Abstract
Background
Constitutive activation of MEK1 (caMEK) can induce the oncogenic transformation of normal intestinal epithelial cells. To define the genetic changes that occur during this process, we used oligonucleotide microarrays to determine which genes are regulated following the constitutive activation of MEK in normal intestinal epithelial cells.
Results
Microarray analysis was performed using Affymetrix GeneChip and total RNA from doxycycline inducible RIEtiCAMEK cells in the presence or absence of doxycycline. MEK-activation induced at least a three-fold difference in 115 gene transcripts (75 transcripts were up-regulated, and 40 transcripts were down-regulated). To verify whether these mRNAs are indeed regulated by the constitutive activation of MEK, RT-PCR analysis was performed using the samples from caMEK expressing RIE cells (RIEcCAMEK cells) as well as RIEtiCAMEK cells. The altered expression level of 69 gene transcripts was confirmed. Sixty-one of the differentially expressed genes have previously been implicated in cellular transformation or tumorogenesis. For the remaining 8 genes (or their human homolog), RT-PCR analysis was performed on RNA from human colon cancer cell lines and matched normal and tumor colon cancer tissues from human patients, revealing three novel targets (rat brain serine protease2, AMP deaminase 3, and cartilage link protein 1).
Conclusion
Following MEK-activation, many tumor-associated genes were found to have significantly altered expression levels. However, we identified three genes that were differentially expressed in caMEK cells and human colorectal cancers, which have not been previously linked to cellular transformation or tumorogenesis.
Background
Mitogen-activated protein kinases (MAPKs) are serine-threonine kinases activated by phosphorylation of specific amino acids in response to extracellular stimuli and have been shown to play an important role in tumorigenesis [1-8]. The first member of this family to be characterized was the extracellular signal-regulated protein kinase (ERK), which is phosphorylated and activated by MAPK/ERK kinase (MEK) [1,2]. The MEK-ERK signaling pathway is one of the downstream targets of oncogenic mutations in ras [1,2] and the increased activity of MEK has been identified in many human malignancies, including colorectal cancer [9]. Constitutive activation of MEK1 signaling can induce the oncogenic transformation of fibroblast [10-12], kidney [13], mammary [14], and intestinal epithelial cells [8,15]. We recently reported that the oncogenic potential of MEK in intestinal epithelial cells was mediated by cyclooxygenase-2 (COX-2) [8]. COX-2 and its derived prostaglandins are also thought to be involved in the development and progression of colorectal cancer [16,17]. The MEK-ERK cascade has been reported to induce increased tumor invasiveness [18,19], pro-cell cycle properties [8,20], angiogenesis [21], anti-apoptosis [8,22], and resistance to some anti-cancer agents [23,24]. However, the precise role of MEK-ERK signaling in intestinal carcinogenesis remains unknown.
In the past few years, newly developed technologies such as gene microarrays [25] have enabled the determination of molecular differences between normal and transformed cells at a genome-wide level. However, since most of these analyses were performed using bulk tissue samples that are composed of multiple cell lineages, the specific roles of identified genes during tumorigenesis are still under investigation. Therefore, the information obtained from a single cell before and after activation of a key signaling pathway during transformation may be a useful strategy for identifying novel targets. We previously established tetracycline regulated constitutively activated MEK1 (caMEK) expressing normal rat intestinal epithelial cells (RIEtiCAMEK cells), and reported that caMEK could induce the transformation of RIE and IEC-6 cells [8]. To clarify the oncogenic potential of MEK-ERK signaling and to identify novel targets of colonic carcinogenesis, we sought to determine the genes involved in caMEK-mediated transformation by gene microarray and RT-PCR analysis.
Results
Microarray results from RIEtiCAMEK cells
Total RNA from RIEtiCAMEK cells with/without doxycycline (DOX) following treatment with 5 mM sodium butyrate (NaB) for 48 hours were submitted for microarray analysis. RIEtiCAMEK cells express high levels of caMEK upon removal of DOX from the culture media and in the presence of NaB. One hundred-fifteen genes were observed (75 genes showed increased expression, while 40 genes were down regulated) with at least a three-fold difference in expression (data not shown).
Confirmation of microarray results by RT-PCR analysis
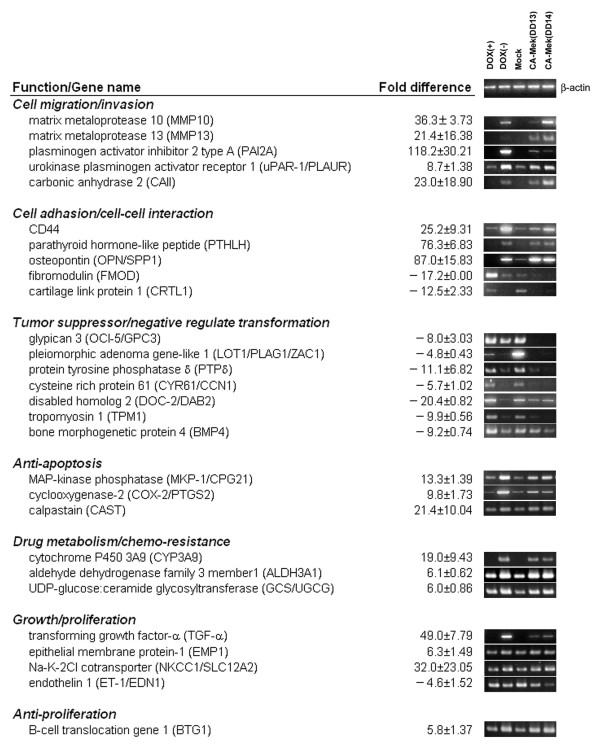
To confirm the differential expression of the genes observed from the microarray results, RT-PCR analysis was performed using gene-specific primers and RNA from MEK-inducible RIEtiCAMEK cells in the presence of NaB. Over 97% of all transcripts (113/115) observed by microarray were verified by RT-PCR analysis from the RIEtiCAMEK cells (data not shown). In order to account for the possibility that transcripts were altered by a histone deacetylase (HDAC) inhibitor which could potentially influence global gene expression [30,31], we also determined the gene profile of other caMEK and empty vector transfected cells in the absence of NaB. Therefore, RT-PCR analysis was performed on constitutively expressing caMEK clones (RIEcCAMEK cells; clone DD13, DD14) [8], as well as empty vector transfected cells (RIE-mock cells) in the absence of NaB. We confirmed 69 genes with altered transcription levels in both cell systems induced by caMEK (Figure 1, 2). However, the altered expression of 46 genes was not confirmed in the second cell system. Therefore, these 46 transcripts may not be regulated by caMEK and are possibly influenced by a HDAC inhibitor. The results from both cell systems indicated that 69 genes may be true targets of MEK-activation in RIE cells. The majority of these differentially expressed genes have previously been implicated in cellular transformation or tumorigenesis, including TGF-α and cyclooxygenase-2 (up-regulated genes) as well as DOC-2/DAB2 (down-regulated gene).
Figure 1.
Altered expression levels of caMEK-regulated genes involved in cell migration/invasion, cell adhesion, tumor suppression, anti-apoptosis, drug metabolism, and growth/proliferation. The microarray results from caMEK expressing cells (DOX(-)) compared to normal cells (DOX(+)) are expressed as fold difference ± S.D. Differentially expressed genes were verified through RT-PCR analysis. β-actin was used to indicate equal template in each lane.
Figure 2.
Altered expression levels of caMEK-regulated genes from transcription factor, signal transduction, metabolic, transportation, cytoskeletal, and other pathways. The microarray results from caMEK expressing cells (DOX(-)) compared to normal cells (DOX(+)) are expressed as fold difference ± S.D. Differentially expressed genes were verified through RT-PCR analysis. β-actin was used to indicate equal template in each lane.
Gene expression analysis in human colon cancers by RT-PCR
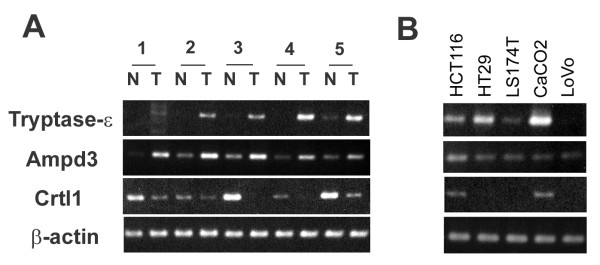
From the results of above experiments, we searched approximately 69 genes using PubMed (National Center for Biotechnology Information) for their involvement in cellular transformation or human cancer. We found that 8 genes (NPPB, PRSS22, CCR1, CTPCH1, P2RY2, AMPD3, CRTL1, AKAP150) did not have clear involvement. We focused on these 8 genes and performed RT-PCR analysis using the samples from 5 human colon cancer cell lines and human colon cancer tissues (tumor and corresponding adjacent normal mucosa from individual patients). Three novel targets were shown to have altered expression levels (Figure 3A,B). Human tryptase-ε/PRSS22, which is highly homologous to rat brain serine protease bsp2, and adenosine monophosphate deaminase 3 (AMPD3) were up-regulated in all 5 human colon cancer tissues compared to the corresponding normal mucosa. These transcripts were also expressed in several different colon cancer cell lines (4 of 5 and 5 of 5 respectively). Conversely, cartilage link protein 1 (CRTL1) was down-regulated in all 5 human colon cancer tissues and was expressed in only two of 5 colon cancer cell lines.
Figure 3.
RT-PCR analysis of human colon cancer tissue and cell lines. (A) RT-PCR analysis was performed on 5 paired normal and tumor human colon cancer tissues. T indicates tumor tissue and N indicates corresponding normal adjacent mucosa. Gene-specific primers for PCR were designed by MacVector 7 software depending on the information from GeneBank. Amplication of the right target DNA was confirmed by sequence analysis. β-actin was used as an internal control to confirm equal amount of the templates. (B) RT-PCR analysis was performed on 5 human colon cancer cell lines (HCT116, HT29, LS174T, CaCO2, LoVo) with the indicated primer sets.
Discussion
Recently, we reported that caMEK signaling is highly oncogenic and induces cellular transformation in rat intestinal epithelial cells [8]. We now show,, through the use of microarray analysis, that many genes associated with cellular transformation have altered expression levels following constitutive MEK activation. MEK-ERK signaling is associated with cell migration, invasion, and metastasis [18,19]. Our array results indicate that 10 transcripts associated with cell migration (e.g. MMP10, MMP13, etc) and adhesion (e.g. PTHLH, OPN, etc) have altered expression levels following MEK activation. Additionally, 7 genes known to possess tumor suppressor function (e.g. GPC3/OCI-5, LOT1/PLAGL1/ZAC1, etc) were down-regulated by MEK-activation. Furthermore, several genes that possess anti-apoptotic or chemo-resistant properties were over-expressed in caMEK expressing clones. The altered expression of transcripts was also seen in genes that are involved in growth and proliferation, transcription, signal transduction, biosynthesis, and the cytoskeleton. Together, this data supports our finding that MEK signaling positively regulates transformation in intestinal epithelial cells.
Among the most interesting findings, surface antigen CD44, complement resistance factor CD55/Daf, and secreted phosphoglycoprotein OPN, all of which are known to be implicated in colorectal cancer [25-32], were also up-regulated by caMEK. All of these results suggest the importance of MEK signaling in the intestinal tumorigenesis. Oncogenic transformation of rat intestinal epithelial cells following MEK-activation may depend on the balance between increased transcription of tumor-promoting genes and reduced levels of tumor suppressor genes.
We also have shown three transcripts that may be involved in human colorectal cancer. Of particular interest are the up-regulation of tryptase-ε/PRSS22 and AMPD3, and the down-regulation of CRTL1. Tryptase-ε/PRSS22 is a member of the chromosome 16p13.3 family of human serine proteases that is preferentially expressed by epithelial cells [36]. The tryptase-ε/PRSS22 gene is expressed in the airways in a developmentally regulated manner and is a major product of several different transformed epithelial cell lines [36]. Malignant cells require a range of proteolytic activities to enable growth, survival, and expansion [37]. Tryptase-ε/PRSS22 may play a role in this process. AMPD3 is one of the isoforms of the AMP deaminase family, which converts AMP to IMP and is a diverse and highly regulated enzyme that is a key component of the adenylate catabolic pathway [38]. This enzyme serves to protect the cell against sharp decreases in the adenylate energy charge by removing AMP generated when the rate of utilization of ATP is suddenly increased [39]. In cancer cells, a marked imbalance in the enzymic pattern of purine metabolism is linked with transformation and/or tumor progression [40]. This enzymatic change of purine metabolism seems to be present in transformed intestinal epithelial cells. CRTL1 (also known as a link protein) is a small glycoprotein of the extracellular matrix that was originally identified for its role in stabilizing aggregates of aggrecan and hyaluronan in cartilage [41]. In addition to being expressed in cartilage, CRTL1 is also immunolocalized in several noncartilaginous tissues [41]. A recent study has suggested that CRTL1 may be a down-stream target of β-catenin in intestinal epithelial cells, which has been implicated early in the progression of colorectal epithelial cells to cancer [42]. Therefore, this gene may also serve a role in preventing tumor formation of intestinal cells. This is the first report which indicates the involvement of these three genes in colorectal cancer.
Conclusion
Although a great body of evidence shows the importance of Ras and its downstream signaling mediators (Raf-MEK-ERK) on colorectal tumor development, the precise role of MEK remains undefined. Our results show that several genes previously known to be implicated in cellular transformation or tumorigenesis were altered following constitutive MEK activation in rat intestinal epithelial cells. Therefore, the MEK-ERK cascade seems to play an important role in intestinal transformation. Also, this is the first report, which indicates the involvement of these three genes in colorectal cancer. Some of the genes acting downstream of this signaling pathway may become useful markers for detection or therapeutic targets for colorectal cancer.
Methods
Cell lines and preparation of total RNA
The RIEtiCAMEK cells, RIEcCAMEK cells, and RIE-mock cells have been previously described [8]. Human colon cancer cell lines, HT29, CaCO2, LS174T, HCT116, and LoVo cells were purchased from American Type Culture Collection (ATCC). The cells were maintained in Eagle's minimal essential medium (Invitrogen, Carlsbad, CA) (CaCO2 and LS174T cells), McCoy 5A medium (Invitrogen) (HT29 and HCT116 cells) or Ham's F12 medium (Invitrogen) (LoVo cells) with 10% heat-inactivated fetal bovine serum (FBS) (Hyclone Laboratories, Logan, UT), and 2 mM L-glutamine. Total RNAs were isolated from each cells using TRIzol reagent (Invitrogen), and were purified by the RNeasy mini kit (Qiagen, Valencia, CA) following treatment with DNase I.
Analysis of gene expression by microarray
Total RNAs were isolated from RIEtiCAMEK cells with/without 2 μg/ml doxycycline (DOX) (BD Bioscience, Palo Alto, CA) following treatment with a histone deacetylase (HDAC) inhibitor (5 mM sodium butyrate (NaB) (Sigma, St.Louis, MO). The HDAC inhibitor can enhance transgene expression under the control of the CMV promoter [26-28], and induces nearly a 3000-fold increase of transgene expression in the cells (data not shown). Samples were sent to Genome Explorations, Inc. (Menphis, TN), where the RNA samples were converted to biotinylated cRNA and hybridized to the Affymetrix (Santa Clara, CA) Rat Genome U34A GeneChip array according to manufacturer's directions. The scanned images were analyzed using Microarray software (Affymetrix). Sample loading and variations in staining were standardized by scaling the average of the fluorescent intensities of all genes on an array to constant target intensity (2500) for all arrays used. The expression data were analyzed as previously described [29]. The signal intensity for each gene was calculated as the average intensity difference, represented by [μ(PM – MM)/(number of probe pairs)], where PM and MM denote perfect-match and mismatch probes. The analysis was performed twice (biological and technical replicates).
Analysis of gene expression by RT-PCR
Single-stranded cDNA was synthesized using oligo-(dT) primer and Superscript II reverse transcriptase (Invitrogen). PCR reactions were done in 50 μL volumes and amplified for 2 minutes at 94°C for initial denaturation, followed by 20–30 cycles at 94°C for 30 seconds, 50–64°C for 30 seconds, and 72°C for 1 minute (the conditions of reaction cycles and annealing temperatures were optimized for each individual pair of primers). PCR products were separated on 1.6–2.0% agarose gels and visualized by ethidium bromide staining. Amplication of the correct target DNA was confirmed by sequence analysis. Gene-specific primers for PCR products were designed by MacVector 7 software (Accelrys, San Diego, CA) using information from GenBank (NCBI). Gene function annotations were obtained from the Affymetrix web site and/or GenBank. RT-PCR analysis was also performed with samples from human colon cancer cell lines and human colon normal and tumor matched cDNA pair panels (BD Bioscience). β-actin was used as an internal control to confirm equal amounts of template.
Abbreviations
caMEK, constitutively activated MEK; COX-2, cyclooxygenase-2; DOX, doxycycline; ERK, extracellular signal-regulated protein kinase; HDAC, histone deacetylase; MAPK, mitogen-activated protein kinase; MEK, mitogen-activated protein kinase kinase; NaB, sodium butyrate
Authors' contributions
KK carried out the molecular genetic studies, designed the study, and drafted the manuscript. MJ analyzed the microarray results. MO and YH carried out the RT-PCR analysis. FGB, MO, SW, and RND conceived the study, participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Grant support
The United State Public Health Services Grants DK 47297, P30CA-68485, DK 62112, and PO1CA-77839 (RND), and Research Fellowships of Uehara Memorial Foundation (KK)
Acknowledgments
Acknowledgements
Authors thank Dr I. Wada, Dr T. Matsuhashi, Dr. J. Oyake, Dr N.Hatakeyama, and Dr R. Ohba for their helpful advice during the study.
Contributor Information
Koga Komatsu, Email: koga-k@qb3.so-net.ne.jp.
F Gregory Buchanan, Email: greg.buchanan@vanderbilt.edu.
Michiro Otaka, Email: otaka@med.akita-u.ac.jp.
Mario Jin, Email: jin@doc.med.akita-u.ac.jp.
Masaru Odashima, Email: odashima@doc.med.akita-u.ac.jp.
Yohei Horikawa, Email: horihori@rnac.ne.jp.
Sumio Watanabe, Email: sumio@doc.med.akita-u.ac.jp.
Raymond N DuBois, Email: raymond.dubois@vanderbilt.edu.
References
- Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/S0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- Davis H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Hoshino R, Chatani Y, Yamori T, Tsuruo T, Oka H, Yoshida O, Shimada Y, Ari-i S, Wada H, Fujimoto J, Kohno M. Constitutive activation of the 41-/43-kDa mitogen-activated protein kinase signaling pathway in human tumors. Oncogene. 1999;18:813–822. doi: 10.1038/sj.onc.1202367. [DOI] [PubMed] [Google Scholar]
- Licato LL, Brenner DA. Analysis of signaling protein kinases in human colon or colorectal carcinomas. Dig Dis Sci. 1998;43:1454–1464. doi: 10.1023/A:1018894227169. [DOI] [PubMed] [Google Scholar]
- Kuno Y, Kondo K, Iwata H, Senga T, Akiyama S, Ito K, Takagi H, Hamaguchi M. Tumor-specific activation of mitogen-activated protein kinase in human colorectal and gastric carcinoma tissues. Jpn J Cancer Res. 1998;89:903–909. doi: 10.1111/j.1349-7006.1998.tb00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Lee JW, Soung YH, Kim SY, Nam SW, Park WS, Kim SH, Yoo NJ, Lee JY. Colorectal tumors frequently express phosphorylated mitogen-activated protein kinase. APMIS. 2004;112:233–238. doi: 10.1111/j.1600-0463.2004.apm11204-0502.x. [DOI] [PubMed] [Google Scholar]
- Komatsu K, Buchannan GF, Katkuri S, Morrow JD, Inoue H, Otaka M, Watanabe S, DuBois RN. Oncogenic potential of MEK1 in rat intestinal epithelial cells is mediated via COX-2. Gastroenterology. 2005;129:577–590. doi: 10.1016/j.gastro.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Bos JL. Ras oncogene in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Mansour SJ, Matten WT, Hermann AS, Candia JM, Rong S, Fukasawa K, Vande Woude GF, Ahn NG. Transformation of mammalian cells by constitutively activate MAP kinase kinase. Science. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- Alessandrini A, Greulich H, Huang W, Erickson RL. Mek1 phosphorylation site mutants activate Raf-1 in NIH3T3 cells. J Biol Chem. 1996;271:31612–31618. doi: 10.1074/jbc.271.49.31612. [DOI] [PubMed] [Google Scholar]
- Montesano R, Soriano JV, Hosseini G, Pepper MS, Schramek H. Constitutively active mitogen-activated protein kinase kinase MEK1 disrupts morphogenesis and induces an invasive phenotype in Madin-Darby canine kidney epithelial cells. Cell Growth Differ. 1999;10:317–332. [PubMed] [Google Scholar]
- Pinkas J, Leder P. MEK1 signaling mediates transformation and metastasis of EpH4 mammary epithelial cells independent of an epithelial to mesenchymal transition. Cancer Res. 2002;62:4781–4790. [PubMed] [Google Scholar]
- Boucher M-J, Jean D, Vezina A, Rivard N. Dual role of MEK/ERK signaling in senescence and transformation of intestinal epithelial cells. Am J Physiol. 2004;286:G736–G746. doi: 10.1152/ajpgi.00453.2003. [DOI] [PubMed] [Google Scholar]
- Gupta RA, DuBois RN. Colorectal cancer preventation and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. 2001;1:11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- Kawai N, Tsujii M, Tsuji S. Cyclooxygenases and colon cancer. Prostaglandins Other Lipid Mediat. 2002;68–69:187–196. doi: 10.1016/S0090-6980(02)00030-8. [DOI] [PubMed] [Google Scholar]
- Taniura S, Nomura K, Ozaki K-I, Tsujimoto M, Kondo T, Kohno M. Prolonged nuclear retention of activated extracellular signal-regulated kinase 1/2 is required for hepatocyte growth factor-induced cell motility. J Biol Chem. 2002;277:28256–28264. doi: 10.1074/jbc.M202866200. [DOI] [PubMed] [Google Scholar]
- Taniura S, Asato K, Fujishiro S-H, Kohno M. Specific blockade of the ERK pathway inhibits the invasiveness of tumor cells: down-regulation of matrix metalloproteinase-3/-9/-14 and CD44. Biochem Biophys Res Commun. 2003;304:801–806. doi: 10.1016/S0006-291X(03)00670-3. [DOI] [PubMed] [Google Scholar]
- Hoshino R, Taniura S, Watanabe K, Kataoka T, Kohno M. Blockade of the extracellular signal-regulated kinase pathway induces marked G1 cell cycle arreat and apoptosis in tumor cells in which the pathway is constitutively activated. Up-regulation of p27Kip1. J Biol Chem. 2001;276:2686–2692. doi: 10.1074/jbc.M006132200. [DOI] [PubMed] [Google Scholar]
- Milanini J, Vinals F, Pouyssegur J, Pages G. p42/p44 MAP kinase module plays a key role in the transcriptional regulation of the vascular endothelial growth factor gene in fibroblasts. J Biol Chem. 1998;273:18165–18172. doi: 10.1074/jbc.273.29.18165. [DOI] [PubMed] [Google Scholar]
- Boucher M-J, Morisset J, Vachon PH, Reed JC, Laine J, Rivard N. MEK/ERK signaling pathway regulates the expression of Bcl-2, Bcl-XL, and Mcl-1 and promotes survival of human pancreatic cancer cells. J Cell Biochem. 2000;79:355–369. doi: 10.1002/1097-4644(20001201)79:3<355::AID-JCB20>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- MacKeigan JP, Collins TS, Ting J-Y. MEK inhibition enhances paclitaxel-induced tumor apoptosis. J Biol Chem. 2000;275:38953–38956. doi: 10.1074/jbc.C000684200. [DOI] [PubMed] [Google Scholar]
- Dent P, Grant S. Pharmacologic interruption of the mitogen-activated extracellular regulated kinase/mitogen-activated protein kinase signal transduction pathway: Potential role in promoting cytotoxic drug action. Clin Cancer Res. 2001;7:775–783. [PubMed] [Google Scholar]
- Schena M, Shalon D, Davis RW, Brown PO. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science. 1995;270:467–470. doi: 10.1126/science.270.5235.467. [DOI] [PubMed] [Google Scholar]
- Cockett MI, Bebbington CR, Yarranton GT. High level expression of tissue inhibitor of metalloproteinases in Chinese hamster ovary cells using glutamine synthetase gene amplification. Biotechnology. 1990;8:662–667. doi: 10.1038/nbt0790-662. [DOI] [PubMed] [Google Scholar]
- Davis SJ, Ward HA, Puklavec MJ, Willis AC, Williams AF, Barclay AN. High level expression in Chinese hamster ovary cells of soluble forms of CD4 T lymphocyte glycoprotein including glycosylation variants. J Biol Chem. 1990;265:10410–10418. [PubMed] [Google Scholar]
- Kim NS, Lee GM. Overexpression of bcl-2 inhibits sodium butyrate-induced apoptosis in Chinese hamster ovary cells resulting in enhanced humanized antibody production. Biotechnol Bioeng. 2000;71:184–193. doi: 10.1002/1097-0290(2000)71:3<184::AID-BIT1008>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Lockhart DJ, Dong H, Byrne MC, Follettie MT, Gallo MV, Chee MS, Mittmann M, Wang C, Kobayashi M, Horton H, Brown EL. Expression monitoring by hybridization to high-density oligonucleotide arrays. Nat Biotechnol. 1996;14:1675–1680. doi: 10.1038/nbt1296-1675. [DOI] [PubMed] [Google Scholar]
- Smith JG, Yokoyama WH, German JB. Butyric acid from the diet: actions at the level of gene expression. Crit Rev Food Sci Nutr. 1998;38:259–297. doi: 10.1080/10408699891274200. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Gabrielson E, Chen W, Anbazhagan R, van Engeland M, Weijenberg MP, Herman JG, Baylin SB. A genomic screen for genes upregulated by demethylation and histone deacetylase inhibition in human colorectal cancer. Nat Genet. 2002;31:141–149. doi: 10.1038/ng892. [DOI] [PubMed] [Google Scholar]
- Okamoto I, Tsuiki H, Kenyon LC, Godwin AK, Emlet DR, Holgado-Madruga M, Lanham IS, Joynes CJ, Vo KT, Guha A, Matsumoto M, Ushio Y, Saya H, Wong AJ. Proteolytic cleavage of the CD44 adhesion molecule in multiple human tumors. Am J Pathol. 2002;160:441–447. doi: 10.1016/S0002-9440(10)64863-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holla VR, Wang D, Brown JR, Mann JR, Katkuri S, DuBois RN. Prostaglandin E2 regulates the complement inhibitor CD55/decay accelerating factor in colorectal cancer. J Biol Chem. 2005;280:476–483. doi: 10.1074/jbc.M407403200. [DOI] [PubMed] [Google Scholar]
- Irby RB, McCarthy SM, Yeatman TJ. Osteopontin regulates multiple functions contributing to human colon cancer development and progression. Clin Exp Metastasis. 2004;21:515–523. doi: 10.1007/s10585-004-2873-4. [DOI] [PubMed] [Google Scholar]
- Agrawal D, Chen T, Irby R, Quackenbush J, Chambers AF, Szabo M, Cantor A, Coppola D, Yeatman TJ. Osteopontin identified as colon cancer tumor progression marker. C R Biol. 2003;326:1041–1043. doi: 10.1016/j.crvi.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Wong GW, Yasuda S, Madhusudhan MS, Li L, Yang Y, Krilis SA, Sali A, Stevens RL. Human tryptase epsilon (PRSS22), a new member of the chromosome 16p13.3 family of human serine proteases expressed in airway epithelial cells. J Biol Chem. 2001;276:49169–49182. doi: 10.1074/jbc.M108677200. [DOI] [PubMed] [Google Scholar]
- Netzel-Arnett S, Hooper JD, Szabo R, Madison EL, Quigley JP, Bugge TH, Antalis TM. Membrane anchored serine proteases: a rapidly expanding group of cell surface proteolytic enzymes with potential roles in cancer. Cancer Metastasis Rev. 2003;22:237–258. doi: 10.1023/A:1023003616848. [DOI] [PubMed] [Google Scholar]
- Sims B, Mahnke-Zizelman DK, Profit AA, Prestwich GD, Sabina RL, Theibert AB. Regulation of AMP deaminase by phosphoinositides. J Biol Chem. 1999;274:25701–25707. doi: 10.1074/jbc.274.36.25701. [DOI] [PubMed] [Google Scholar]
- Chapman AG, Miller AL, Atkinson DE. Role of the adenylate deaminase reaction in regulation of adenine nucleotide metabolism in Ehrlich ascites tumor cells. Cancer Res. 1976;36:1144–1150. [PubMed] [Google Scholar]
- Weber G. Enzymes of purine metabolism in cancer. Clin Biochem. 1983;16:57–63. doi: 10.1016/S0009-9120(83)94432-6. [DOI] [PubMed] [Google Scholar]
- Colas JF, Schoenwolf GC. Localization of cartilage linking protein 1 during primary neurulation in the chick embryo. Brain Res Dev Brain Res. 2003;141:141–148. doi: 10.1016/S0165-3806(03)00011-7. [DOI] [PubMed] [Google Scholar]
- Naishiro Y, Yamada T, Idogawa M, Honda K, Takada M, Kondo T, Imai K, Hirohashi S. Morphological and transcriptional responses of untransformed intestinal epithelial cells to an oncogenic beta-catenin protein. Oncogene. 24:3141–3153. doi: 10.1038/sj.onc.1208517. 2005 Apr 28. [DOI] [PubMed] [Google Scholar]