Abstract
The evolution and persistence of biological cooperation have been an important puzzle in evolutionary theory. Here, we suggest a new approach based on bargaining theory to tackle the question. We present a mechanistic model for negotiation of benefits between a nitrogen-fixing nodule and a legume plant. To that end, we first derive growth rates for the nodule and plant from metabolic models of each as a function of material fluxes between them. We use these growth rates as pay-off functions in the negotiation process, which is analogous to collective bargaining between a firm and a workers' union. Our model predicts that negotiations lead to the Nash bargaining solution, maximizing the product of players' pay-offs. This work introduces elements of cooperative game theory into the field of mutualistic interactions. In the discussion of the paper, we argue for the benefits of such an approach in studying the question of biological cooperation.
Keywords: mutualism, rhizobium–legume symbiosis, cooperative game theory, negotiation, evolution of cooperation
1. Introduction
Mutualistic interactions are ubiquitous in nature and play important roles in many ecosystem processes. Yet, our understanding of how they evolved and are maintained by natural selection has been limited. The apparent dilemma such interactions present to evolutionary theory lies in the fact that partners usually undertake costly actions that do not directly benefit themselves but benefit the other partner. The problem has been studied within three frameworks: evolutionary game theory (Denison 2000; West et al. 2002a), market theory (also called partner selection; Simms & Taylor 2002) and trade advantage models (Schwartz & Hoeksema 1998). Both evolutionary game theory and market theory approaches have focused on how mutualisms are enforced, i.e. how ‘cheaters’ are prevented. On the other hand, trade advantage models aim at predicting under what conditions an exchange of benefits is advantageous, leaving the distribution of the benefits open. We propose a new approach based on bargaining theory that not only both addresses the issue of cheating and whether exchange is advantageous at the same time, but also allows quantitative predictions on the outcome of the interaction to be made. In this paper, we apply the theory to the symbiosis between rhizobia and legumes.
Rhizobium is the general name given to a phylogenetically diverse group of soil bacteria that form nitrogen-fixing symbioses with leguminous plants. This symbiosis is among the most important ecological interactions to humans and ecosystems. It is estimated to globally produce as much nitrogen as fixed by commercial fertilizer production (Gordon et al. 2001), and it represents the most important nitrogen input to many ecosystems. In addition to this immense practical importance, this interaction is an excellent model system in which to study the evolution of mutualisms. Rhizobia are found free living in the soil and reproduce independent of the legumes. However, during the symbiosis, rhizobia and the legume plant coordinate on building a novel plant organ, called the nodule, which fixes nitrogen. Starting with the initiation of the interaction, there is extensive signal exchange between the partners (Lum & Hirsch 2003). The early signals secreted by rhizobia, called nod factors, are not predictive of symbiotic performance, but indicate the ability to nodulate the particular plant species. After a series of coordinated events, rhizobium cells are taken up into plant cells where they differentiate into organelle-like structures called bacteroids (Oke & Long 1999). Bacteroids have two membranes: one derived from the plant, and the other from the rhizobium (Lodwig & Poole 2003). This feature presumably enables both partners to control material flow in and out of the bacteroid. Moreover, recent work has shown that nitrogen fixation is contingent on continuous shuttling of amino acids in and out of the bacteroids (Lodwig et al. 2003). This suggests that coordination is continuing between the bacteroid and plant cell into the nitrogen fixation phase, too. In this manner, we hypothesize that rhizobia and the plant are negotiating the outcome of the symbiosis.
Our model is borrowed with some modifications from bargaining theory in economics. Bargaining theory concerns the interaction of two or more parties which can produce some benefits if they cooperate, but have conflicting interests in the division of these benefits. Thus, they try to agree on a contract on this distribution before embarking on the joint activity. The classic case of such a situation is collective bargaining between a firm and a workers' union. The firm in our case represents an individual plant, and the union a nodule. Proposals on the solution of the bargaining problem pre-date game theory, starting in the 1930s. Early attempts by influential economists Frederik Zeuthen and John Hicks were followed by John Nash's game-theoretic treatments (Nash 1950, 1953). The synthesis paper by John Harsanyi (1956) is a classic reference for the early development of the field. Nash's and Zeuthen's negotiation models make the same prediction on the outcome of the process. They show that the solution to the bargaining problem is unique and maximizes the product of the player's pay-offs. This solution is called the Nash bargaining solution (NBS).
The organization of the paper is as follows. In §2, we derive the growth rates of rhizobium and plant by solving a simple mechanistic model of metabolism. These growth rates are then used as pay-off functions in the bargaining model introduced in §3. We conclude with a discussion on the possible applications of this approach in connection to previous models and argue for the utility of cooperative game theory in studying biological cooperation in §4.
2. Metabolic models
We introduce simple models of how a plant and a nitrogen-fixing nodule function and grow by tracking the dynamics of the metabolites. We assume these dynamics to be happening within a time-scale of seconds to minutes. As a result of these dynamics, the plant and the nodule accumulate biomass at rates rp and rb, respectively. In this setup, the biomass accumulation rate is a proxy for the contribution of any dynamical state to the lifetime fitness of the individual, i.e. we assume that the time-integral throughout an individual's lifetime of rb and rp is proportional to the fitness of an individual rhizobium in a nodule or a plant, respectively. We further assume that the plant and the nodule accumulate biomass on a much slower time-scale (i.e. days) than the metabolite dynamics, meaning that in the time-scale of leaf and nodule growth, the sizes of metabolite pools will be at their equilibrium values for existing leaf and nodule sizes almost all the time. This observation simplifies the analysis significantly: we can simply solve for the equilibrium of the metabolite dynamics while treating the leaf and nodule sizes as constant and use this equilibrium to determine the growth rates of the leaves and the nodule.
(a) Sub-model from the plant's perspective
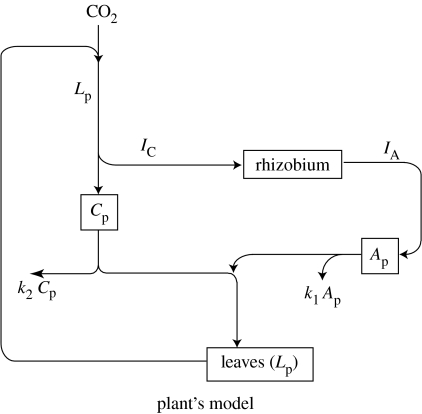
Figure 1 illustrates the sub-model for the plant's functioning. We consider a plant that has leaf area Lp. It produces carbon at a rate proportional to Lp and sends out a fraction up to the nodule, resulting in a flux of IC into the nodule. The remaining part of the photosynthate enters the carbon pool, Cp. Meanwhile, the nodule exports nitrogen into the plant at a rate IA, which enters the nitrogen pool, Ap (for amino acids). New leaf area synthesis then follows from the two pools, at a rate Ap·Cp. Both nitrogen and carbon pools ‘leak’ with rate constants k1 and k2, respectively, accounting for maintenance processes that do not contribute to growth. The growth rate of the leaf area is given by rp. The following differential equations describe this system:
| (2.1) |
| (2.2) |
| (2.3) |
where we set the proportionality constant for the photosynthesis rate equal to 1, for simplicity. In these equations, Lp is a constant, owing to the fast time-scale assumption we made for the metabolite dynamics. We solve for equilibrium by setting the left-hand sides of equations (2.1) and (2.2) equal to zero and calculate rp at this equilibrium from equation (2.3),
| (2.4) |
Figure 1.
Metabolic model from the plant's perspective.
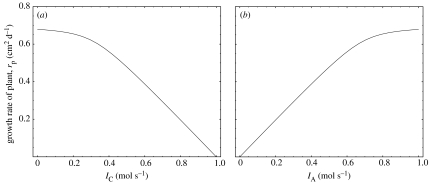
Figure 2 shows the plot of rp versus IC while keeping the nitrogen flux, IA, constant (figure 2a) and vice versa (figure 2b). The most prominent feature of the growth rate function is that the limiting resource switches from being carbon to being nitrogen. This can be seen in figure 2a: for low values of the carbon export rate, IC, the growth rate, rp, does not show strong dependence on it, whereas after a certain threshold, the growth rate decreases linearly with increasing carbon export. The same feature can be observed in figure 2b, where initially the growth rate increases almost linearly with the nitrogen intake, but levels off after a certain threshold. The threshold values for switching from being limiting to not being limiting for each metabolite are determined by the flux of the other metabolite.
Figure 2.
(a) Growth rate of the plant at equilibrium versus IC, the carbon flux that the plant is sending out to rhizobium. The parameters are k1=k2=0.1; Lp=1; IA=0.7. (b) Growth rate drawn versus IA, the amount of nitrogen that the plant is receiving. IC=0.7, other parameters are the same as in (a).
(b) Sub-model from the nodule's perspective
What constitutes a fitness proxy for rhizobia in a nodule is not as obvious as for a plant. Rhizobia in determinate nodules of soybean are known to accumulate large reserves of carbon polymers (e.g. poly-β-hydroxybutyrate (PHB); Denison 2000), which are thought to facilitate reproduction after nodule senescence. Consequently, the size of the PHB reserves can be thought of as a proxy of lifetime fitness for rhizobia in determinate nodules. However, in indeterminate nodules, the bacteroids do not accumulate PHB. Instead, there is a region in the nodule (zones IV and V; Puppo et al. 2005) where the symbiosis breaks down and rhizobia start to live on their own (Denison 2000). As these cells presumably escape to the soil and reproduce after nodule senescence, the size of this region can be taken as a proxy of fitness accrued by the rhizobia in indeterminate nodules. In either of these mechanisms, the allocation of the incoming carbon is either to ‘selfish’ pathways or to nitrogen fixation, and the metabolic model below applies to both. In this paper, we use the term ‘polymer reserves’ for the accumulated fitness, with the understanding that the interpretation of the model for different nodule types can be different.
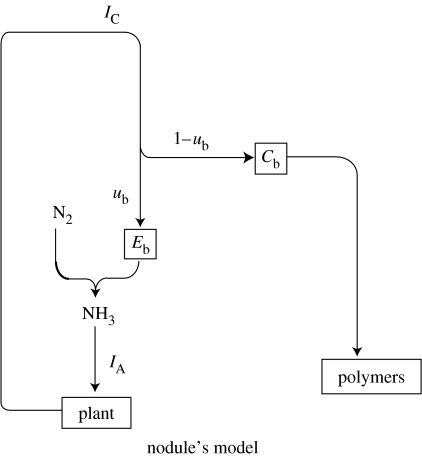
The metabolic model for the nodule is illustrated in figure 3. The nodule receives carbon from the plant at rate IC, from which a fraction ub is used in the Krebs cycle for ATP production, while the rest goes into the carbon pool (Cb). The size of the ATP pool is Eb. Nitrogen fixation follows using the ATP pool and the nitrogen fixed is exported to the plant at a rate IA. At the same time, carbon polymers are synthesized from the rp carbon pool. The growth rate, rb, of the polymer reserves, Sb, gives the fitness accumulation rate. The following set of differential equations describes the nodule's workings:
| (2.5) |
Figure 3.
Metabolic model from the nodule's perspective.
| (2.6) |
| (2.7) |
In these equations, kA and kC are parameters that represent the stoichiometry of the reactions for carbon polymer synthesis and nitrogen fixation and export, with IA=kA·Eb. The solution for equilibrium of equations (2.5) and (2.6) is straightforward and yields for the growth rate rb,
| (2.8) |
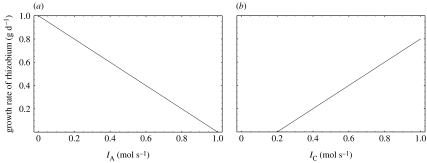
The behaviour of the growth rate with changing IA and IC is depicted in figure 4. Unlike the plant, the nodule's growth rate does not exhibit a switch between the resources limiting it. The reason is that the material flow is linear in the nodule, with only one source of income (IC) and two paths for output (nitrogen fixation and growth). In contrast, the plant has two independent sources of material and two possible outputs, where one of the outputs (growth) requires input from both pools. This feature of the plant model means either of the pools can be limiting for the growth rate.
Figure 4.
(a) Growth rate of the nodule's polymer reserves as a function of IA, nitrogen export rate of the nodule, with IC=1. (b) Growth rate as a function of IC, carbon flux into the nodule with IA=0.2.
3. Negotiation model
In this section, we introduce a model for how the plant and the nodule interact in developmental time-scale to determine the distribution of benefits in the symbiosis. We propose that the development of the interaction should be viewed as continuing negotiations between the partners. This serves as a proximate mechanism of how the nodule and plant benefit from the interaction, and is also where natural selection will act.
We model the negotiation game in discrete periods, which we assume to be significantly longer in duration than the time-scale of metabolite dynamics. This assumption means that we can use the equilibrium solutions of the metabolic models. Each period starts with a certain flow of carbon and nitrogen between the nodule and the plant. In our negotiation model, this pair of fluxes may be considered as a ‘contract’. Such a contract is denoted by R and results in a pair of growth rates, (rb, rp). In each period, one of the players randomly changes one or both of the fluxes up or down. We conjecture that these changes happen as a result of random thermodynamic fluctuations. These random fluctuations, depending on the response of the opponent to them, may result in the contract R changing to some other, , so we call them ‘offers’.
An offer can give rise to three different situations. For a start, it can result in a lower pay-off for the offering player, in which case we assume that the player senses the drop and restores the previous pair of fluxes immediately. We label this case a withdrawn offer, which is essentially the same as no offer being made. Alternatively, if the offer is not withdrawn, there are two possibilities. If the offer also increases the responding player's pay-off, it gets accepted immediately, i.e. the fluxes remain on their new values throughout the period. However, if the responding player's pay-off is lowered by the new fluxes, the responding player, in a ‘shock’ response, shuts down flow of both metabolites, causing both players to experience zero growth rate. This situation, analogous to strikes in union bargaining, continues until one of the players concedes. If the offering player is the first to concede, the fluxes recover to their previous values. If the responding player concedes, the new fluxes take effect.
The situation described above is a war of attrition, with the crucial property of players knowing only their own pay-off functions. To see what the war of attrition results in, take a pair of fluxes giving rise to growth rates R=(rb, rp). Now, suppose the nodule is making the offer, by fluctuating the fluxes to bring the growth rates to , where and . The value of winning in the war of attrition to the nodule is therefore , while the cost it pays for staying in the game is rb, because it could start getting rb immediately by conceding any time. Likewise for the plant, the value is and the cost . A plausible strategy for the players is to stay in the game until it is no longer possible to compensate the suffered cost by winning and growing at the higher rate through the rest of the period. Thus, the fraction of time each player will stay in the game can be calculated by setting equal the cost of staying to the benefit of winning the war of attrition. This condition is given by:
| (3.1) |
| (3.2) |
where p and q are the fractions of period the nodule and the plant stay in the war of attrition, respectively. Rewriting the equations for p and q, we get:
| (3.3) |
If p>q, the nodule wins, and vice versa. Because the nodule is in the offering position, the growth rates will change to R′ if the nodule wins the war of attrition and remains at R if the plant does so. It follows that
| (3.4) |
and rejected otherwise. The same argument can be repeated for the case where the plant is making the offer. The condition for an offer R′ by the plant to be accepted can be shown to be
| (3.5) |
This rule for assigning the winner of the war of attrition is also the evolutionarily stable strategy (ESS) of the general asymmetric war of attrition game (Parker & Rubenstein 1981; Hammerstein & Parker 1982), and the same conditions hold even if the waiting times between successive offers are random (see appendix A).
(a) Direction of movement in negotiation
We want to predict the direction of this negotiation process outlined above. To do that, we look at the three cases separately. If the offer is withdrawn, there is no movement. On the other hand, if the offer is mutually beneficial, the movement increases both players' pay-off. In the third case, whether there is movement or not is determined by equations (3.4) and (3.5). Both of these conditions can be rearranged in the following, which gives the condition for an offer R′ to be accepted, regardless of which player is proposing it:
| (3.6) |
Equation (3.6), along with the rules for the other two cases, implies that the negotiation process can only move in the direction of increasing products of growth rates. This product is called the ‘Nash product’ in cooperative game theory, and the NBS is the unique point which is the maximum of this product. It follows that the equilibrium of the negotiation process will be the NBS. Moreover, since equation (3.6) is true for arbitrary positive pay-off pairs, the NBS is globally stable in the permissible pay-off space.
The NBS can be found by taking the partial derivatives of the product function with respect to IA and IC and equating these to zero. Alternatively, it can easily be computed numerically using software such as Mathematica in a few lines (see our code in the electronic supplementary material). With k1=k2=0.1 and Lp=1, the NBS lies at IA=0.26 and IC=0.74. These are actual fluxes of metabolites predicted by the negotiation model. The growth rates for the nodule and plant are rb=0.48 and rp=0.22, respectively.
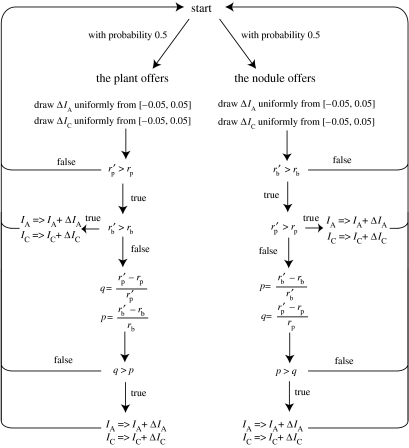
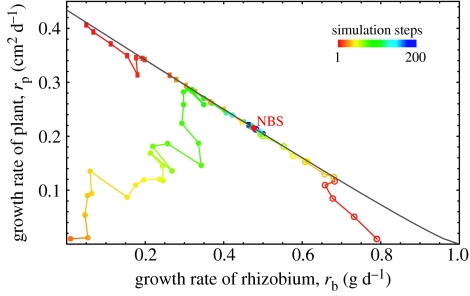
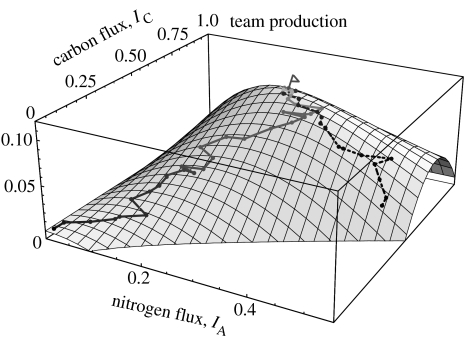
We illustrate how the negotiation works using a simulation in Mathematica (code available in the electronic supplementary material). The process is implemented as successive iterations of the flowchart depicted in figure 5. Sample trajectories of the process in the pay-off space starting from three different initial conditions are shown in figure 6. In this graph, the x- and y-axes are the rhizobium's and the plant's growth rates, respectively. Each point in this pay-off space corresponds to a pair of nitrogen and carbon fluxes. Only the points that lie below the diagonal curve running northwest to southeast are attainable in our game, i.e. there is no pair of fluxes that results in pay-off pairs lying above this curve. This is due to the conflict built into the pay-off functions, which makes it impossible after some point to improve both players' pay-off simultaneously, such that an increase in one player's pay-off necessarily decreases the other player's. This curve is called the Pareto boundary in economics. The NBS, which is the maximum of the product, is marked with a red-filled circle. Note that it lies on the Pareto boundary, so there is no other pay-off pair that is better for both players. One can also follow the trajectory of the negotiations in the flux space, which is shown in figure 7 for the same three runs of the simulation as in figure 6.
Figure 5.
Flowchart of the stochastic simulation.
Figure 6.
Trajectory of negotiations in the growth rate space for three runs of the simulation with different initial conditions (depicted by open circles, filled circles and filled squares). The size of the plant, L, is set equal to 1 for all three runs. The NBS is at rb=0.48 and rp=0.22, corresponding to the fluxes IA=0.26 and IC=0.74. The negotiation process, regardless of the initial conditions, converges to the NBS and stays there. The grey curve depicts the Pareto boundary of the game, which is defined as the set of points upon which it is not possible to increase one player's pay-off without decreasing the other player's.
Figure 7.
The trajectory of negotiations for two of the same runs as in figure 6 plotted in three dimensions, where the vertical axis is the product of the growth rates for the rhizobium and plant. The NBS, which is the maximum of this product, is at IA=0.26 and IC=0.74 and indicated by the little flag. Both trajectories climb uphill to reach this point.
4. Discussion
Our metabolic models, while being simple, succeed in capturing the basic trade-off faced by a nodule and a plant during the interaction. We build our negotiation model upon this mechanistic basis. Our model describes a general process that can unify two of the previous approaches to the rhizobium–legume symbiosis in a single framework. West et al. (2002a) present a model for evolution of plant sanctions and West et al. (2002b) find that given the sanctions, nitrogen fixation becomes evolutionarily stable for rhizobia. On the other hand, the second approach by Simms & Taylor (2002) suggests a somewhat different mechanism termed partner selection, where the nodules are rewarded differentially based on their performance. Both of these approaches have found empirical support (Kiers et al. 2003; Simms et al. 2006). To see how this paper relates to this previous work, we note that both plant sanctions and partner choice hypotheses implicate some sort of evaluation and decision-making mechanism on the part of the plant. Our negotiation model supplies such an evaluation mechanism for both players. It is based on random fluctuations in the material fluxes and requires from the players only the ability to sense their own benefits and react to random changes. The shutting down of fluxes in response to non-beneficial changes can be interpreted as a shock response, similar to heat shock.
In this setup, a simple decision rule is sufficient to show that the dynamics converge to the NBS, where players cooperate and maximize the product of the growth rates. The decision rule requires information about only the player's own pay-off and the expected time to the next offer. It prescribes staying in the war of attrition as long as the expected gain from winning it is positive. Staying in the game longer than that yields a negative expected pay-off to players relative to immediate concession, even if they win the war of attrition. Staying for shorter times might be beneficial if players know the opponent's strategy, but they have no such information. This response rule provides a simple and plausible way of how the players might react in all the possible war of attrition games that can arise during negotiation. The direction of negotiation predicted by this analysis is also consistent with the ESS behaviour in a general war of attrition with incomplete information, as analysed by Parker & Rubenstein (1981) and Hammerstein & Parker (1982).
A major feature of the negotiation model presented here is that it aims to predict the actual material fluxes between the partners, making it immediately testable and readily applicable to practical problems. Specifically, this setup, worked out here for the case of a single nodule and plant, can be extended to the multiple nodule case: a single plant can simultaneously bargain with multiple nodules and allocate resources according to how much it benefits from each of them. The utility of this approach would be that it can be parametrized and used to generate quantitative predictions on the distribution of benefits within a single plant depending on the types and properties of rhizobium strains, potentially guiding development of inoculation procedures for agricultural practice. On the other hand, a direct test of our model can be done in a species like Medicago, where plants inoculated at just one site and grown in test tubes can be used to test the prediction that the benefits are distributed in accordance to the NBS.
McNamara et al. (1999) argue that the outcomes of two-player interactions are determined within the developmental time-scale as a result of a negotiation process. Our approach agrees with this, but differs from their model, in that the negotiation rules are pay-off based and aim for a simple maximization of immediate benefits. This is a common element of developmental strategies in many organisms. For example, plants grow their roots and shoots adaptively by taking into account the amount of resources obtained from different directions. Such general developmental strategies, given an appropriate interaction medium, lead to a negotiation setup and a cooperative outcome. This is a plausible route to the evolution of cooperation, which does not involve any altruism or abstract decision rules.
Finally, we conclude with some comments on the applicability of bargaining theory and cooperative game theory, in general, to biological cooperation. Evolutionary approaches to cooperation have studied whether it can persist in the face of cheaters. Reciprocity is one of the main themes that emerge in models of cooperation (for a review, see Sachs et al. 2004). On the other hand, the phenomenon of coordination, which usually underlies reciprocation, is generally overlooked, even though it is observed commonly in nature (Noë 2006). Most, if not all, cooperative interactions feature elaborate behavioural, cognitive and physiological mechanisms to ensure successful coordination. An example of such a mechanism in our system is the amino acid shuttling between bacteroids and plant cells (Lodwig et al. 2003) that creates a metabolic interdependence between the plant and the nodule. Cooperative game theory is a well-suited framework to study such interactions, since it specifically models situations where players can communicate and coordinate their actions to achieve agreed-upon outcomes. Symbioses like the rhizobium case here are especially suitable to being studied using cooperative games, owing to the intimate physical association between the partners leading to a high potential for coordination.
Previously, we have argued for a two-tier approach using cooperative game theory in investigating reproductive social behaviour (Roughgarden et al. 2006) and here we extend the argument for the utility of the framework to interspecific cooperation. As in Roughgarden et al. (2006), the study presented here models the attainment of a cooperative outcome within a developmental time-scale, but it differs from our previous model in that the negotiation process is derived from an essentially non-cooperative game. During the negotiation, situations of conflict are resolved through a war of attrition, which results in a loss of pay-off relative to immediate settlement for both of the players. In our simulations, this ‘inefficiency’ is around 7% of the players' total pay-off. Even though this figure is rather small, it nonetheless means that mechanisms for resolving conflicts in a more efficient way can be selected for. Such a mechanism might involve each player signalling their staying times in the war of attrition and decide upon the winner without a full-blown conflict. This is a possible route to the evolution of cooperatively playing parties, as in Roughgarden et al. (2006). Cooperative play might thus emerge in evolutionary time-scale as an adaptation to facilitate the attainment of a cooperative outcome within an interaction.
To conclude, we believe that an approach focusing on coordination and communication between parties in cooperative interactions will be more fruitful than considering only individual decision-making. Cooperative game theory complements its non-cooperative counterpart in providing a framework for such an approach. Our model falls in between these two game theory approaches and provides a mechanistic basis for cooperation with coordination within a developmental time-scale. At the evolutionary level, we envision that a research programme focusing on cooperative game theory and the interface with its non-cooperative counterpart will be the basis for investigating the evolution of cooperation and coordination mechanisms, leading to a much more complete and coherent answer to one of the greatest questions in biology.
Acknowledgments
We thank Rob Pringle and Erin Kurten for their helpful discussions during this work and two anonymous referees for their comments on an earlier version of this manuscript.
Appendix A
Appendix A. Random waiting times between offers
The discrete time negotiation setup treats the interval between successive offers as fixed. This condition can be relaxed and the period length can be treated as a random variable, which we call t. Then, the conditions for the maximum time to stay in the game need to be expressed in terms of the expected pay-offs. The maximum strategy that, conditioned on winning the contest, yields a greater expected pay-off to the nodule than immediate concession is given by
| (A1) |
where p is not a fraction of one period but an absolute time measure now. We are calculating p after an offer is made (meaning that rb and rb′ are fixed), so the only random variable in this equation is t, and we can take the rest out of the expectation. If we label the unit in which we measure t, such that , then this equation becomes the same as equation (3.1). The same holds for the plant's maximum staying time, q, as well. Thus, equations (3.1) and (3.2) still apply in a case with random time-intervals between offers.
Supplementary Material
Program for the simulation used in the paper. Prepared in Mathematica 5.0
References
- Denison R.F. Legume sanctions and the evolution of symbiotic cooperation by rhizobia. Am. Nat. 2000;156:567–576. doi: 10.1086/316994. doi:10.1086/316994 [DOI] [PubMed] [Google Scholar]
- Gordon A.J, Lea P.J, Rosenberg C, Trinchant J.-C. Nodule formation and function. In: Lea P.J, Morot-Gaudry J.-F, editors. Plant nitrogen. Springer; Berlin, Germany: 2001. pp. 101–146. [Google Scholar]
- Hammerstein P, Parker G.A. The asymmetric war of attrition. J. Theor. Biol. 1982;96:647–682. doi:10.1016/0022-5193(82)90235-1 [Google Scholar]
- Harsanyi J.C. Approaches to the bargaining problem before and after the theory of games: a critical discussion of Zeuthen's, Hicks' and Nash's theories. Econometrica. 1956;24:144–157. doi:10.2307/1905748 [Google Scholar]
- Kiers E.T, Rousseau R.A, West S.A, Denison R.F. Host sanctions and legume–rhizobium mutualism. Nature. 2003;425:78–81. doi: 10.1038/nature01931. doi:10.1038/nature01931 [DOI] [PubMed] [Google Scholar]
- Lodwig E, Poole P. Metabolism of rhizobium bacteroids. Crit. Rev. Plant Sci. 2003;22:37–78. [Google Scholar]
- Lodwig E.M, Hosie A.H.F, Bourdés A, Findlay K, Allaway D, Karunakaran R, Downie J.A, Poole P.S. Amino-acid cycling drives nitrogen fixation in legume–rhizobium symbiosis. Nature. 2003;422:722–726. doi: 10.1038/nature01527. doi:10.1038/nature01527 [DOI] [PubMed] [Google Scholar]
- Lum M.R, Hirsch A.M. Roots and their symbiotic microbes: strategies to obtain nitrogen and phosphorus in a nutrient-limiting environment. J. Plant Growth Regul. 2003;21:368–382. doi:10.1007/s00344-003-0003-1 [Google Scholar]
- McNamara J.M, Gasson C.E, Houston A.I. Incorporating rules for responding into evolutionary games. Nature. 1999;401:368–371. doi: 10.1038/43869. [DOI] [PubMed] [Google Scholar]
- Nash J. The bargaining problem. Econometrica. 1950;18:155–162. doi:10.2307/1907266 [Google Scholar]
- Nash J. Two person cooperative games. Econometrica. 1953;21:128–140. doi:10.2307/1906951 [Google Scholar]
- Noë R. Cooperation experiments: coordination through communication versus acting apart together. Anim. Behav. 2006;71:1–18. doi:10.1016/j.anbehav.2005.03.037 [Google Scholar]
- Oke V, Long S.R. Bacteroid formation in the rhizobium–legume symbiosis. Curr. Opin. Microbiol. 1999;2:641–646. doi: 10.1016/s1369-5274(99)00035-1. doi:10.1016/S1369-5274(99)00035-1 [DOI] [PubMed] [Google Scholar]
- Parker G.A, Rubenstein D.I. Role assessment, reserve strategy and acquisition of information in asymmetric animal conflicts. Anim. Behav. 1981;29:221–240. doi:10.1016/S0003-3472(81)80170-4 [Google Scholar]
- Puppo A, et al. Legume nodule senescence: roles for redox and hormone signalling in the orchestration of the natural aging process. New Phytol. 2005;165:683–701. doi: 10.1111/j.1469-8137.2004.01285.x. doi:10.1111/j.1469-8137.2004.01285.x [DOI] [PubMed] [Google Scholar]
- Roughgarden J, Oishi M, Akçay E. Reproductive social behavior: cooperative games to replace sexual selection. Science. 2006;311:965–970. doi: 10.1126/science.1110105. doi:10.1126/science.1110105 [DOI] [PubMed] [Google Scholar]
- Sachs J.L, Mueller U.G, Wilcox T.P, Bull J.J. The evolution of cooperation. Q. Rev. Biol. 2004;79:135–160. doi: 10.1086/383541. doi:10.1086/383541 [DOI] [PubMed] [Google Scholar]
- Schwartz M.W, Hoeksema J.D. Specialization and resource trade: biological markets as a model of mutualisms. Ecology. 1998;79:1029–1038. doi:10.2307/176598 [Google Scholar]
- Simms E.L, Taylor D.L. Partner choice in nitrogen-fixation mutualisms of legumes and rhizobia. Integr. Comp. Biol. 2002;42:369–380. doi: 10.1093/icb/42.2.369. doi:10.1093/icb/42.2.369 [DOI] [PubMed] [Google Scholar]
- Simms E.L, Taylor D.L, Povich J, Shefferson R.P, Sachs J.L, Urbina M, Tausczik Y. An empirical test of partner choice mechanisms in a wild legume–rhizobium interaction. Proc. R. Soc. B. 2006;273:77–81. doi: 10.1098/rspb.2005.3292. doi:10.1098/rspb.2005.3292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S.A, Kiers E.T, Pen I, Denison R.F. Sanctions and mutualism stability: when should less beneficial mutualists be tolerated? J. Evol. Biol. 2002a;15:830–837. doi:10.1046/j.1420-9101.2002.00441.x [Google Scholar]
- West S.A, Kiers E.T, Pen I, Denison R.F. Sanctions and mutualism stability: why do rhizobia fix nitrogen? Proc. R. Soc. B. 2002b;269:685–694. doi: 10.1098/rspb.2001.1878. doi:10.1098/rspb.2001.1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Program for the simulation used in the paper. Prepared in Mathematica 5.0