Abstract
The neuropeptides arginine vasotocin (AVT) and arginine vasopressin are key modulators of affiliation and aggression among non-mammalian and mammalian vertebrates, respectively. Here, we explored AVT's effect on aggression in a wild population of beaugregory damselfish, Stegastes leucostictus, a highly territorial species. Aggression by territorial males towards ‘intruders’ (bottled fishes) was assessed before and after each male received intramuscular injections of either AVT, Manning compound (an AVT V1a receptor antagonist), isotocin (the teleost homologue of mammalian oxytocin differing from AVT by two amino acids) or saline (vehicle control). Compared to saline controls, AVT and Manning increased and decreased aggression, respectively, while isotocin had no effect. Response selectivity was further established in a dose–response study that revealed an inverted U-shaped function. Compared to saline controls, aggression levels for low and high AVT doses were similar, while medium dose treatments were significantly greater. This type of behavioural response, the first that we know of for a vertebrate neuropeptide, could depend on the binding of AVT to both V1-type and other AVT or non-AVT receptors. The pattern revealed here for damselfish may be symptomatic of species- and context-dependent specificity of AVT's modulation of aggression across teleosts, as is currently proposed for tetrapods.
Keywords: aggression, vasotocin, V1-type receptor, neuroendocrine, teleost
1. Introduction
The neuropeptide homologues arginine vasotocin (AVT) and arginine vasopressin (AVP) are well documented to modulate both reproductive and aggressive behaviours (reviewed in Goodson & Bass 2001). While these neuropeptides have a fairly conserved effect on reproduction among tetrapods, they have divergent effects on overt aggression. Here, we investigate AVT modulation of aggression in a teleost fish to further establish the evolution of these mechanisms across vertebrate taxa. One hypothesis proposed to explain the diversity of AVT/AVP effects on aggression is the degree to which conspecifics are tolerated during breeding (Goodson 1998b). For example, among birds, even though territorial violet-eared waxbills (Uraeginthus granatina) share a similar ecology and are more closely related to colonial breeding zebra finches (Poephila castanotis) than to territorial field sparrows (Spizella pusilla), AVT inhibits overt aggression in both territorial species (Goodson 1998a,b), but facilitates aggression in the zebra finch (Goodson & Adkins-Regan 1999). Among mammals, AVT/AVP also facilitates aggression in prairie voles (Microtus ochrogaster; Young et al. 1997) that, like zebra finches, share a similar tolerance for conspecifics as indicated by their cooperative breeding and communal group organizations (see Carter et al. 1995; Carter & Roberts 1996; Zann 1996). However, a single hypothesis may not completely explain AVT/AVP's role in modulating aggression across all tetrapods. For example, AVP has no effect on aggression in territorial male montane voles (Microtus montanus; Young et al. 1997), while it facilitates aggression in male golden hamsters (Mesocricetus auratus; Ferris & Delville 1994) that, like montane voles, are considered solitary and territorial (see Johnston 1999). Together, the above observations emphasize the species- and context-dependent specificity of neuroendocrine modulation of aggression and other social behaviours (see Goodson & Evans 2004 and §4).
Adaptive explanations for the evolution of neuroendocrine mechanisms of animal sociality guide, if not require, comparative tests among species with similar and dissimilar ecologies. Teleost fishes provide an excellent opportunity to test hypotheses explaining patterns of neuropeptide action (like the conspecific tolerance hypothesis) because, like tetrapods, they exhibit a wide range of breeding ecologies. To date, it has been difficult to discern any pattern(s) for the effects of AVT on teleost aggression (table 1). While such divergence may represent real species differences, a broad interpretation has been hampered, in part, because these studies focus on teleosts with alternative male reproductive tactics and/or have incomplete tests of both the AVT-dependent selectivity and sensitivity of the response being measured. For example, bluehead wrasse (Thalassoma bifasciatum) is a female-to-male sex changing reef fish with males having two adult phenotypes, initial phase (IP) and terminal phase (TP), that differ dramatically in behavioural, somatic and endocrinological traits (IP males can also transform into TP males; Warner & Schultz 1992). TP males are either territorial (T) and defend a spawning site or are non-territorial (NT) and wander the reef searching for an available territory. AVT has no effect on T-TP male aggression towards T-TP, NT-TP or IP males, but increases NT-TP aggression towards IP males. However, Manning compound, an AVP antagonist specific to the V1a receptor (see Barberis et al. 1999) that is commonly used as an AVT V1 receptor antagonist in teleosts (e.g. Mahlmann et al. 1994; Goodson & Bass 2000a; Guibbolini & Avella 2003), does not show the reciprocal effect. Manning compound decreases T-TP aggression towards IP males, but has no influence on T-TP aggression towards T-TP males (Semsar et al. 2001). The lack of an AVT effect on T-TP male aggression and the lack of a test of Manning compound with NT-TP males that showed an AVT effect bring into question the selectivity of the observed effects specifically on AVT and AVT receptors rather than on a general class of closely related nonapeptides (e.g. isotocin; see below). The territorial amaragosa pupfish (Cyprinodon nevadensis amargosae) also has alternative male reproductive tactics, but not distinct male morphs like IP and TP wrasses. Males spawn either in territories they defend (analogous to T-TP male wrasses) or in loose aggregations (analogous to IP male wrasses). AVT decreases aggression in both wild populations of territorial males and captive individuals, although it is unclear which reproductive tactic the captive males exhibit (Lema & Nevitt 2004). Manning compound, tested only in laboratory-housed males, has no effect on aggression. AVT also elicits a decrease in the aggressive displays of laboratory-housed, non-territorial weakly electric fishes (Bastian et al. 2001), but again the lack of tests using antagonists that are specific for AVT-like receptors generates concerns over the selectivity of the observed effects on AVT alone.
Table 1.
Summary of behavioural effects of AVT and V1a antagonist in teleost studies. (n.a., not applicable because no test conducted;  , inhibits aggression;
, inhibits aggression;  , facilitates aggression;
, facilitates aggression;  , no effect on aggression. See text for more details.)
, no effect on aggression. See text for more details.)
| AVT | Manning compound | |
|---|---|---|
| Thalassoma bifasciatum | ||
| T-TP males |
 a a
|
 b, b,  c c
|
| NT-TP males |
 d d
|
n.a. |
| Cyprinodon nevadensis amargosae | ||
| territorial males |
 e e
|
 f f
|
| non-territorial males | n.a. | n.a. |
| Porichthys notatusg | ||
| type I | ||
| type II |
 h h
|
 h h
|
| Apteronotus leptorhynchus |
 i i
|
n.a. |
Aggression towards all phenotypes (Semsar et al. 2001).
Aggression towards IP males (Semsar et al. 2001).
Aggression towards TP males (Semsar et al. 2001).
Aggression towards IP males (Semsar et al. 2001).
Tested in the laboratory and the wild (Lema & Nevitt 2004).
Tested in the laboratory (Lema & Nevitt 2004).
Tests on fictive vocalizations (Goodson & Bass 2000).
Isotocin was the effective neuropeptide (Goodson & Bass 2000).
Effects on agonistic electrical pulses (Bastian et al. 2001).
Unfortunately, the only teleost case so far where the appropriate tests for the selectivity of AVT effects have been conducted, is in a study of fictive calling in a neurophysiological preparation in the plainfin midshipman fish, Porichthys notatus (Goodson & Bass 2000a), which has alternative male reproductive tactics (reviewed in Bass 1996). Fictive calls are the rhythmic output of the vocal motor system that establishes the temporal features of natural vocalizations (Bass & Baker 1990). AVT inhibits and Manning compound facilitates fictive grunts in type I, territorial males; natural grunts serve an agonistic function (Brantley & Bass 1994). By contrast, neither AVT nor Manning compound has an effect on fictive grunts in type II, sneak-spawning males (Goodson & Bass 2000). If we assume that a decrease in fictive grunts relates to a decrease in overt aggression, then these results are consistent with those for territorial pupfish but not bluehead wrasse, though both midshipman and bluehead wrasse show distinct male morphs (IP and TP wrasses, types I and II midshipman). Alternatively, because aggressive signalling often decreases with fight escalation, while overt attack behaviours increase (see Parker 1974), agonistic signalling and overt aggression may be differentially modulated to allow them to act in opposing directions. Thus, this data could potentially be consistent with bluehead wrasse and not territorial pupfish.
Given the above concerns for teleost studies that either focused on species with alternative male morphs and/or tactics, were conducted on laboratory populations which can introduce other confounding variables (see Fusani et al. 2005) or needed more complete tests of the selectivity of the response on AVT, we aimed to: (i) identify AVT effects on aggressive behaviour in a teleost without alternative reproductive tactics, (ii) conduct studies in a species' natural aquatic habitat to avoid the potential confounding effects of laboratory conditions on behaviour, physiology and neuroendocrine responses to environmental stimuli and (iii) use multiple tests for the selectivity of the response to AVT including dose dependency, an AVT antagonist (Manning compound) and isotocin, the teleost homologue of mammalian oxytocin that differs from AVT by only two amino acids (Bentley 1998).
One candidate teleost group for such studies is the damselfish family Pomacentridae, which contains many behaviourally dynamic species with varying breeding ecologies (see Whiteman & Côté 2004). We chose to test AVT effects on male beaugregory damselfish, Stegastes leucostictus, because all reproducing males of this species permanently and very aggressively defend territories. Given AVT/AVP's effects in several other highly territorial species such as field sparrows, violet-eared waxbills and pupfish (see above), we predicted that AVT would inhibit aggression in the beaugregory damselfish.
2. Material and methods
Beaugregory damselfish inhabit shallow water (0.61–1.83 m), hard bottom areas. Females maintain a general home range within territorial male aggregations. Females spawn in nest sites contained within male territories throughout the year, peaking in summer months (Itzkowtiz 1985). Territory quality varies between males, and male courtship and aggression levels are related to territory quality (Itzkowtiz & Haley 1999; Santangelo et al. 2002). Here, territory quality was standardized by providing males with artificial nest sites made of four 25.5 cm length PVC tubes (7.62 cm in diameter) attached in a ‘+’ design to a plastic base. These artificial sites are known to create high-quality territories for beaugregory damselfish (Itzkowitz 1991; also see Itzkowtiz & Haley 1999; Santangelo et al. 2002) and hence, they will naturally exhibit high levels of aggression.
Experiments were carried out in Pigeon Key, Florida in July 2004 and May 2005. Male aggression levels were tested by placing an ‘intruder’ of equal size (±1.0 cm standard length) in a clear plastic jar and presented to a territory owner approximately 30 cm from the artificial nest site. Territorial males were then observed for 3 min and the number of times they performed aggressive lateral displays and bit the bottle were recorded. This is the common method for assessing damselfish aggression levels (e.g. Santangelo et al. 2002; Cleveland et al. 2003). Bottled intruders were other territorial males within the same community. To control the potential individual intruder differences, a given territorial male was presented with the same intruder for all experimental groups.
Males were ‘pre-tested’ before injections and then tested post-injection with the same intruder at 15, 30, 60, 120 and 240 min. We then compared the change in aggression level from pre-test to post-test for each group at each time point (see similar methods in Lema & Nevitt 2004).
Males were caught with a custom made cast net (i.e. 0.9144 m2 delta mesh net with 113.5 g weights every 15.24 cm around border) and then individually tagged to ensure that no males switched territories within an experiment, and to identify intruders from day to day. After capture, focal males were brought to a raft where they were weighed and given an intramuscular injection of one of the study compounds (see §2a). Injection volumes were 10 μl per gram body weight (gbw). This process never exceeded 3 min. Upon return to their territory, all males resumed normal territory patrolling behaviour within 5 min, though the majority did so within 1 min.
(a) Experiment 1: arginine vasotocin effects on aggression
Owing to the unpredictability of weather conditions within the Florida Keys during summers, this experiment was first carried out in July 2004 (n=3) and then replicated in May 2005 (n=7). Males were combined for the analysis (n=10). All males received all four injection groups, with each injection group and the following intruder presentations separated by 1 day. The injection groups were AVT, Manning compound, isotocin and vehicle control (saline). The order in which males received these injections was balanced across males. AVT doses were based on preliminary data (N. Santangelo 2004, personal observation) and were 0.5 μg per gbw. This dose is half that typically used (e.g. Propper & Dixon 1997; Semsar et al. 2001; Lema & Nevitt 2004). Thus, we also halved the Manning compound dose typically used to 1.61 μg per gbw. The isotocin dose mimicked the AVT dose (0.5 μg per gbw). The observer was blind to the type of injection given each day. Despite their rapid resumption of patrolling behaviour soon after the return to their territories, as well as bites towards intruders, all fish virtually eliminated their lateral displays after injection regardless of injection group. Therefore, we only report overt aggression in terms of bites at the bottled fish as lateral displays appear to be too sensitive to these manipulations. Weight and standard lengths of the fish ranged 14–21 g and 7.5–9.0 cm, respectively.
The statistical design used is a 4 (i.e. four injection groups) within×5 (i.e. five time points) within subject ANOVA (both factors are within subject or repeated measures). Planned comparisons were used to test the above stated hypotheses (Keppel 1991; Zar 1999), namely whether each neuropeptide group at each time point differed from the control group. The Tukey test was used for post hoc comparisons given an overall ANOVA effect. Data were ln(x+1) transformed to generate normal data thus conforming them to parametric assumptions.
(b) Experiment 2: dose dependency of effects of arginine vasotocin
This experiment was carried out in July 2005. Males (n=8) were tested with three different doses of AVT (0.05, 0.5 and 1.0 μg per gbw) and a saline vehicle control. The methodology of injections was similar to experiment 1. Weights and standard lengths of fish ranged 10–18 g and 7.0–8.7 cm, respectively.
The statistical design used is a 4 (i.e. four injection groups) within×5 (i.e. five time points) within subject ANOVA. Again, planned comparisons were used to test whether each dose at each time point differed from the control group. Similar to experiment 1, the Tukey test was used for post hoc comparisons given an overall ANOVA effect and data were ln(x+1) transformed, thus generating normal data.
3. Results
(a) Experiment 1: comparison of arginine vasotocin, Manning compound, isotocin and saline
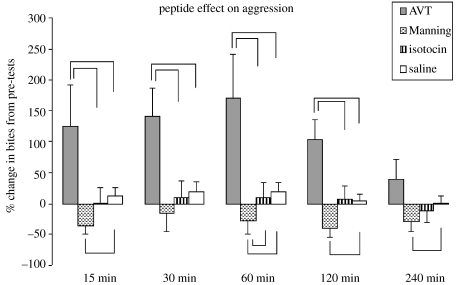
There was a significant overall effect of injection type on the change in aggression from pre-tests (F3,27=12.40, p<0.001; figure 1), but there was neither a significant overall effect of time (F4,36=1.12, p=0.36) nor a significant interaction between injection type and time (F12,108=1.08, p=0.38). Compared to saline injections, AVT significantly increased the number of bites, relative to pre-tests, that territorial males directed at bottled intruders at 15 min (F1,9=5.24, p=0.042), 30 min (F1,9=9.45, p=0.01), 60 min (F1,9=6.10, p=0.036) and 120 min (F1,9=5.71, p=0.041) post-injection, but not at 240 min (F1,9=0.73, p=0.73). Manning compound had the opposite effect: it significantly decreased the number of bites versus saline injections at 15 min (F1,9=11.39, p=0.008), 60 min (F1,9=5.76, p=0.039), 120 min (F1,9=10.04, p=0.011) and 240 min (F1,9=5.85, p=0.038). Although Manning compound did not significantly differ from saline at 30 min (F1,9=4.97, p=0.052), the low p-value indicates a strong trend in this direction. Isotocin had no significant effects on aggression versus saline injections at any time point (15 min, F1,9=1.93, p=0.19; 30 min, F1,9=0.84, p=0.38; 60 min, F1,9=0.524, p=0.489; 120 min, F1,9=0.596, p=0.45; and 240 min, F1,9=0.97, p=0.35).
Figure 1.
Compared to saline controls, AVT significantly increases aggression while the specific V1a receptor antagonist, Manning compound, decreases aggression. Isotocin has no effect on aggression. The mean and s.e. of the per cent change in aggression from pre-tests for peptide and saline control groups are presented. Data presented are actual data; statistics run on transformed lognormal data. All marked comparisons are significantly different at p<0.05.
Post hoc tests revealed that AVT significantly increased aggression versus isotocin at 15 min (Tukey's test, p=0.02), 30 min (Tukey's test, p=0.002), 60 min (Tukey's test, p=0.003) and 120 min (Tukey's test, p=0.033), but not at 240 min (Tukey's test, p=0.78). The change in aggression owing to Manning compound was not significantly different than that seen versus isotocin at 15 min (Tukey's test, p=0.55), 30 min (Tukey's test, p=0.7), 120 min (Tukey's test, p=0.16) or 240 min (Tukey's test, p=0.99). However, Manning compound significantly reduced aggression versus isotocin at 60 min (Tukey's test, p=0.03).
(b) Experiment 2: arginine vasotocin dose-dependent study
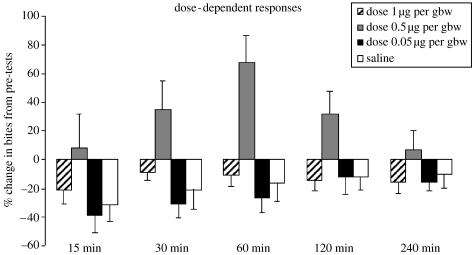
There was no significant overall effect of time on the change in aggression from pre-tests (F4,28=1.83, p=0.14; figure 2), but there was a significant overall effect of dose (F3,21=5.15, p=0.007) and a significant interaction between time and dose (F12,84=1.87, p=0.048). Versus saline injections, 1.0 μg per gbw did not significantly affect the change in aggression at 15 min (F1,7=1.18, p=0.31), 30 min (F1,7=0.87, p=0.38), 60 min (F1,7=0.39, p=0.55), 120 min (F1,7=0.69, p=0.43) or 240 min (F1,7=0.39, p=0.54). Similarly, the 0.05 μg per gbw dose did not produce significant differences versus saline controls across all time points (15 min, F1,7=2.24, p=0.17; 30 min, F1,7<0.001, p=0.99; 60 min, F1,7=1.86, p=0.38; 120 min, F1,7=0.17, p=0.89 and 240 min, F1,7=0.10, p=0.75). The 0.5 μg per gbw dose mimicked the results of experiment 1: it significantly increased aggression versus saline controls at 30 min (F1,7=7.10, p=0.03) and 60 min (F1,7=15.64, p=0.005), but not at 240 min (F1,7=1.94, p=0.20). While this dose significantly increased aggression at 15 and 120 min in experiment 1, here there were no significant differences at 15 min (F1,7=3.19, p=0.11) and 120 min (F1,7=4.88, p=0.06). However, the low p-values indicate strong trends towards an increase in aggression at these time points.
Figure 2.
AVT modulates aggression in a dose-dependent manner that reveals an U-shaped function for three doses (0.05, 0.5 and 1.0 μg per gbw). The mean and s.e. of the per cent change in aggression from pre-tests for different AVT doses and saline control group are presented. As in figure 1, data presented are actual data and statistics run on transformed lognormal data. All marked comparisons are significantly different at p<0.05.
4. Discussion
We demonstrate that AVT facilitates aggression within males of the highly territorial, polygynous beaugregory damselfish. This included multiple tests of the selectivity of the effect on AVT and AVT V1-type receptors, i.e. Manning compound inhibited aggression indicating that AVT's modulation of aggression is, at least in part if not entirely, mediated by a V1-type receptor. The closely related nonapeptide isotocin (see §1) had no significant effect on aggression. Other studies showing aggression facilitation by either AVT or AVP with adequate reciprocal antagonist effects include colonial zebra finches and communal prairie voles, respectively (see §1). The highly aggressive and territorial nature of beaugregory damselfish is opposite of that seen in other species (and consequently opposite of our predictions, see §1). Goodson & Evans (2004) propose that the divergent AVT effects on aggression between territorial and colonial tetrapods may depend on a consistent AVT effect on social stress with the resulting pattern of overt aggression explained by the unique context and species-specific purpose of the aggression. Perhaps a more complete understanding of AVT/AVP's effect (or lack thereof) on other social behaviours such as courtship, mate guarding and parental care within a particular species will further explain those, like damselfish, that diverge from the pattern thus far shown for aggression among many tetrapods.
(a) Arginine vasotocin's behavioural response specificity
AVT effects were observed by 15 min, which is consistent with the time course of AVT effects in field sparrows under semi-natural conditions (Goodson 1998a), but sooner than the earliest time point tested in the field studies of pupfish (Lema & Nevitt 2004; 30 min) and bluehead wrasse (Semsar et al. 2001; 20–90 min). Here, we used intramuscular injections while previous teleost studies used intraperitoneal injections. Intraperitoneal injections should allow quicker uptake of hormone administration owing to the large amount of blood flow through the gut; however, we show that intramuscular injections have effects as rapid, if not sooner, than intraperitoneal injections. Although the dose–response experiment did not show effects at either 15 or 120 min, there were strong trends in this direction. This could be explained by the inability of the smaller sample size in experiment 2 to detect this effect and perhaps these time points are the upper and lower limits of effect duration. However, both experiments show that exogenous AVT facilitates aggression and that this effect persists for 2–4 h. The long duration (at least 4 h) of the Manning compound effect is consistent with that previously reported (Kruszynski et al. 1980; also see Goodson & Bass 2000).
The dose–response experiment supported AVT's specificity for modulating aggression and revealed an apparently novel inverted U-shaped function for neuropeptide modulation of social behaviour. The inverted U-shaped response to AVT may relate, in part, to the methodology followed in this study, such as testing animals in their natural habitat and delivering AVT via intramuscular injections. A wide range of administered AVT doses might reveal its efficacy in modulating behaviour in studies that report an AVT effect in some individuals but not others such as NT-TP and T-TP male wrasses, respectively (see §1). Moreover, AVT studies that have demonstrated dose-dependent effects (e.g. Moore & Miller 1983; Propper & Dixon 1997; Lema & Nevitt 2004) might have also revealed a similar function if they had administered even higher doses. Importantly, here, we reject the possibility that the higher AVT dose in our study elicited toxicity effects negating the facilitating effects of AVT. Males were equally aggressive at high doses as their saline controls and we would expect toxicity effects to either severely reduce or inhibit behaviour altogether, thus producing a significant decrease in aggression compared to controls.
The use of a limited range of doses in other studies may arise, in part, because the AVT doses employed are often adopted from previous studies in different species under different contexts. For example, the AVT dose used to explore bluehead wrasse aggression (Semsar et al. 2001) was based on an earlier report of AVT-stimulated advertisement calling in amphibians (Propper & Dixon 1997). Similarly, the AVT dose used to explore aggression in European starlings, Sturnus vulgaris (Nephew et al. 2005), was based on the effective AVT dose that modulated appetitive sexual behaviour in Japanese quail (Castagna et al. 1998). This is a logical methodology to follow when initiating exploration in a system, but could obscure potential species-specific, fine-scaled differences in neuropeptide effectiveness as shown here for damselfish. We recognize the inherent difficulty in completing an extensive set of physiological manipulations under field conditions as was done here, but such a suite of tests may be necessary to accurately portray any one species' sensitivity to a neurochemical. This also raises the possibility that AVT's influences are behaviour-specific. For example, while we tested AVT's modulation of overt aggression, it probably influences other agonistic actions such as vocalization (reviewed in Goodson & Bass 2001), which damselfish are well known to exhibit during conspecific encounters (Myrberg et al. 1978; Myrberg & Spires 1980). Owing to the nature of fight escalation, the neural modulation of agonistic signalling and overt attack behaviour may be modulated differently (see §1). The results presented here for territorial male damselfish and AVT's inhibition of fictive agonistic grunting in territorial type I male midshipman (Goodson & Bass 2000a) might indicate a simultaneous decrease in agonistic calling in damselfish and an increase in overt aggression in type I midshipman. Differential modulation of these behaviours could be accomplished via either different neurohormones for each behaviour or different mechanisms of a single neurohormone.
Though inverted U-shaped dose functions have not been previously reported for behavioural studies with neuropeptides, they have been observed with steroid hormones. For example, glucocorticoids have been shown to either stimulate or inhibit appetite at low and high doses, respectively (reviewed in Sapolsky et al. 2000). Similarly, the finely tuned AVT response shown here for damselfish might also provide a control mechanism for switching from one behavioural state to another by altering endogenous levels of a single hormone. Behaviourally, this could play a role in encounters that require escalation of aggression, such as potential territory takeovers from rival males or competition for mates (reviewed in Archer 1988; also see Santangelo et al. 2002). For example, AVT showed significant increases in aggression by 15 min with the magnitude of this increase continuing to rise over time, maximizing at 2 h. Males engaged in these encounters would eventually need to revert these aggression levels back to baseline. With an inverted U-shaped dose function, this could be achieved by increasing the circulating AVT levels further. Although the time course of the AVT effects observed in this study may not be of the order of that required under natural circumstances, local endogenous AVT could have even more rapid effects in modulating aggression. For example, in newts, intracerebroventricular AVT administration was more rapid in activating sexual behaviour than systemic intraperitoneal injections (Moore & Miller 1983).
(b) Neuroendocrine mechanisms
Perhaps the most parsimonious explanation at this point for the neuroendocrine basis for an inverted U-shaped dose–response is the unusually high affinity that teleost isotocin receptors have for AVT (relative to AVT receptor affinity for isotocin; Hausmann et al. 1996). Higher concentrations of AVT could potentially bind to either isotocin or other receptor types and negate the effects of bound AVT receptors. Although our results indicate that isotocin does not affect aggression at a dose that is effective for AVT, we cannot conclude that isotocin has no involvement in this mechanism. Dose–response experiments like those conducted here for AVT, together with the use of an isotocin antagonist, might reveal an isotocin effect. An alternative, but not mutually exclusive, hypothesis is that there may be activation of multiple AVT receptor types, analogous to a mechanism proposed to explain inverted U-shaped functions in steroid studies where receptor types may differ in their dose-dependent sensitivity (see Sapolsky et al. 2000). In tetrapods, the multiple physiological effects of AVT/AVP are governed by three receptor types: V1a, V1b and V2 (Strand 1999). As in our study, the V1-type receptor has appropriately been the focus of most others investigating AVT's behavioural mechanisms of action (Goodson & Bass 2001). The V1a receptor is the dominant one expressed in the brain (Strand 1999) and, while the V2 receptor mainly has a peripheral distribution, the V1b receptor is also distributed in the brain (Strand 1999) making it a candidate also to mediate behavioural modulation by AVT. While only V1-type receptors have so far been identified in teleosts (Mahlmann et al. 1994; Warne 2001; Guibbolini & Avella 2003), the presence of multiple AVT receptor subtypes in other taxa at least suggests their presence and possible co-modulation of behaviour among teleosts.
Finally, it remains possible that the AVT effects shown here might also depend upon their interactions with physiological factors other than neuropeptides, such as circulating steroid hormone levels that vary with aggression (reviewed in Adkins-Regan 2005). For example, studies on newts have shown that the effects of AVT and corticosterone on the responsiveness of neurons involved in courtship are dependent on the order in which these neurons are exposed to each hormone and the temporal spacing between exposures (Rose et al. 1995). Although all the subjects in the current study showed the same behavioural response to each treatment group, differences in circulating steroids may contribute to individual variation in the time dependency of behavioural state transitions.
(c) Comparisons between teleosts and tetrapods
The above mechanisms might also explain species- and context-specificity for AVT effects across taxa. However, the available data suggest that there may yet be species-specificity in the neuronal populations that are modulated by AVT/AVP. While both teleosts and tetrapods have hypothalamic AVT/AVP populations, tetrapods also have AVT/AVP neurons in the bed nucleus of the stria terminalis and portions of the amygdala (the ‘extended amygdala’) with their own network of target neurons that may play an essential role in the control of species- and context-dependent effects (Goodson & Bass 2001). While the divergent results across the current and prior AVT behavioural studies on teleosts (see §1) cannot currently be explained on the basis of a non-hypothalamic AVT population (e.g. Goodson & Bass 2000b), the potential for species-specific patterns of AVT receptor distribution that allow AVT to target diverse populations of neurons could provide a mechanism for these divergent behavioural patterns.
(d) Concluding comments
Testing animals in their native habitat and the use of multiple tests of selectivity, including dose–response experiments, are required to adequately explore the hormonal modulation of behaviour. These types of studies will help to clarify the pattern of AVT's influence on social behaviours across teleosts, especially if we focus on closely related teleosts. We chose the Pomacentridae family for this study, in part, because it includes a wide variety of closely related damselfish that exhibit very different breeding ecologies (e.g. Whiteman & Côté 2004), thus enabling the start of a systematic exploration of breeding ecology and species relatedness in the neuropeptide modulation of behaviour in teleosts. This will also facilitate comparisons with birds and mammals where a similar comparative approach has also been undertaken (see Goodson et al. 2005). Finally, we need to explore the effects of other hormones on aggression, such as isotocin and/or corticosteroids, and if there are interactive effects with AVT in modulating behaviour. These types of questions will aid in our understanding of how multiple neuropeptide systems interact to provide the adaptive evolution of animal social behaviour.
Acknowledgments
We thank the Pigeon Key Foundation, their interns, and especially director Sherri Hitz for all the help they provided in completing this project without whom none of this would have been possible at their field station. We thank Luke Remage-Healey, Elizabeth Adkins-Regan, the Adkins-Regan lab group, the Bass lab group for helpful critiques and suggestions during the course of this project, and James Goodson, Ann Magurran and three anonymous reviewers for their many helpful suggestions on an earlier draft. We also thank Margaret Marchaterre for invaluable field support and the late Art Myrberg for directing us to Pigeon Key as a field site. This research adhered to the Association for the Study of Animal Behaviour, Animal Behaviour Society Guidelines for the Use of Animals in Research, the legal requirements of the USA, and was approved by Cornell University IACUC Committee under protocol number 03-21. Research support from NIH (NRSA 1F32 MH069066 to N.S.) and NSF (IBN-9987341, 0516748 to A.H.B.) is gratefully acknowledged.
References
- Adkins-Regan E. Hormones and animal social behavior. Princeton University Press; Princeton, NJ: 2005. Mating, fighting, parenting, and signaling; pp. 34–91. [Google Scholar]
- Archer J. Cambridge University Press; Cambridge, UK: 1988. The behavioral biology of aggression. [Google Scholar]
- Barberis C, Morin D, Durroux T, Mouillac B, Guillon G, Seyer R, Hibert M, Tribollet E, Manning M. Molecular pharmacology of AVP and OT receptors and therapeutic potential. Drug News Perspect. 1999;12:279–292. doi:10.1358/dnp.1999.12.5.863621 [Google Scholar]
- Bass A.H. Shaping brain sexuality. Am. Sci. 1996;84:352–363. [Google Scholar]
- Bass A.H, Baker R. Sexual dimorphisms in the vocal control system of a teleost fish: morphology of physiologically identified neurons. J. Neurobiol. 1990;21:1155–1168. doi: 10.1002/neu.480210802. doi:10.1002/neu.480210802 [DOI] [PubMed] [Google Scholar]
- Bastian J, Schniederjan S, Schniederjan J. Arginine vasotocin modulates a sexually dimorphic communication behavior in the weakly electric fish Apteronotus leptorhynchus. J. Exp. Biol. 2001;204:1901–1923. doi: 10.1242/jeb.204.11.1909. [DOI] [PubMed] [Google Scholar]
- Bentley P.J. The chemical structure, polymorphism, and evolution of hormones. In: Bentley P.J, editor. Comparative vertebrate endocrinology. Cambridge University Press; Cambridge, UK: 1998. pp. 65–176. [Google Scholar]
- Brantley R.K, Bass A.H. Alternative male spawning tactics and acoustic signals in the plainfin midshipman fish Porichtchys notatus Girard (Teleostei, Batrachoididae) Ethology. 1994;96:213–232. [Google Scholar]
- Carter C.S, Roberts R.L. The psychobiological basis of cooperative breeding in rodents. In: Solomon N.G, French J.A, editors. Cooperative breeding in mammals. Cambridge University Press; Cambridge, UK: 1996. pp. 231–266. [Google Scholar]
- Carter C.S, DeVries A.C, Getz L.L. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci. Biobehav. Rev. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. doi:10.1016/0149-7634(94)00070-H [DOI] [PubMed] [Google Scholar]
- Castagna C, Absil P, Foidart A, Balthazart J. Systemic and intracerebroventricular injections of vasotocin inhibit appetitive and consummatory components of male sexual behavior in Japanese quail. Behav. Neurosci. 1998;112:233–250. doi: 10.1037//0735-7044.112.1.233. doi:10.1037/0735-7044.112.1.233 [DOI] [PubMed] [Google Scholar]
- Cleveland A.L, Ludlow A, Itzkowitz M, Draud M, Haley M. Dominance relationships between territorial neighbors in the beaugregory damselfish (Stegastes leucostictus) Behaviour. 2003;140:1021–1037. doi:10.1163/156853903322589623 [Google Scholar]
- Ferris C.F, Delville Y. Vasopressin and serotonin interactions in the control of agonistic behavior. Psychoneuroendocrinology. 1994;19:593–601. doi: 10.1016/0306-4530(94)90043-4. doi:10.1016/0306-4530(94)90043-4 [DOI] [PubMed] [Google Scholar]
- Fusani L, Canoine V, Goymann W, Wikelski M, Hau M. Difficulties and special issues associated with field research in behavioral neuroendocrinology. Horm. Behav. 2005;48:484–491. doi: 10.1016/j.yhbeh.2005.05.005. doi:10.1016/j.yhbeh.2005.05.005 [DOI] [PubMed] [Google Scholar]
- Goodson J.L. Territorial aggression and dawn song are modulated by septal vasotocin and vasoactive intestinal polypeptide in male field sparrows (Spizella pusilla) Horm. Behav. 1998a;34:67–77. doi: 10.1006/hbeh.1998.1467. doi:10.1006/hbeh.1998.1467 [DOI] [PubMed] [Google Scholar]
- Goodson J.L. Vasotocin and vasoactive intestinal polypeptide modulate aggression in a territorial songbird, the violet eared waxbill (Estrildidae: Uraeginthus granatina) Gen. Comp. Endocrinol. 1998b;111:233–244. doi: 10.1006/gcen.1998.7112. doi:10.1006/gcen.1998.7112 [DOI] [PubMed] [Google Scholar]
- Goodson J.L, Adkins-Regan E. Effect of intraseptal vasotocin and vasoactive intestinal polypeptide infusions on courtship song and aggression in the male zebra finch (Taeniopygia guttata) J. Neuroendocrinol. 1999;11:19–25. doi: 10.1046/j.1365-2826.1999.00284.x. doi:10.1046/j.1365-2826.1999.00284.x [DOI] [PubMed] [Google Scholar]
- Goodson J.L, Bass A.H. Forebrain peptide modulation of sexually polymorphic vocal circuitry. Nature. 2000a;403:769–772. doi: 10.1038/35001581. doi:10.1038/35001581 [DOI] [PubMed] [Google Scholar]
- Goodson J.L, Bass A.H. Vasotocin innervation and modulation of vocal-acoustic circuitry in the teleost Porichthys notatus. J. Comp. Neurol. 2000b;422:363–379. doi: 10.1002/1096-9861(20000703)422:3<363::aid-cne4>3.0.co;2-8. doi:10.1002/1096-9861(20000703)422:3<363::AID-CNE4>3.0.CO;2-8 [DOI] [PubMed] [Google Scholar]
- Goodson J.L, Bass A.H. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain. Res. Rev. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. doi:10.1016/S0165-0173(01)00043-1 [DOI] [PubMed] [Google Scholar]
- Goodson J.L, Evans A.K. Neural responses to territorial challenge and nonsocial stress in male song sparrows: segregation, integration, and modulation by a vasopressin V1 antagonist. Horm. Behav. 2004;46:371–381. doi: 10.1016/j.yhbeh.2004.02.008. doi:10.1016/j.yhbeh.2004.02.008 [DOI] [PubMed] [Google Scholar]
- Goodson J.L, Evans A.K, Lindberg L, Allen C.D. Neuro-evolutionary patterning of sociality. Proc. R. Soc. B. 2005;272:227–235. doi: 10.1098/rspb.2004.2892. doi:10.1098/rspb.2004.2892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guibbolini M.E, Avella M. Neurohypophysial hormone regulation of Cl− secretion: physiological evidence for V1-type receptors in sea bass gill respiratory cells in culture. J. Neuroendocrinol. 2003;176:111–119. doi: 10.1677/joe.0.1760111. [DOI] [PubMed] [Google Scholar]
- Hausmann H, Richters A, Kreienkamp H, Meyerhof W, Mattes H, Lederis K, Zwiers H, Richter D. Mutational analysis and molecular modeling of the nonapeptide hormone binding domains of the [Arg8] vasotocin receptor. Proc. Natl Acad. Sci. USA. 1996;93:6907–6912. doi: 10.1073/pnas.93.14.6907. doi:10.1073/pnas.93.14.6907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzkowtiz M. Aspects of the population dynamics and reproductive success in the permanently territorial beaugregory damselfish. Mar. Behav. Physiol. 1985;12:57–69. [Google Scholar]
- Itzkowitz M. Habitat quality and reproductive success in the beaugregory damselfish. Environ. Biol. Fishes. 1991;30:287–293. doi:10.1007/BF02028844 [Google Scholar]
- Itzkowtiz M, Haley M. Are males with more attractive resources more selective in their mate preferences? A test in a polygynous species. Behav. Ecol. 1999;10:366–371. doi:10.1093/beheco/10.4.366 [Google Scholar]
- Johnston R.E. How do hamsters know whose scent is on top and why should it matter? In: Johnston R.E, Müller-Schwarze D, Sorensen P.W, editors. Advances in chemical signals in vertebrates. Kluwer Academic/Plenum Publishers; New York, NY: 1999. pp. 227–238. [Google Scholar]
- Keppel G. 3rd edn. Prentice Hall; Upper Saddle River, NJ: 1991. Design and analysis: a researcher's handbook. [Google Scholar]
- Kruszynski M, Lammek B, Manning M. [1-(β-Mercapto- β,β-cyclopentamethylenepropionic acid),2-(O-methyl)tyrosine]arginine-vasopressin and [1-(β-Mercapto- β,β-cyclopentamethylenepropionic acid)]arginine-vasopressin, two highly potent antagonists of the vasopressor response to arginine-vasopressin. J. Med. Chem. 1980;23:364–368. doi: 10.1021/jm00178a003. doi:10.1021/jm00178a003 [DOI] [PubMed] [Google Scholar]
- Lema S, Nevitt G.A. Exogenous vasotocin alters aggression during agonistic exchanges in male Amargosa River pupfish (Cyprinodon nevadensis amargosae) Horm. Behav. 2004;46:628–637. doi: 10.1016/j.yhbeh.2004.07.003. doi:10.1016/j.yhbeh.2004.07.003 [DOI] [PubMed] [Google Scholar]
- Mahlmann S, Meyerhof W, Hausmann H, Heierhorst J, Schönrock C, Zwiers H, Lederis K, Richter D. Structure, function, and phylogeny of [Arg8]vasotocin receptors from teleost fish and toad. Proc. Natl Acad. Sci. USA. 1994;91:1342–1345. doi: 10.1073/pnas.91.4.1342. doi:10.1073/pnas.91.4.1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore F.L, Miller L.J. Arginine vasotocin induces sexual behavior of newts by acting on cells in the brain. Peptides. 1983;4:97–102. doi: 10.1016/0196-9781(83)90173-0. doi:10.1016/0196-9781(83)90173-0 [DOI] [PubMed] [Google Scholar]
- Myrberg A.A, Spires J.Y. Hearing in damselfishes—an analysis of signal-detection among closely related species. J. Comp. Physiol. 1980;140:135–144. doi:10.1007/BF00606305 [Google Scholar]
- Myrberg A.A, Spanier E, Ha S.J. Temporal patterning in acoustical communication. In: Reese E.S, Lighter F.J, editors. Contrasts in behavior. Wiley; New York, NY: 1978. pp. 137–179. [Google Scholar]
- Nephew B.C, Aaron R.S, Romero M.L. Effects of arginine vasotocin (AVT) on the behavioral, cardiovascular, and corticosterone responses of starlings (Sturnus vulgaris) to crowding. Horm. Behav. 2005;47:280–289. doi: 10.1016/j.yhbeh.2004.11.007. doi:10.1016/j.yhbeh.2004.11.007 [DOI] [PubMed] [Google Scholar]
- Parker G.A. Assessment strategy and the evolution of fighting behaviour. J. Theor. Biol. 1974;47:223–243. doi: 10.1016/0022-5193(74)90111-8. doi:10.1016/0022-5193(74)90111-8 [DOI] [PubMed] [Google Scholar]
- Propper P.R, Dixon T.B. Differential effects of arginine vasotocin and gonadotropin-releasing hormone on sexual behaviors in an anuran amphibian. Horm. Behav. 1997;32:99–104. doi: 10.1006/hbeh.1997.1408. doi:10.1006/hbeh.1997.1408 [DOI] [PubMed] [Google Scholar]
- Rose J.D, Kinnard J.R, Moore F.L. Neurophsiological effects of vasotocin and corticosterone on medullary neurons: implications for hormonal control of amphibian courtship behavior. Neuroendocrinology. 1995;62:406–417. doi: 10.1159/000127030. [DOI] [PubMed] [Google Scholar]
- Santangelo N, Itzkowitz M, Richter M, Haley M.P. Resource attractiveness of the male beaugregory and his decision to court or defend. Behav. Ecol. 2002;13:676–681. doi:10.1093/beheco/13.5.676 [Google Scholar]
- Sapolsky R.M, Romero M, Munck A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. doi:10.1210/er.21.1.55 [DOI] [PubMed] [Google Scholar]
- Semsar K, Kandel F.L.M, Godwin J. Manipulations of the AVT system shift social status and related courtship and aggressive behavior in the bluehead wrasse. Horm. Behav. 2001;40:21–31. doi: 10.1006/hbeh.2001.1663. doi:10.1006/hbeh.2001.1663 [DOI] [PubMed] [Google Scholar]
- Strand F.L. Neuropeptides: regulators of physiological processes. In: Stevens C.F, editor. MIT Press; Cambridge, MA: 1999. [Google Scholar]
- Warne J.M. Cloning and characterization of an arginine vasotocin receptor from the euryhaline flounder Platichthys flesus. Gen. Comp. Endocrinol. 2001;122:312–319. doi: 10.1006/gcen.2001.7644. doi:10.1006/gcen.2001.7644 [DOI] [PubMed] [Google Scholar]
- Warner R.R, Schultz E.T. Sexual selection and male characteristics in the bluehead wrasse, Thalassoma bifasciatum: mating site acquisition, mating site defense, and female choice. Evolution. 1992;46:1421–1442. doi: 10.1111/j.1558-5646.1992.tb01134.x. doi:10.2307/2409947 [DOI] [PubMed] [Google Scholar]
- Whiteman E.A, Côté I.M. Monogamy in marine fishes. Biol. Rev. 2004;79:351–375. doi: 10.1017/s1464793103006304. doi:10.1017/S1464793103006304 [DOI] [PubMed] [Google Scholar]
- Young L.J, Winslow J.T, Nilsen R, Insel T.R. Species differences in V1a receptor gene expression in monogamous and nonmonogamous voles: behavioral consequences. Behav. Neurosci. 1997;111:599–605. doi: 10.1037//0735-7044.111.3.599. doi:10.1037/0735-7044.111.3.599 [DOI] [PubMed] [Google Scholar]
- Zann R.A. Oxford University Press; Oxford, UK: 1996. The zebra finch: a synthesis of field and laboratory studies. [Google Scholar]
- Zar J.H. Prentice Hall; Upper Saddle River, NJ: 1999. Biostatistical analysis. [Google Scholar]
Notice of correction
Table 1 is now presented in its correct form.
31 October 2006