Abstract
Parental investment decisions depend on multiple factors, including the extent that parental care benefits offspring. Humans should show reduced parental effort in environments where parenting cannot improve offspring survival. Data from the standard cross-cultural sample are used to test this prediction. The results show that maternal care was inversely associated with famine and warfare, and also showed a quadratic association with pathogen stress, increasing as pathogen stress increased to moderate levels, but decreasing at higher levels. Age at weaning showed a similar quadratic relation with pathogens. The curvilinear associations between parental effort and pathogen stress may reflect that the saturation point of parental care is a function of environmental hazards. Paternal involvement was also inversely related to pathogen stress. The association between pathogens and paternal involvement was partially mediated by polygyny. In sum, maternal and paternal care appears to have somewhat different relations with environmental hazards, presumably owing to sex-specific tradeoffs in reproductive effort.
Keywords: life history, evolutionary ecology, cross-cultural analysis, parental investment, extrinsic mortality
1. Introduction
Human life history reflects two major decisions: whether to reproduce now or later, and the amount of care to invest in each offspring. These decisions depend on the costs and benefits of mating and parenting effort, which may be influenced by environmental risk. ‘Risk’ can be defined as ‘unpredictable variability in the outcome of an adaptively significant behaviour’ (Winterhalder & Leslie 2002, p. 61). Other definitions view risk as the conditional probability of an outcome (Rothman & Greenland 1998). In life history theory, extrinsic mortality is the risk of dying at a particular age that is shared equally by all members of a population. More specifically, extrinsic mortality is ‘not sensitive to changes in reproductive decisions’ (Stearns 1992, p. 182). Predation is the greatest source of extrinsic mortality for most organisms (see Roff 2002), but there may be other causes. Mortality need not be the only source of risk relevant to life history (Quinlan 2006). Any unavoidable factor that can reduce an organism's reproductive value could be called an ‘extrinsic risk’, and theoretically it should have effects similar to extrinsic mortality.
Extrinsic risk, like extrinsic mortality, is independent of parental care (Harpending et al. 1990; Borgerhoff Mulder 1992; Chisholm 1999; Quinlan 2006). Basic parental care should usually benefit offspring, but extrinsic risk creates substantial diminishing returns to parental effort. There is a point (Smax) beyond which fitness does not respond to additional parental care (figure 1; Pennington & Harpending 1988). Child outcomes are determined by chance once parents reach the ‘saturation’ point at Smax (Pennington & Harpending 1988; Harpending et al. 1990, pp. 254–255). If extrinsic risk is high, then parents reach Smax at lower levels of effort. Hence, channelling resources away from parenting into mating effort or additional offspring should enhance fitness when extrinsic risk is high. But if extrinsic risk is low, then the saturation point of parental care is ‘high’ and responsive parenting can have important influence on children's survival and ultimate success. In sum, tradeoffs between mating and parenting effort or offspring quality and quantity depend on whether environmental hazards can be avoided by increasing parental effort.
Figure 1.
Predicted effect of extrinsic risk on offspring fitness as a function of parental effort. Note: adapted from Pennington & Harpending (1988). Smax (saturation point of parental effort) is a function of extrinsic risk. Smax is the point at which additional parental effort does not improve offspring fitness (i.e. the point at which the derivative of the offspring fitness function equals zero).
Extrinsic or ‘care-independent’ risk in human life history has attracted theoretical interest since the late 1980s (e.g. Harpending et al. 1990; Borgerhoff Mulder 1992; Chisholm 1993); however, empirical work is scarce. Several studies show predicted relations between extrinsic risk and human life-history patterns. Mortality was associated with reproductive timing among urban Americans (Wilson & Daly 1997) and Sub-Saharan Africans (Gant et al. in press), for example. And perception of mortality may influence human reproductive behaviour (Chisholm et al. 2005). This study examines relations between sources of environmental risk and parenting effort cross-culturally. Pathogen stress, famine and warfare may be associated with extrinsic mortality. These sources of extrinsic risk are predicted to be inversely related to parental effort, because parents switch to greater allocations in either mating effort, additional offspring or, if the hazards are severe, to somatic effort (self-preservation) to enhance their fitness.
2. Material and methods
Multiple linear regression analysis in Stata v. 9 was used to test the hypothesis that extrinsic risk is inversely related to parental effort. Parental effort variables were regressed on measures of pathogen stress, famine and warfare from the standard cross-cultural sample (SCCS). The SCCS includes 186 mostly pre-industrial societies with various subsistence strategies, including hunter-gatherers, fishers, pastoralists, horticulturalists and agriculturalists. The analyses here use sub-samples, including 42–85 of the SCCS societies for which relevant data were available.
Predictor variables indicated three sources of environmental risk: (i) total pathogen stress (variable 1260; Low 1988) ranges from 7 to 21 and is the sum of variables 1253–1259 (leishmanias, trypanosomes, malaria, schistosomes, filariae, spirochetes and leprosy), which range from 1 (absent) to 3 (present and serious, widespread, endemic) for each; (ii) severity of famine (variable 1267; Dirks 1993) ranges from 1 (very low) to 4 (very high); famine was reference coded and treated as a categorical variable with ‘very low and low famine severity’ as the reference category; and (iii) overall frequency of warfare (variable 1648; Ember & Ember 1992) ranges from 1 (absent or low) to 18 (occurs constantly). Quadratic terms were entered for each predictor and significant quadratic effects were retained in the models. Alpha was set at 0.05.
There were three measures of parental effort. Two criterion variables were created by summing the standardized values of SCCS variables (Barry & Paxson 1971). Maternal care included three items: (i) mother's sleeping proximity to infant (recoded from variable 23) ranges from 1 (different room) to 3 (same bed); (ii) parental response to infant crying (variable 31) ranges from 1 (indifferent or punitive) to 5 (always speedy, nurturant); and (iii) bodily contact in early infancy (variable 26) ranges from 1 (limited to routine and precautionary care) to 5 (almost constant bodily contact). These three parenting items, largely reflecting maternal behaviours, showed high internal consistency (Cronbach's alpha=0.73). Maternal care ranges from −6.95 to 4.12, with more than 20 different values. Paternal involvement also included three items: (i) father's sleeping proximity to infant (recoded from variable 23) ranges from 1 (different room) to 3 (same bed); (ii) father's involvement in infancy (variable 53) ranges from 1 (distant) to 5 (regularly close); and (iii) father involvement in early childhood (variable 54) ranges from 1 (distant) to 5 (regularly close). The three items also showed high internal consistency (Cronbach's alpha=0.76). Paternal involvement ranges from −6.37 to 5.60, with more than 20 different values. Age at weaning was the third parental effort variable (Barry & Paxson 1971). Age at complete termination of breastfeeding (variable 45) was converted into months by taking the midpoint of the coded intervals.
Control variables were included in each analysis. I added potential controls to linear models with the three extrinsic risk predictors to explore relations with social complexity, economy, subsistence strategy, population size, division of labour by sex, male and female contribution to subsistence, community structure, parenting style, weather patterns, latitude, region, acculturation, modernization and contact with ‘Western-industrial’ populations. Significant control variables were retained in the models, including region (dummy variables for Africa, Eurasia and the Circum-Mediterranean recoded from variable 200; Divale 2004, p. 37), presence of money (dummy variable recoded from variable 17; Murdock & Morrow 1970), endogamy (dummy variable coded from variable 72 indicating more than 60% of marriages within local community; Murdock & Wilson 1972), patrilineal inheritance (dummy variable recoded from variable 279 ‘inheritance of movable property’; Divale 2004, p. 46), modernization (variable 1849; Divale & Seda 2000) ranges from 5 to 35, and population density (variable 64; Murdock & Wilson 1972) ranges from 1 (less than 1 person per 5 square miles) to 7 (more than 500 persons per square mile). Polygyny (variable 79; Murdock & Wilson 1972) was recoded as a dummy variable (1, more than 20% plural wives; 0, less than 20% plural wives) and was included in an additional analysis of paternal involvement to test whether polygyny mediated associations with extrinsic risk. Polygyny may reflect tradeoffs in male reproductive effort. Codes for all variables in the SCCS are available in Divale (2004).
Regional clustering can result in biased standard errors. The SCCS was compiled so that 43% of the groups included did not share common ancestry estimated within 2000 years with any other group in the sample based on linguistic and geographical affinity (Murdock & White 1969). Fifty-seven per cent shared between one and three other members of their linguistic subfamily. Hence, ‘phylogenetic’ relationships were a potential nuisance in these analyses. Robust standard errors for clustered data were computed to adjust for potential intraclass correlation among the clustered societies (Rogers 1993). Variable 1858 (region; Burton 1999) in SCCS was used as the clustering variable.
3. Results
Diagnostic analyses indicated that the models did not deviate appreciably from the assumptions of multiple linear regression. Descriptive statistics for the criterion variables are given in table 1.
Table 1.
Descriptive statistics for parental care variables.
| n | mean | s.d. | min. | max. | |
|---|---|---|---|---|---|
| maternal care | 48 | 0.06 | 2.48 | −6.95 | 4.12 |
| paternal involvement | 42 | −0.39 | 2.71 | −6.37 | 5.60 |
| age at weaning (months) | 85 | 31.14 | 9.08 | 12.00 | 54.50 |
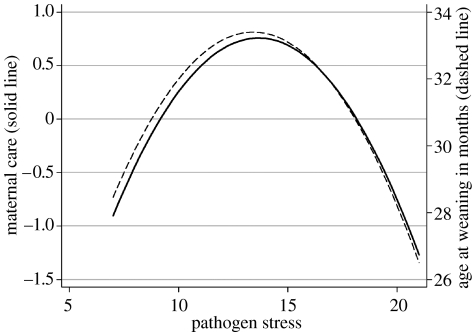
Maternal care, paternal involvement and weaning were associated with environmental risk. Maternal care showed the predicted inverse relations with famine and warfare—as famine severity and prevalence of warfare increased, maternal care decreased (table 2, model 1). These results suggest that famine and warfare are true extrinsic or care-independent risks. Pathogen stress showed a quadratic relationship with maternal care (table 2, model 1; figure 2): maternal care increased as pathogens increased to moderate levels, then decreased at higher pathogen levels. This suggests that the effects of pathogens on child outcomes may depend on parenting at low to moderate levels, but at high levels pathogens may present extrinsic risk. The curvilinear association may indicate that the saturation point of parental care is a function of environmental hazards. African population and endogamy (marriage within the local community) were also statistically significant and included as control variables (table 2, model 1). Other potential controls, including measures of acculturation and modernization, were neither significant nor appreciably altered the associations in the models in table 2.
Table 2.
Multiple linear regression showing associations between environmental risk and parental effort. (B, unstandardized regression coefficient; p, two-tailed significance; s.e., robust standard errors accounting for clustered data; no. of clusters, the number of world regions represented (10 in all cases); n, sample size; R2, variance accounted for by all predictors.)
| B | robust s.e. | p | model statistics | |
|---|---|---|---|---|
| model 1: maternal care | ||||
| pathogen stress | 1.03 | 0.39 | 0.026 | n=48 |
| pathogen stress-squared | −0.04 | 0.01 | 0.020 | R2=0.55 |
| famine severity higha | −1.06 | 0.43 | 0.034 | p=0.000 |
| very higha | −3.55 | 0.61 | 0.000 | |
| warfare | −0.13 | 0.05 | 0.023 | |
| Africa=1, else 0 | 3.45 | 0.81 | 0.002 | |
| Endogamy=1, else 0 | −1.42 | 0.34 | 0.002 | |
| constant | −2.59 | 2.52 | 0.330 | |
| model 2a: paternal involvement | ||||
| pathogen stress | −0.22 | 0.04 | 0.001 | n=42 |
| famine severity higha | −1.86 | 1.85 | 0.341 | R2=0.59 |
| very higha | 0.59 | 1.42 | 0.689 | p=0.000 |
| warfare | 0.03 | 0.04 | 0.565 | |
| patrilineal inheritance=1, else 0 | −2.81 | 0.63 | 0.002 | |
| Eurasia=1, else 0 | 4.07 | 0.69 | 0.000 | |
| constant | 3.42 | 1.28 | 0.025 | |
| model 2b: paternal involvement | ||||
| polygyny >20%=1, else 0 | −1.19 | 1.06 | 0.288 | n=42 |
| pathogen stress | −0.14 | 0.09 | 0.137 | R2=0.61 |
| famine severity higha | −1.98 | 1.87 | 0.316 | p=0.000 |
| very higha | 0.37 | 1.67 | 0.829 | |
| warfare | 0.05 | 0.04 | 0.304 | |
| patrilineal inheritance=1, else 0 | −2.71 | 0.70 | 0.004 | |
| Eurasia=1, else 0 | 3.47 | 0.97 | 0.006 | |
| constant | 2.86 | 1.07 | 0.026 | |
| model 3: age at weaning in months | ||||
| pathogen stress | 3.22 | 1.39 | 0.046 | n=85 |
| pathogen stress-squared | −0.12 | 0.05 | 0.039 | R2=0.33 |
| famine severity higha | 0.52 | 2.64 | 0.847 | p=0.000 |
| very higha | −0.05 | 2.75 | 0.987 | |
| warfare | −0.06 | 0.12 | 0.618 | |
| circum-Mediterranean=1, else 0 | −9.96 | 1.30 | 0.000 | |
| money present=1, else 0 | 6.21 | 1.85 | 0.008 | |
| population density | −2.62 | 0.53 | 0.001 | |
| constant | 19.94 | 9.23 | 0.059 |
Famine severity was treated as reference coded categorical variable with famine severity low/very low as the reference category, hence each level entered the model separately. Control variables are indicated in italics.
Figure 2.
Quadratic associations between pathogen stress and parental effort. Note: predicted parental effort from data in table 2, models 1 (maternal care) and 3 (weaning) adjusted for other variables in table 2.
Paternal involvement showed the predicted inverse relation with pathogen stress (table 2, model 2a). As pathogens increased, paternal involvement decreased. Paternal care was not significantly associated with famine or warfare (table 2, model 2a). The association between pathogen stress and paternal involvement was partially mediated by polygyny; however, polygyny was not a significant predictor (table 2, model 2b; see Low 1988). Patrilineal inheritance (inheritance through male lines) and Eurasian population were included as control variables (table 2, models 2a and 2b). Modernization also showed a significant association with paternal care when included with the other variables (B=0.17, p=0.008, n=32); however, it did not substantially alter the other associations (data not shown). Pathogen stress adjusted for modernization and other controls was a significant predictor of paternal involvement (B=−0.34, p<0.001). Modernization reduced the sample size to 32, making model stability questionable, hence it was excluded from models 2a and 2b in table 2. Polygyny was a significant predictor of paternal involvement when modernization was included (B=−2.03, p=0.027, n=32), and it still mediated the association between pathogen stress and paternal involvement (B=−0.18, p=0.103). Other associations were approximately unchanged.
Age at weaning showed a quadratic relation with pathogen stress (table 2, model 3; figure 2). Age at weaning increased as pathogens increased to moderate levels; however, it decreased at higher pathogen levels. The curvilinear association between weaning and pathogen stress further suggests that the saturation point of parental care is a function of environmental hazards. Famine and warfare were not significantly associated with age at weaning. Circum-Mediterranean, population density and presence of money were included as control variables (table 2, model 3).
4. Discussion
Parental effort was inversely associated with extrinsic risk. Warfare and famine appear to be hazards that are not remedied by parental care. Parental care may improve child outcomes at low to moderate levels of pathogen stress. Weaning age and maternal care increased at low to moderate pathogen levels (which was not predicted), but higher pathogen levels were associated with earlier weaning and less maternal care, consistent with predictions. Paternal involvement decreased as pathogens increased. These results are generally consistent with the life-history prediction of relatively reduced parental effort in environments with high extrinsic risk. The saturation point of human parental effort may be sensitive to environmental hazards.
The SCCS offers useful but limited data. Most parenting measures in the SCCS address infancy and early childhood. Better measures of parental care throughout development would be useful. In some cultures, like suburban North America (which was not included in these analyses), parents do not typically sleep with children and they maintain distant proximity throughout most of the day. Suburban North Americans make substantial indirect parental investment through schools, clothing, etc. Furthermore, differences in early and late childhood and direct and indirect investment and their effects on offspring may prove interesting in themselves. The negative association between paternal involvement and patrilineal inheritance (inheritance through male lines) of movable property (table 2, models 2a and b) suggests tradeoffs between paternal effort in childhood and later investment.
Pathogen stress showed somewhat variable relations with parental effort. At low pathogen levels, parental effort was positively associated with pathogen stress, but parental effort decreased at higher levels. It is difficult to imagine how response to infant crying, maternal co-sleeping and bodily contact with infants could affect exposure to vector-borne pathogens like malaria, etc. measured in the SCCS. Parental care may not affect exposure (as per Harpending et al. 1990), but responsive parenting may reduce severity of infection and increase the likelihood of recovery. Responsive parental care may buffer children from chronic psychosocial stressors, improving immune function (Flinn & England 2003). Children's stress reactivity and hormone levels (cortisol) are positively associated with unresponsive parenting (Gunnar 2000; Gunnar & Donzella 2002; Blunt Bugental et al. 2003). Cortisol levels have been found to be inversely associated with children's immune function and frequency of illness (Flinn 1999). Immune functioning such as IgG1 activity can reduce the severity of vector-borne infections by trypanosomes, for example (Takayanagi et al. 1992), and recovery from trypanosomiasis appears to impart life-time immunity (Khonde et al. 1995). However, at high pathogen levels, survival may be more a matter of chance than child care.
Associations between paternal involvement and pathogen stress raise interpretive issues. Pathogens may create greater sexual selection pressures through female mate choice for heritable immunity (Hamilton & Zuk 1982), leading to higher levels of polygyny associated with reduced paternal investment. This interpretation lacks empirical support. A cross-cultural analysis showed little relationship between indicators of female choice and polygyny (Low 1988, pp. 120–122). In contrast to other animals, human polygyny often appears to entail costs to female reproduction. There is little evidence that women benefit reproductively from polygyny (Strassmann 2000; Josephson 2002). Alternatively, it is possible that pathogen stress increases benefits of genetic diversity in offspring (Low 1988). If so, then pathogen stress should be positively correlated with indicators of multiple mates cross-culturally, which appears to be the case (Low 1988). The question remains whether or not paternal care typically influences children's response to pathogens. Furthermore, this study cannot distinguish whether fathers reduce parental care because they are attempting to produce more offspring to ‘beat the odds’ or to increase genetic diversity in offspring or both. These two possibilities are confounded.
I do not suggest that these findings indicate genetic differences in parenting behaviour in different populations. The environmental hazards measured in this study probably fluctuate too often for selection to create localized genetic adaptations. Rather ‘it is certainly plausible that humans are engineered to sense the state of the environment and to favour … certain behaviours over others depending on their perception of the environment’ (Harpending et al. 1990, p. 255). Such phenotypic plasticity is the hallmark of human adaptation.
Results here may extend to other context-dependent sources of risk. Mortality is only one hazard with potential life-history effects. Other hazards such as substance abuse (Quinlan 2006), HIV infection (Gant et al. in press), crime (Wilson & Daly 1997), imprisonment, etc. may be insensitive to parental care, and hence present ‘fitness cliffs’ (Chisholm 1999) that may affect allocation of effort among mating, parenting and self-preservation.
In sum, these findings suggest an important role for environmental risk in the allocation of human parental effort. Maternal and paternal care appear to have somewhat different relationships with environmental hazards, presumably owing to sex-specific benefits of parenting and mating effort. The sources and nature of such risk warrant further investigation.
Acknowledgments
I would like to thank William Divale who provided access to and assistance with the SCCS. Also I would like to thank Laura Betzig, Henry Harpending and Barry Hewlett who offered helpful comments on an early version of this paper.
References
- Barry, H., III & Paxson, L. M. 1971 Infancy and early childhood: cross-cultural codes 2. Ethnology10, 466–508.
- Blunt Bugental D, Martorell G.A, Barraza V. Hormonal costs of subtle forms of infant maltreatment. Horm. Behav. 2003;43:237–244. doi: 10.1016/s0018-506x(02)00008-9. doi:10.1016/S0018-506X(02)00008-9 [DOI] [PubMed] [Google Scholar]
- Borgerhoff Mulder M. Reproductive decisions. In: Smith E.A, Winterhalder B, editors. Evolutionary ecology & human behavior. Aldine de Gruyter; New York, NY: 1992. pp. 339–374. [Google Scholar]
- Burton M.L. Language and region codes for the Standard Cross-Cultural Sample. Cross Cultur. Res. 1999;33:63–83. [Google Scholar]
- Chisholm J. Death, hope, and sex: life-history theory and the development of reproductive strategies. Curr. Anthropol. 1993;34:1–24. doi:10.1086/204131 [Google Scholar]
- Chisholm J. Cambridge University Press; Cambridge, UK: 1999. Death, hope and sex: steps to an evolutionary ecology of mind and morality. [Google Scholar]
- Chisholm J.S, Quinlivan J.A, Petersen R.W, Coall D.A. Early stress predicts age at menarche and first birth, adult attachment, and expected lifespan. Hum. Nat. 2005;16:233–265. doi: 10.1007/s12110-005-1009-0. [DOI] [PubMed] [Google Scholar]
- Dirks R. Starvation and famine: cross-cultural codes and some hypothesis tests. Cross Cultur. Res. 1993;27:28–69. [Google Scholar]
- Divale W. Codebook of variables for the standard cross-cultural sample. World Cult. J. Cross Cultur. Comp. Res. 2004;14:1–347. [Google Scholar]
- Divale W, Seda A. Cross-cultural codes of modernization. World Cult. 2000;11:153–170. [Google Scholar]
- Ember C.R, Ember M. Codebook for “warfare, aggression, and resource problems: cross-cultural codes”. Behav. Sci. Res. 1992;26:169–186. [Google Scholar]
- Flinn M.V. Family environment, stress, and health during childhood. In: Panter-Brick C, Worthman C, editors. Hormones, health, and behavior. Cambridge University Press; Cambridge, UK: 1999. pp. 105–138. [Google Scholar]
- Flinn M.V, England B.G. Childhood stress: endocrine and immune responses to psychosocial events. In: Wilce J.M, editor. Social & cultural lives of immune systems. Routledge Press; London, UK: 2003. pp. 107–147. [Google Scholar]
- Gant, L., Heath, K. M. & Ejikeme, G. G. In press. Early motherhood, high mortality and HIV/AIDS rates in Sub-Saharan Afirica. J. Health Social Policy [DOI] [PubMed]
- Gunnar M.R. Early adversity and the development of stress reactivity and regulation. In: Nelson C.A, editor. The effects of adversity on neurobehavioral development: Minnesota symposium on child psychology. vol. 31. Lawrence Erlbaum; Mahwah, NJ: 2000. pp. 163–200. [Google Scholar]
- Gunnar M.R, Donzella B. Social regulation of the LHPA axis in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. doi:10.1016/S0306-4530(01)00045-2 [DOI] [PubMed] [Google Scholar]
- Hamilton W.D, Zuk M. Heritable true fitness and bright birds: a role for parasites? Science. 1982;218:384–387. doi: 10.1126/science.7123238. [DOI] [PubMed] [Google Scholar]
- Harpending H, Draper P, Pennington R. Cultural evolution, parental care and mortality. In: Swedlund A.C, Armelgos G.J, editors. Disease in populations in transition: antrhopological and epidemiological perspectives. Bergin & Garvey; New York, NY: 1990. pp. 251–265. [Google Scholar]
- Josephson S. Does polygyny reduce fertility? Am. J. Hum. Biol. 2002;14:222–232. doi: 10.1002/ajhb.10045. doi:10.1002/ajhb.10045 [DOI] [PubMed] [Google Scholar]
- Khonde N, Pepin J, Niyonsenga T, Milord F, De Wals P. Epidemiological evidence for immunity following Trypanosoma brucei gambiense sleeping sickness. Trans. R. Soc. Trop. Med. Hyg. 1995;89:607–611. doi: 10.1016/0035-9203(95)90408-5. doi:10.1016/0035-9203(95)90408-5 [DOI] [PubMed] [Google Scholar]
- Low B. Pathogen stress and polygyny in humans. In: Betzig L, Borgerhoff Mulder M, Turke P, editors. Human reproductive behavior: a Darwinian perspective. Cambridge University Press; Cambridge, UK: 1988. pp. 115–127. [Google Scholar]
- Murdock G.P, Morrow D.O. Subsistence economy and supportive practices: cross-cultural codes. Ethnology. 1970;9:302–330. [Google Scholar]
- Murdock G.P, White D.R. Standard cross-cultural sample. Ethnology. 1969;8:329–369. [Google Scholar]
- Murdock G.P, Wilson S.F. Settlement patterns and community organization: cross-cultural codes. Ethnology. 1972;11:254–295. [Google Scholar]
- Pennington R, Harpending H. Fitness and fertility among the kalahari !Kung. Am. J. Phys. Anthropol. 1988;77:303–319. doi: 10.1002/ajpa.1330770304. doi:10.1002/ajpa.1330770304 [DOI] [PubMed] [Google Scholar]
- Quinlan R.J. Gender and risk in a matrifocal Caribbean community: a view from behavioral ecology. Am. Anthropol. 2006;108:464–479. [Google Scholar]
- Roff D.A. Sinauer; Sunderland, MA: 2002. Life history evolution. [Google Scholar]
- Rogers W. Regression standard errors in clustered samples. Stata Tech. Bull. 1993;13:12–23. [Google Scholar]
- Rothman R.J, Greenland S. Measures of disease frequency. In: Rothman K.J, Greenland S, editors. Modern epidemiology. Lippincott-Raven; Philadelphia, PA: 1998. pp. 29–64. [Google Scholar]
- Stearns S.C. Oxford University Press; Oxford, UK: 1992. The evolution of life histories. [Google Scholar]
- Strassmann B. Polygyny, family structure, and child mortality: a prospective study among the Dogon of Mali. In: Cronk L, et al., editors. Adaptation human behavior: an anthropological perspective. Aldine de Gruyter; New York, NY: 2000. pp. 49–68. [Google Scholar]
- Takayanagi T, Kawaguchi H, Yabu Y, Itoh M, Yano K. Immunological activities of monoclonal IgG1 antibody against Trypanosoma gambiense. Southeast Asian J. Trop. Med. Public Health. 1992;23:297–303. [PubMed] [Google Scholar]
- Wilson M, Daly M. Life expectancy, economic inequality, homicide, and reproductive timing in Chicago neighbourhoods. Br. Med. J. 1997;314:1271–1274. doi: 10.1136/bmj.314.7089.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterhalder B, Leslie P. Risk-sensitive fertility: the variance compensation hypothesis. Evol. Hum. Behav. 2002;23:59–82. doi:10.1016/S1090-5138(01)00089-7 [Google Scholar]