Abstract
A key component of any epidemiological model is the infectious period, which greatly affects the dynamics and persistence of an infection. Social organization, leading to behavioural and spatial heterogeneities among potential susceptibles, interacts with infectious period to create different risk categories within a group. Using the honeybee (Apis mellifera) colony as a social model, a protocol that creates different infectious periods in individual bees and another that follows the diffusion of a transmittable tracer within a colony, we show experimentally how a short infectious period results in an epidemic process with low prevalence confined only to individuals at the outer edge of a group, while a long infectious period results in high prevalence distributed more universally among all the group members. We call this finding an evidence of ‘organizational immunity’ in a social network and propose that the honeybee colony provides a unique opportunity to test its role in social transmission processes.
Keywords: social transmission, social networks, information transfer, trophallaxis, infectious period, honeybees
1. Introduction
The endemic persistence of an infectious disease is largely dependent on the infectious period or how long an infected individual can transmit the infection to other susceptible hosts (Anderson 1992; Keeling & Grenfell 1998; Lloyd 2001). Diseases with long infectious periods tend to have relatively smooth endemic dynamics while those with short periods lead to more pronounced dynamics. Infections with long periods are therefore more likely to be persistent, while those with short periods are more prone to fade outs owing to stochastic extinction. The infectious period is also known to have a significant effect on the spatial spread of a disease (Keeling 1999; Hagenaars et al. 2004), making its role especially important in social contexts. However, until now, these ideas have not been addressed with direct manipulative experiments, probably owing to the obvious scarcity of suitable experimental systems.
The honeybee (Apis mellifera) colony, with its complex social (and correlated spatial) organization, natural susceptibility to many diseases (Bailey 1981) and experimental readiness, provides a rare opportunity to use a bottom-up approach for testing ideas about the epidemiology of social groups (Naug & Camazine 2002). Honeybees live in dense social groups with up to 50 000 individuals. The availability of a large number of susceptible hosts, a stable microclimate provided by social foraging and thermoregulation, and extensive social interactions such as mouth-to-mouth food exchange (trophallaxis), provide an ideal setting for pathogens to survive, multiply and spread. However, individuals in the colony are also segregated based on the tasks they perform (division of labour), correlated with their ages and spatial locations. This segregation presents pathogens with the challenge of negotiating a spatially and behaviourally heterogeneous landscape of hosts if they want to spread, leading to the potential of complex transmission dynamics.
In this paper, we first discuss a method by which one could experimentally manipulate the ‘infectious period’ of an individual bee. Then, ‘infecting’ honeybee colonies with bees having different infectious periods, we study the resulting differences in transmission patterns. In the honeybee colony, each nectar load brought in by a forager is unloaded by one or several receiver bees. Each of these unloaded nectar loads then passes along a chain of one or several individuals before being finally deposited in one or more honey cells, creating a complex transmission network. During this transfer process, a bee temporarily holds the nectar in its foregut (crop)—the only part of the alimentary canal from where nectar can be transferred from one bee to another. Once the nectar moves down from the crop to the mid-gut, it is no longer available for transfer. Previous studies have found that the emptying rate of the crop to the mid-gut is influenced by several factors, such as the season, the hunger and activity level of the bee, the volume and concentration of the nectar load, etc. (Crailsheim 1988). A high sucrose concentration in the nectar results in a low crop-emptying rate and vice versa. Since the crop-emptying rate of a bee determines the duration for which its crop contents are available for transfer to potential recipients, this means that a bee with a low crop-emptying rate can be said to have a long infectious period (LIP) and conversely one with a high crop-emptying rate has a short infectious period (SIP).
2. Material and methods
We assembled a honeybee colony consisting of about 4000 individuals that belonged to six uniquely colour-marked, weekly cohorts of 700 individuals each. We trained 30 marked foragers from the colony to a feeding source, containing 70 ml of sucrose solution containing 1×107 ml−1 of 6μ Polystyrene microsphere particles (Polysciences, PA) as a proxy for pathogen spores. For the LIP treatment, we filled the feeder with a 40% (wt/wt) sucrose solution, while for the SIP treatment we filled it with a 10% solution containing the same concentration of microspheres but of a different colour. By allowing the foragers to return and feed ad libitum at these two types of feeder, we created primary infectives with different lengths of infectious period in the two treatments. After 4 h, by which time the feeder was depleted, we collected a random sample of approximately 150 bees such that there was an approximately equal representation from each of the six age classes. We excluded the foragers which fed at the source from this sample. We performed the two treatments within 2 days of each other and microspheres from the first treatment were not noticeably present in the second. We freeze-killed the extracted bees and dissected their alimentary canals. We homogenized these gut samples in 1 ml of 10% KOH and counted the number of microspheres in each of them using a haemacytometer.
We also put together a similar colony in a two-frame observation hive and subjected it to the same two treatments described earlier (but without using microspheres). The entrance to the hive was designed in a way such that the returning foragers had to perform almost all of their nectar unloading on one side of the frames. For each treatment, we made a video recording of 1 h, using a high-definition video camera (Sony HDR-HC1). We observed these recordings on a digital display, following each forager upon its return from a foraging trip. We measured the duration of each trophallaxis or food transfer initiated by it, which lasted for at least 5 s, and identified the receiver. We followed each of these receivers in turn to similarly quantify the next order of transfer interactions.
3. Results
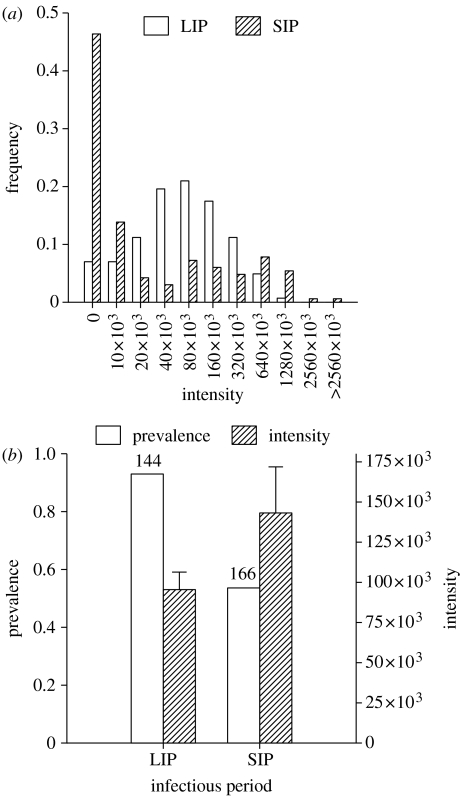
The observed distributions of microspheres among colony members in the two treatments show that a change in infectious period brought about by feeding primary infectives with different sucrose concentrations produced significant changes in the transmission dynamics. A LIP resulting from feeding on a high sucrose concentration resulted in significantly higher prevalence (proportion of individuals infected with particles), lower inter-individual variability in intensity (number of particles per individual) and lower maximum intensity compared with when bees had a SIP from feeding on a low concentration (figure 1). Mean intensity, as expected, was not significantly different in the two treatments given the same concentration of particles or dosage in both. However, given the vastly different shapes of the two distributions, the lower absolute value for mean intensity in the case of LIP should be noted and could be attributed to the observation that receiver bees in the LIP treatment deposited sucrose into honey storage cells with a higher frequency (94 instances) as compared with the SIP treatment (46 instances).
Figure 1.
Colony-level transmission patterns under long and short infectious periods. (a) The distribution resulting from the long infectious period (LIP) treatment is significantly different from that resulting from SIP by Kolmogorov–Smirnov 2-Sample test (Dmax=0.46, n1=143, n2=166, p<0.001). (b) Calculated from these distributions are: (i) prevalence: LIP=0.93, SIP=0.53, G=65.60, d.f.=1, p<0.0001, the number above each respective bar refers to the total number of individuals sampled for the presence of infectious material (microspheres) from which the proportions were calculated, (ii) intensity (mean+s.e.): LIP=9.54×104, SIP=14.31×104, one-way ANOVA after square-root transformation, F=2.75, p=0.09, (iii) coefficient of variation (CV) intensity: LIP=1.36, SIP=2.58 and (iv) maximum intensity: LIP=1.04×106, SIP=3.56×106.
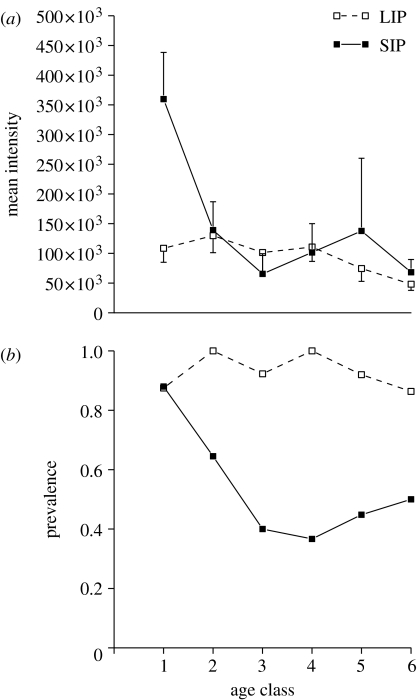
Although the mean intensity is similar in both the treatments, when we consider the transmission pattern with respect to the age of the bees, we note that a LIP resulted in a uniformly low intensity across all age classes, while a SIP caused the oldest bees to have significantly higher intensity than individuals of all other ages (Tukey–Kramer test, LSD=190952.35, p<0.0001; figure 2a). In contrast, prevalence was uniformly higher across all age classes when the infectious period was long but heterogeneous, restricted mostly to the oldest individuals, when infectious period was short (figure 2b).
Figure 2.
Effect of age on transmission patterns under LIP and SIP treatments measured as: (a) intensity (mean±s.e.): LIP: F5,137=1.13, p=0.34; SIP: F5,160=2.32, p=0.04 and (b) prevalence: LIP: GH=5.88, d.f.=5, p=0.31, GP=73.73, d.f.=1, p<0.0001; SIP: GH=23.69, d.f.=5, p=0.0002, GP=28.15, d.f.=1, p<0.0001. The standard error bars for LIP are depicted only for the negative range while those for SIP are depicted for the positive range to avoid overlaps in the figure. The x-axis represents weekly age classes 1–6, with 1 being the oldest and 6 the youngest. The left-to-right order of the x-axis also represents the spatial distribution of ages in the colony, with age class 1 located at the outermost edge and age class 6 at the colony centre, and the direction in which the microspheres enter and travel through the colony.
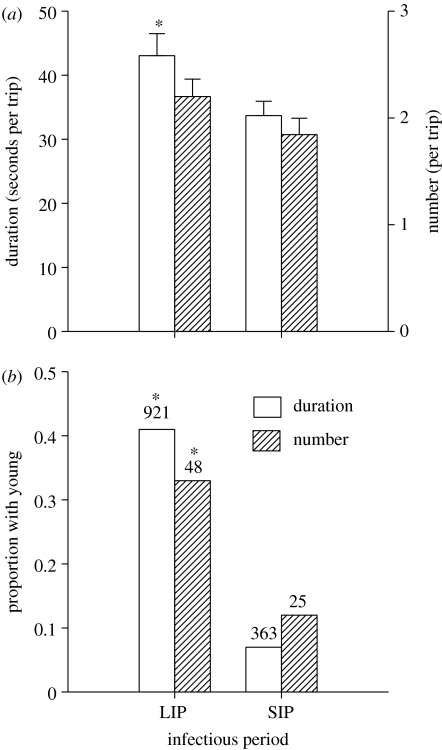
In order to identify the behavioural processes that led to the above patterns, we investigated all trophallactic interactions resulting from each foraging trip, in terms of their duration and the identity of the individuals participating in them. The behavioural data revealed that each foraging trip resulted in a higher total duration of interactions in the LIP treatment than that in the SIP though the number of interactions was similar in the two treatments (figure 3a). We also found a clear age-based pattern in the transfer process with a significantly higher proportion of both the total number and the duration of interactions directed towards younger individuals when the infectious period was long (figure 3b).
Figure 3.
Inter-individual trophallactic interactions among bees in colonies exposed to LIP and SIP treatments. (a) Total duration (mean+s.e.) t131=2.28, p=0.02 and total number (mean+s.e.) t142=1.59, p=0.11; of interactions resulting from each foraging trip, (b) age-dependent pattern of interactions as given by proportions of the transfer process directed by receiver bees towards bees younger than themselves in terms of total duration, G=156.27, d.f.=1, p<0.0001 and number G=4.25, d.f.=1, p=0.03. The number above each respective bar refers to the total duration (seconds) and number of all interactions observed from which the proportions were calculated. Statistical comparisons are between the two treatments for each measure of trophallactic interaction and asterisk represents a statistically significant difference.
4. Discussion
How do these experimental results from the honeybee colony apply to a broader epidemiological context? We see that large changes in transmission dynamics at the group level can result from a change in the infectious period of individuals, a longer infectious period resulting in a higher total trophallactic transfer, in turn leading to a spread of the infection among more individuals. The implications of the observed inverse relationship between prevalence and intensity in the colony are particularly striking. While a LIP results in a large pool of individuals infected to a similar low degree, a SIP produces a few highly infected and a large number of uninfected individuals. The latter resembles a super-spreader situation, where a minority of the individuals could be the major drivers of the future dynamics of the system. Since the inert microspheres in our experiments approximate dormant pathogen spores that are introduced into a population of susceptibles by a few infected individuals, these distributions reflect the initial invasion of a potential infection before the pathogen begins replicating itself. Since the early stages of invasion can determine the success or the failure of a pathogen and set up the scenario for the relatively more deterministic phase of transmission (Keeling 1999), these initial distributions are therefore critical to understanding the final course of transmission dynamics. However, given that the microspheres used in our experiments are inert unlike the spores of an actual pathogen, one must note that the infectious dose received by an individual decays with each step in the transmission chain and the intensity patterns of an actual disease could considerably differ from that observed in our experiment.
If these initial distributions are indeed indicators of the final outcome of the infection process, it suggests that a disease with a LIP will make it relatively easier to detect but more difficult to contain owing to its high prevalence. In contrast, a disease with a SIP will be difficult to spot because infected individuals are relatively rare, but once these few individuals are located, control measures such as quarantine or vaccination would be easier to target. Our results also imply that a change from a SIP to a LIP could be caused by some simple changes in the transmission mechanism, in this case, a change in an environmental variable, causing a disease lurking in the background in a low prevalence state to rapidly erupt into an epidemic of high prevalence.
While changing the infectious period of individual bees causes a change in the prevalence and intensity of the infectious process in the colony, these patterns could be generated by alternate behavioural processes. A longer infectious period could increase the total number of contacts made by an infected individual with susceptibles. Alternately, it could increase the efficacy of each contact in passing on the infection, without causing any change in the contact rate itself. Our behavioural data showing that the number of contacts resulting from each foraged load remains similar across the two treatments though the duration of each contact is longer in the LIP treatment is suggestive of the latter mechanism.
The distribution of the infection among the various age groups in the colony indicates that an infection with a longer period has a higher force, reaching across all age groups, while an infection with a short period is restricted to only older individuals. This pattern suggests that the organizational structure of the colony interacts with the infectious period in a way such that the centripetal transmission wave resulting from a short period is significantly dampened before it reaches the younger individuals, shielding them from contacting such an infection. This phenomenon requires an age-based interaction process in which individuals of each age group interact predominantly with individuals in its adjacent age class. Such age-based interaction could arise as a result of two alternate, though not entirely independent, processes. Individuals could either be spatially segregated in the colony according to their ages with inter-individual interactions following a nearest-neighbour pattern, or individuals could actively seek and interact with other individuals in the adjacent age class even though there is no spatial segregation of ages.
Our observation that a shorter infectious period results in younger individuals participating in a significantly lower proportion of the transfer process suggests that it is indeed age-specific interaction that is responsible for providing protection to the younger individuals. Although our experiment was not designed to distinguish between the two alternate mechanisms that could lead to age-based interaction, a spatial segregation of individuals, with the oldest (that include the foragers) located at the outer edge and the youngest at the centre of the colony, has been suggested in honeybee and other social insect colonies (Bourke & Franks 1995). This is consistent with the idea of the selfish herd (Hamilton 1971) that individuals at the centre of a social group would be less vulnerable to pathogens. While this has been observed in several social groups (Mooring & Hart 1992), most hypotheses concerning the observed spatial organization in social insects are rooted in the idea of optimization of colony work efficiency (Oster & Wilson 1978).
Our results point to how protection of young individuals, who have a higher lifetime ergonomic value than older ones, from infection could have also contributed to the structural organization of insect colonies (Schmid-Hempel 1998; Naug & Camazine 2002). We term this phenomenon of how social organization might interact with epidemiological variables such as infectious period to create different risk categories within a social group as ‘organizational immunity’. This is akin to the concept of herd immunity where the immunological status of the majority reduces transmission of the pathogen to the few remaining susceptible individuals. Assuming that there is a trade-off between transmission rate and virulence of a pathogen, this in turn suggests that pathogens which target individuals that can only be reached by long-distance transmission chains should be selected to have LIPs and lower virulence. In contrast, diseases occurring in individuals accessible by short connections should be selected for SIPs and higher virulence. This implies that the contact structure of a social group and the transmission characteristics of a pathogen probably provide a strong selective force on each other (Ewald 1991; Read & Keeling 2003).
Honeybee colonies commonly experience epidemics of a number of diseases and changes in the environment, such as quality and quantity of nectar availability, are known to produce dramatic changes in colony epidemiology of diseases, such as American and European foulbrood, sacbrood, tracheal mites, etc. (Bailey 1981). While existing explanations for such epidemiological patterns are less than parsimonious, our results show how large changes in colony epidemiology could result from small changes in transmittive properties of individual bees that would accompany environmental variation in nectar quality.
We show that the relative ease with which we can manipulate the transmission characteristics of an individual bee allows us to experimentally simulate transmission processes of various kinds in a honeybee colony. While transmission processes in social groups have attracted considerable theoretical attention, most of the ideas have either remained untested or their tests have been mostly correlative. Our protocol of using inert microspheres as proxy for pathogen spores can produce neutral models of transmission dynamics that occur solely as a result of inter-individual interactions, uninfluenced by any reproductive or behavioural dynamics of an actual host-pathogen interaction. Comparing such models with infectious processes produced by actual pathogens would enable us to separate the characteristics of transmission processes that are consequences purely of the social kinetics from those which arise as a result of interactions between the host and the pathogen. In other words, our experimental technique can provide detailed information about the key role of contact structure in social transmission processes of any kind (e.g. information, resource, disease, etc.), the dynamics of disease processes being in addition influenced by host–pathogen interactions. This, in conjunction with our ability to experimentally alter the social organization of the honeybee colony, presents a unique opportunity to measure the individual and synergistic roles of a large number of putative transmission variables, test the role of social networks and ‘organizational immunity’ in transmission dynamics, and develop testable models of social epidemiology.
In the face of rapid evolution of new strains of resistant pathogens, the inherent limitations of the two main traditional methods of disease control—antimicrobial agents to eliminate pathogens and vaccines to promote host immunological mechanisms—have become increasingly obvious (Cohen 1994; Baquero & Blázquez 1997). Instead, understanding social mechanisms that have been selected to counter pathogen transmission in groups subject to substantial pathogen pressure and promoting them are likely to prove useful in designing cost-effective and high-impact methods to curtail epidemic processes, which is highly relevant to present challenges posed by bioterrorism and emerging infectious diseases (Ferguson et al. 2003; Balthrop et al. 2004; Brookmeyer et al. 2004).
Acknowledgments
This work is supported by a grant from the Ecology of Infectious Diseases Section of the National Science Foundation, USA. D.N. would like to thank Mike Antolin, H. S. Arathi, Chris Brooks, Raghavendra Gadagkar, David Hughes, Janice Moore, Gene Robinson, Mike Wade and three anonymous referees for their valuable comments and Broox Boze, Ann Gibbs and Robert Wildermuth for extracting a large part of the video data.
References
- Anderson R.M. Oxford University Press; Oxford, UK: 1992. Infectious disease of humans. [Google Scholar]
- Bailey L. Academic Press; London, UK: 1981. Honey bee pathology. [Google Scholar]
- Balthrop J, Forrest S, Newman M, Williamson M. Technological networks and the spread of computer viruses. Science. 2004;304:527–529. doi: 10.1126/science.1095845. doi:10.1126/science.1095845 [DOI] [PubMed] [Google Scholar]
- Baquero F, Blázquez J. Evolution of antibiotic resistance. Trends Ecol. Evol. 1997;12:482–487. doi: 10.1016/s0169-5347(97)01223-8. doi:10.1016/S0169-5347(97)01223-8 [DOI] [PubMed] [Google Scholar]
- Bourke A.F.G, Franks N.R. Princeton University Press; Princeton, NJ: 1995. Social evolution in ants. [Google Scholar]
- Brookmeyer R, Johnson E, Bollinger R. Public health vaccination policies for containing an anthrax outbreak. Nature. 2004;432:901–904. doi: 10.1038/nature03087. doi:10.1038/nature03087 [DOI] [PubMed] [Google Scholar]
- Cohen M.L. Emerging problems in antimicrobial resistance. Ann. Int. Med. 1994;24:454–456. doi: 10.1016/s0196-0644(94)70183-0. [DOI] [PubMed] [Google Scholar]
- Crailsheim K. Regulation of food passage in the intestine of the honeybee (Apis meillifera L.) J. Insect Physiol. 1988;34:85–90. doi:10.1016/0022-1910(88)90158-8 [Google Scholar]
- Ewald P.W. Transmission modes and the evolution of virulence, with special reference to cholera, influenza and AIDS. Hum. Nat. 1991;2:1–30. doi: 10.1007/BF02692179. [DOI] [PubMed] [Google Scholar]
- Ferguson N, Keeling M, Edmunds W, Gani R, Grenfell B.T, Anderson R.M, Leach S. Planning for smallpox outbreaks. Nature. 2003;425:681–685. doi: 10.1038/nature02007. doi:10.1038/nature02007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenaars T.J, Donnelly C.A, Ferguson N.M. Spatial heterogeneity and the persistence of infectious diseases. J. Theor. Biol. 2004;229:349–359. doi: 10.1016/j.jtbi.2004.04.002. doi:10.1016/j.jtbi.2004.04.002 [DOI] [PubMed] [Google Scholar]
- Hamilton W.D. Geometry for the selfish herd. J. Theor. Biol. 1971;31:295–311. doi: 10.1016/0022-5193(71)90189-5. doi:10.1016/0022-5193(71)90189-5 [DOI] [PubMed] [Google Scholar]
- Keeling M.J. The effects of local spatial structure on epidemiological invasions. Proc. R. Soc. B. 1999;266:859–867. doi: 10.1098/rspb.1999.0716. doi:10.1098/rspb.1999.0716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling A.J, Grenfell B.T. Effect of variability in infection period on the persistence and spatial spread of infectious diseases. Math. Biosci. 1998;147:207–226. doi: 10.1016/s0025-5564(97)00101-6. doi:10.1016/S0025-5564(97)00101-6 [DOI] [PubMed] [Google Scholar]
- Lloyd A.L. Realistic distributions of infectious periods in epidemic models: changing patterns of persistence and dynamics. Theor. Popul. Biol. 2001;60:59–71. doi: 10.1006/tpbi.2001.1525. doi:10.1006/tpbi.2001.1525 [DOI] [PubMed] [Google Scholar]
- Mooring M.S, Hart B.L. Animal grouping for protection from parasites: selfish herd and encounter-dilution effects. Behavior. 1992;123:173–193. [Google Scholar]
- Naug D, Camazine S. The role of colony organization in pathogen transmission in social insects. J. Theor. Biol. 2002;215:427–439. doi: 10.1006/jtbi.2001.2524. doi:10.1006/jtbi.2001.2524 [DOI] [PubMed] [Google Scholar]
- Oster G.F, Wilson E.O. Princeton University Press; Princeton, NJ: 1978. Caste and ecology in the social insects. [PubMed] [Google Scholar]
- Read J.M, Keeling M.J. Disease evolution on networks: the role of contact structure. Proc. R. Soc. B. 2003;270:699–708. doi: 10.1098/rspb.2002.2305. doi:10.1098/rspb.2002.2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Hempel P. Princeton University Press; Princeton, NJ: 1998. Parasites in social insects. [Google Scholar]