. The stimuli are shown in Appendix A.
RESULTS
Behavioral Results
RT and accuracy results are presented in Table 2. RTs were measured from the onset of the stimulus to the onset of the verbal response. These measurements were made manually off-line using computer software (SoundEdit 16) to mark the digitally recorded waveforms. Only RTs for correct trials were included in the analyses. Occasionally, subjects extended an initial fricative over a prolonged period before continuing with the remainder of the response, suggesting that they had initiated verbalization before fully formulating their answer. When an initial fricative lasted longer than 300 msec, RT was estimated to be 100 msec prior to the onset of the second phoneme. Less than 1% of Gen trials were affected in this manner and they were divided equally between regulars and irregulars. Differences between conditions with regard to RT and accuracy were analyzed by item (t1) and subject (t2) using two-tailed t tests.
Table 2.
The Mean (SD) Response Time (msec) and Accuracy (% Correct) for Different Subsets in the Stimuli
| Group | RT | Accuracy |
|---|---|---|
| GenI | 1123.13 (165.76) | 91.12 (12.13) |
| GenR | 1033.51 (116.45) | 98.24 (5.49) |
| ReadIPres | 801.16 (56.70) | 99.30 (3.83) |
| ReadRPres | 800.13 (51.06) | 99.70 (1.07) |
| ReadIPast | 807.91 (70.47) | 99.60 (1.52) |
| ReadRPast | 803.69 (44.46) | 99.60 (1.22) |
| GenIM | 1115.06 (171.79) | 89.60 (13.61) |
| GenRM | 1056.38 (104.87) | 98.30 (5.65) |
| GenIN | 1095.73 (153.99) | 94.00 (7.25) |
| GenRN | 993.39 (100.58) | 99.00 (3.23) |
| ReadIPastM | 811.31 (78.81) | 99.36 (1.89) |
| ReadRPastM | 802.46 (47.10) | 99.84 (0.80) |
| ReadIPastN | 809.24 (75.72) | 99.84 (0.80) |
| ReadRPastN | 804.08 (42.99) | 99.36 (1.50) |
Gen = Generate; I = Irregular; R = Regular; Pres = Present; M = phonologically matched; N = phonologically mismatched.
Consistent with expectations, RTs were greater when subjects were generating the past tense forms than when they were reading either the present or past tense of verb stems (all p < .0001). With regard to accuracy, subjects were significantly more accurate when reading present or past irregular verb forms than when generating irregulars (all p < .0001). For the regular verbs, the item analyses showed only a nonsignificant trend toward poorer accuracy when generating regulars than when reading the regular present or past tense [t1(53.6) = 1.837, p < .10; t1(54.9) = 1.700, p < .10, respectively]. The difference between these conditions was significant in the subject analyses [t2(24) = 3.673, p < .005; t2(24) = 3.37, p < .005].
In the Gen Irregular versus Gen Regular contrast, both item and subject analyses indicated that subjects were faster [t1(98) = 3.128, p < .005; t2(24) = 4.251, p < .0005] and more accurate [t1(68.3) = 3.781, p < .0005; t2(24) = 6.211, p < .0001] when generating regulars than when generating irregulars. Similar results were obtained when comparing the phonologically mismatched Gen groups [RT: t1(78) = 3.519, p < .001; t2(24) = 5.03, p < .0001; Accuracy: t1(53.9) = 3.985, p < .0005; t2(24) = 5.106, p < .0001]. For the phonologically matched Gen groups, the results of the accuracy analyses were consistent with those from the other Gen comparisons [t1(52) = 3.734, p < .0005; t2(24) = 6.357, p < .0001]. In the RT analyses, the pattern of results was the same, but the magnitude of the differences was substantially less than that seen in the other Gen group contrasts. Specifically, the difference in RT was smaller but still significant in the subject analysis [t2(24) = 2.715, p < .05], and only approached significance in the item analysis [t1(78) = 1.844, p < .10].
The latter finding may be due to a differential relationship between RT and the phonology of the stems for the regular and irregular verbs. In the Gen Regular and the phonologically mismatched Gen Regular conditions, RT was inversely correlated with the number of syllables in the stem (r = −.542, p < .001; r = −.429, p < .01, respectively), indicating that subjects responded faster to multisyllabic regular forms. In contrast, there was no significant relationship between RT and stem syllable length in either the Gen Irregular or the phonologically mismatched Gen Irregular conditions. In the phonologically matched conditions, all of the verb stems and their past tense forms are monosyllabic. Given the strong inverse relationship between syllable length and RT for the regulars, the reduced RT difference between the phonologically matched irregular and regular verbs appears to be due, at least in part, to an increase in RT for the regulars resulting from the elimination of the multisyllabic stems.
There were no significant differences between the Read Regular and Read Irregular present or past tense conditions with regard to either RT or accuracy.
Imaging Results
The imaging results are presented in the form of group maps of contrasts between various conditions, created with a random-effects analysis and thresholded at p < 0.05, corrected for the whole brain volume. Figure 1 shows the results of various condition contrasts. Appendix B provides the locations of peak activations for each contrast.
Figure 1.
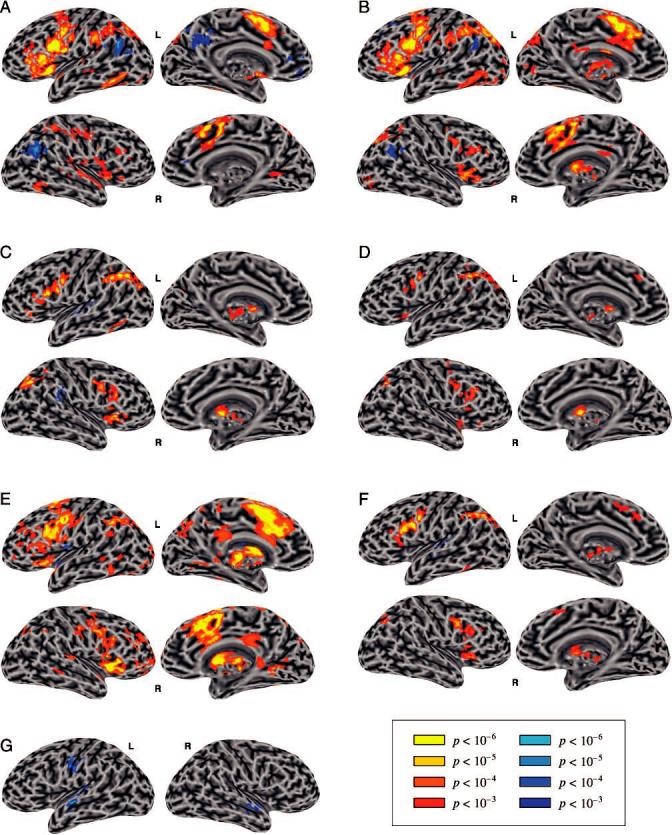
The activation patterns of various contrasts overlaid on an inflated surface model of a brain. The inflated surface reveals the activation hidden in sulci, but can sometimes divide a single cluster in volumetric space into multiple clusters. The activation is thresholded at voxelwise p < .001, and a corrected p < .05. The left hemisphere lateral surface in (C) is slightly rotated to make the superior temporal activation more clearly visible. (A) Generate Regular–Read Regular Present. (B) Generate Irregular–Read Irregular Present. (C) Generate Irregular–Generate Regular. (D) Generate Irregular–Generate Regular (phonologically matched). (E) Areas correlated with RT across conditions. (F) Generate Irregular–Generate Regular (phonologically mismatched). (G) Read Irregular Past–Read Regular Past (phonologically mismatched).
Generate Regular versus Read Regular Present
Comparing areas activated in the Gen task with those activated during the Read task can isolate areas involved in the generation of past tense forms. We first contrast generation of regular past tense with the reading of regular stems (Figure 1A). Extensive frontal activation was observed in the left hemisphere for the Gen Regular condition relative to the Read Regular Present condition. This included the precentral gyrus (BA 6), the IFG (including BA 44, 45, and 47), the anterior insula (BA 13), and the middle frontal gyrus (MFG; BA 46 and 9). The putamen and globus pallidus were activated on the left. Parietal activation was also seen in the left hemisphere, involving the dorsal supramarginal gyrus (BA 40) and the intraparietal sulcus (IPS; BA 7). The frontal and parietal activation associated with the Gen task was less extensive in the right hemisphere, and included the IFG, pre- and postcentral gyri, the anterior insula, and the IPS. Bilateral activation of the supplementary motor area, cingulate gyrus, inferior temporal and fusiform gyri (BA 37), and superior temporal gyrus (STG; BA 22) was also found.
Areas showing higher activation for the Read task included the bilateral angular gyrus (BA 39), the left posterior cingulate gyrus and precuneus, and the bilateral inferior anterior cingulate gyrus (BA 32).
Generate Irregular versus Read Irregular Present
This contrast yielded results strikingly similar to the corresponding regular contrast, with very similar frontal, parietal, temporal, and medial areas activated (Figure 1B). There was more extensive right parietal (IPS) activation compared to the regular contrast. Furthermore, the thalamus and basal ganglia were activated bilaterally and more extensively, including the caudate head and body. Areas showing higher activation for the Read condition were similar to those in the regular contrast.
Generate Irregular versus Generate Regular
The critical comparison involves directly contrasting the generation of irregular and regular past tense forms (Figure 1C). Areas activated bilaterally for irregulars relative to regulars included the IFG and adjacent MFG (BA 44, 45, 46, and 47), the precentral gyrus, the IPS, and the basal ganglia. Left lateralized activation was seen in the dorsal supramarginal gyrus and in the fusiform and posterior inferior temporal gyri. The anterior insula was activated mostly in the right hemisphere.
Higher activation for regular than irregular generation was found in the left dorsal STG, involving the primary auditory areas (Heschl's gyrus) and the planum temporale. Right ventral supramarginal gyrus was also activated more for regulars.
Generate Irregular versus Generate Regular (Phonologically Matched)
The irregular and regular verb groups used in the previous contrast were matched on a number of variables (see Table 3), but not on phonological complexity. As noted before, regular past tense forms tend to be phonologically more complex than irregular past tense forms. In this contrast, we examine the areas activated differentially for irregular and regular generation once the phonological complexity of the past tense form is equated (Figure 1D). This contrast between the phonologically matched groups of irregular and regular verbs yielded similar activation patterns to the previous contrast, but the total extent of the activation was considerably reduced (total positive activation of 16,717 μl compared to 29,414 μl in the previous contrast). Relative to the generation of regular verbs, irregular verbs were associated with greater bilateral activation of the posterior IFG (BA 44), the precentral gyrus, the anterior insula, the IPS, and the basal ganglia. In addition, activation was seen in the left dorsal supramarginal gyrus and in small foci in the left anterior cingulate gyrus, right MFG, right BA 47, and right anterior STG. No areas were activated more for regulars than for irregulars.
Table 3.
The Mean (SD) of Various Measures for Different Subgroups of the Stimuli
| Group | n | F | Syllables | Letters | Phonemes | FER |
|---|---|---|---|---|---|---|
| GenI | 50 | 0.77 (0.61) | 1.30 (0.65) | 4.98 (1.34) | 4.34 (1.38) | 1.12 (1.11) |
| GenR | 50 | 0.74 (0.53) | 1.34 (0.52) | 4.92 (1.50) | 5.02 (1.65) | 1.09 (0.55) |
| ReadIPres | 40 | 0.75 (0.70) | 1.33 (0.62) | 4.98 (1.75) | 4.10 (1.53) | 1.09 (0.62) |
| ReadRPres | 40 | 0.76 (0.48) | 1.35 (0.48) | 4.98 (1.00) | 4.28 (0.96) | 1.14 (0.64) |
| ReadIPast | 40 | 0.75 (0.70) | 1.33 (0.62) | 4.98 (1.76) | 4.15 (1.55) | 1.09 (0.62) |
| ReadRPast | 40 | 0.76 (0.48) | 1.73 (0.78) | 6.83 (0.93) | 5.16 (1.19) | 1.14 (0.64) |
| GenIM | 40 | 0.87 (0.59) | 1.00 (0.00) | 4.48 (0.72) | 4.00 (0.55) | - |
| GenRM | 40 | 0.82 (0.55) | 1.00 (0.00) | 3.98 (0.58) | 4.00 (0.55) | - |
| GenIN | 40 | 0.86 (0.63) | 1.18 (0.44) | 4.78 (0.97) | 3.95 (0.88) | - |
| GenRN | 40 | 0.83 (0.55) | 1.43 (0.55) | 5.18 (1.55) | 5.50 (1.54) | - |
| ReadIPastM | 25 | 0.62 (0.75) | 1.52 (0.71) | 5.72 (1.74) | 4.92 (1.45) | - |
| ReadRPastM | 25 | 0.66 (0.51) | 1.28 (0.46) | 6.40 (0.76) | 4.92 (0.64) | - |
| ReadIPastN | 25 | 0.71 (0.57) | 1.08 (0.27) | 4.32 (1.11) | 3.48 (0.82) | - |
| ReadRPastN | 25 | 0.74 (0.40) | 2.04 (0.79) | 7.16 (0.80) | 6.32 (0.99) | - |
Gen = Generate; I = Irregular; R = Regular; Pres = Present; M = phonologically matched; N = phonologically mismatched; F = Log frequency of the past tense form; FER = Friend-Enemy Ratio; n = number of items in the group.
Areas Correlated with RT
Two condition-specific RT regressors corresponding to Regular and Irregular Gen conditions were included in the regression analysis to identify activation correlated with RT (see Methods). The conjunction of these two regressors indicates areas where activity is modulated by time on task regardless of the condition (Figure 1E). The areas that were correlated with RT overlap with those previously found to be preferentially activated in the Gen Irregular > Gen Regular contrasts, including bilateral frontal (IFG, anterior insula, and precentral gyrus) and parietal (IPS) areas as well as basal ganglia.
Generate Irregular versus Generate Regular (Phonologically Mismatched)
We can further examine the effects of phonological complexity on the activation patterns by contrasting item groups that are intentionally mismatched with regard to this variable (Figure 1F). The activation pattern for the phonologically mismatched contrast was similar to the activation in the phonologically matched contrast, but somewhat more extensive for the irregulars, especially in the left frontal region. The middle and inferior frontal activation for irregulars extended into BA 44 and 45, and left BA 37 was also activated.
Relative to the irregulars, regular past tense generation was associated with a single focus of activation in the left dorsal STG that had not been present in the matched contrast.
Read Irregular Past versus Read Regular Past (Phonologically Matched)
To further examine the areas activated due to differences in phonological complexity between regulars and irregulars, as opposed to those activated due the generation of past tense, we can contrast the reading of the past tense forms using phonologically matched and mismatched groups. In the contrast of reading phonologically matched groups of regular and irregular past tense forms, no areas were differentially activated.
Read Irregular Past versus Read Regular Past (Phonologically Mismatched)
No areas were more activated for reading irregular past tense forms relative to reading regular past tense forms. For the regular forms, activation was greater in the left STG, the left precentral gyrus (BA 4), and the right anterior STG (Figure 1G).
DISCUSSION
Inflection versus Reading
Generating the past tense of verbs places greater demands on attention and working memory compared to reading. The contrasts between these tasks activated extensive frontal. parietal, and cingulate areas bilaterally (Figure 1A and B), many of which have been closely associated with attention, working memory, and response selection processes (see discussion below). The left IFG, including Broca's area (approximately BA 44 and 45), was activated for both regular and irregular inflection relative to reading. This result is not consistent with the proposal that the inflection of regular but not irregular forms relies on a rule-based or grammatical system that is implemented in the left IFG (Ullman, 2004; Musso et al., 2003; Grodzinsky, 2000).
Because the stem must first be read before producing the inflected form, one might expect all areas activated for reading to be activated by inflection. However, clusters in the bilateral angular gyrus (BA 39), as well as in the left posterior cingulate gyrus, precuneus, and ventral anterior cingulate gyrus, showed higher activation for reading compared to inflection in both regular and irregular contrasts. One possibility is that this activation represents “task-induced deactivation” (McKiernan, Kaufman, Kucera-Thompson, & Binder, 2003; Raichle et al., 2001; Binder et al., 1999; Shulman et al., 1997). These areas are among those previously found to show higher activation for a “resting” or “passive stimulation” baseline compared to an attentionally demanding task. One suggestion is that this change in activation represents spontaneous cognitive activity that occurs in these regions during rest (and during relatively simple tasks such as reading common words) that is interrupted by a reallocation of resources to the areas involved in more demanding task performance.
Past Tense Generation: Irregular > Regular
In the analysis directly comparing irregular and regular past tense generation, frontal areas (IFG, inferior frontal sulcus, and precentral gyrus) and parietal regions (dorsal supramarginal gyrus and IPS) were activated bilaterally (Figure 1C). These frontal and parietal areas have been shown to be active in a wide variety of tasks (see Culham & Kanwisher, 2001; Duncan & Owen, 2000 for reviews of parietal and frontal regions, respectively). These areas have also been shown in rhesus monkeys to have rich anatomical connections with each other (Petrides & Pandya, 1984), and activations in these areas are often correlated in functional imaging studies.
These fronto-parietal networks have been implicated in a number of cognitive functions, particularly selective attention and working memory. Multiple lines of evidence including patient lesion and functional imaging studies support the existence of a distributed network of frontal, parietal, and cingulate cortical regions underlying visuospatial attention (Gitelman et al., 1999; for review, see Kastner & Ungerleider, 2000). These regions overlap substantially with those associated with spatial and nonspatial working memory (Kastner & Ungerleider, 2000; LaBar, Gitelman, Parrish, & Mesulam, 1999; D'Esposito et al., 1998). Additionally, these regions have been implicated in resolution of stimulus–response incompatibility (SRI) in multiple modalities (Jiang & Kanwisher, 2003a, 2003b; Schumacher & D'Esposito, 2002; Dassonville et al., 2001). In SRI paradigms, a natural or prepotent mapping needs to be inhibited to allow a less habitual, task-relevant mapping.
Thus, the stronger fronto-parietal activation seen in the current study in association with the generation of irregular past tense forms relative to regular past tense forms may reflect greater processing difficulty due to higher demands for visuospatial attention, interference resolution, and working memory. The greater difficulty of inflecting irregulars is documented in many behavioral studies (Ullman, 1999; Marchman, 1997; Seidenberg, 1992), including the present study, with irregular inflection resulting in longer RTs and higher error rates than regular inflection. The more demanding nature of irregular inflection in English may in part be due to the fact that an overwhelming majority of verbs in English are regular, thus the process of transformation from the present to the past tense is strongly biased toward production of the regular form. The greater fronto-parietal activation during irregular generation may therefore reflect the process of inhibiting the prepotent regular pattern to allow sufficient activation of the appropriate output phonological representation, possibly through additional input from semantics. Continued visual attention to the stimuli and maintenance of the phonological form in working memory during the longer processing interval required for the generation of irregular forms may also contribute to the greater fronto-parietal activations seen in this condition.
Is this interpretation of the IFG activation for irregulars compatible with the DS theory? The DS account suggests that the default rule-based mechanism is inhibited or blocked when the search for an irregular form in lexical memory succeeds, so one could argue that the IFG activation is due to inhibition of the rule-based mechanism. Note that this account refers to a process-specific inhibition that occurs only when the default rule-based path must be blocked. This is in contrast to the aforementioned model, which concerned a more general mechanism for blocking any prepotent response. More importantly, however, this DS interpretation based on inhibition of the rule-based mechanism offers no explanation for why the same areas are also activated for regular generation relative to reading. According to the DS theory, the IFG components of this network (BA 44 and 45) represent a rule-based grammar mechanism and should be activated during regular inflection but inhibited during irregular inflection. To accommodate the observed data within the DS account, therefore, one would need to argue that activation in this region represents both inhibition (in the case of irregulars) and activation (in the case of regulars) of the same neural process.
Other regions activated for irregulars over regulars included the right anterior insula and the adjacent frontal operculum. Recent studies have linked activation in this region with decision-making processes (Binder, Liebenthal, Possing, Medler, & Ward, 2004); it is often activated when a more demanding task is contrasted with an easier task (see Table 2 in Binder et al., 2004). The bilateral basal ganglia, including the thalamus, caudate body, and caudate head, were also activated more for irregular inflection. The basal ganglia are strongly connected to frontal areas of the brain through a number of parallel circuits (Alexander, Crutcher, & DeLong, 1990) and are commonly activated in conjunction with those frontal areas across a variety of tasks. Finally, the fusiform and inferior temporal gyri (BA 37) were activated for irregulars over regulars. This area is associated with visual word-form processing (Cohen et al., 2002; Binder & Mohr, 1992), although whether it is dedicated exclusively to processing visual word forms is questionable (Price & Devlin, 2003). In conjunction with the activation of parietal areas involved in visual attention, and the higher RTs and higher number of errors for irregulars, this activation may be due to greater attention to and deeper orthographic processing of the irregular stems. This fusiform/inferior temporal activation was not present in the phonologically matched contrast, but returned in the phonologically mismatched comparison, suggesting that it is not specific to irregular past tense generation. The posterior temporal area has also been suggested to be a major component of a spatial attention network (Gitelman et al., 1999). As noted previously, the RT difference between irregulars and regulars is substantially less in the phonologically matched condition, suggesting a reduction in the discrepancies regarding attentional demands.
The above interpretation is also supported by the RT conjunction analysis (Figure 1E). The areas modulated by RT regardless of the stimulus condition include the same fronto-parietal areas, as well as the basal ganglia and the inferior temporal/fusiform gyri, suggesting their role in more demanding task performance rather than in processes specific to irregular past tense generation.
Note that the fronto-parietal regions, the fusiform gyrus, and the anterior insula are all activated for regular generation compared to reading, as well as for irregular generation compared to regular generation. This strong similarity in the activation patterns suggests that these areas are not involved in processes specific to irregular or regular past tense generation, but reflect the increasingly demanding nature of these tasks (irregular generation > regular generation > reading).
The greater activation of the IFG and the basal ganglia for irregulars is contrary to the predictions of the declarative/procedural model, which suggests that these regions play a special role in regular inflection. Ullman (2004) suggests that the basal ganglia and the frontal cortex are domain general in the sense that they subserve both linguistic and nonlinguistic processing, but may also contain domain-specific circuits that subserve grammar processing and regular inflection. Here, we find no evidence for such domain-specific circuits governing regular inflection. On the contrary, the results demonstrate that the frontal cortex and basal ganglia are activated more strongly during irregular past tense generation.
Past Tense Generation: Regular > Irregular
Turning to the areas activated more by regulars than irregulars, foci were found in the left dorsal STG and right ventral supramarginal gyrus in the main Gen Regular > Gen Irregular comparison. The left STG and planum temporale are well-established auditory areas that are activated by speech and nonspeech sounds. The specific linguistic function of these areas is not completely understood, but they likely subserve early stages of acoustic to phonetic recoding of speech (Davis & Johnsrude, 2003; Hall, Hart, & Johnsrude, 2003; Binder et al., 2000). As noted before, regular past tense forms tend to be phonologically more complex than irregular forms. Hence, one possibility is that the greater activation in this area for regulars reflects the processing of an auditory input with more complex phonological structure as the subject hears their own spoken response. It follows that this activation should not be present if the regulars and irregulars are matched for phonological complexity.
The results of the phonologically matched Gen Regular > Gen Irregular contrast (Figure 1D), in which regular and irregular past tense forms were matched pairwise on CV structure, support this prediction. No areas were activated more for regular forms. The activation pattern in other areas (those activated more for irregulars) is similar but reduced in extent (see Appendix B for details). A better matching of the difficulty of the stimuli may have led to this reduction in activation for irregulars, as suggested by a smaller difference in RT between the phonologically matched irregular and regular groups.
This decrease in the RT discrepancy also appears to reflect the importance of phonological features in the processing of the regular past tense. Specifically, when syllable length was allowed to vary, the syllable length of the regular stems was found to be strongly inversely correlated with the past tense generation RT, whereas no significant relationship was found between stem syllable length and RT for the irregular verbs. This finding may be due to differences in the typical phonological characteristics of regular and irregular verbs. The majority of irregular verbs are monosyllabic, whereas greater variability is possible with regular verb forms. Thus, greater syllable length may serve as a potent cue that the stem takes the regular past tense ending. The DS account argues against the possibility of any processing advantage for those regular verbs that are more phonologically similar to other regular verbs (Ullman, 1999). In contrast, in the SS perspective, the increased frequency of mappings between longer stems and the regular -ed ending should result in an associative facilitatory effect similar to that observed in the current study. Thus, the strong relationship between RT and stem syllable length for the regulars and the reduction in RT discrepancy when the stems are matched on this variable is more consistent with the latter account.
Another possible explanation for the reduced activation in the phonologically matched analysis is that because these stimulus sets contain fewer items (40 compared to 50 in the main contrast), the activation for regulars is reduced below threshold due to reduced statistical power. The activation for the phonologically mismatched contrast (Figure 1F), which is based on the same number of items as the matched contrast, again shows the STG activation for regulars, further supporting the interpretation of this activation as being related to the phonological complexity of the stimuli.
The hypothesis that this STG activation is simply due to the greater phonological complexity of the regular past tense forms, and not due to the generation of regular past tense, can be further tested by contrasting the reading of regular and irregular past tense forms. In this contrast (Figure 1G), the left STG is again activated for regulars, in addition to the left motor cortex. Relative to the mismatched Gen groups, these two Read groups actually showed a larger discrepancy in the number of syllables in the spoken response. The potentially greater difference in articulatory demands may have resulted in stronger activation in the motor cortex that was present but below threshold in other contrasts. When the past tense forms were matched on phonological complexity (as approximated by the number of phonemes), this temporal and motor cortex activation for regulars disappeared, resulting in no differential activation for either regulars or irregulars.
It is unlikely that the lack of activation for regular past tense generation (after phonological matching) is due to the lack of statistical power resulting from a small number of subjects or a small number of stimuli per condition, or due to overly stringent statistical thresholds. Here, a threshold of voxelwise p < .001 and corrected mapwise p < .05 was used. Reducing the voxelwise threshold to .01 (requiring larger volume correction), or increasing it to .0001 (requiring smaller volume correction), also showed no activation for regulars after correction for multiple comparisons (mapwise p < .05) in the phonologically matched contrasts.
Summary and Conclusions
We examined the activation patterns associated with regular and irregular past tense generation, as well as those for reading the present and past tense forms of regular and irregular verbs. Several results emerge: (1) In agreement with previous studies, irregular past tense generation requires more time and is less accurate than regular generation. (2) Regular and irregular past tense generation activate very similar brain regions compared to the reading of their respective stems. (3) When regular and irregular past tense generation are contrasted directly, no areas are more activated for regulars if the past tense forms are matched on phonological complexity (CV structure). (4) Inflection of irregulars activates inferior frontal and parietal regions, in addition to the anterior insula and basal ganglia, relative to regulars. All these areas are also activated by regular past tense generation compared to reading. Thus, they are not exclusively activated by either regular or irregular generation. (5) These regions activated more strongly by irregulars are commonly associated with executive control, decision-making, and attentional processes, and all were modulated by RT in both regular and irregular generation conditions. (6) The left IFG and Broca's area, regions proposed be involved in grammatical processing and regular rule application by the DS theory, were actually activated more by irregular than by regular inflection, probably reflecting the greater demands on working memory, attention, and prepotent response inhibition in the case of irregulars.
Thus, the results show that the set of brain regions activated during regular past tense generation is a proper subset of those activated during irregular generation. Regardless of the interpretation of the fronto-parietal areas activated more strongly by the irregulars, this result provides only a single dissociation between regular and irregular generation. Hence, DS theories that propose categorically different subsystems for regular and irregular generation are not supported by this result. Furthermore, the areas activated more strongly for irregulars are thought to subserve domain-general processes associated with more demanding tasks. This fact, coupled with the fact that these areas are activated both for regular and irregular generation compared to reading, lends support to an SS theory in which both regular and irregular forms are processed through an integrated system, but may depend more or less heavily on different components of the system (e.g., irregulars on semantics and regulars on phonology to the extent that phonological complexity is mismatched).
From the point of view of DS theory, it is possible to explain these results by suggesting that both regular and irregular past tense subsystems are activated in parallel, and the outcome is differentiated only by which process runs to completion. The computational details of such a parallel processing model are not very clear, but it may be that the differences between regulars and irregulars predicted by such a model are simply not detectable with the spatial and temporal resolution of fMRI. To the extent that fMRI can usefully speak to this debate, however, the evidence at present suggests an integrated system for regular and irregular past tense generation.
METHODS
Subjects
Participants were 25 healthy adults (15 women), 20–47 years of age, with no history of neurological or hearing impairments. The participants were native speakers of English, and all were right-handed according to the Edinburgh Handedness Inventory (Oldfield, 1971). The data from seven other subjects were excluded due to poor behavioral performance. The behavioral performance threshold for inclusion was set at 75% accuracy in both irregular and regular generation. Because we eliminated incorrect trials from the analysis, a large number of errors can affect the statistical reliability of the results. In accordance with a protocol sanctioned by the Medical College of Wisconsin Institutional Review Board, informed consent was obtained from each subject prior to the experiment.
Experimental Paradigm
The subjects performed two basic tasks. In the Gen task, regular and irregular verb stems were presented visually and the subjects said the past tenses of the verbs aloud. In the Read task, subjects read regular and irregular verb forms aloud. PsyScope software (Cohen, MacWhinney, Flatt, & Provost, 1993) was used to present the stimuli. In the Read task, verbs were presented in the present tense for half of the trials (ReadPres condition), and in the past tense for the other half (ReadPast condition). The ReadPres and ReadPast conditions were randomly intermixed. The words were presented in lower case for 1500 msec and were then replaced by a fixation cross. The subjects were instructed to give their response as quickly and accurately as possible.
The Gen and the Read tasks were performed in seven alternating runs, in the sequence R-G-R-G-R-G-R. The instructions “Please read the following words aloud” and “Please say the past tenses of the following words aloud” were displayed before each Read and Gen run, respectively. The order of the stimuli in each run was pseudorandomized, such that there were no more than three consecutive regular or irregular words in any run. To avoid any order effects, three separate pseudorandom orders of the presentation were created, and approximately one-third of the subjects were presented with each order. Consecutive presentation of words that are semantically closely related (e.g., buy and steal) was also avoided. A baseline visual stimulus “+++++” was inserted pseudorandomly between the trials, at an interval varying from two to four trials. The subjects were instructed to remain silent during these baseline trials. A total of 55 images were collected in each run, including 40 task-related (Gen or Read) images and 15 baseline images. An additional image, collected after the presentation of the instruction at the beginning of each run, was discarded.
Before the scan, subjects completed a short practice session outside the scanner for familiarization with the task, consisting of 10 Gen and 10 Read stimuli with five regular and five irregular verbs in each group. These 20 practice verbs were not used during scanning.
The responses of the subjects were transcribed online by an experimenter and were also recorded on digital audio tape. The recordings were referred to in the few cases where the experimenter was uncertain regarding the response of the subject.
Stimuli and Subgroups
One hundred regular and 100 irregular verbs were used in the experiment. Sixty verbs from each group were used in the Gen condition and the other 40 verbs from each group and their past tense forms were used in the Read condition (see Appendix A). For some verbs, both regular and irregular past tense forms are acceptable (e.g., speed → speeded/sped, knit → knitted/knit, dive → dived/dove). No such “doublet” verbs were used in the experiment.
In each of the three Gen runs, there were 20 regular and 20 irregular verb stems, resulting in a total of 60 regular and 60 irregular Gen trials. Similarly, in each of the four Read runs, there were 20 regular and 20 irregular verbs. Half of those verbs were in the ReadPres condition and the other half were in the ReadPast condition, giving a total of 80 ReadPres and 80 ReadPast trials. The presentation of stimuli in the ReadPres and ReadPast conditions was counterbalanced such that for half of the verbs, the present tense was seen first, whereas the past tense was seen first for the other half.
A number of subgroups were created from the Gen and Read stimuli, matched or mismatched on various parameters, to control and examine the effects of various factors that can potentially affect the fMRI and behavioral results. These subgroups are summarized in Table 3. A group of 50 regular and 50 irregular verbs, matched on the number of syllables in the stem and past tense forms, number of letters in the stem, log frequency of the past tense forms in the CELEX database (Baayen, Piepenbrock, and Gulikers, 1995), and FER, was created to examine the main effect of regular versus irregular past tense generation. Representative of typical regular and irregular verbs, the regular and irregular Gen groups were not matched with regard to phonological complexity. Each Gen group was also matched to two 40-item Read groups: (a) a ReadPres set matched on letter length of the stem, number of syllables in the stem, log frequency of the past tense form, and FER; and (b) a ReadPast set matched on number of syllables in the past tense, log frequency of the past tense form, and FER. The ReadPast groups were not matched with regard to phonological complexity.
To examine the effects of phonological complexity, 40 regular and 40 irregular verbs used in the Gen conditions, matched pairwise on CV structure and frequency, were chosen. For a direct comparison, 40 regular and 40 irregular verbs were chosen that were mismatched on phonological complexity (as approximated by the number of phonemes) and matched on frequency. Similar groups of 25 phonologically matched and mismatched verbs were selected from the sets used for the ReadPast condition. It was not possible to pairwise-match the CV structure for these groups, hence, the number of phonemes was used as an approximation of phonological complexity.
Image Acquisition and Analysis
A 1.5-T GE Signa scanner was used to acquire images. Using clustered (or “sparse”) acquisition, one volume of T2*-weighted, gradient-echo, echo-planar images (TE = 40 msec, flip angle = 90°, NEX = 1) was acquired every 7 sec. Acquisition time was 2300 msec, leaving 4700 msec of silence between images, during which the visual stimulus was presented. Volumes were composed of 21 sagittal, contiguous slices with 3.75 × 3.75 × 6.5 mm voxel dimensions. Anatomical images of the entire brain were obtained using a 3-D spoiled-gradient-echo sequence (“SPGR”) as a set of 124 contiguous sagittal slices with 0.9 × 0.9 × 1.2 mm voxel dimensions.
AFNI software package (Cox, 1996) was used for image analysis. Within-subject analysis involved spatial co-registration (Cox & Jesmanowicz, 1999) to minimize motion artifacts, and voxelwise multiple linear regression with reference functions representing the stimulus conditions compared to the baseline. A Gaussian kernel of 5 mm FWHM was used for smoothing prior to the regression analyses. Translation and rotation movement parameters estimated during image registration were included in the regression model to remove residual variance associated with motion-related changes in BOLD signal. General linear tests were conducted to obtain various contrasts between conditions. Incorrect trials were removed from the analysis. The individual statistical maps and the anatomical scans were projected into standard stereotaxic space (Talairach & Tournoux, 1998) by linear resampling, and group maps were created in a random-effects analysis. The group maps were thresholded at voxelwise p < .001, and corrected for multiple comparisons by removing clusters smaller than four voxels (365 μl), to a mapwise two-tailed p < .05. The cluster threshold was determined through Monte Carlo simulations that provide the chance probability of spatially contiguous voxels. Only the voxels inside the brain were used in the analyses, so that fewer comparisons are performed and a smaller volume correction is required.
To estimate the areas whose activation is correlated with time on task regardless of stimulus condition, the analyses were repeated after including two condition-specific regressors with individual trial-by-trial RTs for Gen Regular and Irregular conditions. The RTs were adjusted by removing the mean RT of the condition for each subject. Each regressor represents within-condition RT variance. A conjunction of these two regressors was computed to estimate the areas that are modulated by RT independently of the Gen condition.
APPENDIX A.
(a) Items used in the Generation task. The first 40 items are part of the phonologically matched Generate groups. Membership in other groups is indicated by a “y” in the corresponding column (see Table 2 for legends).
| Regular Verb | CV Structure | F | GenR | GenRN | Irregular Verb | CV Structure | F | GenI | GenIN | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | spray | CCCVC | 0 | y | y | stride | CCCVC | 0.301 | y | |
| 2 | glow | CCVC | 0 | y | bleed | CCVC | 0 | y | ||
| 3 | sway | CCVC | 0 | y | freeze | CCVC | 0 | y | ||
| 4 | flow | CCVC | 0.301 | y | cling | CCVC | 0.301 | y | y | |
| 5 | free | CCVC | 0.301 | y | spin | CCVC | 0.301 | y | ||
| 6 | fry | CCVC | 0.301 | y | y | steal | CCVC | 0.301 | y | y |
| 7 | pray | CCVC | 0.301 | y | y | swear | CCVC | 0.301 | y | y |
| 8 | slow | CCVC | 0.301 | y | y | flee | CCVC | 0.4771 | y | |
| 9 | view | CCVC | 0.4771 | y | y | slide | CCVC | 0.4771 | y | y |
| 10 | dry | CCVC | 0.699 | y | y | fling | CCVC | 0.6021 | y | y |
| 11 | cry | CCVC | 1 | y | y | swing | CCVC | 0.6021 | y | y |
| 12 | stay | CCVC | 1.04 | y | break | CCVC | 1.2041 | y | y | |
| 13 | play | CCVC | 1.3617 | y | y | speak | CCVC | 1.4624 | y | y |
| 14 | try | CCVC | 1.6232 | y | y | bring | CCVC | 1.699 | y | y |
| 15 | crawl | CCVCC | 0.301 | y | y | creep | CCVCC | 0.301 | y | y |
| 16 | slap | CCVCC | 0.301 | y | grind | CCVCC | 0.4771 | y | y | |
| 17 | train | CCVCC | 0.9031 | y | sweep | CCVCC | 0.7782 | |||
| 18 | drop | CCVCC | 1.1761 | y | sleep | CCVCC | 0.8451 | y | ||
| 19 | chew | CVC | 0 | y | bite | CVC | 0.301 | y | y | |
| 20 | weigh | CVC | 0.301 | y | dig | CVC | 0.4771 | |||
| 21 | sigh | CVC | 0.699 | y | ride | CVC | 0.6021 | y | y | |
| 22 | tie | CVC | 0.9031 | y | shake | CVC | 1.2304 | y | y | |
| 23 | show | CVC | 1.3222 | y | buy | CVC | 1.2553 | y | y | |
| 24 | die | CVC | 1.3979 | y | let | CVC | 1.6435 | y | y | |
| 25 | bake | CVCC | 0.301 | y | bind | CVCC | 0.301 | y | ||
| 26 | care | CVCC | 0.6021 | y | weep | CVCC | 0.301 | y | ||
| 27 | guess | CVCC | 0.6021 | cost | CVCC | 0.6021 | ||||
| 28 | wipe | CVCC | 0.6021 | deal | CVCC | 0.699 | y | |||
| 29 | fear | CVCC | 0.7782 | y | hurt | CVCC | 0.7782 | y | ||
| 30 | gain | CVCC | 0.7782 | bend | CVCC | 0.8451 | y | y | ||
| 31 | fill | CVCC | 1.1761 | sell | CVCC | 1.0414 | y | y | ||
| 32 | fail | CVCC | 1.2041 | build | CVCC | 1.3979 | y | |||
| 33 | pull | CVCC | 1.2553 | y | mean | CVCC | 1.4624 | y | y | |
| 34 | like | CVCC | 1.301 | y | y | keep | CVCC | 1.6232 | ||
| 35 | live | CVCC | 1.4472 | y | y | lose | CVCC | 1.6232 | y | |
| 36 | reach | CVCC | 1.4472 | y | hold | CVCC | 1.6335 | y | ||
| 37 | use | CVCC | 1.8692 | y | y | leave | CVCC | 1.9243 | y | y |
| 38 | seem | CVCC | 1.9191 | y | find | CVCC | 1.9823 | |||
| 39 | call | CVCC | 1.9243 | y | y | tell | CVCC | 1.9912 | y | y |
| 40 | link | CVCCC | 0.699 | y | y | burst | CVCCC | 0.4771 | ||
|
| ||||||||||
| 41 | swallow | CCVCVC | 0.4771 | y | y | undertake | VCCVCCVC | 0 | y | |
| 42 | succeed | CVCCVCVC | 0.7782 | y | y | undergo | VCCVCCVCC | 0 | y | |
| 43 | smear | CCVCC | 0 | y | y | teach | CVC | 1.1139 | y | y |
| 44 | shriek | CCVCC | 0 | y | sting | CCVC | 0 | y | ||
| 45 | reveal | CVCVCC | 0.9031 | y | y | stick | CCVC | 0.9542 | y | y |
| 46 | return | CVCVCCC | 1.3424 | y | y | split | CCCVC | 0.4771 | y | y |
| 47 | repeat | CVCVCVC | 0.7782 | y | y | overthrow | VCVCCCV | 0 | y | |
| 48 | realize | CVVCVCC | 1.301 | y | y | oversee | VCVCCV | 0 | y | |
| 49 | provide | CCVCVCVC | 1.1461 | y | y | overhear | VCVCCVCC | 0 | y | y |
| 50 | proceed | CCVCVCVC | 0.4771 | y | y | outgrow | VCCCV | 0 | y | y |
| 51 | extend | VCCVCCVC | 0.8451 | y | y | offset | VCCVC | 0 | y | y |
| 52 | dread | CCVCVC | 0 | y | y | mislead | CVCCVC | 0 | y | y |
| 53 | disappear | CVCVCVCC | 0.8451 | y | y | know | CV | 2.0569 | y | y |
| 54 | confuse | CVCCVCC | 0.301 | y | y | hear | CVCC | 1.7559 | y | y |
| 55 | complete | CVCCCVCVC | 0.7782 | y | y | forget | CVCCVC | 0.7782 | y | y |
| 56 | combine | CVCCVCC | 0.9031 | y | y | fly | CCV | 0.7782 | y | y |
| 57 | blast | CCVCCVC | 0 | y | y | fight | CVC | 0.8451 | y | y |
| 58 | attach | VCVCC | 0.9031 | y | y | fall | CVC | 1.4314 | y | y |
| 59 | agree | VCCVC | 1.301 | y | y | beat | CVC | 0.7782 | y | y |
| 60 | achieve | VCVCC | 1.0792 | y | y | arise | VCVC | 0.6021 | y | y |
APPENDIX A.
(b) The items used in the Reading task. A “y” in a column indicates membership in that group (see Table 2 for legends).
| Regular Verb | Past | ReadRPastM | ReadRPastN | Irregular Verb | Past | ReadIPastM | ReadIPastN | |
|---|---|---|---|---|---|---|---|---|
| 1 | admit | admitted | y | eat | ate | y | ||
| 2 | appear | appeared | y | blow | blew | y | ||
| 3 | attend | attended | y | bear | bore | y | ||
| 4 | blink | blinked | y | y | breed | bred | y | y |
| 5 | clean | cleaned | y | y | catch | caught | y | y |
| 6 | clear | cleared | y | y | do | did | y | |
| 7 | compare | compared | y | drink | drank | y | ||
| 8 | dare | dared | y | drive | drove | y | y | |
| 9 | divide | divided | y | feed | fed | y | ||
| 10 | earn | earned | y | feel | felt | y | ||
| 11 | end | ended | y | forgive | forgave | y | ||
| 12 | fold | folded | y | grow | grew | y | ||
| 13 | follow | followed | y | hide | hid | y | ||
| 14 | glare | glared | y | y | lend | lent | y | y |
| 15 | greet | greeted | y | y | meet | met | y | |
| 16 | heat | heated | y | y | overdo | overdid | y | |
| 17 | intend | intended | y | overtake | overtook | y | ||
| 18 | invite | invited | y | rewrite | rewrote | y | y | |
| 19 | kick | kicked | y | rise | rose | y | ||
| 20 | last | lasted | y | y | see | sat | y | |
| 21 | leak | leaked | y | seek | saw | |||
| 22 | match | matched | y | send | sent | y | ||
| 23 | need | needed | y | shoot | shot | y | y | |
| 24 | permit | permitted | y | slay | slew | y | ||
| 25 | pick | picked | y | sling | slung | y | y | |
| 26 | receive | received | y | seek | sought | y | ||
| 27 | refuse | refused | y | spend | spent | y | y | |
| 28 | remind | reminded | y | spoonfeed | spoonfed | y | ||
| 29 | reply | replied | y | stand | stood | y | ||
| 30 | scratch | scratched | y | string | strung | y | y | |
| 31 | seal | sealed | y | swim | swam | y | y | |
| 32 | spare | spared | y | y | throw | threw | y | |
| 33 | stare | stared | y | underwrite | underwrote | y | ||
| 34 | stun | stunned | y | y | undo | undid | y | y |
| 35 | treat | treated | y | y | uphold | upheld | y | |
| 36 | trim | trimmed | y | withdraw | withdrew | y | ||
| 37 | trust | trusted | y | withhold | withheld | y | ||
| 38 | turn | turned | y | win | won | y | ||
| 39 | visit | visited | y | wear | wore | y | ||
| 40 | wink | winked | y | wring | wrung | y |
APPENDIX B.
The locations of peaks in the Talairach and Tournoux (1988) atlas. Only the peaks separated by other peaks by at least 20 mm are reported. (a) Generate Regular-Read Regular Present
| Structure | Approximate BA | z-score | x | y | z |
|---|---|---|---|---|---|
| L prCG | 6 | 7.1 | −49 | 1 | 24 |
| R SFG | 6 | 6.7 | 2 | 7 | 48 |
| L Putamen | 6.0 | −24 | 11 | 8 | |
| L ITG | 37 | 5.4 | −49 | −57 | −12 |
| L IPS | 40 | 5.2 | −54 | −33 | 35 |
| R prCG | 6 | 5.1 | 43 | −8 | 33 |
| L IPS | 40 | 5.0 | −33 | −46 | 39 |
| R Insula | 13 | 5.0 | 39 | 18 | −3 |
| L SPG | 7 | 4.9 | −27 | −66 | 43 |
| R FG | 37 | 4.7 | 42 | −53 | −18 |
| L IFG | 47/45 | 4.7 | −45 | 22 | 2 |
| R IPS | 40 | 4.6 | 44 | −38 | 45 |
| R STG | 22 | 4.6 | 63 | −8 | −2 |
| R STG | 22 | 4.5 | −44 | −25 | 2 |
| L CG | 24 | 4.4 | −5 | 14 | 27 |
| L IOG | 18 | 4.4 | −37 | −91 | −14 |
| R STG | 41/13 | 4.2 | 40 | −31 | 16 |
| R SPG | 7 | 4.2 | 24 | −67 | 51 |
| L Putamen | 4.0 | −21 | −7 | 17 | |
| R MTG | 22 | 3.9 | 56 | −36 | 5 |
| R MFG | 46 | 3.9 | 47 | 30 | 16 |
|
| |||||
| L AG | 39 | −5.6 | −54 | −62 | 26 |
| R AG | 39 | −5.0 | 45 | −64 | 25 |
| L Precuneus | 7 | −4.2 | −7 | −61 | 38 |
| L AC | 32 | −4.0 | −5 | 44 | −7 |
| L PHG | 36 | −4.0 | −31 | −25 | −12 |
SFG = superior frontal gyrus; MFG = middle frontal gyrus; IFG = inferior frontal gyrus; prCG = precentral gyrus; poCG = postcentral gyrus; STG = superior temporal gyrus; MTG= middle temporal gyrus; ITG = inferior temporal gyrus; FG = fusiform gyrus; SPG = superior parietal gyrus; IPS = intraparietal sulcus; SG = supramarginal gyrus; AG = angular gyrus; LG = lingual gyrus; MOG = middle occipital gyrus; IOG = inferior occipital gyrus; PHG = parahippocampal gyrus; CG = cingulate gyrus; N = nucleus.
APPENDIX B.
(b) Generate Irregular-Read Irregular Present
| Structure | Approximate BA | z-score | x | y | z |
|---|---|---|---|---|---|
| L prCG | 6 | 6.7 | −50 | 2 | 26 |
| L Insula | 13 | 6.2 | −31 | 13 | 7 |
| R SFG | 6 | 6.2 | 2 | 7 | 47 |
| L SG | 40 | 5.6 | −40 | −45 | 33 |
| L prCG | 6 | 5.5 | −36 | −10 | 38 |
| L SFG | 6 | 5.4 | −14 | 0 | 62 |
| R Caudate | 5.3 | 12 | 1 | 11 | |
| L CG | 24 | 5.2 | −7 | −3 | 26 |
| L Precuneus/IPS | 7/19 | 5.1 | −23 | −72 | 28 |
| R Insula | 13 | 5.0 | 44 | 13 | 2 |
| L Lentiform N | 5.0 | −13 | −1 | 1 | |
| R IPS | 7 | 5.0 | 27 | −65 | 27 |
| R Red N | 4.8 | −3 | −19 | −16 | |
| L LG | 18 | 4.8 | −17 | −67 | 0 |
| L IPS | 40 | 4.8 | −62 | −38 | 39 |
| R prCG | 6 | 4.8 | 39 | −9 | 34 |
| L FG/ITG | 37 | 4.7 | −40 | −53 | −14 |
| L STG | 22 | 4.6 | −46 | −22 | 2 |
| R SPG | 7 | 4.4 | 25 | −66 | 51 |
| R MFG | 46 | 4.3 | 46 | 25 | 23 |
| L MOG | 19 | 4.2 | −30 | −90 | 9 |
| L Cuneus | 19 | 4.1 | −10 | −88 | 32 |
| R prCG | 6 | 4.0 | 41 | −9 | 56 |
| R MOG | 18 | 4.0 | 30 | −83 | −9 |
| L PC | 23 | 4.0 | −6 | −33 | 20 |
| R FOG | 11 | 3.8 | 25 | 41 | −8 |
|
| |||||
| L MFG | 8 | −4.4 | −22 | 21 | 38 |
| R AG | 39 | −4.4 | 53 | −55 | 25 |
| L AG | 39 | −4.2 | −53 | −66 | 33 |
APPENDIX B.
(c) Generate Irregular–Generate Regular
| Structure | Approximate BA | z-score | x | y | z |
|---|---|---|---|---|---|
| R Caudate | 5.6 | 7 | 3 | 6 | |
| L IPS | 40 | 5.5 | −32 | −56 | 39 |
| L IFG | 9 | 5.5 | −48 | 5 | 23 |
| R IPS | 19 | 5.4 | 32 | −64 | 39 |
| R IFG/Insula | 13 | 5.2 | 35 | 23 | 6 |
| R prCG | 6 | 5.1 | 46 | 2 | 33 |
| R MOG | 19 | 4.8 | −29 | −74 | 20 |
| L ITG | 37 | 4.4 | −43 | −53 | −5 |
| L IFG | 45/47 | 4.1 | −48 | 29 | 5 |
| L prCG | 6 | 4.0 | −45 | −6 | 40 |
| L Thalamus | 3.9 | −12 | −27 | 7 | |
|
| |||||
| L STG | 41 | −4.2 | −54 | −17 | 8 |
| R SG | 40 | −4.1 | 63 | −38 | 27 |
APPENDIX B.
(d) Generate Irregular–Generate Regular (phonologically matched)
| Structure | Approximate BA | z-score | x | y | z |
|---|---|---|---|---|---|
| R Caudate | 5.6 | 5 | 1 | 7 | |
| L IPS | 40 | 5.3 | −38 | −52 | 37 |
| R IPS | 7 | 4.9 | 34 | −64 | 44 |
| L prCG | 6 | 4.9 | −45 | −1 | 34 |
| R IFG/Insula | 13/47 | 4.6 | 43 | 13 | −9 |
| R prCG | 6 | 4.5 | 33 | −8 | 40 |
| R MFG | 46 | 4.5 | 46 | 23 | 28 |
| L CG | 32/8 | 4.3 | −9 | 26 | 39 |
| L MOG | 19 | 4.1 | −29 | −73 | 20 |
| L Thalamus | 4.0 | −7 | −19 | 1 | |
| L IFG | 45/47 | 3.9 | −44 | 30 | 4 |
APPENDIX B.
(e) Areas Correlated with RT Across Conditions
| Structure | Approximate BA | z-score | x | y | z |
|---|---|---|---|---|---|
| L SFG | 6 | 6.8 | −6 | 7 | 49 |
| R IFG/Insula | 13/47 | 6.2 | 32 | 19 | 4 |
| L Putamen | 6.0 | −15 | 4 | 10 | |
| R Caudate | 6.0 | 13 | 7 | 6 | |
| L prCG | 6 | 5.9 | −41 | −4 | 40 |
| R Red N | 5.8 | 4 | −25 | −1 | |
| L CG | 32 | 5.6 | −1 | 21 | 35 |
| R SFG | 6 | 5.6 | 12 | −1 | 64 |
| L IFG/Insula | 47/13 | 5.2 | −45 | 14 | −5 |
| L IPS | 40 | 5.0 | −39 | −52 | 38 |
| R LG | 19 | 5.0 | 17 | −52 | −4 |
| R prCG | 6 | 5.0 | 45 | 1 | 32 |
| L Culmen | 4.8 | −12 | −54 | −9 | |
| R SFG | 9 | 4.8 | 32 | 48 | 28 |
| R STG | 22 | 4.7 | 54 | −24 | 7 |
| R IPS | 40 | 4.7 | 39 | −54 | 39 |
| R Culmen | 4.6 | 32 | −59 | −28 | |
| L prCG | 4 | 4.6 | −58 | −3 | 20 |
| L Cerebellum | 4.4 | −13 | −76 | −35 | |
| L MFG | 46 | 4.4 | −45 | 21 | 22 |
| R CG | 23 | 4.4 | 1 | −30 | 26 |
| R poCG | 7 | 4.3 | 8 | −48 | 67 |
| L FG | 37 | 4.3 | −49 | −56 | −9 |
| R IFG | 46 | 4.3 | 47 | 41 | 1 |
| L Precuneus | 7 | 4.1 | −12 | −54 | 60 |
| R SFG | 10 | 4.1 | 23 | 46 | −3 |
| R STG | 22 | 4.0 | 41 | −16 | −9 |
| L MFG | 10 | 3.9 | −30 | 52 | 1 |
| R Cuneus | 19 | 3.9 | 6 | −87 | 36 |
| L prCG | 4 | 3.8 | −8 | −30 | 69 |
| L PHG | 36 | 3.8 | −28 | −32 | −19 |
|
| |||||
| L Insula | 13 | −4.5 | −41 | −10 | 15 |
| L Insula | 13 | −3.8 | −40 | −3 | −6 |
APPENDIX B.
(f) Generate Irregular–Generate Regular (phonologically mismatched)
| Structure | Approximate BA | z-score | x | y | z |
|---|---|---|---|---|---|
| L prCG | 6 | 6.0 | −48 | 6 | 26 |
| L IPS | 40 | 5.6 | −33 | −57 | 39 |
| R prCG | 6 | 5.1 | 46 | 2 | 32 |
| R IFG/Insula | 13 | 4.7 | 34 | 25 | 5 |
| L prCG | 6 | 4.6 | −36 | −4 | 40 |
| R IPS | 7 | 4.6 | 32 | −65 | 39 |
| R Caudate | 4.5 | 10 | 7 | 17 | |
| L Thalamus | 4.4 | −4 | −20 | 2 | |
| L ITG | 37 | 4.1 | −47 | −52 | −19 |
| L MOG | 19 | 4.0 | −30 | −73 | 20 |
| L SFG | 8 | 3.9 | −10 | 25 | 40 |
| R SFG | 6 | 3.9 | 2 | 9 | 47 |
|
| |||||
| L STG | 41 | −4.2 | −50 | −18 | 4 |
APPENDIX B.
(g) Read Irregular Past–Read Regular Past (phonologically matched): None. (h) Read Irregular Past–Read Regular Past (phonologically mismatched)
| Structure | Approximate BA | z-score | x | y | z |
|---|---|---|---|---|---|
| L prCG | 4 | −5.5 | −49 | −12 | 46 |
| L STG | 22 | −5.5 | −55 | −11 | 3 |
| R STG | 22 | −4.5 | 54 | −8 | −7 |
| L STG | 41 | −4.5 | −39 | −36 | 11 |
Acknowledgments
Supported by National Institute of Neurological Diseases and Stroke grant R01 NS33576 and National Institutes of Health General Clinical Research Center grant M01 RR00058. We thank anonymous reviewers for their helpful comments.
APPENDIX
See Appendix A and Appendix B in the Figures and Tables section.
Footnotes
The data reported in this experiment have been deposited in the fMRI Data Center (www.fmridc.org). The accession number is 2-2005-119G2.
The FER of a verb is defined as follows. The verbs that rhyme with a given verb and form their past tenses in the same way are called “friends” of that verb (e.g., sleep and weep are friends). The verbs that rhyme but form their past tenses in a different way are the “enemies” of that verb (e.g., drink, blink, and think are enemies of each other). The FER of a verb is log(f)/log(e), where f is the sum of the frequencies of the past tense forms of the friends of the verb, and e is the corresponding value for the enemies. The effects of FER manipulation will be reported elsewhere.
REFERENCES
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits—Parallel substrates for motor, oculomotor, prefrontal and limbic functions. In: Uylings HBM, Van Eden CG, DeBruin JPC, Corner MA, Feenstra MGP, editors. Progress in brain research. Vol. 85. Elsevier; New York: 1990. pp. 119–146. [PubMed] [Google Scholar]
- Baayen RH, Piepenbrock R, Gulikers L. The CELEX Lexical Database [CD-ROM] Philadelphia: 1995. [Google Scholar]
- Beretta A, Campbell C, Carr TH, Huang J, Schmitt LM, Christianson K, Cao Y. An ER-fMRI investigation of morphological inflection in German reveals that the brain makes a distinction between regular and irregular forms. Brain and Language. 2003;85:67–92. doi: 10.1016/s0093-934x(02)00560-6. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study. Journal of Cognitive Neuroscience. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Springer JA, Kaufman JN, Possing ET. Human temporal lobe activation by speech and nonspeech sounds. Cerebral Cortex. 2000;10:512–528. doi: 10.1093/cercor/10.5.512. [DOI] [PubMed] [Google Scholar]
- Binder JR, Liebenthal E, Possing ET, Medler DA, Ward WD. Neural correlates of sensory and decision processes in speech perception. Nature Neuroscience. 2004;7:295–301. doi: 10.1038/nn1198. [DOI] [PubMed] [Google Scholar]
- Binder JR, Mohr JP. The topography of callosal reading pathways. A case-control analysis. Brain. 1992;115:1807–1826. doi: 10.1093/brain/115.6.1807. [DOI] [PubMed] [Google Scholar]
- Bird H, Lambon Ralph MA, Seidenberg MS, McClelland JL, Patterson K. Deficits in phonology and past-tense morphology: What's the connection? Journal of Memory and Language. 2003;48:502–526. [Google Scholar]
- Burzio L. Missing players: Phonology and the past-tense debate. Lingua. 2002;112:157–199. [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J. PsyScope: A new graphic interactive environment for designing psychology experiments. Behavioral Research Methods, Instruments, and Computers. 1993;25:257–271. [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex? Functional properties of the visual word form area. Brain. 2002;125:1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A. Real-time 3D image registration of functional MRI. Magnetic Resonance in Medicine. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Culham JC, Kanwisher NG. Neuroimaging of cognitive functions in human parietal cortex. Current Opinions in Neurobiology. 2001;11:157–163. doi: 10.1016/s0959-4388(00)00191-4. [DOI] [PubMed] [Google Scholar]
- Dassonville P, Lewis SM, Zhu XH, Ugurbil K, Kim SG, Ashe J. The effect of stimulus-response compatibility on cortical motor activation. Neuroimage. 2001;13:1–14. doi: 10.1006/nimg.2000.0671. [DOI] [PubMed] [Google Scholar]
- Davis MH, Johnsrude IS. Hierarchical processing in spoken language comprehension. Journal of Neuroscience. 2003;23:3423–3431. doi: 10.1523/JNEUROSCI.23-08-03423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Cognitive Brain Research. 1998;7:1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- Dhond RP, Marinkovic K, Dale AM, Witzel T, Halgren E. Spatiotemporal maps of past-tense verb inflection. Neuroimage. 2003;19:91–100. doi: 10.1016/s1053-8119(03)00047-8. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Kim YH, Meyer JR, Mesulam M. A large-scale distributed network for covert spatial attention: Further anatomical delineation based on stringent behavioural and cognitive controls. Brain. 1999;122:1093–1106. doi: 10.1093/brain/122.6.1093. [DOI] [PubMed] [Google Scholar]
- Grodzinsky Y. The neurology of syntax: Language use without Broca's area. Behavioral and Brain Sciences. 2000;23:1–71. doi: 10.1017/s0140525x00002399. [DOI] [PubMed] [Google Scholar]
- Hall DA, Hart HC, Johnsrude IS. Relationships between human auditory cortical structure and function. Audiology and Neuro-Otology. 2003;8:1–18. doi: 10.1159/000067894. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Brown C, Hagoort P, Herzog H, Sach M, Seitz R. A PET study of cerebral activation patterns induced by verb inflection. Neuroimage. 1997;5:S548. [Google Scholar]
- Jaeger JJ, Lockwood AH, Kemmerer DL, Van Valin RD, Jr., Murphy BW, Khalak HG. A positron emission tomographic study of regular and irregular verb morphology in English. Language. 1996;72:451–497. [Google Scholar]
- Jiang Y, Kanwisher N. Common neural mechanisms for response selection and perceptual processing. Journal of Cognitive Neuroscience. 2003a;15:1095–1110. doi: 10.1162/089892903322598076. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Kanwisher N. Common neural substrates for response selection across modalities and mapping paradigms. Journal of Cognitive Neuroscience. 2003b;15:1080–1094. doi: 10.1162/089892903322598067. [DOI] [PubMed] [Google Scholar]
- Joanisse M, Seidenberg MS. Impairments in verb morphology after brain injury: A connectionist model. Proceedings of the National Academy of Sciences, U.S.A. 1999;96:7592–7597. doi: 10.1073/pnas.96.13.7592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annual Review of Neuroscience. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Mesulam M. Neuroanatomic overlap of working memory and spatial attention networks: A functional MRI comparison within subjects. Neuroimage. 1999;10:695–704. doi: 10.1006/nimg.1999.0503. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Braber N, McClelland JL, Patterson K. What underlies the neuropsychological pattern of irregular > regular past-tense verb production? Brain and Language. 2005;93:106–119. doi: 10.1016/j.bandl.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Lavric A, Pizzagalli D, Forstmeier S, Rippon G. A double-dissociation of English past-tense production revealed by event-related potentials and low-resolution electromagnetic tomography (LORETA) Clinical Neurophysiology. 2001;112:1833–1849. doi: 10.1016/s1388-2457(01)00615-0. [DOI] [PubMed] [Google Scholar]
- Marchman VA. Children's productivity in the English past tense: The role of frequency, phonology, an neighborhood structure. Cognitive Science. 1997;21:283–303. [Google Scholar]
- Marslen-Wilson WD, Tyler LK. Rules, representations, and the English past-tense. Trends in Cognitive Sciences. 1998;2:428–435. doi: 10.1016/s1364-6613(98)01239-x. [DOI] [PubMed] [Google Scholar]
- McClelland JL, Patterson K. Rules or connections in past-tense inflections: What does the evidence rule out? Trends in Cognitive Sciences. 2002;6:465–472. doi: 10.1016/s1364-6613(02)01993-9. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaing. Journal of Cognitive Neuroscience. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Munte TF, Say T, Clahsen H, Schiltz K, Kutas M. Decomposition of morphologically complex words in English: Evidence from event-related brain potentials. Cognitive Brain Research. 1999;7:241–253. doi: 10.1016/s0926-6410(98)00028-7. [DOI] [PubMed] [Google Scholar]
- Musso M, Moro A, Glauche V, Rijntjes M, Reichenbach J, Buchel C, Weiller C. Broca's area and the language instinct. Nature Neuroscience. 2003;6:774–781. doi: 10.1038/nn1077. [DOI] [PubMed] [Google Scholar]
- Newman A, Izvorski R, Davis L, Neville H, Ullman MT. Distinct electrophysiological patterns in the processing of regular and irregular verbs. Journal of Cognitive Neuroscience. 1999:47–48. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Patterson K, Lambon Ralph MA, Hodges JR, McClelland JL. Deficits in irregular past-tense verb morphology associated with degraded semantic knowledge. Neuropsychologia. 2001;39:709–724. doi: 10.1016/s0028-3932(01)00008-2. [DOI] [PubMed] [Google Scholar]
- Penke M, Weyerts H, Gross M, Zander E, Munte TF, Clahsen H. How the brain processes complex words: An event-related potential study of German verb inflections. Cognitive Brain Research. 1997;6:37–52. doi: 10.1016/s0926-6410(97)00012-8. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. Journal of Comparative Neurology. 1984;228:105–116. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- Pinker S. Rules of language. Science. 1991;253:530–535. doi: 10.1126/science.1857983. [DOI] [PubMed] [Google Scholar]
- Pinker S. Words and rules: The ingredients of language. HarperCollins; New York: 1999. [Google Scholar]
- Pinker S, Ullman MT. The past and future of the past tense. Trends in Cognitive Sciences. 2002;6:456–463. doi: 10.1016/s1364-6613(02)01990-3. [DOI] [PubMed] [Google Scholar]
- Plunkett K, Nakisa RC. A connectionist model of the Arabic plural system. Language and Cognitive Processes. 1997;12:807–836. [Google Scholar]
- Price CJ, Devlin JT. The myth of the visual word form area. Neuroimage. 2003;19:473–481. doi: 10.1016/s1053-8119(03)00084-3. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences, U.S.A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Fornells A, Clahsen H, Lleo C, Zaake W, Munte TF. Event-related brain responses to morphological violations in Catalan. Cognitive Brain Research. 2001;11:47–58. doi: 10.1016/s0926-6410(00)00063-x. [DOI] [PubMed] [Google Scholar]
- Rumelhart D, McClelland J. Parallel distributed processing: Explorations in the microstructure of cognition. MIT Press; Cambridge: 1986. On learning the past tenses of English verbs; pp. 216–271. [Google Scholar]
- Sach M, Seitz RJ, Indefrey P. Unified inflectional processing of regular and irregular verbs: A PET study. NeuroReport. 2004;15:533–537. doi: 10.1097/00001756-200403010-00030. [DOI] [PubMed] [Google Scholar]
- Schumacher EH, D'Esposito M. Neural implementation of response selection in humans as revealed by localized effects of stimulus-response compatibility on brain activation. Human Brain Mapping. 2002;17:193–201. doi: 10.1002/hbm.10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenberg MS. Connectionism without tears. In: Davis S, editor. Connectionism: Theory and practice. Oxford University Press; New York: 1992. pp. 84–137. [Google Scholar]
- Seidenberg MS, Arnoldussen A. The brain makes a distinction between hard and easy stimuli: Comments on Beretta et al. Brain & Language. 2003;85:527–530. doi: 10.1016/s0093-934x(03)00065-8. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Corbetta M, Buckner RL, Raichle ME, Fiez JA, Miezin FM, Petersen SE. Top-down modulation of early sensory cortex. Cerebral Cortex. 1997;7:193–206. doi: 10.1093/cercor/7.3.193. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme Medical; New York: 1988. [Google Scholar]
- Tyler LK, deMornay-Davies P, Anokhina R, Longworth C, Randall B, Marslen-Wilson WD. Dissociations in processing past tense morphology: Neuropathology and behavioral studies. Journal of Cognitive Neuroscience. 2002;14:79–94. doi: 10.1162/089892902317205348. [DOI] [PubMed] [Google Scholar]
- Ullman MT. Acceptability ratings of regular and irregular past tense forms: Evidence for a dual-system model of language from word frequency and phonological neighbourhood effects. Language and Cognitive Processes. 1999;14:47–67. [Google Scholar]
- Ullman MT. Contributions of memory circuits to language: The declarative/procedural model. Cognition. 2004;92:231–270. doi: 10.1016/j.cognition.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Ullman MT. Evidence that lexical memory is part of the temporal lobe declarative memory, and that grammatical rules are processed by the frontal/basal-ganglia procedural system. Brain and Language. doi: 10.1162/jocn.1997.9.2.266. in press. [DOI] [PubMed] [Google Scholar]
- Ullman MT, Bergida R, O'Craven K. Distinct fMRI activation patterns for regular and irregular past tense. Neuroimage. 1997;5:S549. [Google Scholar]
- Ullman MT, Corkin S, Coppola M, Hickok G, Growdon JH, Koroshetz WJ, Pinker S. A neural dissociation within language: Evidence that the mental dictionary is part of declarative memory, and that grammatical rules are processed by the procedural system. Journal of Cognitive Neuroscience. 1997;9:266–276. doi: 10.1162/jocn.1997.9.2.266. [DOI] [PubMed] [Google Scholar]


