Abstract
Manipulative parasites can alter the phenotype of intermediate hosts in various ways. However, it is unclear whether such changes are just by-products of infection or adaptive and enhance transmission to the final host. Here, we show that the alteration of serotonergic activity is functionally linked to the alteration of specific behaviour in the amphipod Gammarus pulex infected with acanthocephalan parasites. Pomphorhynchus laevis and, to a lesser extent, Pomphorhynchus tereticollis altered phototactism, but not geotactism, in G. pulex, whereas the reverse was true for Polymorphus minutus. Serotonin (5-hydroxytryptamine, 5-HT) injected to uninfected G. pulex mimicked the altered phototactism, but had no effect on geotactism. Photophilic G. pulex infected with P. laevis or P. tereticollis showed a 40% increase in brain 5-HT immunoreactivity compared to photophobic, uninfected individuals. In contrast, brain 5-HT immunoreactivity did not differ between P. minutus-infected and uninfected G. pulex. Finally, brain 5-HT immunoreactivity differed significantly among P. tereticollis-infected individuals in accordance with their degree of manipulation. Our results demonstrate that altered 5-HT activity is not the mere consequence of infection by acanthocephalans but is specifically linked to the disruption of host photophobic behaviour, whereas the alteration of other behaviours such as geotactism may rely on distinct physiological routes.
Keywords: host manipulation by parasites, phototactism, geotactism, intermediate amphipod host
1. Introduction
Parasites with complex life cycles can manipulate the physiology and the behaviour of their intermediate hosts in ways that enhance their own transmission to final hosts (Moore 2002; Thomas et al. 2005). Although evidence of manipulation exists for various behaviours (Moore 2002), the precise mechanisms by which a parasite can interfere with its intermediate host's neuronal function to cause change in behaviour are particularly difficult to identify. However, there is growing evidence that the biogenic amine serotonin (5-hydroxytryptamine, 5-HT) is involved in several host–parasite systems in which infected hosts show altered behaviour. 5-HT has been implicated in the altered phenotype of amphipod hosts infected with both acanthocephalan and trematode parasites (Helluy & Holmes 1990; Maynard et al. 1996; Helluy & Thomas 2003; Poulin et al. 2003) as well as in sticklebacks parasitized by a tapeworm larva (Overli et al. 2001) and rodents infected with nematodes (Terenina et al. 1997). However, it remains unclear whether change in serotonergic activity is a general feature of a host infected by manipulative parasites or is linked to the alteration of specific behaviours in relation to the trophic transmission of the parasite to its final host.
Invertebrates present several advantages for neuroethological studies of host manipulation by parasites as they have simpler nervous systems and more stereotyped behaviours than vertebrates (Helluy & Holmes 2005). The freshwater amphipod Gammarus pulex (crustacean) is regularly infected with acanthocephalan parasites that use either fish or birds as final hosts. Previous studies (Bakker et al. 1997; Cézilly et al. 2000) have demonstrated specific changes in the behaviour of infected gammarids in relation to the type of final host. Such modified behaviours are observed only after the cystacanths have become infective to the definitive host (F. Cézilly & M.-J. Perrot-Minnot 2006, unpublished results; see also Bethel & Holmes 1974), indicating that the observed alterations of infected hosts are the consequence and not the cause of infection. In various rivers of Burgundy (Eastern France), G. pulex can be infected by two fish acanthocephalans, Pomphorhynchus laevis and Pomphorhynchus tereticollis (see Perrot-Minnot (2004) for discussion of taxonomic status), and one bird acanthocephalan, Polymorphus minutus. The two fish parasites tend to modify the reaction to light in their intermediate host, although the effect is less pronounced in P. tereticollis (Perrot-Minnot 2004; M.-J. Perrot-Minnot 2006, unpublished data). Uninfected individuals are strongly photophobic, whereas those carrying infection tend to swim in illuminated areas. Such a change in behaviour makes infected gammarids more vulnerable to predation by fish (Bakker et al. 1997; F. Cézilly 2006, unpublished data). Polymorphus minutus reverses the geotactism of its intermediate host, making it swim closer to the surface, where it presumably becomes an easy prey for waterbirds, the parasite's final hosts. Although the physiological mechanisms by which the three parasite species achieve specific manipulation remain so far unknown, 5-HT has previously been shown to modify phototactic behaviour in crustaceans (McPhee & Wilkens 1989).
We have examined the possibility that altered serotonergic activity in the brain of gammarids infected with P. laevis, P. tereticollis or P. minutus might be functionally and specifically linked to changes in the host behaviour. We first quantified alterations in the host behaviour associated with each parasite, and following the injection of serotonin in the uninfected hosts. Using immunocytochemistry and confocal microscopy, we then measured serotonergic (5-HT) immunoreactivity in the brains of infected and non-infected gammarids. If 5-HT is functionally and specifically related to parasite-induced changes in phototactism, serotonin injection should mimic altered phototactism, but not geotactism in uninfected individuals, and the altered phototactism in Pomphorhynchus-infected individuals should correlate with changes in brain 5-HT activity.
2. Material and methods
(a) Collection and maintenance of Gammarus pulex
Sampling took place in Burgundy (Eastern France) in July 2004 for injections only, and from February to July 2005 for behavioural assays and measures of 5-HT activity and neuronal architecture. Gammarids infected with the three parasite species and uninfected individuals were collected during each sampling period. The infected gammarids could easily be recognized from the bright yellow–orange colour of the cystacanths visible through the host's translucid cuticle. Pomphorhynchus laevis infections were obtained from the river Ouche at the Parc de la Colombière, while both P. tereticollis and P. minutus infections were obtained from the river Bèze at Noiron-sur-Bèze. The uninfected individuals from each site were also sampled for site-specific controls. To standardize the conditions of behavioural experiments, gammarids were maintained in laboratory aquariums containing aerated water at 14°C, under a constant photoperiod regime of light : dark 12 h: 12 h, for 24 h prior to experiments. Rotted elm leaves were provided as food ad libitum.
(b) Behavioural assays
(i) Phototactism
Individual gammarids, harbouring a single parasite and uninfected controls (site specific), were placed in a horizontal, half light, half dark, 23×3 cm plastic tube containing aerated dechlorinated UV-treated water at 14°C. After an acclimation period of 5 min, the position of individual gammarids was noted every 30 s for 5 min and scored as 0 (dark zone) or 1 (light zone). Behaviour was therefore scored as 0 (strongly photophobic) to 10 (strongly photophilic).
(ii) Geotactism
Individual gammarids were placed within a vertical plastic column (28×4.5 cm diameter) filled with aerated dechlorinated UV-treated water at 14°C and covered with a small net that animals could cling to. Black screens were placed above and below the columns to produce a non-direct uniform light. The water column was graded into five layers allowing a simple scoring method. Following an acclimation period of 5 min, the position of the individual was noted every 30 s for 5 min, and scored from 1 (bottom layer) to 5 (top layer). The overall performance ranged from 10 (strong positive geotactism) to 50 (negative geotactism). Once assayed, individuals were maintained in separate vials until dissection and the sample sizes were shown within individual figures.
As a previous study (Bauer et al. 2000) failed to show sex-specific effects of host behaviour manipulations, all the behavioural experiments examined individuals of both sexes. All comparisons were performed between individuals collected at the same period of the year.
(c) Injections
To determine if 5-HT could be involved in the altered behaviour of infected G. pulex, we carried out a series of injection experiments that delivered excess, but not lethal (Helluy & Holmes 1990) levels of 5-HT (5-hydroxytryptamine hydrochloride, Sigma). Parallel injections of octopamine (Sigma) were performed as a control. The uninfected gammarids were immobilized on wet modelling clay and viewed at 30×. Sharpened steel wire was then used to puncture the cuticle of the third coxal plate. Neurotransmitters (1 μl) were then delivered via a Hamilton 33 gauge syringe, through the puncture hole, at a concentration of 5 μg μl−1 (diluted in filtered Crustacean Ringer solution; Van Harreveld 1936). Sham injections were administered as mentioned earlier, but neurotransmitters were omitted. After injection, individuals were left in aerated dechlorinated UV-treated water at 14°C for 1 h before their behaviour was assayed; the effects of exogenous neurotransmitters have been shown to diminish after this time (Helluy & Holmes 1990).
(d) 5-HT activity and neuronal architecture
(i) Brain preparation and immunocytochemistry
Brains of infected/uninfected male G. pulex were dissected in refrigerated crustacean Ringer solution, leaving the most anterior sections of the cuticle to maintain the structural integrity of the brain tissues. On dissection, sex, infection status and infection type were verified. Brains were fixed overnight at 4°C in 4% paraformaldehyde in phosphate buffered saline (PBS). Brains were rinsed copiously in PBS with 0.2% Triton X-100 (PBSTX) and incubated for 4 h in 4% goat serum (Zymed) at 24°C. After rinsing with PBSTX (3×5 min), the brains were incubated overnight at 4°C with a rabbit anti-5-HT (Sigma) primary antibody diluted to 1 : 500 in PBSTX. The brains were then rinsed (PBSTX 3×5 min) and incubated for 4 h at 24°C with an Alexa Fluor 488 goat anti-rabbit secondary antibody (Molecular probes) diluted to 1 : 50 in PBSTX. Once rinsed (PBSTX, 3×5 min), the brains were mounted in 90% glycerol and viewed with a Leica TCS SP2 AOBS confocal microscope. An argon laser (100 mW), at 25% intensity, was used to visualize Alexa Fluor 488. Since all the brains could not be processed in a single block, each experimental block consisted of brains from both the infected and the uninfected individuals collected at the same time in the same river. Sample sizes are shown within the individual figures.
(e) Image analysis and optical microdensitometry
Individual brains were scanned within a single frame using a 20× objective. The imaging of each brain consisted of 50 regularly spaced scans (adapted from Helluy & Thomas 2003). Scans were taken in the horizontal plane and encompassed the entire brain. Maximum transformation images (composite of all the 50 scans) created within Leica Confocal Software‐LITE (LCS-LITE) were used to determine the presence of morphological differences between parasitized and unparasitized brains. To compare the brain serotonergic activity between infected and uninfected individuals, we measured the level of labelling within the region encompassing the tritocerebrum from the lateral to the medial projections and ventrally to include the tritocerebral giant neuron (TGN) cell body. This region was chosen as it is readily identified and its distinct boundaries allow delineation by the polygon tool within the stack profile function of LCS-lite. This delineation formed an approximate triangle using the medial and lateral projections of the TGN as anterior landmarks with the TGN cell body acting as a posterior landmark. The use of this function allows the estimation of pixel intensity within a given area, and therefore the estimation, though on an arbitrary scale, of 5-HT within individual brains.
(f) Statistical analysis
All the statistical analyses were carried out using Jmp software (v. 5, SAS Institute, Cary, NC, USA). Wilcoxon–Mann–Whitney tests, Kruskal–Wallis tests and post hoc comparisons were used to assess behavioural differences, as behavioural scores were not normally distributed. Moses tests were used to assess differences in the dispersion between groups (Siegel & Castellan 1988). ANOVA models, including infection status, experimental block (to correct for potential variability inherent in the immunocytochemical process) and the interaction term, were used to determine the differences between levels of 5-HT labelling. Post hoc (multiple comparison) tests were used to determine 2×2 differences. The data were log transformed, prior to ANOVA analysis, to meet normality criteria.
3. Results
(a) Altered host phototactic behaviour
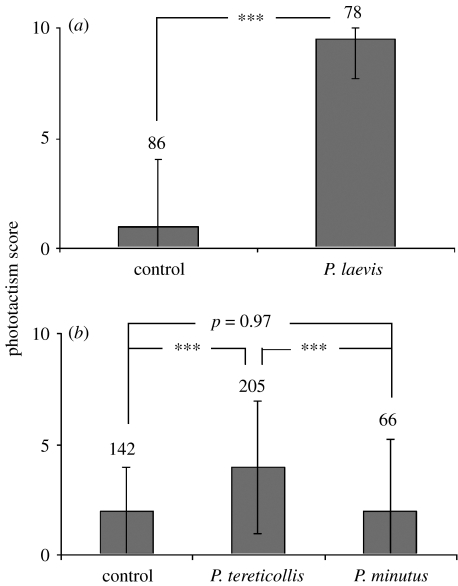
Analysis of samples collected in the river Ouche (figure 1a) showed reversed phototactic behaviour in P. laevis-infected G. pulex compared to site-specific uninfected controls (Wilcoxon–Mann–Whitney test: Z=8.7, nu=86, ni=78, p<0.0001). Analysis of samples collected in the river Bèze (figure 1b) demonstrated reduced photophobia in P. tereticollis-infected individuals compared to both site-specific uninfected controls (multiple comparison test: nu=142, ni=205, p<0.0001) and P. minutus-infected individuals (multiple comparison test: nP.tereticollis=205, nP.minutus=66, p<0.0001), whereas no difference was detected between the latter two groups (multiple comparison test: nu=142, ni=66, p=0.97). There was no significant difference between the sampling sites in the reaction to light of uninfected individuals (Wilcoxon–Mann–Whitney test: Z=−1.17 nBèze=142, nOuche=86, p=0.24). On average, P. tereticollis-infected individuals were significantly less photophilic than P. laevis-infected individuals (Wilcoxon–Mann–Whitney test: Z=7.72, nP.tereticollis=205, nP.laevis=78, p<0.0001). In addition, behavioural manipulation was more variable among the individuals infected with P. tereticollis compared to those infected with P. laevis (Moses test, p<0.0001). However, additional experiments showed that phototactic behaviour was, to some extent, repeatable at 1 h intervals within P. tereticollis-infected individuals (Spearman's rank-correlation coefficient: rs=0.397, N=73, p=0.0007).
Figure 1.
Phototactic behaviour in acanthocephalan-infected and uninfected (site specific) G. pulex. (a) Samples collected in the river Ouche; (b) samples collected in the river Be`ze. Figures show median phototactism scores, interquartile range, sample size and significance as determined by Wilcoxon–Mann–Whitney two-sample test (P. laevis), Kruskal–Wallis one-way test (P. tereticollis and P. minutus) and 2×2 significance as determined by post hoc tests, ***p≤0.0001.
(b) 5-HT-induced alteration of host phototactic behaviour
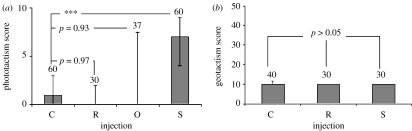
Significant differences were found between 5-HT-injected G. pulex and octopamine-injected, Ringer-injected uninfected and uninjected-uninfected G. pulex (Kruskal–Wallis test: χ2=42.5, ncontrol=60, n5-HT=60, noctopamine=37, nRinger=30, p<0.0001; figure 2a). G. pulex injected with octopamine or Ringer solution showed no alteration in phototactic behaviour, and did not differ from non-injected individuals (multiple comparison test: p=0.93 and 0.97, respectively; figure 2a). Only injection of 5-HT had a significant effect on phototactism (multiple comparison test, p<0.0001), resulting in a dramatic shift from photophobic to photophilic behaviour (figure 2a) that mimicked the behaviour of G. pulex infected with P. tereticollis or P. laevis. In contrast, injections of 5-HT (figure 2b) had no effect on geotactic behaviour (Wilcoxon–Mann–Whitney test: χ2=42.3, ncontrol=40, nRinger=30, n5-HT=30, p=0.12).
Figure 2.
(a) Phototactic behaviour of uninfected and non-injected G. pulex (C) and those injected with 1 μl Ringer solution (R), octopamine (5 μg μl−1) (O) or 5-HT (5 μg μl−1) (S). (b) Geotactic behaviour of non-injected G. pulex (C) and those injected with 1 μl Ringer solution (R) or 5-HT (5 μg μl−1) (S). Figures show median phototactism/geotactism scores, associated interquartile range, sample size and significance as determined by Kruskal–Wallis one-way test and 2×2 significance as determined by post hoc tests, ***p≤0.0001.
(c) Neuronal architecture and brain 5-HT activity
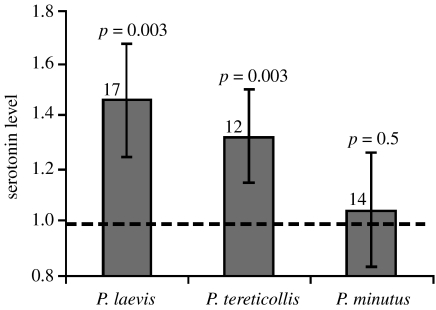
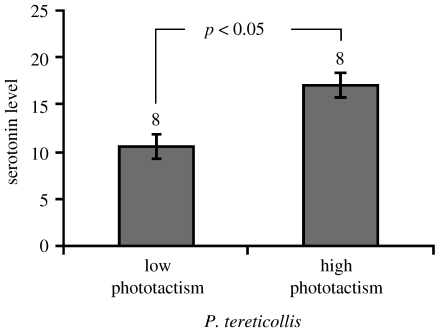
Side-by-side examination of selected confocal scans did not reveal any gross anatomical differences between the brains of uninfected G. pulex and those of individuals infected with any of the three acanthocephalan parasites (figure 3). To test for differences within the fine structure of the brain, we measured the length of TGN axons from the cell body to the lateral projection. No differences were found; brains from individuals infected with any of the three acanthocephalan parasites show similar TGN axon length to that of their site-specific controls (p>0.05 for all the comparisons, data not shown). We used optical densitometry of the tritocerebral region of infected and uninfected individuals to determine the presence of differences in brain 5-HT immunoreactivity. We found significant increases in the level of 5-HT immunoreactivity within the brains of both P. tereticollis-infected (+33%, ANOVA; F1,24=10.46, p=0.003) and P. laevis-infected G. pulex (+46%, ANOVA; F1,28=10.46, p=0.003), compared to uninfected individuals (figure 4). Furthermore, we found that brain 5-HT immunoreactivity was on average 62% higher in P. tereticollis-infected G. pulex that were strongly photophilic (phototactism scores ranging between 8 and 10) compared to P. tereticollis-infected G. pulex that showed no sign of phototactic modification (scores between 0 and 2; ANOVA; F1,14=5.24, p=0.038; figure 5). In striking contrast, we found no significant difference in 5-HT immunoreactivity in the brains of Gammarids infected with P. minutus compared to the uninfected ones (ANOVA (nested experimental block): F1,40=0.06, p=0.5; figure 3).
Figure 3.
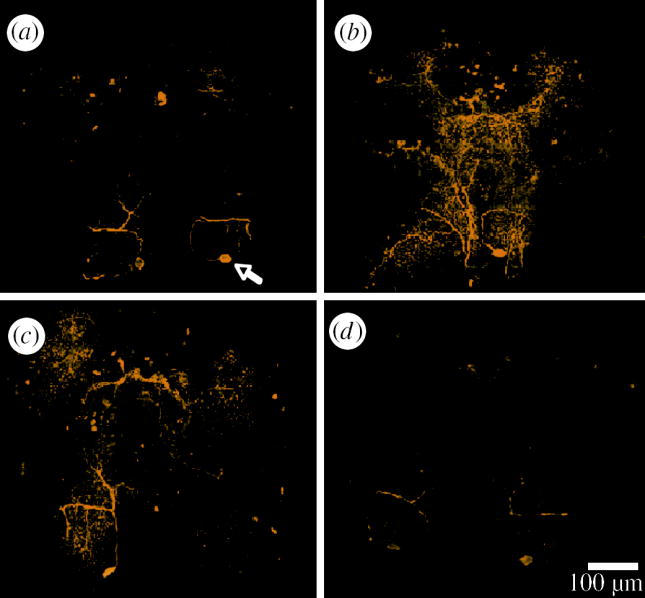
Images show 5-HT immunoreactivity (yellow) within the brains of (a) uninfected (arrow shows position of TGN cell body) and (b) P. laevis-, (c) P. tereticollis- and (d) P. minutus-infected individuals. No differences, gross or fine, in brain anatomy from infected and uninfected individuals were observed. Bar shows 100 μm.
Figure 4.
Optical densitometric measures of 5-HT immunoreactivity within the tritocerebral region of G. pulex infected with P. laevis, P. tereticollis and P. minutus, shown as relative to uninfected controls (dotted line). Only the brains of those individuals showing extreme alteration of normal phototactic behaviour were examined here. Figures show standardized means against site-specific controls, associated 95% CI, sample size and significance as determined by ANOVA. No effect of experimental block was found (p=0.05 for all).
Figure 5.
Optical densitometric measures of 5-HT immunoreactivity within the tritocerebral region of G. pulex infected with P. tereticollis showing altered and unaltered phototactic behaviour. Figure shows mean values, associated 95% CI, sample size and significance as determined by ANOVA.
4. Discussion
We employed quantification of stereotyped behaviours, injection of neurotransmitters and brain immunocytochemistry to compare the respective effects of three acanthocephalan parasites on their common intermediate host. Our study clearly establishes that the alteration of brain 5-HT activity is not a general feature of infection, but is concomitant with the alteration of a particular behaviour, i.e. phototactism. It must be emphasized that the observed increase in immunostaining does not necessarily coincide with a decrease in the release of 5-HT because this depends on the turnover rate of secretory products. However, combining proper observations, experimental design and immunocytochemistry provides strong arguments for a direct link between 5-HT and manipulation (de Jong-Brink & Koene 2005). Importantly, the alteration of both brain serotonergic activity and phototactic behaviour was associated only with infection by fish acanthocephalans. It has been previously shown that altered phototactism increases the vulnerability of G. pulex to fish predators (Bakker et al. 1997). Bird acanthocephalans, on the other hand, tend to induce reverse geotactism in their amphipod intermediate hosts (Cézilly et al. 2000), presumably to enhance trophic transmission to appropriate final hosts. We found that 5-HT had no influence on geotactism and that gammarids infected with a bird acanthocephalan showed no sign of altered 5-HT activity in their brain.
Compared to previous attempts, the present study provides unambiguous evidence for a link between altered behaviour and 5-HT in amphipods infected with manipulative parasites. Simple injections causing uninfected amphipods to behave like infected ones (Helluy & Holmes 1990) cannot alone provide firm evidence for a direct role of 5-HT in manipulation by parasites because the possibility remains that 5-HT injections mimic the altered behaviour without actually being involved (Maynard et al. 1996). Two studies resorting to immunocytochemistry (Maynard et al. 1996; Helluy & Thomas 2003) are suggestive of a link between 5-HT and manipulation, but both have limitations. A significant increase in the number of swellings was established from paired comparisons between the nerve cords of six G. lacustris infected by Polymorphus paradoxus and those of six uninfected gammarids (Maynard et al. 1996). Such swellings were interpreted as varicosities inside which 5-HT was stored, though conclusive evidence was lacking. Interestingly, the increase in swellings ranged from 1.5 to 7, but no information was provided as to whether such variation was related to variation in the degree of manipulation. Recently, 5-HT immunoreactivity in the brain of Gammarus insensibilis infected by the trematode Microphallus papillorobustus was assessed in two regions of the gammarid brain by measuring the percentage of the surface area of these regions that was labelled for 5-HT. This method showed that in infected gammarids the level of 5-HT immunoreactivity is decreased in the optic neuropils, but increased in the olfactory lobes (Helluy & Thomas 2003) as well as in the tritocerebrum (S. Helluy 2006, personal communication). In addition, infection with M. papillorobustus inflicts a severe alteration of neuronal architecture (Helluy & Thomas 2003), and it is therefore difficult to establish whether the altered behaviour is the result of altered 5-HT activity or that of mechanical damage, though the absence of contact with the TGN makes direct mechanical damage unlikely (Helluy & Thomas 2003).
Here, we used a more reliable estimate of 5-HT immunoreactivity, based upon variation in the overall intensity of staining and larger sample size. Additionally, contrary to M. papillorobustus that encysts in the brain of its amphipod host, acanthocephalans develop in the amphipod's haemocoel as free-floating ovoid cystacanths, which cause no mechanical damage. Indeed, close inspection with confocal imaging did not reveal any significant alteration of neuronal architecture in the brains of G. pulex infected with P. tereticollis, P. laevis or P. minutus, indicating that observed changes in 5-HT activity and behaviour were independent of any possible mechanical damage caused by infection. Finally, variation in the degree of manipulation observed in P. tereticollis-infected G. pulex was mirrored in the degree of altered 5-HT in the brain, thus providing conclusive evidence for a functional link between 5-HT and manipulation. All the infected individuals used in our experiments harboured mature infective cystacanths as determined from visual inspection after dissection of hosts. However, to further ensure that no difference in maturity was responsible for the observed variation in the degree of manipulation among P. tereticollis-infected G. pulex, we examined the ability of P. tereticollis cystacanths to devaginate their proboscis. Upon ingestion by an appropriate final host, acanthocephalan cystacanths must rapidly evert their proboscis to hook onto the wall of the intestinal tract of the final host inside which they will mature and reproduce. To mimic this passage, we extracted mature P. tereticollis cystacanths from infected gammarids (whose phototactic score had been previously measured), and exposed them to fish bile at 14°C in the absence of light. All cystacanths examined (n=28) everted their proboscis within 24 h, independent of the level at which they manipulated host behaviour.
It is still unclear how acanthocephalan parasites cause a change in brain 5-HT activity. Although the presence of biogenic amines has been demonstrated in acanthocephalans (Budziakowski et al. 1983), it seems unlikely that acanthocephalans are capable of directly releasing 5-HT in large quantities in the nervous system of their hosts. Instead, acanthocephalans might act directly on genes that are involved either in the production of serotonin or in the differential expression of 5-HT receptors in the brain. Alternatively, parasites may regulate either the secretion of other neuromodulators that alter the secretion of 5-HT or the expression of their receptors. Interestingly, it has been shown that G. pulex infected with P. laevis show elevated glycogen levels (Plaistow et al. 2001). 5-HT is precisely known to regulate blood glucose level in crustaceans (Bauchau & Mengeot 1966; Luschen et al. 1993; Glowik et al. 1997) and 5-HT receptors involved in the regulation of blood glucose levels in the crayfish, Procambarus clarkiii, have been pharmacologically characterized (Lee et al. 2000). It is well documented that 5-HT receptors are highly conserved in arthropods (Tierney 2001), and there is some indication that conservation extends to effector molecules (Sosa et al. 2004). Comparing the expression of such receptors between uninfected gammarids and gammarids infected with P. laevis or P. tereticollis might in the near future, provide a way to better understand the mode of action of manipulative parasites. A putative receptor of serotonin has recently been cloned from crayfish, prawn, Macrobrachium rosenbergii, and spiny lobster, Panulirus interruptus, making possible the quantification of its expression (Sosa et al. 2004; Spitzer et al. 2005).
The variability in behavioural manipulation observed in gammarids infected with P. tereticollis may, however, remain open to alternative explanations. First, some hosts might be resistant to manipulation. Variation in the ability of P. laevis to manipulate the phototactic behaviour and immune response of closely related intermediate host species has previously been reported (Bauer et al. 2000; Rigaud & Moret 2003), but intraspecific variation in the ability of amphipods to resist manipulation attempts by acanthocephalans remains unexplored. Second, some individual parasites might be more competent than others at manipulating their hosts. This is to be expected if manipulation incurs a cost to parasites. However, the existence of costs to manipulation awaits empirical evidence (Poulin et al. 2005; Thomas et al. 2005). Obviously, host ability to resist manipulation and parasite ability to manipulate may combine to provide observed patterns of variation in the degree of manipulation. Third, different parasites may rely on different temporal strategies of manipulation. For instance, some parasites may induce chronic manipulation, whereas others may manipulate their hosts on an intermittent basis. Long-term measures of the repeatability of altered behaviour could be useful to distinguish between these two options. Alternatively, quantitative comparisons of the density of 5-HT receptors between infected and uninfected hosts could be performed. Based on the evidence from other systems (Cooper et al. 2001), it would be expected that chronically elevated 5-HT levels in infected individuals would result in compensatory downregulation of the 5-HT receptors.
Another possibility, which may explain variation in the level of behavioural manipulation, is that caused by vertically transmitted intracellular parasites. A recent study by Haine et al. (2005) showed that P. minutus-infected Gammarus roeseli exhibit altered geotactic behaviour, but when G. roeseli were found to harbour infections of P. minutus along with intracellular microsporidia infections, this behavioural alteration was ‘sabotaged’—co-infected individuals exhibited unaltered, normal geotactic behaviour. However, this possibility is unlikely to explain the variation seen here, since vertically transmitted microsporidia were never found in G. pulex in Burgundy (T. Rigaud, personal communication).
Comparative studies of the physiological mechanisms underlying manipulation between different host–parasite systems remain scarce. They may however provide important information in the future (Moore 2002; Thomas et al. 2005). In particular, mapping the various physiological routes used by parasites onto a phylogeny (Moore & Gotelli 1996) might help our understanding of the evolution of host manipulation by parasites. In addition, elucidating the mechanisms by which manipulation is achieved might contribute to a better understanding of the functioning of the nervous system of arthropods. In this framework, the study of interactions between crustacean species and acanthocephalans parasites might prove particularly rewarding.
Acknowledgments
We are particularly grateful to Simone Helluy for advice and helpful discussions. We thank Josette Relot and Claude Humbert for assistance with confocal microscopy, Jean-François Ferveur for advice at the initial stage of the study as well as Stephane Cornet and Thomas Ropion for technical assistance. We also thank three anonymous referees for helpful comments on previous versions of the paper. The study was funded by the Conseil Re´gional de Bourgogne through a post-doc grant to L.T.
References
- Bakker T.C.M, Mazzi D, Zala S. Parasite-induced changes in behaviour and color make Gammarus pulex more prone to fish predation. Ecology. 1997;78:1098–1104. doi:10.2307/2265861 [Google Scholar]
- Bauchau A.G, Mengeot J.C. Sérotonine et glycémie chez les crustacés. Experientia. 1966;22:238–239. doi: 10.1007/BF01900932. doi:10.1007/BF01900932 [DOI] [PubMed] [Google Scholar]
- Bauer A, Trouve S, Gre´goire A, Bollache L, Ce´zilly F. Differential influence of Pomphorhynchus laevis (Acanthocephala) on the behaviour of native and invader gammarid species. Int. J. Parasitol. 2000;30:1453–1457. doi: 10.1016/s0020-7519(00)00138-7. doi:10.1016/S0020-7519(00)00138-7 [DOI] [PubMed] [Google Scholar]
- Bethel W.M, Holmes J.C. Correlation of development of altered evasive behavior in Gammarus lacustris (Amphipoda) harboring cystacanths of Polymorphus paradoxus (Acanthocephala) with the infectivity to the definitive host. J. Parasitol. 1974;60:272–274. [PubMed] [Google Scholar]
- Budziakowski M.E, Mettrick D.F, Webb R.A. Aminergic neurons in the anterior nervous system of the rat acanthocephalan Moniliformis dubius. J. Neurobiol. 1983;14:313–325. doi: 10.1002/neu.480140406. doi:10.1002/neu.480140406 [DOI] [PubMed] [Google Scholar]
- Cézilly F, Grégoire A, Bertin A. Conflict between co-occurring manipulative parasites? An experimental study of the joint influence of two acanthocephalan parasites on the behaviour of Gammarus pulex. Parasitology. 2000;120:625–630. doi: 10.1017/s0031182099005910. doi:10.1017/S0031182099005910 [DOI] [PubMed] [Google Scholar]
- Cooper R.L, Chase R.J, Tabor J. Altered responsiveness to 5-HT at the crayfish neuromuscular junction due to chronic p-CPA and m-CPP treatment. Brain Res. 2001;916:143–151. doi: 10.1016/s0006-8993(01)02885-2. doi:10.1016/S0006-8993(01)02885-2 [DOI] [PubMed] [Google Scholar]
- de Jong-Brink M, Koene J.M. Parasitic manipulation: going beyond behaviour. Behav. Process. 2005;68:229–233. doi: 10.1016/j.beproc.2004.08.014. doi:10.1016/j.beproc.2004.08.014 [DOI] [PubMed] [Google Scholar]
- Glowik R.M, Golowasch J, Keller R, Marder E. d-glucose-sensitive neurosecretory cells of the crab Cancer borealis and negative feedback regulation of blood glucose level. J. Exp. Biol. 1997;200:1421–1431. doi: 10.1242/jeb.200.10.1421. [DOI] [PubMed] [Google Scholar]
- Haine E.R, Boucansaud K, Rigaud T. Conflict between parasites with different transmission strategies infecting an amphipod host. Proc. R. Soc. B. 2005;272:2505–2510. doi: 10.1098/rspb.2005.3244. doi:10.1098/rspb.2005.3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helluy S, Holmes J.C. Serotonin, octopamine, and the clinging behavior induced by the parasite Polymorphus paradoxus (Acanthocephala) in Gammarus lacustris (Crustacea) Can. J. Zool. 1990;68:1214–1220. [Google Scholar]
- Helluy S, Holmes J.C. Parasitic manipulation: further considerations. Behav. Process. 2005;68:205–210. doi: 10.1016/j.beproc.2004.08.011. doi:10.1016/j.beproc.2004.08.011 [DOI] [PubMed] [Google Scholar]
- Helluy S, Thomas F. Effects of Microphallus papillorobustus (Platyhelminthes: Trematoda) on serotonergic immunoreactivity and neuronal architecture in the brain of Gammarus insensibilis (Crustacea: Amphipoda) Proc. R. Soc. B. 2003;270:563–568. doi: 10.1098/rspb.2002.2264. doi:10.1098/rspb.2002.2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.Y, Yau S.M, Liau C.S, Huang W.J. Serotonergic regulation of blood glucose levels in the crayfish, Procambarus clarkii: site of action and receptor characterization. J. Exp. Zool. 2000;286:596–605. doi: 10.1002/(sici)1097-010x(20000501)286:6<596::aid-jez6>3.0.co;2-s. doi:10.1002/(SICI)1097-010X(20000501)286:6<596::AID-JEZ6>3.0.CO;2-S [DOI] [PubMed] [Google Scholar]
- Luschen W, Willig A, Jaros P.P. The role of biogenic amines in the control of blood glucose levels in the decapod crustacean, Carcinus maenas L. Comp. Biochem. Physiol. 1993;105C:291–296. [Google Scholar]
- Maynard B.J, DeMartini L, Wright W.G. Gammarus lacustris harboring Polymorphus paradoxus show altered patterns of serotonin-like immunoreactivity. J. Parasitol. 1996;82:663–666. doi:10.2307/3283801 [PubMed] [Google Scholar]
- McPhee M.J, Wilkens J.L. Serotonin, but not dopamine or octopamine, modifies locomotor and phototaxic behaviour of the crab, Carcinus maenas. Can. J. Zool. 1989;67:391–393. [Google Scholar]
- Moore J. Oxford University Press; Oxford, UK: 2002. Parasites and the behaviour of animals. [Google Scholar]
- Moore J, Gotelli N.J. Evolutionary patterns of altered behavior and susceptibility in parasitized hosts. Evolution. 1996;50:807–819. doi: 10.1111/j.1558-5646.1996.tb03890.x. doi:10.2307/2410853 [DOI] [PubMed] [Google Scholar]
- Overli O, Pall M, Borg B, Jobling M, Winberg S. Effects of Schistocephalus solidus infection on brain monoaminergic activity in female three-spined sticklebacks Gasterosteus aculeatus. Proc. R. Soc. B. 2001;268:1411–1415. doi: 10.1098/rspb.2001.1668. doi:10.1098/rspb.2001.1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot-Minnot M.J. Larval morphology, genetic divergence, and contrasting levels of host manipulation between forms of Pomphorhynchus laevis (Acanthocephala) Int. J. Parasitol. 2004;34:45–54. doi: 10.1016/j.ijpara.2003.10.005. doi:10.1016/j.ijpara.2003.10.005 [DOI] [PubMed] [Google Scholar]
- Plaistow S.J, Troussard J.P, Cézilly F. The effect of the acanthocephalan parasite Pomphorhynchus laevis on the lipid and glycogen content of its intermediate host Gammarus pulex. Int. J. Parasitol. 2001;31:346–351. doi: 10.1016/s0020-7519(01)00115-1. doi:10.1016/S0020-7519(01)00115-1 [DOI] [PubMed] [Google Scholar]
- Poulin R, Nichol K, Latham A.D. Host sharing and host manipulation by larval helminths in shore crabs: cooperation or conflict? Int. J. Parasitol. 2003;33:425–433. doi: 10.1016/s0020-7519(03)00002-x. doi:10.1016/S0020-7519(03)00002-X [DOI] [PubMed] [Google Scholar]
- Poulin R, Fredensborg B.L, Hansen E, Leung T.L. The true cost of host manipulation by parasites. Behav. Process. 2005;68:241–244. doi: 10.1016/j.beproc.2004.07.011. doi:10.1016/j.beproc.2004.07.011 [DOI] [PubMed] [Google Scholar]
- Rigaud T, Moret Y. Differential phenoloxidase activity between native and invasive gammarids infected by local acanthocephalans: differential immunosuppression? Parasitology. 2003;127:571–577. doi: 10.1017/s0031182003004050. doi:10.1017/S0031182003004050 [DOI] [PubMed] [Google Scholar]
- Siegel S, Castellan N.J. 2nd edn. McGraw-Hill; New York, NY: 1988. Nonparametric statistics for the behavioral sciences. [Google Scholar]
- Sosa M.A, Spitzer N, Edwards D.H, Baro D.J. A crustacean serotonin receptor: cloning and distribution in the thoracic ganglia of crayfish and freshwater prawn. J. Comp. Neurol. 2004;473:526–537. doi: 10.1002/cne.20092. doi:10.1002/cne.20092 [DOI] [PubMed] [Google Scholar]
- Spitzer N, Antonsen B.L, Edwards D.H. Immunocytochemical mapping and quantification of expression of a putative type 1 serotonin receptor in the crayfish nervous system. J. Comp. Neurol. 2005;484:261–282. doi: 10.1002/cne.20456. doi:10.1002/cne.20456 [DOI] [PubMed] [Google Scholar]
- Terenina N.B, Asatryan A.M, Movsesyan S.O. Content of serotonin in brain and other tissues of rats with experimental trichinellosis. Dokl. Akad. Nauk. 1997;335:412–413. [PubMed] [Google Scholar]
- Thomas F, Adamo S, Moore J. Parasitic manipulation: where are we and where should we go? Behav. Process. 2005;68:185–199. doi: 10.1016/j.beproc.2004.06.010. doi:10.1016/j.beproc.2004.06.010 [DOI] [PubMed] [Google Scholar]
- Tierney A.J. Structure and function of invertebrate 5-HT receptors: a review. Comp. Biochem. Physiol. A. 2001;128:791–804. doi: 10.1016/s1095-6433(00)00320-2. doi:10.1016/S1095-6433(00)00320-2 [DOI] [PubMed] [Google Scholar]
- Van Harreveld A. A physiological solution for freshwater crustaceans. Proc. Soc. Exp. Biol. 1936;34:428–432. [Google Scholar]