Abstract
Viral diseases are a key constraint in the production of staple food crops in lesser developed countries. New and improved disease control methods are developed and implemented without consideration of the selective pressure they impose on the virus. In this paper, we analyse the evolution of within-plant virus titre as a response to the implementation of a range of disease control methods. We show that the development of new and improved disease control methods for viral diseases of vegetatively propagated staple food crops ought to take the evolutionary responses of the virus into consideration. Not doing so leads to a risk of failure, which can result in considerable economic losses and increased poverty. Specifically in vitro propagation, diagnostics and breeding methods carry a risk of failure due to the selection for virus strains that build up a high within-plant virus titre. For vegetatively propagated crops, sanitation by roguing has a low risk of failure owing to its combination of selecting for low virus titre strains as well as increasing healthy crop density.
Keywords: evolutionary ecology, plant disease virus, mathematical model, cassava mosaic virus, sweet potato virus disease
1. Introduction
Diseases caused by plant viruses in cassava (Manihot esculenta), sweet potato (Ipomoea batatas) and plantain (Musa spp.) are among the main constraints to sustainable production of these vegetatively propagated staple food crops in lesser developed countries (Dahal et al. 1997; Rybicki & Pietersen 1999; Gibson & Aritua 2002; Thresh & Cooter 2005). New viral strains frequently emerge (Rybicki & Pietersen 1999; Boulton 2003; Varma & Malathi 2003; Seal et al. 2006); some with devastating consequences, such as the current pandemic of a new strain of the virus causing cassava mosaic disease (CMD) in Africa (Gibson et al. 1996).
Large investments are underway (Anonymous 2005a,b; Barnett et al. 2005) to alleviate poverty and malnutrition by developing new or more effective disease control strategies. These strategies include plant breeding for resistance to the virus (Fargette et al. 1996; Aritua et al. 1998; Morales 2001; Lapidot & Friedmann 2002; Diaz-Pendon et al. 2004; Ariyo et al. 2005; Thresh & Cooter 2005), the control of vectors (Palumbo et al. 2001), as well as crop sanitation through removal of diseased plants (Gibson & Aritua 2002; Thresh & Cooter 2005) and improved selection/production of planting material (Thro et al. 1999; Feng et al. 2000; Monger et al. 2001; Gibson & Aritua 2002; Alleman et al. 2004) for these vegetatively propagated crops.
In the context of staple crops, surprisingly little attention has been given to the inadvertent selection for increased virulence by disease control, although disease control is known to exert selection pressure on pathogen populations (Dieckmann et al. 2002). For example, the manner in which the virus titre builds up in the plant during systemic infection is known to vary widely between strains (Varma & Malathi 2003; Jimenez-Martinez & Bosque-Perez 2004; Seal et al. 2006) and selection imposed by new or more effective disease control can select strains that build up a higher virus titre. Low virus titre is associated with low symptom severity, which in turn makes it more likely that infected plants are not recognized and are used for vegetative propagation. Vectored virus transmission is more likely at higher within-plant virus titre. By affecting transmission routes in different ways, it follows that alternative strategies for the control of disease may lead to the selection of virus strains, which build up to different virus titres in the plant. Thus, the rationale for this paper is that disease control could favour strains building up a higher virus titre with an associated increase in plant damage. Here, we consider the evolution of virus titre for a given cultivar–virus combination under constant conditions, in which we assume that virus titre is monotonically related to plant damage. This is a simplification of natural systems, but it serves to illustrate the underlying principles of how inadvertent selection of pathogens can occur and hence the need to take this form of selection pressure into consideration before implementation of wide scale disease control methods.
In this paper, we use a theoretical evolutionary ecology approach (Dieckmann et al. 2002) to show which disease control strategies lead to the evolution of strains with increased virus titre in the plant. From this, we identify strategies that are suited to sustainable crop production and those for which there is a risk of failure. Specifically, we use a combination of an epidemiological model together with the analysis of evolutionary stable strategies (ESSs; Maynard Smith 1982; Eshel 1983) to analyse the differential effects of disease control on the evolution of virus titre in vegetatively propagated crops. We show how this approach, linking transmission mode and disease control with virus titre, can be applied to an example of disease control in staple food crops to identify strategies with high or with low risk of failure. Whether or not a particular strategy for control of plant viruses is implemented by farmers depends on a wide range of socio-economic and cultural drivers (Thresh & Cooter 2005) in addition to the effectiveness of control. Nevertheless, we contend that in allowing an assessment of the risk of long-term failure of control, the methods developed in this paper have an important role to play in the selection and implementation of control strategies for virus diseases of vegetatively propagated crops.
Our work is of a generic nature and applies to a wide range of vegetatively propagated crops, but we use CMD of cassava and sweet potato virus disease (SPVD) of sweet potato as exemplars. Both cassava and sweet potato are major staple food crops in many parts of the tropics. CMD, a major constraint to cassava production in Africa, India and Sri Lanka, is caused by viruses of the genus Begomovirus (family Geminiviridae; Thresh & Cooter 2005, and references therein). These viruses are transmitted: (i) by the whitefly Bemisia tabaci (Homoptera: Aleyrodidae), a polyphagous herbivorous insect feeding on phloem sap and (ii) through cuttings from infected plants used to establish a new crop. CMD causes characteristic leaf symptoms which are easily recognized by farmers and there is a general relationship between symptom severity and decrease in yield (Thresh & Cooter 2005). SPVD is caused by dual infections of the whitefly (B. tabaci) borne sweet potato chlorotic stunt virus (SPCSV; Crinivirus; Closteroviridae) and the aphid (Myzus persicae, Aphis gossypii) borne sweet potato feathery mottle virus (SPFMV; Potyvirus; Potyviridae; Gibson et al. 2004). The disease is also transmitted through cuttings used for planting (see also §4 for further notes on these diseases).
2. Material and methods
(a) Epidemiological model
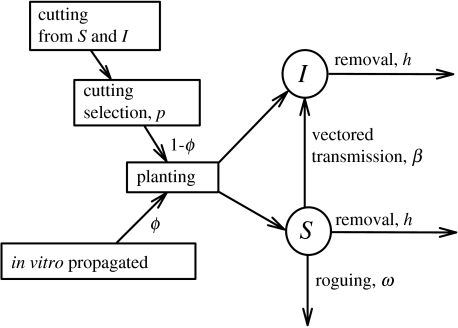
The epidemiological model is formulated as a SIR model for the change in disease status of plants from susceptible, S, to infected, I, and removed, R. Removal occurs by death or by sanitation (figure 1). New susceptible plants enter the system by continual replanting at rate, σ. The crop is planted from in vitro propagated virus-free material, from cuttings taken from a previous crop or from a combination of these methods. Some cuttings from infected plants may be healthy due to reversion (an ill chosen word, signifying the virus becoming only partially systemic when virus titre is low) with probability r. Cuttings are selected visually or using diagnostic methods, and discarded with probability p, if recognized to be infected. Crop plants are removed from the system after 1/η time units, which is either harvest time or the end of their productive lifetime. Infectives derive either from infective cuttings or through virus transmission by insect vectors. Vectored transmission is governed by the transmission rate, β. Roguing removes infected plants from the system at rate, ω. This leads to (table 1, figure 1) the model equations:
the internal steady state (S*, I*) of this system of differential equations exists if R0>1, with . The stability of the internal steady state is checked by simulation.
Figure 1.
Epidemiology of virus diseases in crops propagated by cuttings from previous crop or by in vitro methods. The circled letters represent the state variables, density of healthy, S, and infected, I, plants. Symbols are explained in table 1 and the text.
Table 1.
Model parameters, their interpretation and default value.
| parameter | description | default value |
|---|---|---|
| η | harvest rate | 0.002a |
| σ | planting rate | 0.0015b |
| ϕ | fraction planted from in vitro propagated, virus free, material | 1.0–0.0c |
| xhalf | cutting selection parameter | ∞d |
| rs | reversion parameter | 10.0e |
| ωs | roguing rate parameter | 0.005f |
| βs | transmission rate parameter | 0.025g |
Holt & Chancellor (1997) use 0.002–0.004 d−1 as default range for cassava. Sweet potato has a similar cropping period as cassava and η=0.002 is an appropriate value. However, the results presented here do not change qualitatively for this change in parameter value.
Planting density of cassava is 0.5 plants m−2 (Holt & Chancellor 1997). The maximum density in the absence of disease is σ/η. Thus σ=0.0015. For sweet potato, the planting density is 4 plants m−2 giving σ=0.015 (Gibson & Aritua 2002). We use the values for the cassava and note here that the results presented do not change qualitatively with larger values of σ.
African small holders replant cassava and sweet potato with cuttings only from previous crop. Commercial sweet potato growers in China use disease-free material from in vitro propagation programmes (Feng et al. 2000). This parameter thus varies between 0 and 1.
African small holders do not apply cutting selection in cassava on a standard basis. For sweet potato, some cutting selection takes place. Farmers recognize 10–50% of the plants that are infected (Aritua et al. 1998; Gibson & Aritua 2002). Around 40% of the farmers realize that the disease can be transmitted by cuttings (Aritua et al. 1998). This parameter thus has values between ∞ and approximately 0.7. In our analysis, we vary this parameter between 0 and ∞.
For the default parameter set, xESS-default=0.255, which using rs=10 implies that the probability for a cutting from an infected plant to be reversed is exp (−0.255×10)≈0.08. Fondong et al. (2000) measured 5–40% reversion depending on the susceptibility of the cultivar and our parameter value rs=10 falls in this range. For sweet potato, reversion has not been described in the literature.
No quantitative data available. Roguing is applied in some places in cassava and sweet potato. Collaborators of the tropical whitefly IPM project have observed small holder farmers in Africa pulling out plants exhibiting symptoms of infection. Further young plants that are infected are known to grow slowly, and therewith are overgrown by healthy plants (compensatory growth). For a value of ωs=0.005, for xESS=0.255, ca 10%, , of the infected plants die or are removed before harvest.
For xESS=0.255 and using our default value of βs=0.025, we find β=0.0064. This is a realistic value for both cassava and sweet potato. Cassava, there are around 50 whitefly per plant, V, (Legg 1995) inoculation rate is 0.008, λ1, (Holt & Chancellor 1997) acquisition rate is 0.008, λ2, (Holt & Chancellor 1997) duration of viruliferous period is 4.5 days, δ(1/(rate of leaving viruliferous state (0.1, Dubern 1994)+ insect death rate (0.12; Holt & Chancellor 1997))). β is equal to V(σ/η) λ1 λ2 δ≈0.007. Sweet potato: experiments from Gibson et al. (2004) show a rate of increase, r, of 0.024 per day. In the model dI/dt=βSI−ηI which gives an initial rate of increase of r=βS−η, where S=σ/η. Substituting all other default parameter value yields β=0.0064.
(b) Evolving trait
The within-plant virus titre varies widely among virus strains (Varma & Malathi 2003; Jimenez-Martinez & Bosque-Perez 2004; Seal et al. 2006), making it possible to analyse the effects of the selection pressure associated with different disease control strategies on the evolution of virus titre in these virus populations. Thus, the evolving trait is the within-plant virus titre, x, that builds up in the plant with systemic infection.
(c) Model parameters and their relation to virus titre (figure 2)
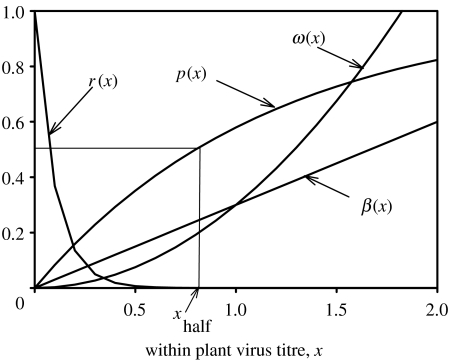
Figure 2.
The dependence of four epidemiological parameters on the within-plant virus titre. r(x) is the probability that a cutting taken from an infected crop plant does not carry the virus (is reversed), p(x) is the probability that an infected cutting is correctly detected as infected, ω(x) is the roguing rate and β(x) is the transmission rate. xhalf is the virus titre at which half of the infected cuttings are correctly detected as infected.
Virus titre influences key epidemiological parameters notably the transmission rate, plant death rate and reversion (Foxe & Rochow 1975; Jimenez-Martinez & Bosque-Perez 2004). The virus titre also interacts with disease control by affecting symptom severity, which affects the roguing rates and the detection rates of infected cuttings. The dependence of the transmission rate, β, the roguing rate, ω, the probability of detecting an infected cutting, p and the reversion ratio, r, on within-plant virus titre, x, are modelled as β(x)=βsx, ω(x)=ωsx2, p(x)=1−exp (−(ln (2)/xhalf)x) and r(x)=exp (−rsx), respectively (figure 2). No experimental data exist to underpin the functions r(x), p(x) and ω(x), and these were chosen to reflect the accepted common wisdom of increasing roguing rate, increasing probability of detecting an infected cutting and decreasing reversion with increasing virus titre. The only well-studied example of the relationship between transmission rate and virus titre (Escriu et al. 2000) relates to cucumber mosaic virus transmitted by A. gossypii and shows a saturating transmission rate for larger virus titres. However, note that this does not contradict our assumption of a linear relation between transmission rate and x, as x can be scaled to match the relation of Escriu et al. (2000) without changing the qualitative behaviour of the other functions. Reduction of the roguing rate corresponds to reducing the parameter ωs, and reducing the transmission rate corresponds to reducing the parameter βs. The parameter xhalf is the virus titre for which 50% of the infected cuttings are recognized as such, and we use xhalf to parametrize the accuracy of screening cuttings, with large values of xhalf representing low-accuracy screening. Note that the precise form of the relationships is not important for the qualitative relationships for the ESS for virus titre (xESS) demonstrated here, provided the death rate ω(x) increases faster than the transmission rate β(x) at least for higher values of plant virus titre.
(d) Mapping disease control onto model parameters
To study the effect of disease control on the evolution of the virus titre, we related each disease control method to its effect on parameter values of the model. The introduction of in vitro propagated planting material affects the parameter, ϕ, the fraction planted with propagated material (figure 1). The accuracy of cutting selection is parametrized by the virus titre at which 50% of the infected cuttings are recognized as infected, xhalf (figure 2). More efficient roguing programmes will cause the roguing rate ω to increase more steeply with virus titre (figure 2). Methods controlling transmission of the virus by the vector result in a smaller value of the transmission rate parameter, β (figure 2). The value of this parameter also describes the introduction of resistance that reduces the acquisition of the virus by vectors feeding on infected plants and/or the reduction of the inoculation of healthy plants by viruliferous vectors, which will be termed transmission resistance. The cultivars that express less severe symptoms for a given virus titre, which we term here tolerance (Restif & Koella 2004), are described by a reduced value of the parameter ω (figure 2).
(e) Parameter values
The parameter values, given and motivated in table 1, are chosen to represent the CMD and the SPVD pathosystems.
(f) Evolutionary dynamics
Assume that the resident virus population builds up virus titre x*. The fitness of an invader building up titre x, W(x, x*), is given by
where S* and I* are the steady-state densities of S and I when x=x*. The mutant invader will spread when W>0 and will die out when W<0. The ESS value of x, xESS, is calculated from W(xESS, xESS)=0 and (∂W/∂x)=0 at x=x*=xESS (Maynard Smith 1982). An ESS is attracting; it is a continuously stable strategy (CSS; Eshel 1983), when W(x, x*)>0 for x∈(x*, xESS) when x*<xESS, and W(x, x*)>0 for x∈(xESS, x*) when x*>xESS. The criteria for ESS and CSS were evaluated numerically.
3. Results and discussion
Our work shows that there is an ESS (Maynard Smith 1982) for the virus titre. This corresponds to the value of the virus titre that if the resident virus strain builds up this ESS titre, no other virus strains causing a different titre can invade. Our results also imply that the evolutionary endpoint of the system (Eshel 1983) is the virus strain associated with the ESS virus titre. The evolutionary stable virus titre and the healthy plant density at this stable state are plotted in figures 3 and 4 as functions of the parameters describing the disease control options.
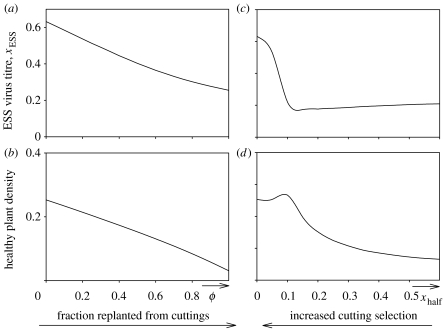
Figure 3.
(a and b) The evolutionary stable virus titre, xESS, and the density of healthy plants as a function of the fraction of the crop planted from in vitro propagated and virus free material, ϕ and (c and d) as function of the virus titre at which half of the infected cuttings is correctly detected as infected, xhalf.
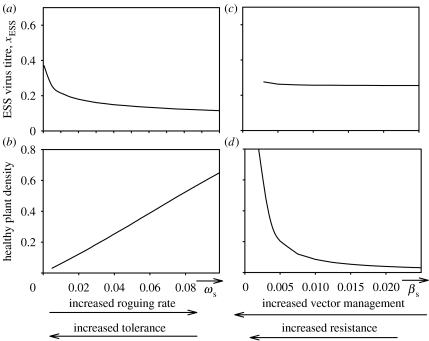
Figure 4.
(a and b) The evolutionary stable virus titre, xESS, and the density of healthy plants as a function of the fraction of the parameter describing the increase in roguing rate with virus titre, ωs, and (c,d) as a function of the parameter describing the increase of transmission rate with virus titre, βs. The parameters ωs and βs are defined in §2c.
(a) In vitro propagation
In vitro propagation programmes for sweet potato (Feng et al. 2000) and cassava (Thro et al. 1999; Alleman et al. 2004) are being developed, but the consequences of the selection that these impose on the virus have not so far been considered. Our results show that although the introduction of in vitro propagated material increases the density of healthy plants (figure 3b), it also selects for virus strains that build up an increased titre in infected plants (figure 3a). It follows that the robustness of this strategy as a means of sustainable disease control depends upon balancing the advantage in generating more healthy plants with the risks associated with higher virus titres. This risk depends upon the occurrence of higher virus titre strains within the pathogen population, but if they arise our model shows that they will be selected.
(b) Cutting selection
Selection of asymptomatic planting material is routinely practised by visual assessment in sweet potato (Gibson & Aritua 2002) while visual inspection of cuttings of cassava plants has been suggested but is not widely adopted (Thresh & Cooter 2005). Several projects are also developing molecular diagnostic techniques to detect virus infection efficiently in the pre-symptomatic stage (Monger et al. 2001). Presently used for research, these methods might be of value in the selection of virus-free cuttings for sweet potato crops in China, but will very likely not be cost effective in subsistence systems in Africa. Low-accuracy screening of cuttings (xhalf large), whether by visual assessment or by other forms of diagnosis, imposes selection on the virus to evolve strains that build up a slightly lower titre (figure 3c, where accurate screening corresponds to smaller xhalf values). Healthy plant density increases under this low-accuracy screening (figure 3d). However, if the accuracy of the screening is high (xhalf small), this disease control method imposes selection on the virus to evolve strains associated with high virus titres. Moreover, this occurs without the increase in healthy plant density associated with low-accuracy screening. Again, we note that the risk of failure of this disease control strategy depends upon the occurrence of higher virus titre strains within the virus population. We conclude that the risk of failure of the introduction of cutting selection is large when selection is accurate.
(c) Sanitation
Roguing of infected plants is a well-known disease control method, though less widely used, for virus disease control in cassava and sweet potato. Several studies have shown this disease control to be effective in reducing the density of diseased plants (Thresh & Cooter 2005), although the evolutionary response of the virus has not been considered. Our results show that roguing selects for virus strains associated with a lower virus titre as well as increasing the density of healthy plants (figure 4a,b). Introduction of disease control by roguing thus has a small risk of failure, but widespread adoption of the technique will depend upon farmer perceptions of the value of the technique for efficient and long-term disease control relative to logistical and cost constraints associated with organizing labour (Thresh & Cooter 2005).
(d) Breeding for resistance and tolerance
Breeding for resistance is widely advocated as the key strategy for disease control in cassava and sweet potato (Fargette et al. 1996; Aritua et al. 1998; Morales 2001; Lapidot & Friedmann 2002; Diaz-Pendon et al. 2004; Ariyo et al. 2005; Thresh & Cooter 2005). Although at present there is little evidence of resistance breakdown (Thresh & Cooter 2005) in cassava and sweet potato, the appearance of virulent virus strains is a problem in many crops worldwide as it can render the resistance redundant. Our results show that resistance reducing transmission of infection does not impose selection on the virus to evolve strains, which build up a different virus titre and, at the same time, increases the density of healthy plants (figure 4c,d) and in this sense, the risk of failure of the introduction of this type of resistance is small. The situation is different for tolerance (in which plants with high virus titre remain symptomless), with a risk of failure due to the selection for strains with higher virus titres, as well as a reduction in the density of healthy plants (figure 4a,b).
(e) Vectors
Control of vectors does not put a selection pressure on the virus to evolve to higher within-plant virus titre and at the same time increases the density of healthy plants (figure 4c,d). Thus, vector control has a small risk of failure due to selection for more damaging virus strains. However, the reduction of vector populations in the field has proven to be difficult and is, as yet, seldom used in cassava and sweet potato (Palumbo et al. 2001; Thresh & Cooter 2005). Other methods to control vector populations efficiently, but not leading to environmental damage, can however be a useful component in the development of sustainable disease control (Thresh & Cooter 2005).
(f) Notes on the exemplar diseases
Originally two virus species causing CMD were recognized in Africa, named East African mosaic virus and African cassava mosaic virus, which are geographically separated (Swanson & Harrison 1994). The recent highly damaging (Legg & Thresh 2000) Uganda variant (EACMV-UG) is an interspecific recombinant strain of the East African mosaic virus (Zhou et al. 1997). All our parameter estimates relate to the African cassava mosaic virus. SPVD in Africa is caused by dual infection with SPCSV and SPFMV (Brown et al. 1995). Although an oversimplification of the situation for this particular disease complex, we considered dual infection by these two viruses to be causal and estimated parameters on this basis. We note here again that our work is of a generic nature and applies to a wide range of vegetatively propagated crops and these two are exemplars only. Changing the parameter values (over a wide range around the default values, +100% and −100%) influences the quantitative behaviour of the model, but does not alter the qualitative effects of disease management on the evolution of virus titre and the associated response on healthy plants.
Our results show that it depends on the precise mechanisms through which resistance is expressed whether or not introduction of crop resistance has a risk of failure due to selection for high titre strains. However, we note here that our study is restricted to a subset of the possible mechanisms. For cassava and sweet potato, some resistant cultivars are known to suppress virus multiplication rate or within-plant virus movement (Thresh & Cooter 2005). These types of resistance cannot be properly accommodated in the present model structure and further research is needed in this area. For tomato (Solanum lycopersicon) crops resistance to tomato yellow leaf curl virus, a preliminary study was done by Van den Bosch et al. (2006).
Our results show that in the long term, only one strain building up the ESS virus titre will be present in the system. It is well known that under field conditions, a mixture of virus strains is found for both diseases. The model in its simplicity necessarily describes a homogeneous system with no variability in disease control, no variability in parameter values between sites or years and no influence of possible other host species. Under field conditions, this variability exists and facilitates the presence of a number of strains. The ESS then refers to the virus titre that will allow a strain to become the dominant and most sustainable one. However, also note that strains are often distinguished on the basis of genetic, molecular or epidemiological characteristics, which have no relation with virus titre build up. The grouping of these strains by virus titre may well reveal only a small number of groups to be present.
(g) The feasibility of virulence management
The practical feasibility of virulence management has been challenged by Ebert & Bull (2003). They question the existence of trade-off relations between transmission and longevity. Their main concern is that such trade-offs are simply assumed to exist without further knowledge about the mechanisms generating the trade-off. As discussed in Alizon & van Baalen (2005), and references they cite, this criticism is not entirely justified in many cases, and in our case this criticism simply does not apply. The virus titre and its well-documented effects on symptom severity, acquisition rate and reversion constitute the mechanism required by Ebert & Bull (2003) to justify the use of a trade-off relation.
Whether or not the rate of change and the magnitude of the change in pathogen virulence induced by disease management measures are large enough to be of practical relevance (Ebert & Bull 2003) is a point of concern also for the work presented in this paper. Recently, Day & Proulx (2004) developed a methodology to study short-term evolutionary trends using a quantitative genetics approach, which could be useful in estimating rates, and we envisage further development of our work in this direction. Given that different virus strains are known to cause different virus titres, we are less concerned in our case about the magnitude of the possible changes in virulence.
In summary, the concerns expressed by Ebert & Bull (2003) apply to ESS model approaches in general and thus apply as well to the work presented here. However, in the model presented, the existence of a trade-off and the practical relevance of the magnitude of the shift in virulence are, to our opinion, not major concerns.
4. Concluding remarks
The transmission of a virus through the use of infected cuttings from the previous crop as planting material and transmission through a herbivorous insect vector map onto vertical and horizontal disease transmission modes, respectively. Lipsitch et al. (1996) showed that the classical view (Ewald 1987; Ewald & Schubert 1989) that horizontal transmission promotes high pathogen virulence and vertical transmission promotes low pathogen virulence is not generally correct. They showed that the effect of transmission mode on virulence critically depends on the mechanisms responsible for transmission in combination with the trade-offs operating in the system under consideration. Therefore, to determine the effect of various disease control methods on the selection of virus strains in these vegetatively propagated crops, we extended their work by analysing the underlying routes of virus transmission and the extent to which these can be adjusted by practical plant disease control. In so doing, we studied the consequences of disease control on the evolution of the virus.
Specifically, we have shown that research aimed at the development of new or improved disease control has to take into account the selection imposed on the virus and the consequences of evolutionary responses of the virus on crop damage. Failure to recognize this may lead to considerable economic losses and increased poverty. Specifically, in vitro propagation and diagnostic methods presently receiving a great deal of attention (Thro et al. 1999; Feng et al. 2000; Monger et al. 2001; Alleman et al. 2004) have a risk of failure due to the selection of virus strains building up a high within-plant virus titre. Moreover, we have shown that sanitation by roguing is not associated with such a risk, because this disease control method selects for low virus titre strains as well as increasing healthy crop density. We have also shown that breeding for resistance, as opposed to tolerance, does not have the risk of failure due to select for high virus titre.
Acknowledgments
This work was funded by the Crop Protection Programme (CPP) of the Department for International Development (DFID). Rothamsted Research and CAG receive support from the Biotechnology and Biological Research Council (BBSRC) of the United Kingdom.
References
- Alleman J, Laurie S.M, Thiart S, Vorster H.J. Sustainable production of root and tuber crops (potato, sweet potato, indigenous potato, cassava) in southern Africa. S. Afr. J. Bot. 2004;70:60–66. [Google Scholar]
- Alizon S, van Baalen M. Emergence of a convex trade-off between transmission and virulence. Am. Nat. 2005;165:E155–E167. doi: 10.1086/430053. doi:10.1086/430053 [DOI] [PubMed] [Google Scholar]
- Anonymous 2005a Tropical whitefly IPM Project. http://www.tropicalwhiteflyipmproject.cgiar.org/ [DOI] [PubMed]
- Anonymous 2005b Rockefeller foundation http://www.rockfound.org/
- Aritua V, Alicai T, Adipala E, Gibson R.W. Aspects of resistance to sweet potato virus disease in sweet potato. Ann. Appl. Biol. 1998;132:387–398. [Google Scholar]
- Ariyo O.A, Dixon A.G.O, Atiri G.I. Whitefly Bemisia tabaci (Homoptera: Aleyrodidae) infestation on cassava genotypes grown at different ecozones in Nigeria. J. Econ. Entomol. 2005;98:611–617. doi: 10.1093/jee/98.2.611. [DOI] [PubMed] [Google Scholar]
- Barnett, T., et al 2005 Foresight infectious diseases in Africa: using science to fight the evolving threat. Office of Science and Technology, DTI/Pub 7735/0.2k/02/05/NP. Available at http://www.foresight.gov.uk
- Boulton M.I. Geminiviruses: major threats to world agriculture. Ann. Appl. Biol. 2003;142:143. doi:10.1111/j.1744-7348.2003.tb00239.x [Google Scholar]
- Brown J.K, Frohich D.R, Rosell R.C. The sweetpotato or silverleaf whiteflies: biotypes of Bemisia tabaci or a species complex? Annu. Rev. Entomol. 1995;40:511–534. doi:10.1146/annurev.en.40.010195.002455 [Google Scholar]
- Dahal G, Hughes J.d'A, Thottappilly G, Lockhart B.E.L. Status of banana streak badnavirus in Africa: problems and future research needs. Integr. Pest Manag. Rev. 1997;3:1–13. [Google Scholar]
- Day T, Proulx S.R. A general theory for the evolutionary dynamics of virulence. Am. Nat. 2004;163:E40–E63. doi: 10.1086/382548. doi:10.1086/382548 [DOI] [PubMed] [Google Scholar]
- Diaz-Pendon J.A, Truniger V, Nieto C, Garcia-Mas J, Bendahmane A, Aranda M.A. Advances in understanding recessive resistance to plant viruses. Mol. Plant Pathol. 2004;5:223–233. doi: 10.1111/j.1364-3703.2004.00223.x. doi:10.1111/j.1364-3703.2004.00223.x [DOI] [PubMed] [Google Scholar]
- Dieckmann U, Metz J.A.J, Sabelis M.W, Sigmund K, editors. Adaptive dynamics of infectious diseases: in pursuit of virulence management. Cambridge University Press; Cambridge, UK: 2002. [Google Scholar]
- Dubern J. Transmission of African cassava mosaic geminivirus by whitefly (Bemisia tabaci) Trop. Sci. 1994;34:82–91. [Google Scholar]
- Ebert D, Bull J.J. Challenging the trade-off model for the evolution of virulence: is virulence management feasible? Trends Microbiol. 2003;11:15–20. doi: 10.1016/s0966-842x(02)00003-3. doi:10.1016/S0966-842X(02)00003-3 [DOI] [PubMed] [Google Scholar]
- Escriu F, Perry K.L, Garcia-Arenal F. Transmissibility of cucumber mosaic virus by Aphis gossypii correlates with viral accumulation and is affected by the presence of its satellite RNA. Phytopathology. 2000;90:1068–1072. doi: 10.1094/PHYTO.2000.90.10.1068. [DOI] [PubMed] [Google Scholar]
- Eshel I. Evolutionary and continuous stability. J Theor. Biol. 1983;103:99–111. doi: 10.1006/jtbi.1996.0312. doi:10.1016/0022-5193(83)90201-1 [DOI] [PubMed] [Google Scholar]
- Ewald P.W. Transmission modes and evolution of the parasitism–mutualism continuum. Ann. NY Acad. Sci. 1987;503:295–306. doi: 10.1111/j.1749-6632.1987.tb40616.x. [DOI] [PubMed] [Google Scholar]
- Ewald P.W, Schubert J. Vertical and vector-borne transmission of insect endocytobionts, and the evolution of the begnigness. In: Schwemmler W, editor. CRC handbook of insect endocytobiosis: morphology, physiology, genetics and evolution. CRC Press; Boca Raton, FL: 1989. pp. 21–35. [Google Scholar]
- Fargette D, Colon L.T, Bouveau R, Fauquet C. Components of resistance of cassava to African cassava mosaic virus. Eur. J. Plant Pathol. 1996;102:645–654. doi:10.1007/BF01877245 [Google Scholar]
- Feng G, Yifu G, Pinbo Z. Production and development of virus-free sweet potato in China. Crop Prot. 2000;19:105–111. doi:10.1016/S0261-2194(99)00085-X [Google Scholar]
- Fondong V.N, Thresh J.M, Fauquet C. Field experiments in Cameroon on cassava mosaic virus disease and the reversion phenomenon in susceptible and resistant cassava cultivars. Integr. J. Pest Manag. 2000;46:211–217. doi:10.1080/096708700415553 [Google Scholar]
- Foxe M.J, Rochow W.F. Importance of virus source leaves in vector specificity of barley yellow dwarf virus. Phytopathology. 1975;65:1124–1129. [Google Scholar]
- Gibson R.W, Aritua V. The perspective of sweet potato chlorotic stunt virus in sweet potato production in Africa, a review. Afr. Crop Sci. J. 2002;10:281–310. [Google Scholar]
- Gibson R.W, Aritua V, Byamukama E, Mpembe I, Kayongo J. Control strategies for sweet potato virus disease in Africa. Virus Res. 2004;100:115–122. doi: 10.1016/j.virusres.2003.12.023. doi:10.1016/j.virusres.2003.12.023 [DOI] [PubMed] [Google Scholar]
- Gibson R.W, Legg J.P, Otim-Nape G.W. Unusually severe symptoms are a characteristic of the current epidemic of mosaic virus disease of cassava in Uganda. Ann. Appl. Biol. 1996;128:479–490. [Google Scholar]
- Holt J, Chancellor T.C.B. A model of plant virus disease epidemics in asynchronously-planted cropping systems. Plant Pathol. 1997;46:490–501. doi:10.1046/j.1365-3059.1997.d01-36.x [Google Scholar]
- Jimenez-Martinez E.S, Bosque-Perez N.A. Variation in barley yellow dwarf virus transmission efficiency by Rhopalosiphum padi (Homoptera: Aphididae) after acquisition from transgenic and nontransformed wheat genotypes. J. Econ. Entomol. 2004;97:1790–1796. [PubMed] [Google Scholar]
- Lapidot M, Friedmann M. Breeding for resistance to whitefly-transmitted geminiviruses. Ann. Appl. Biol. 2002;140:109–127. doi:10.1111/j.1744-7348.2002.tb00163.x [Google Scholar]
- Legg, J. P. 1995 The ecology of Bemisia tabaci (Gennardius, Homoptera: Alerodidae), vector of African cassava mosaic geminivirus in Uganda. Ph.D. thesis, University of Reading, UK.
- Legg J.P, Thresh J.M. Cassava mosaic virus disease in East Africa: a dynamic disease in a changing environment. Virus Res. 2000;71:135–149. doi: 10.1016/s0168-1702(00)00194-5. doi:10.1016/S0168-1702(00)00194-5 [DOI] [PubMed] [Google Scholar]
- Lipsitch M, Siller S, Nowak M.A. The evolution of virulence in pathogens with vertical and horizontal transmission. Evolution. 1996;50:1729–1741. doi: 10.1111/j.1558-5646.1996.tb03560.x. doi:10.2307/2410731 [DOI] [PubMed] [Google Scholar]
- Maynard Smith J. Cambridge University Press; Cambridge, UK: 1982. Evolution and the theory of games. [Google Scholar]
- Monger W.A, Seal S, Cotton S, Foster G.D. Identification of different isolates of Cassava brown streak virus and development of a diagnostic test. Plant Pathol. 2001;50:768–775. doi:10.1046/j.1365-3059.2001.00647.x [Google Scholar]
- Morales F.J. Conventional breeding for resistance to Bemisia tabaci-transmitted geminiviruses. Crop Prot. 2001;20:825–834. doi:10.1016/S0261-2194(01)00114-4 [Google Scholar]
- Palumbo J.C, Horowitz A.R, Prabhaker N. Insecticidal control and resistance management of Bemisia tabaci. Crop Prot. 2001;20:739–765. doi:10.1016/S0261-2194(01)00117-X [Google Scholar]
- Restif O, Koella J.C. Concurrent evolution of resistance and tolerance to pathogens. Am. Nat. 2004;164:90–102. doi: 10.1086/423713. doi:10.1086/423713 [DOI] [PubMed] [Google Scholar]
- Rybicki E.P, Pietersen G. Plant virus disease problems in the developing world. Virus Res. 1999;53:127–175. doi: 10.1016/s0065-3527(08)60346-2. [DOI] [PubMed] [Google Scholar]
- Seal S.E, van den Bosch F, Jeger M.J. Factors influencing Begomovirus evolution and their increasing global significance: implications for sustainable control. Crit. Rev. Plant Sci. 2006;25:23–46. doi:10.1080/07352680500365257 [Google Scholar]
- Swanson M.M, Harrison B.D. Propeties, relationships and distribution of cassava mosaic geminiviruses. Trop. Sci. 1994;34:15–25. [Google Scholar]
- Thresh J.M, Cooter R.J. Strategies for controlling cassava mosaic disease in Africa. Plant Pathol. 2005;54:587–614. doi:10.1111/j.1365-3059.2005.01282.x [Google Scholar]
- Thro A.M, Roca W.M, Restrepo J, Caballero H, Poats S, Escobar R, Mafla G, Hernandez C. Can in vitro biology have farm-level impact for small-scale cassava farmers in Latin America? In Vitro Cell. Dev. Biol. Plant. 1999;35:382–387. [Google Scholar]
- Van den Bosch F, Akudibilah G, Seal S, Jeger M. Host resistance and the evolutionary response of plant viruses. J. Appl. Ecol. 2006;43:506–516. doi:10.1111/j.1365-2664.2006.01159.x [Google Scholar]
- Varma A, Malathi V.G. Emerging geminivirus problems: a serious threat to crop production. Ann. Appl. Biol. 2003;142:145–164. doi:10.1111/j.1744-7348.2003.tb00240.x [Google Scholar]
- Zhou X, Liu Y, Calvert L, Munoz D, Otim-Nape G.W, Robinson D.J, Harrison B.D. Evidence that DNA-A of a geminivirus associated with severe cassava mosaic disease in Uganda has arisen by interspecific recombination. J. Gen. Virol. 1997;78:2101–2111. doi: 10.1099/0022-1317-78-8-2101. [DOI] [PubMed] [Google Scholar]