Abstract
The fastest swimming fishes in relation to size are found among coral reef fish larvae on their way to settle on reefs. By testing two damselfishes, Chromis atripectoralis and Pomacentrus amboinensis, we show that the high swimming speeds of the pre-settlement larvae are accompanied by the highest rates of oxygen uptake ever recorded in ectothermic vertebrates. As expected, these high rates of oxygen uptake occur at the cost of poor hypoxia tolerance. However, hypoxia tolerance is needed when coral reef fishes seek nocturnal shelter from predators within coral colonies, which can become severely hypoxic microhabitats at night. When the larvae settle on the reef, we found that they go through a striking respiratory transformation, i.e. the capacity for rapid oxygen uptake falls, while the ability for high-affinity oxygen uptake at low oxygen levels is increased. This transition to hypoxia tolerance is needed when they settle on the reef; this was strengthened by our finding that small resident larvae of Acanthochromis polyacanthus, a damselfish lacking a planktonic larval stage, do not display such a transition, being well adapted to hypoxia and showing relatively low maximum rates of oxygen uptake that change little with age.
Keywords: respirometry, coral reef, oxygen consumption, hypoxia tolerance, damselfish, fish larvae
1. Introduction
Pre-settlement larvae of coral reef fishes have extraordinary swimming capacities in relation to their size, reaching maximum sustained swimming speeds (Ucrit) of 30–50 body lengths per second (BL s−1) when raced in swim flumes (Stobutzki & Bellwood 1994; Pain 1997; Fisher et al. 2005). This impressive swimming performance occurs at the end of their pelagic phase when they need to reach and settle on suitable coral habitats (Stobutzki & Bellwood 1994; Leis & Carson-Ewart 1997; Leis in press). In general, larvae of temperate fishes do not reach swimming speeds greater than 4–5 BL s−1 (Blaxter 1986; Meng 1993), which may relate to their larger size relative to many coral reef fish larvae (Bellwood & Fisher 2001). Indeed, most adult fishes, including salmonids, cannot attain sustained swimming speeds greater than 5–7 BL s−1, and those best known for exceptional swimming performance, which include swordfish (Xiphias), tunas (Thunnus and Euthynnus) and the inconnu (Stenodus leucichthys), may reach maximum sustained speeds of 12–20 BL s−1 (Aleyev 1977; Beamish 1978).
In small larval fishes, swimming appears to be primarily aerobic, and it can be sustained only if it is fully aerobic and does not lead to a build-up of lactate (Goolish 1991). Thus, the extremely high sustained swimming speeds of coral reef fish larvae imply that they can achieve very high rates of maximum oxygen uptake (MO2max). On the other hand, it was recently reported that adult coral reef fishes are hypoxia tolerant, being able to maintain their routine rate of oxygen consumption (MO2) even at relatively severe hypoxia (oxygen levels of 15–30% of air saturation; Nilsson & Östlund-Nilsson 2004). Indeed, coral reef fishes display a degree of hypoxia tolerance which is similar to that of fishes living in tropical freshwater habitats that often become severely hypoxic (Chapman et al. 1995; Nilsson 1996; Val et al. 1998). A reason for the generalized hypoxia tolerance of coral reef fishes is that they seek nocturnal shelter within coral colonies that can become severely hypoxic at night owing to the respiration of the coral and associated organisms (Goldshmidt et al. 2004; Nilsson et al. 2004). Hypoxia tolerance may also be needed to allow survival in hypoxic tide pools on the reef at night (see Nilsson & Östlund-Nilsson 2006 for review).
High aerobic capacities of very active fish species appear to preclude hypoxia tolerance, and vice versa (see Burggren et al. 1991 for review). Thus, species known for highly active lifestyles and top swimming performance are unable to tolerate low oxygen levels. For example, salmonids cannot maintain their resting MO2 if the [O2] in the ambient water falls below 50% of air saturation (Davis 1975) and for tuna, water [O2] below 60% of air saturation is lethal (Gooding et al. 1981).
Hitherto, there have been no published measurements of MO2max in swimming coral reef larvae. We have now constructed a miniature swim respirometer with a volume that is small enough to allow the oxygen consumption of these minute fishes to be detected, and at the same time has the capacity to produce water speeds exceeding the maximum sustained swimming speed of coral reef fish larvae. Using this respirometer, we have examined the oxygen uptake during swimming in pre- and post-settlement larvae and juveniles of two abundant Indo-Pacific coral reef fishes of the damselfish family (Pomacentridae), Chromis atripectoralis and Pomacentrus amboinensis. Among the coral reef fish larvae, pre-settlement stage damselfishes are among those maintaining the highest spontaneous swimming speeds when followed by divers in situ (Leis & Carson-Ewart 1997). In Ucrit measurements of racing fishes in a swim flume, C. atripectoralis showed a mean Ucrit of 33 BL s−1, with some individuals reaching Ucrit over 50 BL s−1 (Fisher et al. 2005). Pomacentrus amboinensis is a more average performer among the pre-settlement larvae of coral reef fishes, showing sustained speeds of 24–30 BL s−1 (Stobutzki & Bellwood 1994; Fisher et al. 2005).
In addition, we have used closed respirometry on pre- and post-settlement larvae and juveniles of both species to examine their hypoxia tolerance. Finally, we have performed comparative measurements of the same variables in Acanthochromis polyacanthus, which is the only coral reef damselfish on the Great Barrier Reef showing parental care, thus having resident larvae lacking a planktonic stage (Randall et al. 1997). This trait coincides with a comparatively poor swimming performance of its resident larvae, which only at ‘pre-settlement size’ reach an average maximum sustained swimming speed of 12 BL s−1 (Fisher et al. 2005).
2. Material and methods
(a) Animals
All the experiments were carried out during December–January at Lizard Island Research Station (LIRS; www.lizardisland.net.au) on the northern Great Barrier Reef (14°40′ S 145°28′ E), Australia.
Pre-settlement larvae C. atripectoralis and P. amboinensis were captured near LIRS at night over a sandy bottom about 100 m from the reef using light traps (Doherty 1987, as modified by Stobutzki & Bellwood 1997). The pre-settlement larvae were run in the respirometers within 3–30 h of capture. It should be pointed out that since these larvae were captured while apparently heading for the reef, they can be considered to be settlement stage larvae, particularly 30 h after capture. However, for simplicity, we have denoted here all individuals in this group as pre-settlement larvae.
Small post-settlement larvae were obtained by feeding light-trapped larvae in aquaria for 5 or more days. Larger post-settlement larvae and juveniles of C. atripectoralis and P. amboinensis, and larvae and juveniles of A. polyacanthus, were captured by diving with scuba in the lagoon near LIRS, over branching coral at a depth of 2–5 m. They were captured with a hand net after lightly anaesthetizing them with clove oil, as previously described (Östlund-Nilsson & Nilsson 2004). In this study, C. atripectoralis denotes a possible mixture of two closely related species, C. atripectoralis and Chromis viridis, since these cannot be clearly distinguished at the larval stage (Leis & Carson-Ewart 1997).
The fishes were kept in shaded outdoor aquaria, continuously supplied with water pumped in directly from the ocean (29–31°C). The water oxygen level varied between 80 and 105% of air saturation. They were fed daily with live brine shrimp nauplii, but were starved for 24 h before closed respirometry. All the experiments were carried out in shaded daylight (10.00–18.00 h).
(b) The swim respirometer
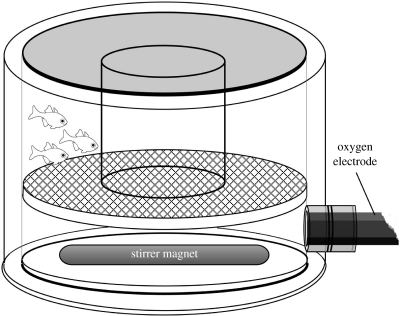
The respirometer chamber consisted of a Perspex cylinder (80 mm inner diameter, 50 mm internal height) with a total water volume of 250 ml (figure 1). The chamber could be opened at the bottom, where a 5 mm thick circular Perspex lid was fitted. An o-ring provided the seal between the lid and the chamber wall. The tip of an oxygen electrode (OXI 340i from WTW, Germany) was inserted 10 mm above the bottom of the chamber. The two o-rings provided the seal for the electrode. A removable wire mesh (0.25 mm stainless steel wire, 2 mm mesh width) was positioned horizontally in the middle of the chamber. The mesh was glued to a thin Perspex ring that fitted tightly to the inside of the cylinder. A PVC jar (34 mm diameter, 30 mm height) was glued to the centre of this mesh. In this way, a circular swim chamber was created, with the PVC jar serving as the inner wall and the Perspex cylinder as the outer wall. The mesh served as the floor of the swim chamber. Water was set in motion by a 6 cm long stirring magnet (cylindrical, 8 mm diameter) put in the compartment below the mesh. The respirometer was placed on the bottom of a 50 l aquarium, below which a magnetic stirrer was placed to drive the magnet in the respirometer. The fishes were put in the chamber while it was held upside down, whereupon the ring with the mesh, the stirrer magnet and finally the lid were put in place. This procedure was performed with the respirometer submerged in the aquarium so that air bubbles could be readily avoided. Water temperature was 29–31°C.
Figure 1.
The small swim respirometer constructed for this study. Water was set in motion by a magnetic stirrer and the fall in water [O2] was followed by an oxygen electrode. See text for details.
The water speed was regulated with the magnetic stirrer. As soon as the water was set in motion, the fishes started swimming against the current, apparently guided by landmarks provided by, e.g. the oxygen electrode and the edges of the surrounding aquarium. The speed was set to a point where it was assumed that the fishes swam at the maximum speed. This was performed by setting the water speed to a level where the fishes just barely could maintain steady positions in the chamber. At a slightly higher speed, the fishes were no longer able to maintain position for more than a few seconds. At slightly slower water speeds, the fishes were not seen loosing position and passively following the flow, indicating that they did their best to swim against the current. Water [O2] was recorded for 10 min, during which a linear fall in water [O2] was seen. To increase the rate of oxygen fall, and therefore the sensitivity of the method, the fishes were run in groups of three to five individuals. During the runs, water [O2] never fell below 90% of air saturation. No systematic effort was made to calibrate the speed of the water, as this will be heterogeneous owing to the small size and circular shape of the chamber (different inner and outer circumferences). However, following the movement of particles in the respirometer with a video camera showed that the water mass could be made to move at approximately 5 rev s−1, indicating that water speeds of 50 and 125 cm s−1 could be achieved near the inner and outer wall of the chamber, respectively. Attempting to reach higher speeds caused the stirrer magnet to wobble. For C. atripectoralis, the water speed had to be set very close to the maximal rate of the apparatus, suggesting that they were swimming at their Ucrit (36 cm s−1 according to Fisher et al. 2005). Thus, this swim respirometer appears to provide a way to measure MO2 in small fishes swimming at their maximum speed, but it is not suited for measuring the maximum swimming speeds per se. For the latter purpose, larger linear chambers should be used.
(c) Closed respirometry
Routine MO2 and the hypoxia tolerance of pre-settlement larvae were examined with closed respirometry at 29–31°C, as described by Nilsson & Östlund-Nilsson (2004). The respirometers for larvae consisted of 10–30 ml glass vials, while 150 ml Plexiglas cylinders were used for larger juveniles. The experiments were terminated when the fishes showed the first signs of agitation or problems with maintaining equilibrium. As in our previous study (Nilsson & Östlund-Nilsson 2004), the [O2] at this end point was denoted [O2]out, and MO2, in milligram O2 kg−1 h−1 (fish wet weight), was plotted against water [O2], measured in percentage of air saturation. From this curve, we determined the critical oxygen concentration [(O2)crit], which is the lowest [O2] where the fish is able to maintain its routine MO2. The values of routine MO2 (table 2) were determined between 60 and 100% of air saturation.
Table 2.
Hypoxia tolerance of pre-settlement and post-settlement larvae and juveniles of C. atripectoralis and P. amboinensis, and of resident larvae and juveniles of A. polyacanthus. ([O2]crit is the lowest water [O2] where the routine MO2 can be maintained, while [O2]out is the [O2] where the fishes showed signs of agitation and were taken out of the respirometer. Values are means±s.d. from 6 to 11 fishes. *p<0.05; **p<0.01; ***p<0.001 (Wilcoxon test) compared to the conspecific pre-settlement larvae (or small larvae of A. polyacanthus.)
| [O2]crit | [O2]out | ||||
|---|---|---|---|---|---|
| routine MO2 | |||||
| species | mass (mg) | (mg kg−1 h−1) | (% air saturation) | ||
| C. atripectoralis pre-settlement | 18.7±1.5 | 2286±520 | 45.0±8.9 | 17.1±2.8 | |
| C. atripectoralis post-settlement | 23.4±2.6*** | 1526±257*** | 34.5±3.4** | 19.4±3.8 | |
| C. atripectoralis juveniles | 71.5±18.5*** | 1577±156*** | 25.4±3.0*** | 12.7±2.5** | |
| P. amboinensis pre-settlement | 32.7±4.8 | 2201±405 | 43.3±5.6 | 14.8±3.2 | |
| P. amboinensis post-settlement | 59.0±18.0* | 1621±263** | 32.1±4.3** | 12.9±4.5 | |
| P. amboinensis juveniles | 102.3±20.2*** | 1145±83*** | 31.4±1.6*** | 14.1±2.9 | |
| A. polyacanthus small larvae | 14.8±2.9 | 2553±673 | 28.3±2.5 | 16.0±1.6 | |
| A. polyacanthus large larvae | 44.8±15.5*** | 2072±304 | 31.2±4.2 | 14.8±2.3 | |
| A. polyacanthus juveniles | 134.4±21.9*** | 1561±185*** | 28.7±6.5 | 12.4±2.9 | |
[O2] is given as percentage of air saturation which can easily be converted to oxygen partial pressure (100%=151 mmHg) or weight-based concentration (100%=6.0 mg O2 l−1 at 30°C in seawater). All the values are means±s.d. The study followed ethical guidelines given by the University of Queensland.
3. Results
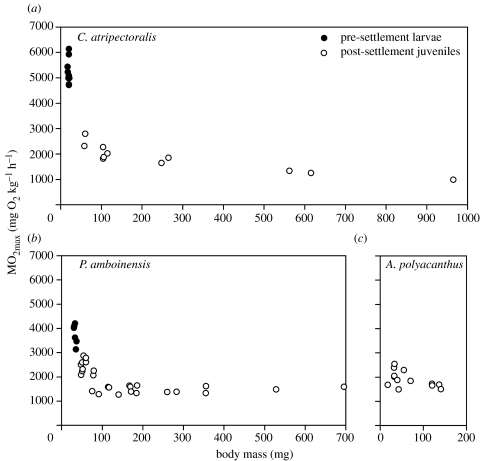
The MO2max for swimming pre-settlement larvae of C. atripectoralis (weighing 17–21 mg) was 5250±497 mg kg−1 h−1 (range 4715–6135). This was significantly higher than the MO2max of 2181±360 mg kg−1 h−1 (range 1817–2791) measured in post-settlement juveniles (weighing 58–115 mg; table 1).
Table 1.
Rate of O2 consumption at maximum swimming speeds in pre- and post-settlement larvae of C. atripectoralis and P. amboinensis, and in small and large larvae of A. polyacanthus. (Values are means±s.d. from five to nine replicates (three to five fishes per replicate). *p<0.05; **p<0.01; ***p<0.001 (Wilcoxon test) compared to the conspecific pre-settlement larvae (or small larvae of A. polyacanthus.)
| species | mass (mg) | MO2max (mg kg −1 h−1) |
|---|---|---|
| C. atripectoralis pre-settlement | 19.4±1.3 | 5250±497 |
| C. atripectoralis post-settlement | 91.2±25.3*** | 2181±360*** |
| P. amboinensis pre-settlement | 32.9±2.4 | 3753±418 |
| P. amboinensis post-settlement | 50.3±2.4*** | 2433±284** |
| A. polyacanthus small larvae | 29.8±6.9 | 2090±321 |
| A. polyacanthus large larvae | 73.7±38.3* | 1834±284 |
For pre-settlement larvae of P. amboinensis (weighing 30–37 mg), the MO2max was 3753±418 mg kg−1 h−1 (range 3129–4204). This was also significantly higher than the MO2max of 2433±284 mg kg−1 h−1 (range 2080–2876) measured for post-settlement juveniles (weighing 47–54 mg; table 1).
By contrast, small resident larvae of A. polyacanthus (weighing 17–39 mg) had a relatively low MO2max of 2090±321 mg kg−1 h−1 (range 1677–2542), which did not differ significantly from 1834±284 mg kg−1 h−1 (range 1492–2292) measured in larger resident larvae (weighing 43–141 mg; table 1).
Figure 2a,b illustrates the drastic reduction in MO2max that was found to occur after settlement. For P. amboinensis, a clear transition in the MO2max had taken place when the weight was about doubled. Pomacentrus amboinensis larvae that we fed ad libitum with live brine shrimp nauplii showed a doubling in weight within about 10 days. For C. atripectoralis, the more limited data do not allow us to pin point precisely at which weight or age the transition starts, although the magnitude of the transition appears to be even more drastic than in P. amboinensis, and most of the change had occurred before the larvae had reached a weight of about 60 mg.
Figure 2.
Relationship between body mass and oxygen consumption at maximum swimming speed of larvae and juveniles of (a) C. atripectoralis (weighing 17–965 mg), (b) P. amboinensis (weighing 30–528 mg) and (c) A. polyacanthus (weighing 17–141 mg). The latter species lacks a planktonic larval stage.
In A. polyacanthus (figure 2c), there was no indication of any rapid change in MO2max with age. In the swim respirometer, it was obvious that A. polyacanthus is not such a good swimmer. To maintain their position in the chamber, the magnetic stirrer had to be set at a much slower speed than for the other species. Indeed, A. polyacanthus larvae did apparently put about the same effort into swimming against the current in the swim respirometer as they put into spontaneous activity in the closed respirometer, because there were no significant differences between MO2max (table 1) and MO2 (table 2) measured in the larvae of this species.
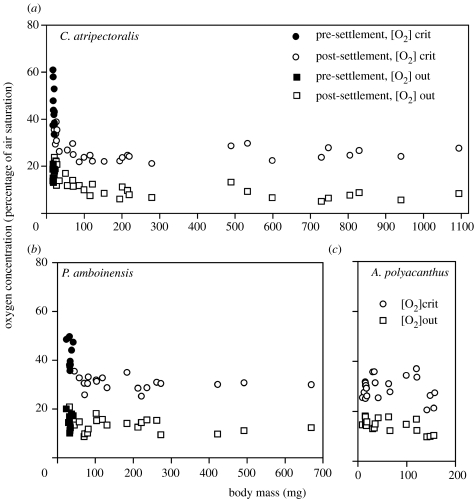
The [O2]crit values recorded in the closed respirometry experiments (table 2) were strikingly high (around 45% of air saturation) in the pre-settlement larvae of C. atripectoralis and P. amboinensis, revealing a low hypoxia tolerance compared with that in the post-settlement larvae. Indeed, the age/mass-related fall in [O2]crit in these species (figure 3a,b) virtually paralleled the changes seen in MO2max (figure 2a,b). In C. atripectoralis, a highly significant fall in [O2]crit had already occurred after 5 days in captivity, when their weight had increased from 18.7 to 23.4 mg (table 2). Similarly, in P. amboinensis, a significant fall in [O2]crit had occurred after about 10 days, corresponding to a doubling in weight. There was a striking variability in the [O2]crit of pre-settlement larvae, indicating that a transition to hypoxia tolerance may already have started at the time of capture. As with MO2max, the resident larvae of A. polyacanthus displayed no significant age/mass-related change in [O2]crit (table 2 and figure 3c). Here, even the smallest specimen, having a mass of 9 mg, was quite hypoxia tolerant, showing a [O2]crit of 25.5% of air saturation.
Figure 3.
Relationship between body mass and hypoxia tolerance of larvae and juveniles of (a) C. atripectoralis (weighing 17–1094 mg), (b) P. amboinensis (weighing 23–668 mg) and (c) A. polyacanthus (weighing 9–157 mg). The latter species lacks a planktonic larval stage. [O2]crit is the lowest water [O2] where the routine MO2 can be maintained, while [O2]out is the [O2] where the fishes showed signs of agitation and were taken out of the respirometer.
In C. atripectoralis or P. amboinensis, the [O2]out values (table 2 and figure 3a,b) did not show such a marked fall with age/mass as the [O2]crit values did, indicating a quite high anaerobic capacity already at the time of settlement. However, the [O2]out of juvenile C. atripectoralis was still significantly lower than the values displayed by the younger groups. For A. polyacanthus, [O2]out showed no significant change with age/mass (table 2 and figure 3c).
4. Discussion
The mean MO2max of 5250 and 3753 mg kg 1 h−1 presently measured for pre-settlement larvae of C. atripectoralis and P. amboinensis, respectively, are to our knowledge the highest rates of O2 consumption ever measured in fishes or in any other ectothermic vertebrate. The previous record holders among the fishes are the tunas, which can reach a MO2max of 2500 mg kg−1 h−1, which is two to five times that of the other active fishes like salmonids (Korsmeyer et al. 1996). The pre-settlement larvae of C. atripectoralis reached a mean MO2max that was 1.4 times higher than that of P. amboinensis. This parallels the difference in the maximum sustained swimming speed (in BL s−1) attained by the pre-settlement larvae of these two species, which is also 1.4 times higher in C. atripectoralis than in P. amboinensis, when measured in the same study (Fisher et al. 2005).
The results show that both C. atripectoralis and P. amboinensis undergo a transition in aerobic capacity, resulting in a drastic fall in MO2max and [O2]crit when the larvae settle on the reef. The change is highly significant within a few days and it is possibly initiated as soon as the larvae reach the reef. A study of the swimming performance (Ucrit) of damselfishes at the same locality suggested that there is a similar rapid reduction in the maximum sustainable swimming speed when the larvae settle on the reef (Stobutzki & Bellwood 1994), and such a fall in Ucrit is also seen in laboratory-reared damselfish larvae when they reach the post-settlement size (Bellwood & Fisher 2001).
Most probably, the respiratory transition that occurs upon settlement is needed to allow the fishes to tolerate hypoxia at the cost of a lower MO2max and reduced sustained swimming performance. As mentioned, adult coral reef fishes, in general, are hypoxia tolerant (Nilsson & Östlund-Nilsson 2004), and this hypoxia tolerance allows the fishes to seek shelter in coral colonies at night, where [O2] may fall below 20% of air saturation (Goldshmidt et al. 2004; Nilsson et al. 2004). This interpretation of the results fits well with the observed lack of transitional changes in A. polyacanthus, a species with no planktonic stage. Thus, even the smallest larvae of A. polyacanthus were found to be hypoxia tolerant and have a low O2max.
Interestingly, the pre-settlement larvae of C. atripectoralis and P. amboinensis had a low [O2]out that showed little or no change after settlement, suggesting that their glycolytic capacity for anaerobic ATP production is quite high already at the time of settlement. This could function to buy them time, by allowing them to immediately seek nocturnal shelter in hypoxic coral habitats, while waiting for the ability of high-affinity oxygen uptake in hypoxia to improve. The drawback with relying on anaerobic glycolysis for hypoxic survival is that it results in a build-up of lactate, the main anaerobic end product in vertebrates. Apart from the resultant acidosis, which can be a threat to survival, the lactate has to be oxidized when oxygen levels increase again, causing an oxygen debt that slows down the recovery from hypoxia.
Therefore, surviving hypoxia merely through anaerobic glycolysis is not a good long-term solution, and there is probably a selection pressure for acquiring a low [O2]crit as soon as possible. Indeed, that this may be performed sooner than later was suggested by the large variability in [O2]crit values in the pre-settlement larvae, particularly in C. atripectoralis (figure 3a). These larvae were light-trapped on their way to settle on the reef, and some of them may have already started the transition to increased hypoxia tolerance. This could also explain the strikingly high individual variability in maximum sustained swimming speed previously observed, which for newly light-tapped C. atripectoralis and P. amboinensis range from 20 to 57 and 15 to 46 BL s−1, respectively (Fisher et al. 2005).
The apparent trade-off between a high MO2max and hypoxia tolerance seen in fishes is thought to be related to the opposing demands on the oxygen-carrying properties of haemoglobin. Thus, fishes adapted to survive at low oxygen levels have haemoglobins with very high O2 affinities, while haemoglobins of highly active fishes show much lower O2 affinities than those of sedentary species (reviewed by Burggren et al. 1991). The reason is that a high oxygen affinity leads to reduced rates of O2 downloading in the tissues. More precisely, O2 has to be downloaded at a low partial pressure, leading to a small pressure gradient from blood into the mitochondria, and therefore a slow O2 delivery. Fishes are well known for possessing multiple haemoglobin isoforms, and there are examples of ontogenetic changes in the expression of haemoglobin isoforms in fishes (reviewed by Jensen et al. 1998). Thus, one may speculate that when the fish larvae settle on a coral reef, there is a change in the expression of haemoglobin isoforms from forms with low O2 affinities to forms with higher O2 affinities that allow hypoxia tolerance, which inevitably results in a reduced maximum rate of O2 downloading and a lowered maximum sustained swimming speed. It may be possible to examine the changes in the expression of haemoglobin isoform of coral reef fish larvae at the mRNA level, but the small size of the larvae will probably preclude a study of the O2 affinity of their haemoglobins. Another possibility is that the intracellular milieu changes in the erythrocytes in a way that gives the haemoglobins a higher oxygen affinity after reef settlement. Intracellular levels of H+, Cl−, CO2 and organic phosphates are all well known to affect haemoglobin oxygen affinity in vertebrates (Jensen et al. 1998).
There is a possibility that the very high MO2 measured at maximum aerobic swimming speeds in the pre-settlement larvae were underestimates of the maximum rate of O2 uptake by their respiratory system. Goolish (1991) suggested that the part of the muscle mass that is used for aerobic swimming in adult fishes is often too small to demand the full scope of the respiratory system. However, Goolish (1991) also suggested that most of the swimming in larval fishes is done by aerobic muscles, and that the full activity of these muscles demands O2 uptake to occur at the maximum rate. This is probably particularly true for the pre-settlement larvae of coral reef fishes because they are able to maintain extremely high swimming speeds for a long time, making it evident that this is fully aerobic swimming. By contrast, anaerobic burst swimming can only be maintained for a few seconds (Plaut 2001). Observations of spontaneous swimming of pre-settlement coral reef fish larvae suggest that they are almost constantly swimming at high speeds, although rarely at their Ucrit (Fisher & Bellwood 2003). When followed by divers in situ, coral reef fish larvae of 60 taxa were found to swim at 20 cm s−1 on an average, while their mean Ucrit was 36 cm s−1 (Leis & Fisher 2006). Moreover, their endurance is remarkable; when fed, coral reef fish larvae are even able to grow while swimming constantly in a flume at about 10 BL s−1, and they can continue swimming at this speed for at least a week (Leis & Clark 2005). Indeed, it has become clear that the considerable swimming abilities of marine fish larvae, in general, and coral reef fish larvae, in particular, have a much more profound influence on their movement and distribution in the ocean than previously assumed (Leis in press).
To conclude, the high swimming speeds of pre-settlement larvae of coral reef fishes are supported by very high rates of O2 uptake, at least in the two species studied. To our knowledge, these are the highest rates of O2 uptake measured in ectothermic vertebrates. While this capacity will help the pre-settlement larvae to reach their coral reef habitats, it comes at the cost of a low tolerance to hypoxia. To be able to profit from the protection offered by moving into coral colonies at night, where the water can become severely hypoxic, they have to alter the properties of their respiratory system to allow hypoxia tolerance. This transition leads to a reduction in both their MO2max and, as previously shown, the maximum swimming speed they can sustain. Although they are then no longer champion swimmers, post-settlement larvae and adults of damselfishes are still good swimmers, being able to reach sustained swimming speeds of about 10 BL s−1 (Stobutzki & Bellwood 1994).
Acknowledgments
This study was financed by The University of Oslo and The Research Council of Norway. We thank the personnel at LIRS for their enthusiastic help and great hospitality, and John Reierstad at the University of Oslo for drawing figure 1.
References
- Aleyev Y.G. Dr. W. Junk b.v. Publishers; The Hague, The Netherlands: 1977. Nekton. [Google Scholar]
- Beamish F.W.H. Swimming capacity. In: Hoar W.S, Randall D.J, editors. Fish Physioloy VII. Locomotion. Academic Press; New York, NY: 1978. pp. 101–187. [Google Scholar]
- Bellwood D.R, Fisher R. Relative swimming speeds in reef fish larvae. Mar. Ecol. Prog. Ser. 2001;211:299–303. [Google Scholar]
- Blaxter J.H.S. Development of sense organs and behaviour of teleost fish larvae with special reference to feeding and predator avoidance. Trans. Am. Fish. Soc. 1986;115:98–114. doi:10.1577/1548-8659(1986)115<98:NLFCDO>2.0.CO;2 [Google Scholar]
- Burggren W, McMahon B, Powers D. Respiratory functions of blood. In: Prosser C.L, editor. Environmental and metabolic animal physiology. Wiley-Liss; New York, NY: 1991. pp. 437–508. [Google Scholar]
- Chapman L.J, Kaufman L.S, Chapman C.A, McKenzie F.E. Hypoxia tolerance in twelve species of East African cichlids: potential for low oxygen refugia in Lake Victoria. Conserv. Biol. 1995;9:1274–1288. doi: 10.1046/j.1523-1739.1995.9051262.x-i1. doi:10.1046/j.1523-1739.1995.9051274.x [DOI] [PubMed] [Google Scholar]
- Davis J.H. Minimal dissolved oxygen requirements of aquatic life with emphasis on Canadian species: a review. J. Fish. Res. Board Can. 1975;32:2295–2332. [Google Scholar]
- Doherty P.J. Light-traps: selective but useful devices for quantifying the distribution and abundances of larval fishes. Bull. Mar. Sci. 1987;41:423–431. [Google Scholar]
- Fisher R, Bellwood D.R. Undisturbed swimming behaviour and nocturnal activity of coral reef fish larvae. Mar. Ecol. Prog. Ser. 2003;263:177–188. [Google Scholar]
- Fisher R, Leis J.M, Clark D.L, Wilson S.K. Critical swimming speeds of late-stage coral reef fish larvae: variation within species, among species and between locations. Mar. Biol. 2005;147:1201–1212. doi:10.1007/s00227-005-0001-x [Google Scholar]
- Goldshmidt R, Holzman R, Weihs D, Genin A. Aeration of coals by sleep-swimming fish. Limnol. Oceanogr. 2004;49:1832–1839. [Google Scholar]
- Gooding R.M, Neill W.H, Dizon A.E. Respiration rates and low-oxygen tolerance limits in skipjack tuna Katsuwonus pelamis. Fish. Bull. 1981;79:31–48. [Google Scholar]
- Goolish E.M. Aerobic and anaerobic scaling in fish. Biol. Rev. 1991;66:33–56. [Google Scholar]
- Jensen F.B, Fago A, Weber R.E. Fish physiology. vol. 17. Academic Press; San Diego, CA: 1998. Hemoglobin structure and functions. [Google Scholar]
- Korsmeyer K.E, Dewar H, Lai N.C, Graham J.B. Tuna aerobic swimming perormance: physiological and environmental limits based on oxygen supply and demand. Comp. Biochem. Physiol. 1996;113B:45–56. [Google Scholar]
- Leis, J. M. In press. Are larvae of demersal fishes plankton or nekton? Adv. Mar. Biol.51 [DOI] [PubMed]
- Leis J.M, Carson-Ewart B.M. In situ swimming speeds of the late pelagic larvae of some Indo-Pacific coral-reef fishes. Mar. Ecol. Prog. Ser. 1997;159:165–174. [Google Scholar]
- Leis J.M, Clark D.L. Feeding greatly enhances endurance swimming of settlement-stage reef-fish larvae (Pomacentridae) Ichthyol. Res. 2005;52:185–188. doi:10.1007/s10228-004-0265-z [Google Scholar]
- Leis, J. M. & Fisher R. 2006 Swimming speed of settlement-stage reef-fish larvae measured in the laboratory and in the field: a comparison of critical speed and in situ speed. In Proc. 10th Int. Coral Reef Symposium, Okinawa, Japan 28 June–2 July 2004 (ed. Y. Suzuki, T. Nakamori, M. Hidaka, H. Kayanne, B. E. Casareto, K. Nadaoka, H. Yamano & M. Tsuchiya), pp. 438–445. Tokyo, Japan: Japanese Coral Reef Society.
- Meng L. Sustainable swimming speeds of striped bass larvae. Trans. Am. Fish. Soc. 1993;122:702–708. doi:10.1577/1548-8659(1993)122<0702:SSSOSB>2.3.CO;2 [Google Scholar]
- Nilsson G.E. Brain and body oxygen requirements of Gnathonemus petersii, a fish with an exceptionally large brain. J. Exp. Biol. 1996;199:603–607. doi: 10.1242/jeb.199.3.603. [DOI] [PubMed] [Google Scholar]
- Nilsson G.E, Östlund-Nilsson S. Hypoxia in paradise: widespread hypoxia tolerance in coral reef fishes. Proc. R. Soc. B. 2004;271(Suppl. 3):S30–S33. doi: 10.1098/rsbl.2003.0087. doi:10.1098/rsbl.2003.0087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson G.E, Östlund-Nilsson S. Hypoxia tolerance in coral reef fishes. In: Val A.L, de Almeida-Val V.M.F, Randall D.J, editors. Fish physiology. vol. 21. Academic Press/Elsevier; London, UK: 2006. pp. 583–596. [Google Scholar]
- Nilsson G.E, Hobbs J.-P, Munday P.L, Östlund-Nilsson S. Coward or braveheart: extreme habitat fidelity through hypoxia tolerance in a coral-dwelling goby. J. Exp. Biol. 2004;207:33–39. doi: 10.1242/jeb.00713. doi:10.1242/jeb.00713 [DOI] [PubMed] [Google Scholar]
- Östlund-Nilsson S, Nilsson G.E. Breathing with a mouth full of eggs: respiratory consequences of mouthbrooding in cardinalfish. Proc. R. Soc. B. 2004;271:1015–1022. doi: 10.1098/rspb.2004.2700. doi:10.1098/rspb.2004.2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain S. Swimming for dear life. New Sci. 1997;2099:28–32. [Google Scholar]
- Plaut I. Critical swimming speed: its ecological relevance. Comp. Biochem. Physiol. A. 2001;131:41–50. doi: 10.1016/s1095-6433(01)00462-7. doi:10.1016/S1095-6433(01)00462-7 [DOI] [PubMed] [Google Scholar]
- Randall J.E, Allen R.G, Steene R.C. Crawford Hourse Publishing; Bathurst, Australia: 1997. Fishes of the Great Barrier Reef and Coral Sea. [Google Scholar]
- Stobutzki I.C, Bellwood D.R. An analysis of the sustained swimming abilities of pre- and post-settlement coral reef fishes. J. Exp. Mar. Biol. Ecol. 1994;175:275–286. doi:10.1016/0022-0981(94)90031-0 [Google Scholar]
- Stobutzki I.C, Bellwood D.R. Sustained swimming abilities of the late pelagic stages of coral reef fishes. Mar. Ecol. Prog. Ser. 1997;149:35–41. [Google Scholar]
- Val A.L, Silva M.N.P, Almeida-Val V.M.F. Hypoxia adaptation in fish of the Amazon: a never-ending task. S. Afr. J. Zool. 1998;33:107–114. [Google Scholar]