Abstract
The beads in the wing scales of pierid butterflies play a crucially important role in wing coloration as shown by spectrophotometry and scanning electron microscopy (SEM). The beads contain pterin pigments, which in Pieris rapae absorb predominantly in the ultraviolet (UV). SEM demonstrates that in the European subspecies Pieris rapae rapae, both males and females have dorsal wing scales with a high concentration of beads. In the Japanese subspecies Pieris rapae crucivora, however, only the males have dorsal wing scales studded with beads, and the dorsal scales of females lack beads. Microspectrophotometry of single scales without beads yields reflectance spectra that increase slightly and monotonically with wavelength. With beads, the reflectance is strongly reduced in the UV and enhanced at the longer wavelengths. By stacking several layers of beaded scales, pierid butterflies achieve strong colour contrasts, which are not realized in the dorsal wings of female P. r. crucivora. Consequently, P. r. crucivora exhibits a strong sexual dichroism that is absent in P. r. rapae.
Keywords: pterin pigments, structural colour, small white, scattering, wing reflectance
1. Introduction
The colours of butterflies are usually determined by the scales that shingle the wings (Nijhout 1991). Numerous studies have been devoted to iridescent wings where coherent scattering occurs by regularly arranged, nanosized structures in the wing scales (e.g. Ghiradella 1984; Vukusic et al. 1999; Kinoshita & Yoshioka 2005). However, the colour of most butterflies results from selective absorption of incoherently scattered light by pigments in the wing scales. Each wing scale is the cuticular product of a single cell, with a rather flat, unstructured lower scale leaf and a highly structured upper leaf, consisting of longitudinal ridges connected by crossribs, which in pierids are adorned with granules (Ghiradella 1998). Wavelength-dependent scattering by these structures, together with the spectral absorption by the pigments they may contain, result in the wing colours. Butterflies of the family Pieridae are divided into two broad groups based on their colour: the sulphurs (the Coliadinae, with predominantly yellow to orange wings) and the whites (the Pierinae, with mostly white wings).
The wing pigments of pierid butterflies, first analysed in the brimstone (Gonepteryx rhamni; Hopkins 1895), were appropriately called pterins. Various UV- and blue-green-absorbing pterins were later characterized in the orange sulphur, Colias eurytheme (Watt 1964), and virtually exclusively UV-absorbing pigments were encountered in the small white, Pieris rapae (Makino et al. 1952). The pterins were concluded to be located in granules, studded at the scale crossribs (Yagi 1954). Transmission and scanning electron microscopical photographs of the granules indicated, however, that they were void (Stavenga et al. 2004), leading to the assumption that the pterin pigments were dispersed in the scale structures, as is the case for the pigments in the scales of other butterfly families, where granules are absent. Furthermore, spectrophotometric measurements demonstrated that the granules enhance the wing reflectance of pierids by scattering, and therefore the granules were called beads (Stavenga et al. 2004).
Chemical removal of the beads from the wing scales of C. eurytheme severely reduced the long-wavelength reflectance, confirming the general scattering function of the beads, but the bead removal also caused an increase in short-wavelength reflectance, indicating that the beads absorb short-wavelength light, by a pigment (Rutowski et al. 2005). This finding suggests a dual function for the beads, namely to enhance long-wavelength reflectance and simultaneously to suppress short-wavelength reflectance, thus creating colour contrast.
Here, we investigate this hypothesis by relating the reflectance spectra to the density of beads in the small white P. rapae, and we compare the European subspecies P. r. rapae with the Japanese subspecies P. r. crucivora. The general appearance of pierid wing reflectance spectra is low reflectance at short wavelengths and high reflectance at long wavelengths, which is a cumulative effect of multilayers of scales (Stavenga et al. 2006). In P. r. rapae both sexes conform to this rule, as both combine low reflectance in the UV with a brilliant diffuse white coloration, but in P. r. crucivora this is only the case for the male. The reflectance of the dorsal wings of female P. r. crucivora is substantial in the UV, but it gradually rises with longer wavelengths, where it still remains much lower than the wing reflectance of the males (Obara & Majerus 2000). Pieris rapae crucivora thus features a distinct sexual dichroism (Obara 1970), which is absent in P. r. rapae (Obara & Majerus 2000). The bead density of the dorsal wing scales of female P. r. crucivora is low (Hidaka & Okada 1979) and correlates with the reflectance characteristics. Our findings provide further insight into the optical mechanisms of butterfly wing coloration, which will presumably be useful in enhancing our understanding of butterfly speciation and development.
2. Material and methods
(a) Animals
Specimens of the European small white butterfly, subspecies P. r. rapae, were obtained from a continuous culture (with non-diapausing pupae) maintained by Dr J. J. van Loon, Entomology Department of the Agricultural University in Wageningen, The Netherlands (Hopkins & van Loon 2001). Japanese small whites, P. r. crucivora, were obtained from Prof. K. Arikawa, University of Yokohama, Japan (Arikawa et al. 2005). Three males and three females of each subspecies were investigated.
(b) Spectrophotometry
Reflectance spectra of intact wings were measured with a reflection probe connected to a fibre optic spectrometer (SD2000, Avantes, Eerbeek, The Netherlands), using a deuterium/halogen light source. On the dorsal as well as ventral side of both forewings and hindwings, three locally adjacent areas indicated with a number in the insets of figure 1a,b were investigated. The three reflectance spectra were averaged. The fibre aperture (half-angle of the maximum cone of light) is about 12°, and the sampled area had a diameter of about 1 mm. Spectra of the abwing (upper) side of single scales, taken from the same areas and glued to the tip of a micropipette, were measured with a microspectrophotometer, consisting of a xenon light source, a Leitz Ortholux microscope and the fibre optic spectrometer. The microscope objective was an Olympus 20×, NA 0.25. A white reflectance standard (Spectralon, Labsphere, North Sutton, NH, USA) served as a reference.
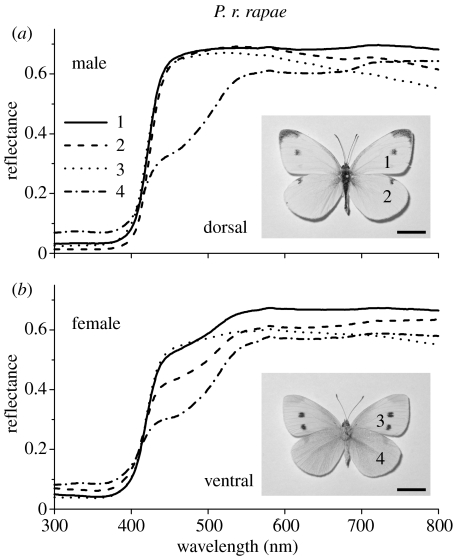
Figure 1.
Reflectance spectra of the wings of Pieris rapae rapae measured with a reflection probe connected to a fibre optic spectrometer from restricted areas (indicated by a number) of the dorsal forewing (1), dorsal hindwing (2), ventral forewing (3) and ventral hindwing (4) of (a) a male and (b) a female. The inset in (a) shows the dorsal side of a male P. r. rapae and the inset in (b) presents the ventral side of a female. The reflectance spectrum of the ventral hindwing of the male is lower than the other spectra in the blue–green wavelength range, resulting in a yellowish colour. The female wings are generally slightly yellower than those of the male. The indicated wing locations were the same for both male and female. Scale bars, 1 cm.
(c) Scanning electron microscopy
The wing areas from which reflectance spectra were measured were prepared for scanning electron microscopy (SEM) by sputtering the samples with paladium for 5 min with 800 V and 200 mTorr (Hummer, Technics, Alexandria, VA, USA). The scale anatomy was then investigated with a Philips XL-30, using a voltage of 3 kV.
3. Results
Small white butterflies, P. rapae, have white wings with a black dorsal wing tip and a few characteristic black dots, which are sex dependent (Obara & Majerus 2000). Male P. r. rapae in particular have very white dorsal wings, as is directly recognizable from the reflectance spectra (figure 1a). In the visible wavelength range the reflectance is high, approximately 70%, while the reflectance in the UV is not more than a few per cent. The reflectance of the male ventral hindwings is depressed in the blue–green, which correlates with a higher, although still minor reflectance in the UV (figure 1a). In female P. r. rapae, the reflectance spectra of both dorsal and ventral wings feature a blue–green depression (figure 1b), similar to that in the reflectance spectrum of the ventral hindwing of the male (figure 1a). The modest reflectances in the UV of the female wings are only slightly larger than the reflectances of the male dorsal wings.
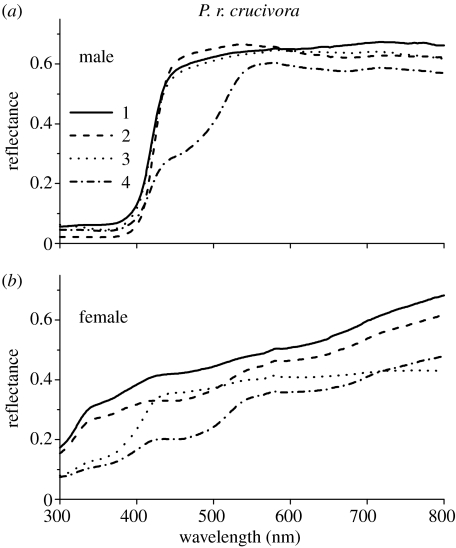
The situation is different in the Japanese subspecies P. r. crucivora. For the human observer, the visual appearance of the male is similar to that of the male (and female) P. r. rapae, but the wings of the female P. r. crucivora are greyer and very slightly brownish (Obara & Majerus 2000). The reflectance spectra of the male P. r. crucivora are indeed very similar to those of the male P. r. rapae (figure 2a). The reflectance spectra of the female increase monotonically from 10–20% in the UV to about 20–50% in the visible wavelength range (figure 2b). Pieris rapae crucivora hence features a marked sexual dichroism (Obara 1970), certainly from the viewpoint of the butterflies, which detect UV light well (Arikawa et al. 2005). Compared with the sexual dichroism of P. r. crucivora, that of P. r. rapae is much reduced.
Figure 2.
Reflectance spectra of the wings of Pieris rapae crucivora measured from the dorsal forewing (1), dorsal hindwing (2), ventral forewing (3) and ventral hindwing (4) of (a) a male and (b) a female; see insets in figure 1a,b. The reflectances in the UV of the dorsal forewing and hindwing of the male are considerably lower than those of the female, while the reflectances are higher at the longer wavelengths, yielding a distinct sexual dichroism.
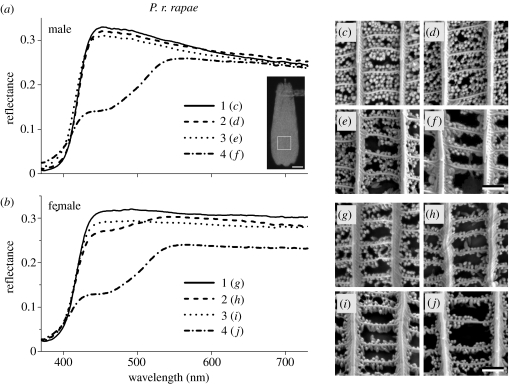
To investigate the physical origin of the different coloration and reflectance spectra, we have examined the anatomical structure of scales in the same wing areas as shown in figures 1 and 2. The reflectance measured from single scales is low in the UV, only a few per cent, and higher in the visible wavelength range, with a maximum of about 30% (figure 3a). SEM shows that the scales of both male and female P. r. rapae in the dorsal as well as ventral wing areas are densely packed with beads (figure 3c–j). Quite notably, however, is the slightly lower bead density of the scales of the ventral hindwings in both the male (figure 3f) and the female (figure 3j), which correlates with the lower reflectance of single scales from the hindwings (figure 3a,b). The depression of the reflectance spectra of the latter area in the blue–green correlates with the yellowish colour (see figure 1).
Figure 3.
Reflectance spectra of (a) male and (b) female Pieris rapae rapae and (c)–(j) SEM of single scales. The reflectance spectra 1–4 are from single scales, taken from wing locations shown by the numbers in the insets of figure 1a,b, of the dorsal forewing (1), dorsal hindwing (2), ventral forewing (3) and ventral hindwing (4). The single-scale reflectance spectra were measured from an area indicated by the square in the inset in (a). The spectra of (a) correspond to the male SEM photographs (c)–(f), and the spectra of (b) correspond to the female SEM photographs (g)–(j), as indicated. Scale bars, 20 μm (inset (a)) and 1 μm ((c)–(j)).
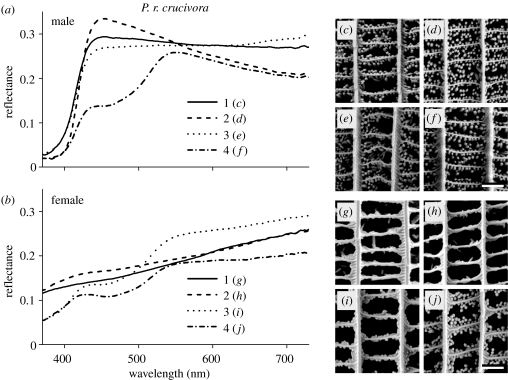
The reflectance of single scales from the dorsal wings of male P. r. crucivora is also only a few per cent in the UV and maximally about 30% in the visible wavelength range, as in P. r. rapae (figure 4a). In addition, the scales from the ventral hindwing have a lower reflectance in the blue–green compared with the scales from the other wing areas (figure 4a). The density of beads in the scales of male P. r. crucivora is high (figure 4c–f). In female P. r. crucivora, the characteristics of scales from the dorsal forewing and hindwing dramatically differ. The single-scale reflectance spectra rise almost linearly from 10% in the UV to about 20% in the red (figure 4b), and beads appear to be fully lacking dorsally (figure 4g,h). The ventral forewing and hindwing scales generally carry a low, although somewhat variable concentration of beads (figure 4i,j). The reflectance in the UV is about 5% and can rise to about 25% in the red (figure 4b).
Figure 4.
Reflectance spectra of (a) male and (b) female Pieris rapae crucivora and (c)–(j) SEM of single scales. The reflectance spectra 1–4 are from single scales taken from about the same locations at the dorsal forewing (1), dorsal hindwing (2), ventral forewing (3) and ventral hindwing (4) as given in figure 1. They correspond to the SEM photographs (male: (c)–(f); female: (g)–(j)) as indicated. Scale bar, 1 μm.
4. Discussion
The colour of butterfly wings commonly results from the combined optical effects of light scattering on the scales and wing substrate and absorption by pigments deposited in these structures. Coherent scattering on regularly arranged lamellae in the scale ridges is a dominant factor for the coloration of many male pierids of the subfamily Coliadinae, creating bright UV iridescence (Ghiradella et al. 1972), but this phenomenon is not featured by the Pierinae, the subfamily of P. rapae. In a comparative study on butterfly wing reflectance, we found that incoherent scattering on the scale beads substantially contributes to the brightness of the small white's wings in the long-wavelength range (Stavenga et al. 2004), a conclusion supported by Rutowski et al. (2005), who studied the wing reflectance of the orange sulphur, C. eurytheme. We demonstrate here that the beads are absent in the dorsal wing scales of female P. r. crucivora, resulting in a lower reflectance at long wavelengths, compared with males (figure 2). The female wing reflectance is higher in the UV, however, providing strong evidence for the view that the beads contain a UV-absorbing pigment. The present results show that an increased bead density is related to a decrease in UV reflectance and an increase in long-wavelength reflectance.
In previous works, the beads were called pigment granules (Yagi 1954; Waku & Kitagawa 1986; Hidaka & Okada 1979; Ghiradella 1998), because they were assumed to contain the pterin pigments that cause the distinct yellow colour of sulphurs (Hopkins 1895; Watt 1964). The pterins of P. r. crucivora, leucopterin and (iso)xanthopterin (Makino et al. 1952) absorb exclusively in the UV. Broadband-absorbing pigments, the melanins, are expressed in the darkly coloured scales (Nijhout 1991), which together create the black spots of P. rapae wings. The black scales do not contain beads (Hirata & Uehara 1959; Allyn & Downey 1977; Stavenga et al. 2004), meaning that the melanin pigment is distributed in the scale surfaces, ridges and/or crossribs. This must also hold for the pigments in the scales of butterflies from families other than the Pieridae, because they also do not have beads.
The beads of the white pierid scales appeared in transmission electron microscopical photographs as clear bodies (Stavenga et al. 2004), which seemed to indicate that they were empty, suggesting that the pterin pigments exist similarly distributed throughout the scales as the melanin. The optical function of the beads thus seemed to be exclusively to be that of strong scatterers. Rutowski et al. (2005) however showed that alkalic treatment of the scales simultaneously extracts pterins and removes beads, resulting in an increased reflectance in the short-wavelength range and a decreased reflectance at longer wavelengths. They hence concluded that the beads, in addition to being scatterers, contain the pterin pigments. Furthermore, Morehouse et al. (submitted) found that the scale reflectance increases proportionally to the number of beads. The high reflectance in the UV of depigmented C. eurytheme wings (fig. 1b of Rutowski et al. 2005) resembles that of the dorsal wings of female P. r. crucivora (figure 2b). The latter wings have beadless scales, and we thus conclude that the present results underscore the findings of Rutowski et al. (2005).
The reflectance spectra of female wings are rather featureless (figure 2b), rising slightly with increasing wavelength. The presence of beads strongly decreases the reflectance in the UV and enhances the longer-wavelength reflectance (figures 3 and 4). The function of the beads thus appears to be twofold. By concentrating a pigment with an appropriate absorbance band in numerous small, nanosized granules, the scale reflectance at short wavelengths is reduced. Since the beads create an additional scattering medium with a refractive index distinctly higher than that of air, the reflectance at wavelengths outside the absorption band is increased.
Several components determine the reflectance of butterfly wings, including the assembly of scales together with the wing substrate (Stavenga et al. 2006). In the case of the female P. r. crucivora, the reflectance of the intact dorsal forewing is 40–50% between 400 and 600 nm (figure 2b), whereas the reflectance of single scales of the dorsal forewing is 15–20% in that wavelength range (figure 4b). The enhanced wing reflectance results from the presence of a stack of 2–3 scales on both sides of the intact wing. Studding a scale with pigmented beads, which occurs heavily in male dorsal scales, depresses the reflectance in the UV to a few per cent and increases the reflectance in the red to about 30%. For a single scale these changes may still seem to be minor, but in the intact wing the effects are multiplied, resulting in maximum reflectances of about 70%. This high reflectance of the intact wings is predominantly determined by the scales on the illuminated side of the wing, but the scales on the opposite side can also contribute substantially, as has been analysed in detail for P. r. rapae (Stavenga et al. 2006). Whereas the dorsal wing reflectance of female P. r. crucivora, with unpigmented scales, gradually and slightly increases from short to long wavelengths, the reflectance spectrum of the pigmented wings of male P. r. crucivora changes abruptly at about 400 nm, and similar steep spectral changes are seen in the reflectance spectra of male and female P. r. rapae. (We note here that the reflectance spectra of figures 1 and 2 are in agreement with those reported by Obara & Majerus (2000, fig. 1), except that they erroneously interchanged the reflectance spectra for the ventral and dorsal surfaces.)
The colours, and accordingly the reflectance spectra, of the dorsal and ventral wings differ slightly in the studied butterflies, i.e. in both sexes of the two P. rapae subspecies. In many sulphurs, the Coliadinae, the difference between dorsal and ventral wings is much more extreme. The sulphurs have extensively beaded scales (Rutowski et al. 2005), but the pigments absorb well into the visible wavelength range, causing a yellow or orange colour (Watt 1964). The resulting colour contrast is enhanced in the dorsal wings of males of many sulphur species by a brilliant iridescence, which is restricted to the UV (Ghiradella et al. 1972; Silberglied & Taylor 1973; Kemp et al. 2005). Such an additional coloration in the UV only works in combination with a yellow, orange or red background. In white Pieridae, an additional UV reflectance would reduce colour contrast, and therefore iridescence is not found in the white wings of Pieris species.
In P. rapae, the ventral hindwings are yellowish, because the reflectance in the blue is suppressed. The ventral wing reflectance spectrum can be easily explained by assuming that scales with a blue-absorbing pterin occur simultaneously with scales containing the generally occurring UV-absorbing pterin. However, the reflectance spectra of single scales from the ventral hindwings have the same biphasic shape as those measured from the intact ventral hindwing. The scales hence must express (at least) two types of pterin, absorbing in the UV and blue, respectively. Detailed measurements on ventral scales from the same area show that they are variably coloured; some are white, others are rather yellow or yellowish. Apparently, the relative expression of the different pterins is not constant. Furthermore, the reflectance spectrum depends on the location of measurement, which correlates with the local density of beads.
As the reflectance spectra of male and female P. r. crucivora only differ strongly in the UV, no striking colour difference is seen by a human observer. Nevertheless, for the Japanese small white butterflies, males and females will have distinctly different colours, because the butterflies possess a visual system with an rich repertoire that is unsurpassed, especially in the short-wavelength range. Both male and female have three types of photoreceptors each with a short-wavelength-absorbing rhodopsin, peaking in the UV, violet and blue, respectively (Arikawa et al. 2005). In males of the Japanese subspecies P. r. crucivora, the receptors with a violet-absorbing rhodopsin are modified into a double-peaked blue receptor by a UV-absorbing, whitish-fluorescing pigment (Arikawa et al. 2005). Presumably, the short-wavelength receptors serve in the sensitive discrimination of the male and female wing colours. Pieris rapae crucivora males can already discriminate females on their slightly different ventral coloration (Obara 1970). The dorsal sexual dichroism of P. r. crucivora will strongly facilitate the intraspecies recognition.
Visual discrimination of females is clearly also done by male P. r. rapae, but this is relatively poor, and presumably therefore resting P. r. rapae males, when erroneously approached by other males, when searching for females, elicit a male-characteristic flutter response, which then results in no copulatory attempts. The fluttering response thus plays an important role in mate recognition, i.e. it functions as a ‘mechanical isolation mechanism’ (Obara & Majerus 2000). Male P. r. crucivora also exhibit the flutter response, but this can be considered as rather redundant, because sexual discrimination is readily achieved visually (Obara 1970). Obara & Majerus (2000) therefore hypothesized that the flutter response of male P. r. crucivora is a relic of P. r. rapae, which is thus assumed to be evolutionarily ancestral. The suppressed expression of pterin pigments and the resulting absence of beads in the scales on the dorsal wings of female P. r. crucivora, which causes the strong sexual dichroism and easy sexual discrimination of P. r. crucivora, hence may be subject to evolutionary forces.
Acknowledgments
Drs H. Ghiradella, R. Rutowski and N. I. Morehouse read the manuscript and offered valuable suggestions for improvement. Prof J. Th. M. de Hosson of the Materials Science Department, University of Groningen, provided excellent SEM facilities. Financial support was given by the EOARD (grant no. 063027).
References
- Allyn A.C, Downey J.C. Observations on male U-V reflectance and scale structure in Phoebis (Pieridae) Bull. Allyn Mus. 1977;42:1–20. [Google Scholar]
- Arikawa K, Wakakuwa M, Qiu X, Kurasawa M, Stavenga D.G. Sexual dimorphism of short-wavelength photoreceptors in the small white butterfly, Pieris rapae crucivora. J. Neurosci. 2005;25:5935–5942. doi: 10.1523/JNEUROSCI.1364-05.2005. doi:10.1523/JNEUROSCI.1364-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiradella H. Structure of iridescent lepidopteran scales: variations on several themes. Ann. Entomol. Soc. Am. 1984;77:637–645. [Google Scholar]
- Ghiradella H. Hair, bristles and scales. In: Locke M, editor. Microscopic anatomy of invertebrates. vol. 11A. Wiley-Liss; New York, NY: 1998. pp. 257–287. [Google Scholar]
- Ghiradella H, Aneshansley D, Eisner T, Silberglied R, Hinton H.E. Ultraviolet reflection of a male butterfly: interference color caused by thin-layer elaboration of wing scales. Science. 1972;178:1214–1217. doi: 10.1126/science.178.4066.1214. [DOI] [PubMed] [Google Scholar]
- Hidaka T, Okada M. Sexual difference in wing scales of the white cabbage butterfly, Pieris rapae crucivora, as observed under a scanning electron microscope. Zool. Mag. 1979;79:181–184. [Google Scholar]
- Hirata K, Uehara J. Studies on the structure of scales and hairs of insects II. Microstructures of scales of the blank female of the butterfly, Colias erate poliographus Motschulsky. Sci. Rep. Kagoshima Univ. 1959;8:155–174. [Google Scholar]
- Hopkins F.G. The pigments of the Pieridae: a contribution to the study of excretory substances which function in ornament. Phil. Trans. R. Soc. B. 1895;186:661–682. [Google Scholar]
- Hopkins R.J, van Loon J.J.A. The effect of host acceptability on oviposition and egg accumulation by the small white butterfly, Pieris rapae. Physiol. Entomol. 2001;26:149–157. doi:10.1046/j.1365-3032.2001.00228.x [Google Scholar]
- Kemp D.J, Rutowski R.L, Mendoza M. Colour pattern evolution in butterflies: a phylogenetic analysis of structural ultraviolet and melanic markings in North American sulphurs. Evol. Ecol. Res. 2005;7:133–141. [Google Scholar]
- Kinoshita S, Yoshioka S. Structural colors in nature: the role of regularity and irregularity in the structure. ChemPhysChem. 2005;6:1–19. doi: 10.1002/cphc.200500007. [DOI] [PubMed] [Google Scholar]
- Makino K, Satoh K, Koiki M, Ueno N. Sex in Pieris rapae L. and the pteridin content of their wings. Nature. 1952;170:933–934. doi: 10.1038/170933a0. doi:10.1038/170933a0 [DOI] [PubMed] [Google Scholar]
- Morehouse, N. I., Vukusic, P. & Rutowski, R. L. Submitted. Pterin pigment granules are responsible for both broadband light scattering and wavelength selective absorption in pierid butterflies. Proc. R. Soc. B. [DOI] [PMC free article] [PubMed]
- Nijhout H.F. Smithsonian Institution Press; Washington, DC: 1991. The development and evolution of butterfly wing patterns. [Google Scholar]
- Obara Y. Studies on the mating behavior of the white cabbage butterfly, Pieris rapae crucivora Boisduval. III. Near-ultraviolet reflection as the signal of intraspecific communication. Z. Vergl. Physiol. 1970;69:99–116. doi:10.1007/BF00340912 [Google Scholar]
- Obara Y, Majerus M.E.N. Initial mate recognition in the British cabbage butterfly, Pieris rapae rapae. Zool. Sci. 2000;17:725–730. doi:10.2108/zsj.17.725 [Google Scholar]
- Rutowski R.L, Macedonia J.M, Morehouse N, Taylor-Taft L. Pterin pigments amplify iridescent ultraviolet signal in males of the orange sulphur butterfly, Colias eurytheme. Proc. R. Soc. B. 2005;272:2329–2335. doi: 10.1098/rspb.2005.3216. doi:10.1098/rspb.2005.3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberglied R, Taylor O.R. Ultraviolet differences between the sulphur butterflies, Colias eurytheme and C. philodice, and a possible isolating mechanism. Nature. 1973;241:406–408. doi: 10.1038/241406a0. doi:10.1038/241406a0 [DOI] [PubMed] [Google Scholar]
- Stavenga D.G, Stowe S, Siebke K, Zeil J, Arikawa K. Butterfly wing colours: scale beads make white pierid wings brighter. Proc. R. Soc. B. 2004;271:1577–1584. doi: 10.1098/rspb.2004.2781. doi:10.1098/rspb.2004.2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavenga D.G, Giraldo M.A, Hoenders B.J. Reflectance and transmittance of light scattering scales stacked on the wings of pierid butterflies. Opt. Express. 2006;14:4880–4890. doi: 10.1364/oe.14.004880. doi:10.1364/OE.14.004880 [DOI] [PubMed] [Google Scholar]
- Vukusic P, Sambles J.R, Lawrence C.R, Wootton R.J. Quantified interference and diffraction in single Morpho butterfly scales. Proc. R. Soc. B. 1999;266:1403–1411. doi:10.1098/rspb.1999.0794 [Google Scholar]
- Waku Y, Kitagawa M. Developmental process of scale pigment granules in the cabbage butterfly, Pieris rapae crucivora. Jpn J. Appl. Ent. Zool. 1986;30:35–42. [Google Scholar]
- Watt W.B. Pteridine components of wing pigmentation in the butterfly Colias eurytheme. Nature. 1964;201:1326–1327. doi: 10.1038/2011326b0. doi:10.1038/2011326b0 [DOI] [PubMed] [Google Scholar]
- Yagi N. Note of electron microscope research on pterin pigment in the scales of pierid butterflies. Annot. Zool. Jpn. 1954;27:113–114. [Google Scholar]