Abstract
Correlations between species richness and climate suggest non-random occupation of environmental space and niche evolution through time. However, the evolutionary mechanisms involved remain unresolved. Here, we partition the occupation of environmental space into intra- and inter-clade components to differentiate a model based on pure conservation of ancestral niches with higher diversification rates in the tropics, and an adaptive radiation model based on shifts in adaptive peaks at the family level allowing occupation of temperate regions. We examined these mechanisms using within- and among-family skewness components based on centroids of 3560 New World bird species across four environmental variables. We found that the accumulation of species in the tropics is a result of both processes. The components of adaptive radiation have family level skewness of species' distributions strongly structured in space, but not phylogenetically, according to the integrated analyses of spatial filters and phylogenetic eigenvectors. Moreover, stronger radiation components were found for energy variables, which are often used to argue for direct climatic effects on diversity. Thus, the correspondence between diversity and climate may be due to the conservation of ancestral tropical niches coupled with repeated broad shifts in adaptive peaks during birds' evolutionary history more than by higher diversification rates driven by more energy in the tropics.
Keywords: bird richness, bioclimatic envelope, environmental space, latitudinal gradients, macroevolution, niche conservatism
1. Introduction
The existence of geographical gradients in species richness, and the consequent skewed distribution of richness towards the tropics, is one of the best-documented ecological patterns (Hawkins 2001; Whittaker et al. 2001; Willig et al. 2003; Orme et al. 2005), and it suggests that the occupation of environmental space and niche evolution throughout time have not been random (Davies et al. 2005). Richness is well known to be correlated with climate (Hawkins et al. 2003a), but such correlations, however strong, do not by themselves reveal the evolutionary processes that must influence biodiversity patterns (Ricklefs 2004).
The accumulation of species in the tropics suggests variation in diversification rates (the difference between speciation and extinction rates) along environmental gradients. For example, the tropics may be richer owing to higher diversification rates because more available energy in warm climates buffers extinctions and allows higher population persistence, shorter generation times, higher mutation rates or faster physiological processes (Willig et al. 2003). Alternatively, phylogenetic niche conservatism (Wiens 2004; Wiens & Donoghue 2004; Wiens & Graham 2005) proposes that once an ancestral species appears in a given environment, descendants will tend to ‘inherit’ this niche, creating a bias in the occupation of environmental space by clades. If most ancestral clades arose in the tropics, niche conservatism will cause species to accumulate there.
Since the relative position of a species in the environmental space (e.g. its bioclimatic envelope) can be thought of as a species-level quantitative trait, we propose that it is possible to evaluate these historical components using analytical methods developed to examine macroevolutionary trends (McShea 1994, 1996; Gould 2002). This approach has been used to evaluate patterns in body size evolution and other quantitative traits (Maurer 1998; Maurer et al. 1992), but it has not been used to understand the patterns of occupation of environmental space and, consequently, as a tool to test the relative roles of alternative historical hypotheses developed to explain why there are more species in the tropics (Ricklefs 2004; Currie et al. 2004).
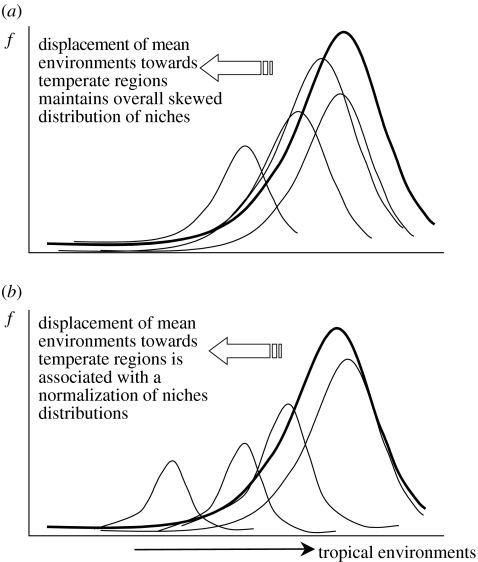
There are two basic macroevolutionary processes that can define the latitudinal richness gradient of a group of organisms. First, diversification rates in tropical regions may be higher (owing to higher speciation and/or lower extinction) across all clades comprising a large taxonomic group. Under this process, all the clades will have a similarly skewed distribution towards this region (figure 1a). High diversification rates may occur, because increasing productivity in tropical regions buffers extinction or because high levels of available energy accelerate speciation. These non-mutually exclusive mechanisms would generate a common spatial pattern in richness, and both can be characterized by a single ‘tropical attractor’, leading to an accumulation of species across all clades there. Thus, adaptation to non-tropical conditions is a process operating at relatively low-hierarchical levels (populations, species or eventually small and ‘young’ clades), and shifts towards temperate regions will occur independently within clades under a strong species-level niche conservatism process (Wiens & Donoghue 2004). Even when some groups of species, or subclades, occupy regions in a more extreme part of the environmental space (i.e. the temperate zone), higher diversification rates in tropical conditions will generate a skewed distribution of species' centroids.
Figure 1.
Alternative macroevolutionary processes generating the extra-tropical to tropical gradient in species richness. (a) If there are greater diversification rates in the tropics, generating a skewed distribution of an entire group of organisms in the environmental space, then all subgroups (clades) within this large group will show a similar skewed distribution. (b) Differential occupation of environmental space could reflect adaptive radiation giving rise to clades situated at a given ‘distance’ from the group-wide ancestral peak, after which adaptation will position the species following a Gaussian distribution around the new adaptive peaks.
Alternatively, the differential occupation of tropical space may not be generated by hierarchically lower-level shifts in all clades, but instead by a shift of one or more ancestral species to the temperate zone, followed by adaptive radiation centred at the new ecological position. After the initial colonization of the extra-tropics, niche conservatism would generate a Gaussian distribution of the derived species forming a new clade around this new peak. An overall skewed distribution towards the tropics would then be a consequence of an ancestral (tropical) condition, in which new adaptive peaks (i.e. clade centres) tend to appear more frequently near the overall ancestral tropical peak. But the distribution of species' centroids in the environmental space within these new clades would be skewed only if the new peak is close to the ancestral environmental conditions, owing to a lack of conditions more extreme than the ancestral ones (nothing is more tropical than the tropics). However, as long as a new peak originates at a large ‘distance’ from the ancestral conditions in the environmental space, adaptive radiation occurs and gives rise to new species following a Gaussian distribution around this new adaptive peak (figure 1b).
The latter process can explain richness gradients if most extant vertebrate families arose in the warm and wet climates of the Cretaceous–Eocene (i.e. in a particular region of the environmental space; see Wiens & Donoghue 2004). Thus, diversification leading to the occupation of newly created temperate environments generated by Oligocene–Miocene cooling in these regions (Wilf et al. 2003; Hawkins et al. 2006; McKenna & Farrell 2006) would occur by broad shifts in adaptive peaks of specific lineages and not by the independent adaptation of species to the new conditions within all lineages. Thus, the ancestral species that shifted in the environmental space will give rise to entire lineages whose new species have niches normally distributed around the new adaptive peak (Schluter 2000). Thus, the distribution of species in the environmental space would be a consequence of large-scale processes at higher clade levels, characterized by a skewed distribution of adaptive peaks themselves (i.e. clade means) and not by the skewed distribution of all species within these clades generated by a higher intrinsic rate of tropical diversification.
This allows us to partition the evolutionary trend underlying the occupation of the temperate zone into inter-clade (adaptive radiation in a subset of clades outside of ancestral conditions) and intra-clade processes (tropically biased diversification in all clades). Of course, these mechanisms are not mutually exclusive, and, indeed, we might expect both to be operating. In the context of the latitudinal diversity gradient, we can evaluate this by quantifying how much variation along the environmental space can be explained by the higher rates of tropical diversification of all clades in the tropics versus the variation explained by the appearance of new clades in the temperate zone, followed by their adaptive radiation to these new conditions.
Birds represent an excellent model group to test these ideas, as they have a well-documented latitudinal diversity gradient, with most species occurring in the wet tropics, and numerous ecological and historical mechanisms have been proposed to explain their richness gradient (Rahbek & Graves 2001; Jetz & Rahbek 2002; Hawkins et al. 2003b; Orme et al. 2005). Here, we used New World birds to partition the distribution of species' centroids into components of skewness, allowing us to quantify the relative roles of inter- and intra-clade diversification processes generating richness gradients. Simulations of these two processes of temperate region occupation along the defined environmental space are used to assess the power of the method used to detect these macroevolutionary components of diversification.
2. Material and methods
(a) Data
We divided the New World into 4220 grid cells of 1°×1° latitude/longitude and recorded the presence of each of the 3560 bird species (Ridgley et al. 2003) using Environmental Systems Research Institute, Inc.® ArcView v. 3.1 scripts. For each grid cell, we also recorded values of four environmental variables: (i) actual evapotranspiration (AET, in mm), defined as the amount of water used to meet the environmental energy demand; (ii) net primary productivity (NPP, in g C m−2 yr−1), defined as the total weight of carbon produced by plants per unit time; (iii) mean annual temperature (TEMP, in °C); and (iv) range in elevation (RELEV, in m; see Hawkins et al. 2003a,b, 2005).
Species' centroids in each environmental variable were obtained by averaging the values of these environmental data for those cells in which species is present. We followed Maurer (1998) and only families with more than 20 species were conservatively included in the analyses. Families were defined taxonomically according to Sibley & Monroe (1990).
(b) The analysis of skewness
The test of the alternative diversification models starts by evaluating the skewness of the statistical distribution of the coordinates of 3650 species' centroids along the four environmental variables (AET, TEMP, NPP and RELEV). The skewness of a distribution is measured by the coefficient g1, given as
where Yj is the quantitative variable of interest (i.e. the species' centroid along one of the environmental dimensions) for the jth species; , the mean across all n species; and s3, the cube of the standard deviation. A positive value of g1 indicates skewness to the right, a negative value skewness to the left, whereas g1=0 indicates a normal distribution.
Here, we used a more complex model, called analysis of skewness (ANSKEW hereafter; Wang 2001), to partition variation in skewness within families, between families and a heteroscedasticity component, along species' centroids for each environmental variable. These components quantify the proportion of variation in skewness within and between families that can distinguish between tropical diversification within clades and adaptive radiation across clades. ANSKEW is an improved form of McShea's subclade test (McShea 1994, 1996; Maurer 1998) that allows a quantitative evaluation of the so-called ‘passive’ and ‘driven’ trends explaining macroevolutionary patterns (which are associated here with adaptive radiation and biased tropical diversification models, respectively).
More formally, ANSKEW allows partitioning of the skewness into components defined by different sources of variation, analogous to an analysis of variance. The total skewness (SCT) of a variable (i.e. coordinates of species' centroids in the environmental space) is then given by
where Yij is the quantitative variable of interest (i.e. the species' centroid along a environmental dimension) for the jth species in the ith clade (bird family); and , the mean among all species and clades (the grand mean). This SCT is analogous to the g1 coefficient described above. However, it is also possible to partition this SCT into between- (SCB) and within-clade (SCW) skewness by
and
where is the mean of the ith clade. There is also a heteroscedasticity component (SCH) given by
SCH combines variability of the ith clade about the mean (the second term of SCH), weighted by the ‘distance’ to the overall mean (the first term of SCH). Thus, SCH will be large when clades in the tail of the overall distribution are more variable than clades close to the grand mean. For example, in our analyses, this component was low for NPP, which explains why it has a higher active component in relation to energy variables (AET and TEMP; discussed in §3).
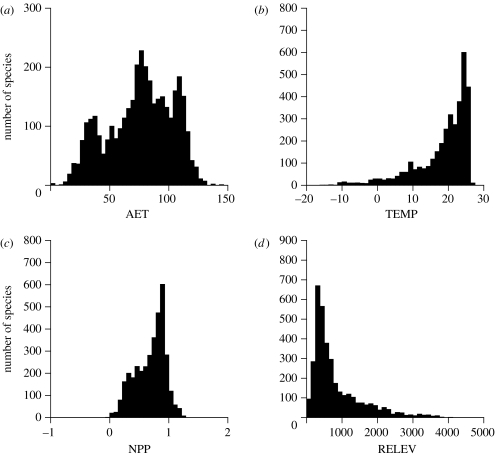
In general, SCB, SCW and SCH can be expressed as proportions of SCT. A driven trend (i.e. tropical diversification) would be characterized by a high SCW relative value, since the overall skewness is largely determined by within-clade processes. Since ANSKEW was developed for right-skewed traits and our environmental variables were usually left-skewed, species' centroids were multiplied by −1 prior to the ANSKEW (except for ELEV; figure 2).
Figure 2.
The distribution of 3560 species' centroids in four environmental dimensions (actual evapotranspiration (AET), mean annual temperature (TEMP), range in elevation (RELEV) and net primary productivity (NPP)). The distribution of (a) AET is multimodal, with a significant left skewness of g1=−0.30, revealing an aggregation of species at higher AET values. The distributions of (b) TEMP and (c) NPP show a similar pattern of aggregation in tropical conditions of high temperature (g1=−1.59) and high NPP (g1=−0.48), whereas (d) RELEV shows a right skewness (1.69), indicating more species' centroids in areas with moderately low relief.
(c) Simulations and statistical tests of analysis of skewness
In a purely statistical framework, if species' positions in the environmental space are randomized with respect to clades, SCW relative values (i.e. SCW/SCT) will approach 1.0, since SCT will be due entirely to within-clade variation. Thus, we first used 10 000 bootstrap samples to obtain a confidence interval around components, assuming that all evolution is due to individual species' adaptive responses in the environmental space, governed by a tropical attractor. The null statistical hypothesis being tested is then the absence of adaptive radiation outside tropical conditions along a given environmental condition. In this case, the expected driven component in ANSKEW, corresponding to the purely tropical diversification model, would be 1.0.
On the other hand, previous papers on macroevolutionary dynamics (McShea 1994; Wang 2001) have argued that passive trends should be viewed as a null model against which the driven trends must be tested. Consequently, the expected component of tropical diversification model would be close to zero, exactly the opposite to what is found using a purely statistical bootstrapping strategy in ANSKEW. Therefore, it is necessary to devise an evolutionary scenario that generates null expectations of ANSKEW components by defining how adaptive peaks (i.e. clade means) move in the environmental space through time. The idea is to define the overall expectations of SCB, SCH and SCW under a more realistic null evolutionary model that captures the basic features of the theoretical processes of diversification previously discussed.
The simple model used (Maurer et al. 1992; McShea 1998) can be viewed initially as an approximation of passive occupation of environmental space under an adaptive radiation model. For each bird family, we simulated the distribution of species' centroids in a one-dimensional environmental space, with a minimum boundary at zero and increasing towards infinity (but in practice limited by the parameters of the simulation, usually creating environmental spaces ranging from 0 to 1.0). The ancestral species arises in a variable position along this space and by cladogenesis (using a punctuated equilibrium model; see Maurer et al. 1992) gives rise to a new species whose environmental centroid is at a given distance away in this space. At each time-step, this distance is obtained as a number chosen from a random distribution with a mean of zero and a standard deviation of 0.1. The relationship between the average distance and the length of the environmental space can be viewed as a ‘species level’ heritability of the environmental centroid (see Jablonski 1987; Hunt et al. 2005; Diniz-Filho 2004; but see Webb & Gaston 2004), and increasing the variance of this distribution will only increase the total length of occupation of the environmental space. This shift in space is, in principle, completely random in direction, thus species can shift upward or downward along the environmental space (i.e. there is no diversification bias towards the tropics). However, if the value of the new species lies below the zero boundary, it will assume the ancestral condition of the clade (the barrier is not defined here as an absorbing state, but as a reflecting state favouring strong niche conservatism). For a new family, the new ancestral centroid can appear anywhere within the limits of the environmental space previously occupied by all other species.
Thus, we varied two parameters in this model: (i) the position of the ancestral species in relation to the ancestral barrier, ranging from 0.1 to 0.5 (a little less than half of the maximum practical occupation of the environmental space); and (ii) the probability of direction of the shift in the environmental space, called here ‘tropical bias’ (TB) parameter. If TB=0.5, new species can increase or decrease their values in the environmental space with respect to the clade, whereas higher values of TB indicate that the probability of increase will be greater than the probability of decreasing values. Thus, if we assume arbitrarily that higher values in space state indicate tropical conditions (as in the real data), values of TB larger than 0.5 will create the tropical diversification model, in which the tropical conditions can be interpreted as a diversification attractor in the space state, generating higher speciation rates.
Average ANSKEW components were then calculated for each of the 500 simulations generated for different combinations of these parameters, using the same sample sizes as the real data (3650 species unevenly distributed into 33 families). We then mapped the upper 95% limit for the passive component of ANSKEW in the parameter space tested.
(d) Understanding analysis of skewness in the context of latitudinal richness gradients
The magnitude of the ANSKEW components determines the relative roles of tropical diversification (the driven trend) and adaptive radiation (the passive trend) generating accumulation of species in tropical regions. However, beyond that, it is also important to understand how the adaptive radiation component (the among-family component) is structured in bird families along the different environmental dimensions. Thus, we evaluated spatial and phylogenetic patterns in the g1 coefficients of each family along each environmental variable, using an integrated analytical strategy combining two different methods that are based on extracting eigenvectors from spatial and phylogenetic distance matrices.
Spatial patterns in g1 were investigated using a principal coordinate analysis of neighbour matrix (PCNM; see Borcard & Legendre 2002; Borcard et al. 2004; Diniz-Filho & Bini 2005; Dray et al. 2006), which is based on extracting eigenvectors from a truncated geographical distance matrix (in km) among family centroids, given by the spatial midpoint of the species' ranges within each family. These eigenvectors describe spatial structures in data and can be used as predictors in a multiple regression framework to quantify the magnitude of spatial patterns in g1 for each family.
On the other hand, phylogenetic patterns were evaluated using a technique analogous to PCNM, called phylogenetic eigenvector regression (PVR; Diniz-Filho et al. 1998). Eigenvectors of a phylogenetic distance matrix among the bird families were also extracted and used as predictors in a multiple regression, in which g1 is the response variable. Phylogenetic distances were computed based on Sibley & Ahlquist's (1990) ‘tapestry’ phylogeny based on DNA hybridization, with branch lengths expressed by ΔT50H units (see Bennett & Owens (2002) for a recent discussion on using this phylogeny in bird comparative analyses). Owing to the recent advances in bird phylogenetic reconstruction, we also repeated PVR based on a combined phylogeny fusing Sibley & Ahlquist's (1990) phylogeny for non-passerines and a phylogeny for passerines based on nuclear genes RAG-1 and RAG-2 (Barker et al. 2004; see Hawkins et al. (2005, 2006) for details on using and comparing results for these alternative phylogenies in biogeographical analyses of New World birds).
An integrated spatial and phylogenetic ANSKEW pattern along bird families is then possible, because eigenvectors obtained in PCNM and PVR can be used simultaneously as predictors in a multiple regression model that considers g1 for each environmental dimension in different bird families as a response variable. Partial regressions (see Legendre & Legendre 1998; Desdevises et al. 2003; Hawkins et al. 2003b) were used to quantify phylogenetic and spatial effects on skewness, in addition to their interaction. For each environmental variable, the unexplained variation in g1 (d) is given by: 1–R2Total, where R2Total is the coefficient of determination of the regression with both spatial and phylogenetic eigenvectors (resulting in component a+b+c), where the effects of the space (a+b) and phylogeny (b+c) are given by the R2 from the regressions which consider only the spatial and phylogenetic eigenvectors as predictors, respectively. The comparison of these three components allows us to estimate the unique effects of space (a) and phylogeny (c) on skewness. The component (b) can be interpreted as the portion of the variance in g1 sharing common spatial and phylogenetic structures.
3. Results
The distribution of the species' centroids along environmental space defined by four variables is skewed, with peaks in the tropics (i.e. in areas of high productivity and temperatures) and in regions with moderately low ranges in elevation (figure 2). The distribution with respect to AET is multimodal but also skewed, with more species in warm, wet climates. The ANSKEW components indicate that diversity arises from a balance between tropical diversification and adaptive radiation (table 1). Adaptive radiation into extra-tropical environments (i.e. cool or less productive areas) explains on average 38% of the variation of species' positions in niche space, although there is wide variation among the four environmental variables. The values were greater than 50% along water–energy and temperature gradients (AET and TEMP), but only 29% along the productivity gradient (NPP). This is also consistent with displacement by adaptive radiation towards temperate regions, since the ANSKEW component for latitudinal centroids of the species is also very high, around 82% (but with a high heteroscedasticity component of 62%). On the other hand, range in elevation, which describes deviations from warm environments at higher altitudes, shows the lowest level of adaptive radiation. Net primary productivity possesses a relatively high between-family skewness component, consistent with the adaptive radiation of entire families, but the overall radiation component is low because the families tend to be less variable along this environmental dimension when compared with variation along the other climate variables.
Table 1.
Results of analysis of skewness (ANSKEW; see Wang 2001) based on 33 families of New World birds, showing the between- and within-clade components (SCB and SCW), heteroscedasticity (SCH) and the percentage of variation along environmental state space that can be explained by tropical diversification and by adaptive radiation. (All the adaptive radiation components were significant at p<0.0001 according to 10 000 bootstrap resamples, because tropical diversification components are always lower than the expected value of ca 0.995.)
| environmental dimension | SCB | SCW | SCH | tropical diversification | adaptive radiation |
|---|---|---|---|---|---|
| AET | 0.223 | 0.476 | 0.300 | 47.64 | 52.36 |
| TEMP | 0.222 | 0.431 | 0.346 | 43.13 | 56.86 |
| RELEV | 0.002 | 0.849 | 0.153 | 84.96 | 15.04 |
| NPP | 0.149 | 0.709 | 0.141 | 70.99 | 29.05 |
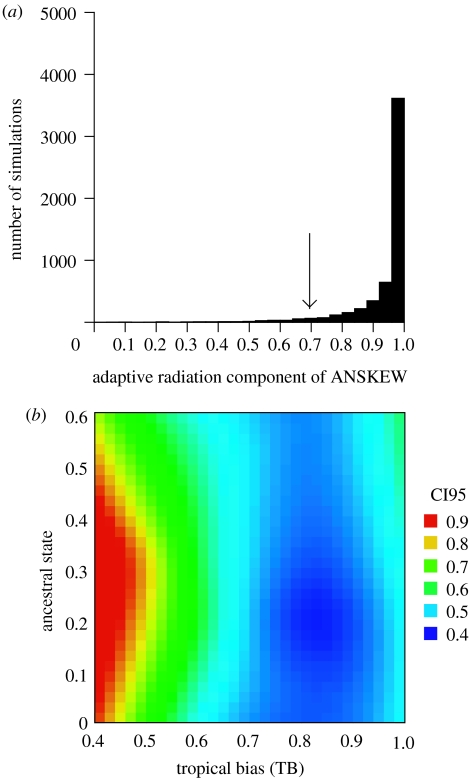
Simulation analyses support the ability of ANSKEW to partition the two components of diversification (figure 3). Under a pure passive process of adaptive radiation towards the temperate zone starting with tropical ancestors, the ANSKEW component would rarely be lower than 75%, even under a very restricted ancestral condition. Thus, even the highest adaptive radiation component of ANSKEW found for birds (ca 56% for variation of species' centroids on the temperature axis) is much lower than critical limits of our simulations under a pure adaptive radiation process. This supports the conclusion that TB has a significant role in the latitudinal gradient. Actually, increasing the tropical diversification parameter in the simulation from 0.5 (pure adaptive radiation) to 0.9 (a large bias towards tropical diversification) decreases this critical level to ca 45%, although this limit is also slightly affected by the position of the ancestral species in the environmental space (figure 3b).
Figure 3.
Summary of the results of simulations of a simple adaptive radiation process as a null model for passive trends towards temperate diversification. (a) The distribution of the adaptive radiation component ((SCB+SCH)/SCT) for an ancestral condition of 0.1, with no tropical diversification bias (TB=0.5). The arrow indicates the lower 95% of the component. Thus, under tropical origin and no diversification bias, few adaptive radiation components are lower than 0.7, reinforcing the power of ANSKEW to detect this. (b) Landscape of lower 95% confidence intervals of adaptive radiation components varying the ancestral condition and the tropical diversification (TB) parameter. Increasing TB produces, as expected, smaller adaptive radiation components.
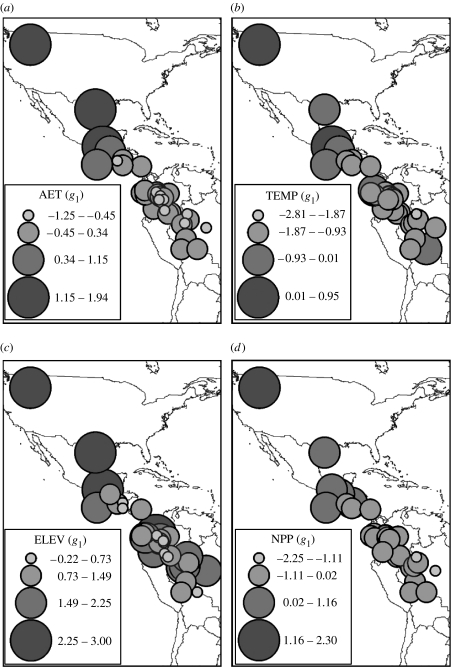
Evaluation of spatial and phylogenetic patterns in skewness among families was based on a combination of PCNM and PVR, and five and three eigenvectors were extracted from spatial and phylogenetic distance matrices, respectively, based on broken-stick distribution of eigenvalues. PCNM revealed that there are strong spatial effects in three of the four environmental dimensions, with R2 higher than 60% for AET, NPP and temperature (table 2). Families whose geographical midpoints are more tropical tend to have more skewed distributions of species' centroids than temperate families, which tend to have more Gaussian (or even negatively skewed) distributions (figure 4a–c). However, range in elevation does not closely follow this pattern (figure 4c), with a relatively low spatial pattern and a R2 of 0.316 (p=0.056).
Table 2.
Coefficients of determination of regression models of g1 on spatial predictors (), phylogenetic predictors () and both ( or the components a+b+c of the decomposition). (Variability strictly attributed to space (a) and phylogeny (c) are also shown. Component (b) indicates the fraction shared simultaneously by spatial and phylogenetic effects.)
| space (a) | shared (b) | phylogeny (c) | ||||
|---|---|---|---|---|---|---|
| AET | 0.767 | 0.183 | 0.792 | 0.609 | 0.158 | 0.025 |
| TEMP | 0.597 | 0.188 | 0.636 | 0.448 | 0.149 | 0.039 |
| ELEV | 0.316 | 0.318 | 0.454 | 0.136 | 0.180 | 0.138 |
| NPP | 0.619 | 0.163 | 0.649 | 0.486 | 0.133 | 0.030 |
Figure 4.
Spatial patterns of g1 for the 33 bird families analysed. Families whose geographical midpoints are situated in tropical areas tend to be negatively skewed for AET, TEMP and NPP, hence deviations towards temperate region change the centroid's distribution within families towards more Gaussian (or even right-skewed) shapes. Along ELEV, spatial patterns are weaker, owing to its smaller niche conservatism component (table 2).
On the other hand, phylogenetic components obtained from PVR based on Sibley & Ahlquist's (1990) phylogeny were usually very low for all environmental dimensions, with the minor exception of range in elevation (table 2). This suggests that adaptive radiation towards temperate zone starting from tropical ancestrals has occurred multiple times and independently within different clades, although the adaptive radiation component for changes in elevational range was slightly structured in phylogeny. The results from PVR based on the combination of Sibley & Ahlquist (1990) and Barker et al. (2004) phylogenies were qualitatively identical.
Partial regressions showed that these spatial and phylogenetic components were independent from each other (table 2), with a relatively small interaction between them (component b). The components of spatial variation in g1 alone are much higher than phylogenetic components for all environmental dimensions, except for range in elevation.
4. Discussion
The balance between biased tropical diversification and adaptive radiation probably explains why it has been difficult to test historical scenarios using direct comparisons of evolutionary rates (Bromham & Cardillo 2003). Attempts to quantify upward-biased speciation rates in the tropics have generated contradictory results, despite the plausibility of historical processes and differential diversification rates as an explanation for the latitudinal diversity gradient (Ricklefs 2004; Currie et al. 2004). However, no difference between diversification rates of temperate and tropical birds along the energy gradients is expected if species richness along this environmental dimension has a relatively high adaptive radiation component. On the other hand, heterogeneous rates of molecular evolution recently found between tropical and temperate species (Cardillo et al. 2005) are indeed expected along environmental dimensions with a relatively low adaptive radiation component, such as in the case of NPP and range in elevation.
Range in elevation, which describes deviations from warmer environments at higher altitudes within both the temperate zone and the tropics, shows the lowest level of adaptive radiation. This indicates that adaptation to higher altitudes involves species-specific processes of directional selection driving species, or eventually genera, of multiple clades (families) out of overall ancestral conditions (lowlands), whereas broad shifts in adaptive peaks at higher hierarchical levels may be more common with respect to energy inputs. This high component of driven diversification in mountains is indeed expected if we consider, for example, that geological events associated with the origin of the Andes generated diversification in multiple clades, and the region is characterized by a large number of small-ranged, endemic species (Hawkins & Diniz-Filho 2006). Despite this, even the small adaptive radiation component towards higher mountains has a low but significant phylogenetic component, indicating that this preferentially occurred in some closely related families, such as more recent passerines families that diversified in the Andean region (Barker et al. 2004).
It is also important to note that these patterns make sense if we track the space–time variation in skewness variation among families, which can help us understand the processes underlying clade dynamics. Since adaptive radiation involves clade-scale shifts in adaptive peaks towards temperate regions, skewness coefficients of families should possess a strong spatial component. As expected, we found strong correlations between skewness coefficients and latitude in three of the four environmental dimensions, indicating that families whose geographical midpoints are more tropical have more left-skewed distributions of species' centroids, whereas more temperate families (especially in the Northern Hemisphere) have more Gaussian (or even negatively skewed) distributions. That skewness with respect to range in elevation shows a weaker geographical component is not unexpected, since mountains occur at all latitudes in the New World, and diversification is not strongly associated with diversification by adaptive radiation. Thus, variation in skewness of environmental variables with a higher adaptive radiation component is more strongly spatially structured than those with a stronger TB component.
An additional prediction is that if adaptive radiation contains a strong phylogenetic signal, then the occupation of the temperate zone by birds occurred temporally as a stepwise evolutionary process of displacement in adaptive peaks away from an ancestral (tropical) state. Thus, only younger, more derived families would tend to occupy temperate regions. However, phylogenetic components of adaptive radiation are much lower than spatial ones for all the four environmental dimensions, except range in elevation. This suggests that niche evolution and adaptive radiation into the temperate zones by a passive diffusion process cannot be interpreted as a simple stepwise process in time. Rather, this indicates that it occurred independently multiple times during the evolution of birds, although shifts from lowlands to mountains have a small (but statistically significant) phylogenetic component, as discussed above. Low phylogenetic components of skewness, as calculated here, are also expected under strong processes of post-speciation range shifts, as a direct consequence of geographical range evolutionary lability (Diniz-Filho & Tôrres 2002; Gaston 2003).
Our results are consistent with known spatial patterns of New World bird species richness for basal and derived clades (Gaston & Blackburn 1996; Ricklefs 2002; Hawkins et al. 2006; see also Jablonski 1991; McKenna & Farrell 2006). Under a process dominated by adaptive radiation and clade-level niche conservatism, we would predict that: (i) basal clades reflect strongly their initial adaptive peak and have a clear latitudinal gradient in richness, and (ii) derived groups should have no clear spatial pattern, since species would be normally distributed around the new adaptive peaks outside the tropics. However, our previous analyses based on patterns of richness in derived and basal bird groups in the New World (Hawkins et al. 2006; see also Gaston & Blackburn 1996) suggested an intermediate scenario, clearly supporting prediction (i), but not entirely prediction (ii). Although spatial autocorrelation analyses have indicated a less spatially structured pattern of species richness in the most derived groups, a tropical peak in richness still exists, albeit it is in the Andes (i.e. in cooler climates) rather than the warmer lowlands.
It is important to stress that we used here New World bird families as clades, which is not strictly correct, since many families have species outside of this region. ANSKEW is robust to this assumption (Wang 2001), except if there is a systematic bias towards niche displacement in other regions of the world, biasing the within-family relative centroids. This error is expected to be low here, since the distribution of species in the New World covers a very broad latitudinal extent. We also assume that bird families defined by Sibley & Ahlquist (1990) are monophyletic, which is debatable, despite a relatively high agreement between alternative phylogenies at this taxonomic level (Barker et al. 2004). Nevertheless, our results are stable to changes in phylogenetic definitions, since analyses repeated at the order level gave almost identical results. Alternatively, using genera as clades would strongly reduce sample sizes within groups and generate high errors in estimates of the coefficients of skewness (g1), providing less comparable within-clade components. In addition, deleting families with fewer than 20 species, as suggested by Maurer (1998), generates a slightly conservative estimate of adaptive radiation, since some small families, such as Paridae, provide examples of this process, but were not included in our analysis owing to their low richness.
The simulations performed here showed that the ANSKEW method is capable of capturing variation in the parameters of our simulations according to the predictions, especially the changes in the components when increasing the tropical diversification parameter. Thus, we believe that this is a promising methodological approach for understanding species dynamics in the context of historical explanations for broad-scale richness gradients. Of course, because our simulations are very simple, it is not straightforward to directly associate the relative magnitudes of simulated and real components of ANSKEW and, under a pattern-oriented modelling framework (see Grimm et al. 2005), to interpret these parameters in ecologically realistic way. Even so, the main point is that the simulation of the diversification processes according to the conceptual models of niche conservatism and adaptive radiation produced coherent results in ANSKEW, so our analysis of real data using this approach must truly reflect balances between these processes during bird diversification in New World.
Our approach indicates how broad-scale gradients in species richness have been generated by a balance between higher diversification rates in the tropics and adaptive radiation away from ancestral tropical conditions. However, it is intriguing that the strongest adaptive radiation component appears with respect to energy variables, which are commonly used to explain the climatic effects on diversity associated with higher diversification rates (Hawkins et al. 2003a,b; Currie et al. 2004). According to our analyses, a correspondence between the species richness and temperature is probably determined by the maintenance of ancestral niches and broad shifts in adaptive shifts during birds' evolutionary history more than by faster rates of diversification owing to more available energy in tropical environments.
Acknowledgments
We thank Trevor Price, Brian Maurer, Marcel Cardillo, Robert Colwell, Daniel McShea and two anonymous reviewers for their comments on previous versions of the manuscript. J.A.F.D.F., L.M.B. and T.F.L.V.B.R. have been continuously supported by many CNPq fellowships and grants. All the computations were performed in Dell Precision 450 series Workstations provided by CNPq/SECTEC-GO (PRONEX Project no. 23234156).
References
- Barker F.K, Cibois A, Schilker P, Feinstein J, Cracraft J. Phylogeny and diversification of the largest avian radiation. Proc. Natl Acad. Sci. USA. 2004;101:11 040–11 045. doi: 10.1073/pnas.0401892101. doi:10.1073/pnas.0401892101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett P.M, Owens P.F. Oxford University Press; Oxford, UK: 2002. Evolutionary ecology of birds. [Google Scholar]
- Borcard D, Legendre P. All-scale spatial analysis of ecological data by means of principal coordinates of neighbour matrices. Ecol. Model. 2002;153:51–68. doi:10.1016/S0304-3800(01)00501-4 [Google Scholar]
- Borcard D, Legendre P, Avois-Jacquet C, Tuomisto H. Dissecting the spatial structure of ecological data at multiple scales. Ecology. 2004;85:1826–1832. [Google Scholar]
- Bromham L, Cardillo M. Testing the link between the latitudinal gradient in species richness and rates of molecular evolution. J. Evol. Biol. 2003;16:200–207. doi: 10.1046/j.1420-9101.2003.00526.x. doi:10.1046/j.1420-9101.2003.00526.x [DOI] [PubMed] [Google Scholar]
- Cardillo M, Orme C.D.L, Owens I.P.F. Testing for latitudinal bias in diversification rates: an example using New World birds. Ecology. 2005;86:2278–2287. [Google Scholar]
- Currie D.J, et al. Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness. Ecol. Lett. 2004;7:1121–1134. doi:10.1111/j.1461-0248.2004.00671.x [Google Scholar]
- Davies T.J, Grenyer R, Gittleman J.L. Phylogeny can make the mid-domain effect an inappropriate null model. Biol. Lett. 2005;1:143–146. doi: 10.1098/rsbl.2005.0297. doi:10.1098/rsbl.2005.0297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desdevises Y, Legendre P, Azouzi L, Morand S. Quantifying phylogenetically-structured environmental variation. Evolution. 2003;57:2647–2652. doi: 10.1111/j.0014-3820.2003.tb01508.x. doi:10.1554/02-695 [DOI] [PubMed] [Google Scholar]
- Diniz-Filho J.A.F. Macroecology and the hierarchical expansion of evolutionary theory. Global Ecol. Biogeogr. 2004;13:1–5. doi:10.1111/j.1466-882X.2004.00066.x [Google Scholar]
- Diniz-Filho J.A.F, Bini L.M. Modelling geographical patterns in species richness using eigenvector-based spatial filters. Global Ecol. Biogeogr. 2005;14:177–185. doi:10.1111/j.1466-822X.2005.00147.x [Google Scholar]
- Diniz-Filho J.A.F, Tôrres N.M. Phylogenetic comparative methods and the geographic range size–body size relationship in new world terrestrial carnivora. Evol. Ecol. 2002;16:351–367. doi:10.1023/A:1020210321776 [Google Scholar]
- Diniz-Filho J.A.F, Sant'Ana C.E.R, Bini L.M. An eigenvector method for estimating phylogenetic inertia. Evolution. 1998;52:1247–1262. doi: 10.1111/j.1558-5646.1998.tb02006.x. doi:10.2307/2411294 [DOI] [PubMed] [Google Scholar]
- Dray S, Legendre P, Peres-Neto P.R. Spatial modeling: a comprehensive framework for principal coordinate analysis of neighbor matrices (PCNM) Ecol. Model. 2006;196:483–493. doi:10.1016/j.ecolmodel.2006.02.015 [Google Scholar]
- Gaston K.J. Oxford University Press; Oxford, UK: 2003. The structure and dynamics of geographic ranges. [Google Scholar]
- Gaston K.J, Blackburn T.M. The tropics as a museum of biological diversity: an analysis of the New World avifauna. Proc. R. Soc. B. 1996;263:63–68. [Google Scholar]
- Gould S.J. Harvard University Press; Cambridge, MA: 2002. The structure of evolutionary theory. [Google Scholar]
- Grimm V, et al. Pattern-oriented modeling of agent-based complex systems: lessons from ecology. Science. 2005;310:987–991. doi: 10.1126/science.1116681. doi:10.1126/science.1116681 [DOI] [PubMed] [Google Scholar]
- Hawkins B.A. Ecology's oldest pattern? Trends Ecol. Evol. 2001;16:470. doi:10.1016/S0169-5347(01)02197-8 [Google Scholar]
- Hawkins B.A, et al. Energy, water, and broad-scale geographic patterns of species richness. Ecology. 2003a;84:3105–3117. [Google Scholar]
- Hawkins B.A, Porter E.E, Diniz-Filho J.A.F. Productivity and history as predictors of the latitudinal diversity gradient of terrestrial birds. Ecology. 2003b;84:1608–1623. [Google Scholar]
- Hawkins B.A, Diniz-Filho J.A.F, Soeller S.A. Water links the historical and contemporary components of the Australian bird diversity gradient. J. Biogeogr. 2005;32:1035–1042. doi:10.1111/j.1365-2699.2004.01238.x [Google Scholar]
- Hawkins B.A, Diniz-Filho J.A.F, Jaramillo C, Soeler S.A. Post-Eocene climate change, niche conservatism, and the latitudinal diversity gradient of New World birds. J. Biogeogr. 2006;33:770–780. doi:10.1111/j.1365-2699.2006.01452.x [Google Scholar]
- Hawkins B.A, Diniz-Filho J.A.F. Beyond Rapoport's rule: evaluating range size patterns of New World birds in a two-dimensional framework. Global Ecol. Biogeogr. 2006;15:461–469. doi:10.1111/j.1466-822x.2006.00243.x [Google Scholar]
- Hunt G, Roy K, Jablonski D. Species-level heritability reaffirmed: a comment on “On the heritability of geographic range sizes”. Am. Nat. 2005;166:129–135. doi: 10.1086/430722. doi:10.1086/430722 [DOI] [PubMed] [Google Scholar]
- Jablonski D. Heritability at the species level—analysis of geographic ranges of cretaceous molluscs. Science. 1987;238:360–363. doi: 10.1126/science.238.4825.360. [DOI] [PubMed] [Google Scholar]
- Jablonski D. The tropics as a source of evolutionary novelty through geological time. Nature. 1991;364:142–144. doi:10.1038/364142a0 [Google Scholar]
- Jetz W, Rahbek C. Geographic range size and determinants of avian species richness. Science. 2002;297:1548–1551. doi: 10.1126/science.1072779. doi:10.1126/science.1072779 [DOI] [PubMed] [Google Scholar]
- Legendre P, Legendre L. 2nd edn. Elsevier; Amsterdam, The Netherlands: 1998. Numerical ecology. [Google Scholar]
- McKenna D.D, Farrell B.D. Tropical forests are both evolutionary cradles and museums of leaf beetle diversity. Proc. Natl Acad. Sci. USA. 2006;103:10 947–10 951. doi: 10.1073/pnas.0602712103. doi:10.1073/pnas.0602712103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShea D.W. Mechanisms of large-scale evolutionary trends. Evolution. 1994;48:1747–1763. doi: 10.1111/j.1558-5646.1994.tb02211.x. doi:10.2307/2410505 [DOI] [PubMed] [Google Scholar]
- McShea D.W. Metazoan complexity and evolution: is there a trend? Evolution. 1996;50:477–492. doi: 10.1111/j.1558-5646.1996.tb03861.x. doi:10.2307/2410824 [DOI] [PubMed] [Google Scholar]
- McShea D.W. Dynamics of diversification in space state. In: McKinney M.L, Drake J.A, editors. Biodiversity dynamics. Columbia University press; New York, NY: 1998. pp. 91–108. [Google Scholar]
- Maurer B.A. The evolution of body size in birds. I. Evidence of non-random diversification. Evol. Ecol. 1998;12:925–934. doi:10.1023/A:1006512121434 [Google Scholar]
- Maurer B.A, Brown J.H, Rusler R.D. The micro and macro in body size evolution. Evolution. 1992;48:939–953. doi: 10.1111/j.1558-5646.1992.tb00611.x. doi:10.2307/2409748 [DOI] [PubMed] [Google Scholar]
- Orme C.D.L, et al. Global hotspots of species richness are not congruent with endemism or threat. Nature. 2005;436:1016–1019. doi: 10.1038/nature03850. doi:10.1038/nature03850 [DOI] [PubMed] [Google Scholar]
- Rahbek C, Graves G.R. Multiscale assessment of patterns of avian species richness. Proc. Natl Acad. Sci. USA. 2001;98:4534–4539. doi: 10.1073/pnas.071034898. doi:10.1073/pnas.071034898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs R.E. Splendid isolation: historical ecology of the South American passerine fauna. J. Avian Biol. 2002;33:207–211. doi:10.1034/j.1600-048X.2002.330301.x [Google Scholar]
- Ricklefs R.E. A comprehensive framework for global patterns in biodiversity. Ecol. Lett. 2004;7:1–15. doi:10.1046/j.1461-0248.2003.00554.x [Google Scholar]
- Ridgley R.S, Allnutt T.F, Brooks T, McNicol D.K, Mehlman D.W, Young B.E, Zook J.R. NatureServe; Arlington, VA: 2003. Digital distribution maps of the birds of the Western Hemisphere, version 1.0. [Google Scholar]
- Schluter D. Oxford University Press; Oxford, UK: 2000. The ecology of adaptive radiation. [Google Scholar]
- Sibley C.G, Ahlquist J.E. Yale University Press; New Haven, CT: 1990. Phylogeny and classification of birds. [Google Scholar]
- Sibley C.G, Monroe B.L., Jr . Yale University Press; New Haven, CT: 1990. Distribution and taxonomy of birds of the world. [Google Scholar]
- Wang S.C. Quantifying passive and driven large-scale evolutionary trends. Evolution. 2001;55:849–858. doi: 10.1554/0014-3820(2001)055[0849:qpadls]2.0.co;2. doi:10.1554/0014-3820(2001)055[0849:QPADLS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Webb T.J, Gaston K.J. Heritability of geographic range sizes revisited: a reply to Hunt et al. Am. Nat. 2004;161:553–558. doi: 10.1086/430726. doi:10.1086/368296 [DOI] [PubMed] [Google Scholar]
- Whittaker R.J, Willis K.J, Field R. Scale and species richness: towards a general, hierarchical theory of species diversity. J. Biogeogr. 2001;28:453–470. doi:10.1046/j.1365-2699.2001.00563.x [Google Scholar]
- Wiens J.J. Speciation and ecology revisited: phylogenetic niche conservatism and the origin of species. Evolution. 2004;58:193–197. doi: 10.1111/j.0014-3820.2004.tb01586.x. doi:10.1554/03-447 [DOI] [PubMed] [Google Scholar]
- Wiens J.J, Donoghue M.J. Historical biogeography, ecology and species richness. Trends Ecol. Evol. 2004;19:639–644. doi: 10.1016/j.tree.2004.09.011. doi:10.1016/j.tree.2004.09.011 [DOI] [PubMed] [Google Scholar]
- Wiens J.J, Graham C.H. Niche conservatism: integrating evolution, ecology, and conservation biology. Annu. Rev. Ecol. Evol. Syst. 2005;36:519–539. doi:10.1146/annurev.ecolsys.36.102803.095431 [Google Scholar]
- Wilf P, Cúneo N.R, Johnson K.R, Hicks J.F, Wing S.L, Obradovich J.D. High plant diversity in Eocene South America: evidence from Patagonia. Science. 2003;300:122–125. doi: 10.1126/science.1080475. doi:10.1126/science.1080475 [DOI] [PubMed] [Google Scholar]
- Willig M.R, Kaufman D.M, Stevens R.D. Latitudinal gradients of biodiversity: pattern, process, scale and synthesis. Annu. Rev. Ecol. Syst. 2003;34:273–309. doi:10.1146/annurev.ecolsys.34.012103.144032 [Google Scholar]