Abstract
The two sibling bat species Myotis myotis and Myotis blythii occur in sympatry over wide areas of Southern and Central Europe. Morphological, ecological and previous genetic evidence supported the view that the two species constitute two well-differentiated groups, but recent phylogenetic analyses have shown that the two species share some mtDNA haplotypes when they occur in sympatry. In order to see whether some genetic exchange has occurred between the two species, we sequenced a highly variable segment of the mitochondrial control region in both species living in sympatry and in allopatry. We also analysed the nuclear diversity of 160 individuals of both species found in two mixed nursery colonies located north and south of the Alps. MtDNA analysis confirmed that European M. blythii share multiple, identical or very similar haplotypes with M. myotis. Since allopatric Asian M. blythii presents mtDNA sequences that are very divergent from those of the two species found in Europe, we postulate that the mitochondrial genome of the European M. blythii has been replaced by that of M. myotis. The analysis of nuclear diversity shows a strikingly different pattern, as both species are well differentiated within mixed nursery colonies (FST=0.18). However, a Bayesian analysis of admixture reveals that the hybrids can be frequently observed, as about 25% of sampled M. blythii show introgressed genes of M. myotis origin. In contrast, less than 4% of the M. myotis analysed were classified as non-parental genotypes, revealing an asymmetry in the pattern of hybridization between the two species. These results show that the two species can interbreed and that the hybridization is still ongoing in the areas of sympatry. The persistence of well-differentiated nuclear gene pools, in spite of an apparent replacement of mitochondrial genome in European M. blythii by that of M. myotis, is best explained by a series of introgression events having occurred repeatedly during the recent colonization of Europe by M. blythii from Asia. The sharp contrast obtained from the analysis of mitochondrial and nuclear markers further points to the need to cautiously interpret results based on a single class of genetic markers.
Keywords: hybridization, introgression, mtDNA, microsatellites, phylogeography, speciation
1. Introduction
The role of hybridization in evolution is still debated among biologists. For botanists, it is clear that hybridization plays a role in creating new diversity either by introgression of single alleles, or by the establishment of recombinant genotypes as a new species (Barton 2001). Zoologists traditionally view hybridization as a rare phenomenon, an ‘evolutionary dead end’ of no particular significance in evolution, except to reinforce reproductive isolation of species through selection against hybrids (Dowling & Secor 1997). Some recent reviews and theoretical studies have tried to reconcile these different views, in particular by jointly considering the effects of gene flow and selection (Barton 2001; Morjan & Rieseberg 2004; Seehausen 2004), i.e. those species may evolve collectively at major loci through the spread of favourable alleles, while simultaneously diverging at other loci owing to drift and local adaptation.
We report in this paper a study on the genetic relationship between two sibling mouse-eared bat species, Myotis myotis (Borkhausen, 1797) and Myotis blythii (Tomes, 1857). These two species occur sympatrically over most of Southern and Central Europe and often form mixed roosting groups (Ruedi et al. 1990; Arlettaz et al. 1991). Although both species are difficult to distinguish morphologically and qualify as sibling species, a combination of several morphological measurements generally allows an unambiguous discrimination (Ruedi et al. 1990; Arlettaz et al. 1991). Two allozyme loci also clearly distinguish the two species, as no hybrid genotypes were found in more than 300 individuals analysed in sympatry (Ruedi et al. 1990; Arlettaz et al. 1997b). The two species were also shown to differ in their ecology and physiology, i.e. they generally use different feeding habitats, occupy distinct food niches (Arlettaz et al. 1997a) and their parturition time differs (Arlettaz et al. 2001). Given these multiple differences and fossil records, Arlettaz et al. (1997b) proposed a scenario of allopatric speciation for the two species, where the ancestral Myotis population would have become subdivided during a glacial period of the Pleistocene. The ancestral M. myotis would have occupied the western range and adapted to forested habitats of the Mediterranean region, while the ancestral M. blythii found in the eastern part of the range would have adapted to Asian steppic environments. Under this scenario, no gene flow would have been possible between the two species until M. blythii reinvaded Europe, probably as late as during the Holocene (Piksa & Woloszyn 2001).
At the intraspecific level, a phylogeographical study of M. myotis has revealed that mtDNA diversity is highly structured geographically, with the Alps constituting a major barrier to dispersal (Castella et al. 2001; Ruedi & Castella 2003). While nuclear diversity is not as highly structured, the Alps still constitutes a significant barrier to gene flow (Castella et al. 2001). However, a recent study of mtDNA diversity raised the possibility of past gene flow between M. myotis and M. blythii. Indeed, Castella et al. (2000) showed that some M. blythii from Spain shared the same mitochondrial cytochrome b haplotype as that found in most Iberian M. myotis. The analysis of another mtDNA gene (nd1) further revealed that lineages from both species did not constitute monophyletic units (Mayer & Helversen 2001). This unexpected sharing of mtDNA lineages between both species could be explained by the retention of some ancestral polymorphism, incomplete lineage sorting, or recent introgression between M. myotis and M. blythii.
In order to investigate more precisely the nature of genetic interactions between these two species, we sequenced the mtDNA control region of additional M. blythii sampled across other areas of sympatry to refine the phylogeographical picture of the M. myotis–M. blythii complex in Europe. We also investigated the pattern of nuclear diversity at five microsatellite markers in two mixed nursery colonies of Switzerland and Italy, to see if the observed mtDNA lineage sharing could be confirmed at the nuclear level and if recent traces of interspecific gene flow could be found between the two sister species.
2. Material and methods
(a) Sampling and DNA amplifications
To investigate the degree of overlap between mitochondrial lineages in Europe, we extended the extensive sampling of M. myotis mtDNA control region sequences already available (see Ruedi & Castella 2003) with 7 samples of M. myotis from Greece and Bulgaria and with 21 samples of M. blythii from Spain, France, Italy, Bulgaria and Greece (table 1). Both species are known to live in sympatry in all these areas. As no M. myotis occur in central Asia, four M. blythii from Kirghiztan were also analysed to act as an allopatric outgroup.
Table 1.
Sequence variation and lineage identification of 32 M. myotis and M. blythii analysed for 308 bp of mtDNA HVII. Haplotypes H1–H43 have already been reported in M. myotis (see Ruedi & Castella 2003), while H58–H75 are new variants. (Dots represent the same nucleotide as in H1, and hyphens represent indels. Only variations of the initial 180 bp are shown here, but the full length table is available in the electronic supplementary material.)
| polymorphic positions | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 5 | 6 | 7 | 9 | 9 | 9 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| 0 | 3 | 4 | 7 | 9 | 6 | 8 | 1 | 2 | 5 | 1 | 1 | 2 | 2 | 2 | 2 | 3 | 4 | 4 | 5 | 6 | 6 | 7 | 7 | 7 | |||||
| species | origin | field number | haplo-type | haplo-group | 8 | 9 | 1 | 2 | 3 | 4 | 0 | 5 | 6 | 9 | 1 | 5 | 3 | 7 | 8 | ||||||||||
| M. myotis | Bulgaria | BSM13, 14 | H1 | A | G | A | A | A | T | T | G | T | T | G | — | T | A | A | A | C | A | T | A | A | A | G | A | T | G |
| M. myotis | Bulgaria | BSM4 | H33 | A | · | · | · | · | · | · | · | C | · | · | — | · | · | · | · | · | · | · | · | · | · | · | · | · | · |
| M. myotis | Greece | M749 | H1 | A | · | · | · | · | · | · | · | · | · | · | — | · | · | · | · | · | · | · | · | · | · | · | · | · | · |
| M. blythii | Spain | M122, 135 | H1 | A | · | · | · | · | · | · | · | · | · | · | — | · | · | · | · | · | · | · | · | · | · | · | · | · | · |
| M. blythii | Spain | MbB | H27 | A | · | · | · | · | · | · | · | · | · | · | — | · | · | G | · | · | · | · | · | · | · | · | · | · | · |
| M. blythii | Spain | M165 | H63 | A | · | · | · | · | · | · | · | · | C | · | — | · | · | · | · | · | · | · | · | · | · | · | · | · | · |
| M. blythii | Spain | M235 | H64 | A | · | · | · | · | · | · | · | · | · | · | — | · | · | G | · | · | · | · | · | · | · | · | · | · | · |
| M. blythii | Spain | M265 | H65 | A | · | · | · | · | · | · | · | C | · | · | — | · | · | G | · | · | · | · | · | · | · | · | · | · | · |
| M. blythii | France | V46, 47 | H1 | A | · | · | · | · | · | · | · | · | · | · | — | · | · | · | · | · | · | · | · | · | · | · | · | · | · |
| M. blythii | France | EMC—3 | H27 | A | · | · | · | · | · | · | · | · | · | · | — | · | · | G | · | · | · | · | · | · | · | · | · | · | · |
| M. blythii | Italy | M634 | H13 | C | · | · | · | · | · | · | · | C | · | A | — | C | · | · | · | · | · | · | G | · | · | · | · | C | T |
| M. blythii | Italy | Pi40 | H15 | C | · | · | · | G | · | · | · | C | · | A | — | . | · | · | · | · | · | · | G | · | · | · | · | · | · |
| M. blythii | Italy | M633 | H66 | C | · | · | · | G | · | · | · | C | · | A | — | . | · | · | · | · | · | · | G | · | · | · | · | C | A |
| M. blythii | Italy | Pi95 | H69 | C | · | · | · | · | · | · | · | C | · | A | — | C | · | · | · | · | · | · | G | · | · | · | · | C | T |
| M. blythii | France | EMC—2 | H62 | C | · | · | · | G | · | · | · | C | · | A | — | · | · | · | · | · | · | · | G | · | · | · | · | C | · |
| M. myotis | Greece | M752 | H34 | D | A | · | · | · | · | · | · | C | · | A | — | · | · | G | · | · | · | · | · | G | G | · | · | · | · |
| M. blythii | Greece | M759, 760 | H34 | D | A | · | · | · | · | · | · | C | · | A | — | · | · | G | · | · | · | · | · | G | G | · | · | · | · |
| M. myotis | Bulgaria | BSM3 | H35 | D | A | · | · | · | · | · | · | C | · | A | — | · | · | G | · | · | · | · | · | G | G | · | · | · | · |
| M. myotis | Bulgaria | BSM1 | H58 | D | A | · | · | · | · | · | · | C | · | A | — | · | · | G | · | · | G | · | · | G | G | · | · | · | · |
| M. blythii | Bulgaria | BSMb10 | H59 | D | A | · | · | · | · | · | · | C | · | A | — | · | · | G | · | · | · | · | · | G | G | · | · | · | A |
| M. blythii | Bulgaria | BSMb12 | H61 | D | A | · | · | · | · | · | · | C | · | A | — | · | · | G | · | · | · | · | · | G | G | · | · | · | · |
| M. blythii | Bulgaria | BSMb11 | H60 | F | · | · | G | · | · | · | · | C | · | A | — | · | · | G | · | · | · | · | G | G | G | · | · | · | · |
| M. blythii | Greece | M819 | H67 | F | · | G | G | · | · | · | · | C | · | A | — | . | · | G | · | · | · | · | G | G | G | · | · | · | · |
| M. blythii | Greece | M839 | H68 | F | · | · | G | · | · | · | · | C | · | A | — | . | · | G | · | · | · | · | G | G | G | · | · | · | · |
| M. blythii | Kirghiztan | Mb1 | H72 | Kgz | A | · | G | · | C | C | · | C | · | A | C | C | G | G | G | T | · | C | T | · | · | A | G | · | A |
| M. blythii | Kirghiztan | Mb2 | H73 | Kgz | A | · | G | · | C | C | A | C | · | A | C | C | G | G | G | T | · | C | T | · | · | · | G | · | A |
| M. blythii | Kirghiztan | Mb4 | H74 | Kgz | A | · | · | · | C | C | A | C | · | A | C | C | G | G | G | T | · | C | T | · | · | A | G | · | A |
| M. blythii | Kirghiztan | Mb5 | H75 | Kgz | A | · | · | · | C | C | A | C | · | A | C | C | G | G | G | T | · | C | T | · | · | A | G | · | A |
The nuclear diversity was investigated in two mixed nursery colonies located in Southern Switzerland (Valais) and in Northern Italy (Aglié). Forty bats of each species were sampled and analysed in each colony, thus constituting a total of 160 individuals. The Swiss bats were actually sampled in two neighbouring colonies (at Raron and Naters), 14 km apart, but we considered them as a single colony since previous ring studies (R. Arlettaz 2004, personal communication) had shown that the same individuals used both colonies, and because there was no significant genetic differentiation between them (FST=0.008, p>0.10, as previously determined by Castella et al. 2001). Species identification was made in the field using morphological criteria (ear length, tooth row length, presence or absence of a white spot between the ears) defined by Arlettaz et al. (1991). Note that a few (less than 5%) individuals that could not be unambiguously identified with these external criteria were released without being sampled.
The tissue samples were obtained using a sterile biopsy punch of the wing membrane (Worthington Wilmer & Barratt 1996). The wing biopsies were fixed in 90% ethanol at ambient temperature and then stored at −20°C until DNA extraction. Based on whole DNA extracts, 308 bp of the second hypervariable domain (HVII) of the control region (D-loop) were amplified and sequenced, as described by Castella et al. (2001). The bats from the mixed colonies were genotyped at the five microsatellite loci that successfully amplified for both species (C113, D9, E24, H29 and H19; Castella & Ruedi 2000). Seven other loci developed for M. myotis could not be used because they failed to amplify in M. blythii or gave uninterpretable banding patterns.
(b) Measure of interbreeding
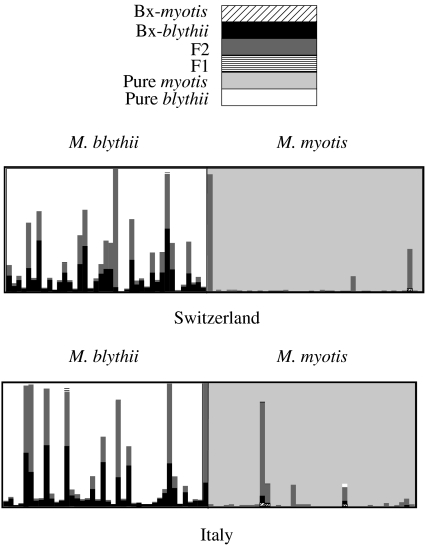
The five-locus genotypes of the 160 bats sampled in the two areas of sympatry were analysed with the program Newhybrids (Anderson & Thompson 2002), which implements a Bayesian method aimed at detecting the presence of hybrids from a sample of individuals of mixed origins. In more detail, the program estimates the allele frequencies in the two parental populations and for each genotype its posterior probability of being pure or hybrid origin. While it is in principle possible to consider as many hybrid categories as needed (F1, F2, F3, backcrosses, etc.), the available data may not be sufficient to reliably distinguish between these numerous categories, and it seems more appropriate to concentrate on the hybrid categories which could be formed by the last two generations of interbreeding: F1, F2 and the backcross of F1 with the two parental species. It is then possible to assign to each hybrid category a Q-value, which is defined here as the proportion of the genome of an individual being of M. myotis origin (e.g. the Q-value of a F1 individual is 0.5). Following Anderson & Thompson (2002), the prior distribution of allele frequencies at the lth locus was assumed to be Dirichlet distributed with parameters λl,j=1/Kl, where Kl is the number of alleles observed at the lth locus and j varies between 1 and Kl. Newhybrids obtained the posterior distributions based on a Markov chain Monte Carlo procedure, with a burn-in of 10 000 steps, followed by a sampling period of 10 000 steps, as recommended by the authors of the program. The convergence of the Markov chain was checked by visual inspection over several independent runs. We slightly modified the format of the output of Newhybrids to make it compatible with the program Distruct (Rosenberg 2004), which allows a graphical representation of the posterior probabilities to be obtained (figure 2).
Figure 2.
Results of the Newhybrids analysis of 160 multilocus genotypes of bats sampled in two mixed nursery colonies in Italy and Switzerland. One vertical bar represents one individual, and the length of the different coloured sections of the bar is proportional to the posterior probability to belong to each hybrid category (see colour legend). The morphological identity and origin of the sampled bats are indicated on the top and bottom labels of the graph, respectively.
By simulation, we tested whether the results observed with Newhybrids could have been observed by chance alone in the absence of any interbreeding between the two species. Using the program Simcoal (Excoffier et al. 2000), we simulated the evolution of two allopatric species which had been separated 5000 generations ago. The effective size of the two species was set to 5000 individuals, after some adjustment, since this combination of divergence time and population size led to an empirical FST value of about 18%, which is the level of differentiation observed between the two species for the five microsatellites studied here (see §3). The nuclear diversity at five microsatellite loci for 40 diploid individuals per species was generated by setting the mutation rate to μ=5×10−4 per locus (Ellegren 2004) without specifying any range constraint for the alleles. We performed a total of 1000 simulations, which were all analysed with Newhybrids, under the same settings as described earlier. The partition of genetic variance within and between species was estimated by an analysis of molecular variance (AMOVA) analysis using the program Arlequin v.3.0 (Excoffier et al. 2005), with 10 000 permutations computed for significance values.
3. Results
(a) mtDNA diversity
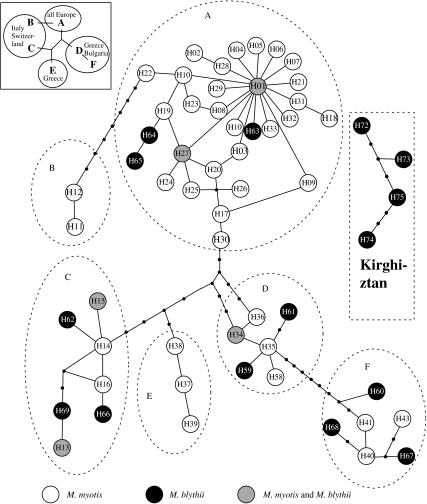
Information on the 32 M. myotis and M. blythii sequenced in this paper for the mitochondrial HVII region (308 bp) is reported in table 1 and electronic supplementary material. Haplogroups were defined following Ruedi & Castella (2003). Figure 1 is a maximum parsimony network obtained with the program TCS v.1.18 (Clement et al. 2000), showing the relationships among 21 HVII sequences of M. blythii, 7 sequences of M. myotis from Greece, Bulgaria, Italy, France and Spain, 4 sequences of M. blythii from Kirghiztan and more than 460 previously published sequences of M. myotis (Castella et al. 2001; Ruedi & Castella 2003). It shows that all 16 different haplotypes of European M. blythii are intermingled within those of M. myotis in the network and do not form a distinct cluster. All M. blythii haplotypes are indeed identical to or within one to three mutations from those existing in M. myotis. Figure 1 also shows that both species share the same local lineage in places where they occur in sympatry, i.e. in Greece (H34), Italy (H13, H15), France (H27, H1) or in Spain (H1). Thus, the European sequences of M. blythii present the same phylogeographical characteristics as those of M. myotis, including a similar geographical distribution of major haplogroups (figure 1 and Ruedi & Castella 2003). In contrast, sequences of the four Asian M. blythii from Kirghiztan were very divergent (23–33 mutations) and could not be unambiguously connected to a particular point of the parsimony network (figure 1). Overall, mean sequence divergence within Europe regardless of the species was 2.87%, compared with an average divergence of 8.91% between European and Asian samples.
Figure 1.
Parsimony network of the newly sequenced haplotypes of M. blythii and M. myotis (table 1) incorporated into the existing M. myotis database from Ruedi & Castella (2003). A connecting line between haplotypes represents one mutation and small black dots represent missing (inferred) haplotypes. Black haplotypes are those found only in M. blythii, white haplotypes only in M. myotis and grey haplotypes in both species. The four Kirghize haplotypes, differing by 23–33 mutations from European haplotypes, did not connect to the TCS network according to the 95% parsimony interval. The geographical distribution of the major haplogroups (letters A–F) is indicated in the inset sketch.
(b) Nuclear DNA diversity
The nuclear diversity at the five microsatellite loci assayed in both species was comparable, but not identical (table 2). For instance, at the locus C113, all 80 M. myotis were fixed for the seven-repeat allele, while M. blythii individuals were polymorphic for the six-, seven- and eight-repeat alleles. For the locus E24, M. blythii showed alleles varying by 2 bp (as expected for this TC-dinucleotidic locus, Castella & Ruedi 2000) as well as by 1 bp, while only 2 bp repeat alleles were found in M. myotis. Therefore, both species do share some alleles, but some are private to M. blythii. The comparison of the overall nuclear diversity within and between species located north and south of the Alps revealed a drastically different pattern of gene flow than that exhibited by mtDNA. The level of genetic differentiation, as measured by FST, was much higher between species within locality (FST=0.20, p<0.001 in Italy and FST=0.17, p<0.001 in Switzerland), than between populations within species (FST=0.023, p<0.001 for M. myotis and FST=0.017, p=0.004 in M. blythii). Populations of M. myotis and M. blythii are not only significantly differentiated north and south of the Alps, but differences between species are also similar in each area and about 10 times larger than at the intraspecific level.
Table 2.
Mean number of alleles (Na), observed (Ho) and expected heterozygosity (He) measured at five microsatellite loci genotyped in two populations each of M. myotis and M. blythii (n=80 bats per species). (FCT is the proportion of genetic variance due to the effect of species in an AMOVA design; these values are not significant (p=0.33), except for the overall measure (p=0.004).)
| M. myotis | M. blythii | ||||||
|---|---|---|---|---|---|---|---|
| locus | Na | Ho | He | Na | Ho | He | FCT |
| C113 | 1 | — | — | 3 | 0.29 | 0.41 | 0.77 |
| D9 | 17 | 0.61 | 0.88 | 17.5 | 0.59 | 0.92 | 0.03 |
| E24 | 17.5 | 0.94 | 0.92 | 29.5 | 0.97 | 0.95 | 0.04 |
| H29 | 11.5 | 0.88 | 0.87 | 11 | 0.53 | 0.87 | 0.06 |
| H19 | 9 | 0.78 | 0.78 | 7.5 | 0.35 | 0.81 | 0.01 |
| overall | 10.2 | 0.79 | 0.87 | 13.8 | 0.60 | 0.78 | 0.17 |
(c) Measure of interbreeding
The Newhybrids analysis was performed separately in the Aglié and Raron–Naters samples to provide two replicates (figure 2). The results suggest that most bats morphologically identified as M. myotis are indeed classified as purebred M. myotis. Their posterior probability of being purebred M. myotis is greater than 0.97 in 73 cases, greater than 0.81 in four cases, and there are only two individuals that could be convincingly classified as F2 hybrids (i.e. with less than 0.5 posterior probability of being purebred). In contrast, at least 14 bats morphologically identified as M. blythii are likely to be hybrids (either F2 or backcrosses F1–M. blythii), using the same posterior probability threshold of 0.5. Overall, we find that the average probability of M. blythii individuals being of hybrid origin is 25%, while it is only 4% in M. myotis. Both the average level of hybridization and the asymmetry of hybridization between M. myotis and M. blythii are similar in the two areas of sympatry sampled. We also note that no F1 and no backcrosses F1–M. myotis were detected (figure 2). In addition, since a few morphologically ambiguous individuals were not sampled (see §2), the true frequency of hybrids is probably underestimated.
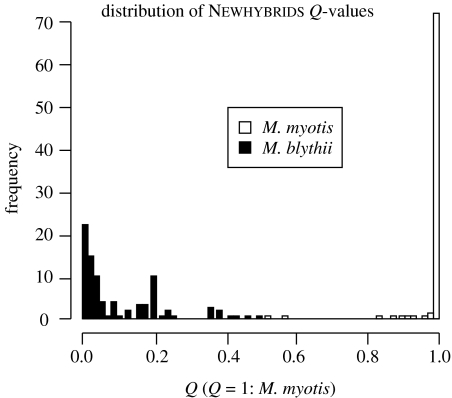
We report in figure 3 the distribution of Q-values, which is the expected proportion of the genome of an individual being of pure M. myotis ancestry. Newhybrids estimates, for each individual, the probability of the different hybrid categories which can be easily translated into Q-values as , where P(Ck) and qk are the probabilities to belong to the hybrid category Ck and the q-value of the Ck hybrid category, respectively. Consistent with the results of figure 2, figure 3 shows that most M. myotis individuals can be considered as non-hybrids, whereas M. blythii samples appear much more introgressed by M. myotis genes. Overall, we find that M. blythii have a proportion of Q=9.3% of their genes coming from M. myotis, while the latter species has integrated only about 2.1% of M. blythii genes.
Figure 3.
Distribution of Q-values, i.e. the posterior probability of being a pure parental M. myotis, inferred in two mixed nursery colonies of M. myotis and M. blythii in the Alps.
(d) Simulation study
The analysis of simulated samples drawn from two completely isolated population with the same level of divergence than that between M. myotis and M. blythii (FST=0.18) with Newhybrids reveals that 96.4% of individuals (mean of 1000 replicates) are correctly identified as being pure parental genotypes. The incorrectly classified individuals (3.6% of cases) are mostly identified as backcrosses with their own species (1.8%) or as F2s (1.3%) and more rarely as F1s (0.2%), or as backcrosses with the other species (0.1%). Thus, these results clearly show that the Newhybrids program does not infer an excess of false hybrid individuals on the basis of the multi-locus genotypes obtained by typing as few as five microsatellites. Our results are in keeping with another simulation study (Vähä & Primmer 2006) which showed that the few misclassifications of Newhybrids were essentially backcross genotypes considered as purebred parental individuals.
4. Discussion
(a) Mitochondrial replacement
The following surprising features characterize the mitochondrial DNA variation of the two sibling species of bats sampled in Europe (figure 1 and Castella et al. 2000; Mayer & Helversen 2001): (i) compared to the extensive haplotype database already accumulated in M. myotis (Ruedi & Castella 2003), no new divergent lineage has been found in M. blythii sampled in Europe, whereas those from Kirghiztan (located thousands of kilometres away from the nearest M. myotis) do differ extensively (greater than 7% genetic dissimilarity) from any European samples; (ii) contrary to the general pattern found for the highly variable control region in other species, European M. myotis and M. blythii share several identical haplotypes; (iii) haplotypes of each species sampled in the same geographical area are similar (or even identical), regardless of taxonomic boundaries. Therefore, in Europe, both species share mitochondrial lineages as if they were a single species. Shared ancestral polymorphism or incomplete lineage sorting is commonly invoked to account for non-monophyletic relationships among closely related species (e.g. Donnelly et al. 2004). However, multiple ancestral sequences are very unlikely to persist in each species through random lineage sorting and if that pattern occurred by chance, no obvious correlation with geographical location is expected (Powell 1983). Likewise, positive selection of M. myotis-like haplotypes could favour their quick replacement in M. blythii individuals, but this would imply that selective sweeps happened several times with different variants in European M. blythii. Although we do not dismiss this possibility, it appears more probable that the original mitochondrial genome of M. blythii found today in Asia has been repeatedly replaced through hybridization during the recent range expansion of that species into Europe. The necessarily low density of the invading M. blythii compared with the resident M. myotis could have favoured heterospecific matings in the early stages of their secondary contact. This phenomenon, known as Hubbs' principle, has being documented in several animal species such as fishes (Hubbs 1955) or birds (Grant & Grant 1992; Randler 2002). Taking into account the fact that gene flow is highly male-biased in Myotis bats (Castella et al. 2001), this early demographic imbalance would have favoured the loss of female-transmitted genes of the invading species. Recently, simulations of human spatial expansions (Currat & Excoffier 2004) suggested that the genome of an invading species may indeed quickly get invaded by the genome of the resident species if both can hybridize. This is expected owing to the progressive dilution of the invading genome in spatially expanding populations. These simulation results fit with our observation that M. blythii is the invading species and suggest that the original M. blythii mtDNA genome has been replaced by the local lineages of M. myotis during their expansion into Europe, until the original genes have finally been lost. Under this model, the correlated phylogeographical patterns observed in both species for mtDNA would result from a succession of mtDNA genome replacements during the westwards colonization of Europe by M. blythii.
(b) Asymmetric nuclear DNA introgression
In sharp contrast to mtDNA, sympatric populations of M. myotis and M. blythii differ markedly at the nuclear DNA level, with levels of genetic differentiation being an order of magnitude higher between species than within species (FST=0.18 versus FST=0.02, respectively). This pattern of differentiation is highly consistent in the two areas of sympatry located south and north of the Alps we examined. This confirms an earlier body of evidence showing that interspecific gene flow is necessarily very limited to maintain the numerous phenotypic, behavioural and allozymic differences that characterize each species (Ruedi et al. 1990; Arlettaz et al. 1997a,b). Although most bats sampled in the same nursery colony were purebred parental forms (figure 3), the Bayesian analyses of individual genotypes revealed that 2 out of 80 bats identified morphologically as M. myotis and 14 out of 80 M. blythii were classified as hybrids with high posterior probability (figure 2). This represents 10% of all bats genotyped, which is well beyond the expected 4% misclassification rate suggested by our simulations. Although no F1 hybrids were detected, either because they are effectively rare in the mixed colonies, or perhaps because the few atypical individuals excluded during the sampling process were those F1s (see §2), our results not only demonstrate that some interspecific gene flow is still ongoing, but also that gene introgression is highly asymmetrical (figure 3). Indeed, all second-generation hybrids were in the direction of M. blythii, while no backcross in the reverse direction was detected (figure 2). Different pre- and post-zygotic mechanisms such as assortative mating, female choice, partial sterility, or biased survival of hybrids might account for asymmetric gene introgression between two species (Chan & Levin 2005). Since almost nothing is known about their mating systems, further studies are needed to elucidate the causes of the observed asymmetric gene introgression between M. myotis and M. blythii.
(c) Genome porosity between biological species
The pattern of ongoing nuclear gene introgression between M. myotis and M. blythii was consistent in both areas of sympatry sampled (figure 2) and raises the question of how two biological species can maintain their genetic integrity in face of such levels of hybridization. Given a rate h of successful hybridization, Clarke et al. (1996) showed that differences between nuclear allele frequencies should fall to 2% after 2/h generation. In the case of M. myotis and M. blythii, with about 10% inferred hybrids, gene pools would quickly homogenize, which is not the case, suggesting that hybrids are counter selected. The level of selection against hybrids can be roughly estimated if one assumes that there is a single locus affecting the fitness of hybrids. Assuming equilibrium between migration and selection, Barton & Bengtsson (1986) have indeed shown that the effective migration rate me at a neutral locus located r units of recombination from the selected locus is me=mr(1−s)/[s+r(1−s)], where m is the true migration rate and 1−s is the fitness of hybrids. If we assume that me determines the level of differentiation between the two species as FST=1/(1+4Nme)=0.18 and that the fraction of introgressed genes of M. myotis in M. blythii Q=0.093 represents the migration rate m before selection, we can estimate, for a given deme size N, the selection coefficient s as (Q−me)/(Q+me)=0.78 (for N=100). Thus, such a selection coefficient would be sufficient to explain the strong differentiation observed between species despite the relatively high levels of ongoing gene flow we have measured. This level of selection against hybrids is certainly an underestimate as the observed level of gene flow has already been reduced by selection against hybrids. For instance, if Q was initially as high as 20%, s would be equal to 0.89 (still for N=100), implying that about 90% of the hybrids would be directly eliminated by selection. Note that such a high mortality of hybrids would explain why we do not observe (or overlooked) any F1 in our samples. However, it appears actually more probable that several genes are involved in maintaining interspecific barriers (e.g. Wu 2001), which are more efficient in preventing gene flow between species during the speciation process. Along these lines, a recent simulation study of a spatial expansion process with possible hybridization has shown (Eswaran et al. 2005) that if eight or more loci are controlling phenotypic differences between species, very little introgression would be possible between the two species. Thus, it appears that the simultaneous complete replacement of M. blythii mtDNA and the maintenance of a strong nuclear differentiation between species can be well explained by the presence of several nuclear loci being counter-selected in hybrid individuals irrespective of their mtDNA background.
(d) Conclusions
A variety of other organisms including plants (Petit et al. 2004), insects (Powell 1983), fishes (Bernatchez et al. 1995), or mammals (Tegelström 1987) show evidence of mitochondrial genome replacement, even away from any current zone of hybridization (Melo-Ferreira et al. 2005). Frequency-dependent genome replacement proposed for Myotis could also apply to other historic range expansions of closely related species. As this process would occur essentially during the early stages of spatially expanding populations, present events of hybridization are difficult to distinguish from more ancient ones with mtDNA markers alone. Thus, the combination of nuclear and mitochondrial DNA markers provides a better approach to tease apart these different perspectives on gene flow.
The sharp contrast of results obtained from the mitochondrial and nuclear markers further points to the need to interpret cautiously results based on a single class of genetic markers. This is especially relevant for the estimation of gene flow among populations (Castella et al. 2001), for species being managed, or for taxonomic applications based on single-gene sequences, like the bar-coding of animal life (Hebert et al. 2003). We also show that patterns of intra- and interspecific gene flow need to be interpreted not only in the context of current processes potentially influencing the extent of introgression, but also by taking into account historical factors such as demographic expansions to fully understand the evolutionary importance of hybridization in natural populations.
Although bats represent a quarter of all mammalian species (Simmons 2005), hybridization has been documented only twice in this group (Webb & Tidemann 1995; Hoffmann et al. 2003), suggesting that hybridization is a rare phenomenon in bats. Previous attempts have failed to detect hybrid genotypes in the genus Myotis (Herd & Fenton 1983; Ruedi et al. 1990). The availability of better, highly variable nuclear markers (microsatellites) and of more powerful Bayesian methods to distinguish parental and hybrid genotypes (Vähä & Primmer 2006) may now change our perception of natural hybridization, in bats and in other animals or plants, and provide better opportunities to detect speciation genes (Wu 2001).
Acknowledgments
Acknowledgements for facilitating sampling and permits to access to the colonies: Raphael Arlettaz, Massimo Bertozzi, Philippe Christe, Emmanuel Cosson, Paulo Debernardi, Carlos Ibañez, Tea Ivanova, Javier Juste, Elena Patriarca, Dino Scaravelli and Björn Siemers. Most sequences were done by Vincent Castella, to whom we are most grateful for help. We thank Alex Kohler, Ghislaine Rigoli and Simon Walther for their help in the lab. We thank Eric Anderson, Pierre Taberlet, Raphaël Arlettaz and Rémy Petit for their discussions early in the development of the manuscript. Eric Anderson also provided valuable support for using the Newhybrids program. P.B. was supported by Swiss NSF grants no. 31-61458.00 to M.R. and L.E. received support from Swiss NSF grant no. 3100A0-100800.
Supplementary Material
Sequence variation and lineage identification of the 32 M. myotis and M. blythii analysed for 308 bp of mtDNA HVII. Haplotypes H1 to H43 have already been reported in M. myotis (see Ruedi and Castella, 2003), while H58 to H75 are new variants. Dots represent the same nucleotide as in H1, and hyphens represent indels.
References
- Anderson E.C, Thompson E.A. A model-based method for identifying species hybrids using multilocus genetic data. Genetics. 2002;160:1217–1229. doi: 10.1093/genetics/160.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlettaz R, Ruedi M, Hausser J. Field morphological identification of Myotis myotis and Myotis blythi (Chiroptera, Vespertilionidae): a multivariate approach. Myotis. 1991;29:7–16. [Google Scholar]
- Arlettaz R, Perrin N, Hausser J. Trophic resource partitioning and competition between the two sibling bat species Myotis myotis and Myotis blythii. J. Anim. Ecol. 1997a;66:897–911. doi:10.2307/6005 [Google Scholar]
- Arlettaz R, Ruedi M, Ibañes C, Palmeirim J, Hausser J. A new perspective on the zoogeography of the sibling mouse-eared bat species Myotis myotis and M. blythii: morphological, genetical and ecological evidence. J. Zool. 1997b;242:45–62. [Google Scholar]
- Arlettaz R, Christe P, Lugon A, Perrin N, Vogel P. Food availability dictates the timing of parturition in insectivorous mouse-eared bats. Oikos. 2001;95:105–111. doi:10.1034/j.1600-0706.2001.950112.x [Google Scholar]
- Barton N.H. The role of hybridization in evolution. Mol. Ecol. 2001;10:551–568. doi: 10.1046/j.1365-294x.2001.01216.x. doi:10.1046/j.1365-294x.2001.01216.x [DOI] [PubMed] [Google Scholar]
- Barton N, Bengtsson B.O. The barrier to genetic exchange between hybridising populations. Heredity. 1986;57:357–376. doi: 10.1038/hdy.1986.135. [DOI] [PubMed] [Google Scholar]
- Bernatchez L, Glemet H, Wilson C.C, Danzmann R.G. Introgression and fixation of Arctic char (Salvelinus alpinus) mitochondrial genome in an allopatric population of brook trout (Salvelinus fontinalis) Can. J. Fish. Aquat. Sci. 1995;52:179–185. [Google Scholar]
- Castella V, Ruedi M. Characterisation of highly variable microsatellite loci in the bat Myotis myotis (Chiroptera: Vespertilionidae) Mol. Ecol. 2000;9:1000–1002. doi: 10.1046/j.1365-294x.2000.00939-6.x. doi:10.1046/j.1365-294x.2000.00939-6.x [DOI] [PubMed] [Google Scholar]
- Castella V, Ruedi M, Excoffier L, Ibáñez C, Arlettaz R, Hausser J. Is the Gibraltar Strait a barrier to gene flow for the bat Myotis myotis (Chiroptera: Vespertilionidae)? Mol. Ecol. 2000;9:1761–1772. doi: 10.1046/j.1365-294x.2000.01069.x. doi:10.1046/j.1365-294x.2000.01069.x [DOI] [PubMed] [Google Scholar]
- Castella V, Ruedi M, Excoffier L. Contrasted patterns of mitochondrial and nuclear structure among nursery colonies of the bat Myotis myotis. J. Evol. Biol. 2001;14:708–720. doi:10.1046/j.1420-9101.2001.00331.x [Google Scholar]
- Chan K.M.A, Levin S.A. Leaky prezygotic isolation and porous genomes: rapid introgression of maternally inherited DNA. Evolution. 2005;59:720–729. doi:10.1554/04-534 [PubMed] [Google Scholar]
- Clarke B, Johnson M.S, Murray J. Clines in the genetic distance between two species of island land snails: how ‘molecular leakage’ mislead us about speciation. Phil. Trans. R. Soc. B. 1996;351:773–784. [Google Scholar]
- Clement M, Posada D, Crandall K. TCS: a computer program to estimate gene genealogies. Mol. Ecol. 2000;9:1657–1660. doi: 10.1046/j.1365-294x.2000.01020.x. doi:10.1046/j.1365-294x.2000.01020.x [DOI] [PubMed] [Google Scholar]
- Currat M, Excoffier L. Modern humans did not admix with Neanderthals during their range expansion into Europe. PLoS Biol. 2004;2:2264–2274. doi: 10.1371/journal.pbio.0020421. doi:10.1371/journal.pbio.0020421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly P, Pinto J, Girod R, Besansky N.J, Lehmann T. Revisiting the role of introgression vs shared ancestral polymorphisms as key processes in shaping genetic diversity in the recently separated sibling species of the Anopheles gambiae complex. Heredity. 2004;92:61–68. doi: 10.1038/sj.hdy.6800377. doi:10.1038/sj.hdy.6800377 [DOI] [PubMed] [Google Scholar]
- Dowling T.E, Secor C.L. The role of hybridization and introgression in the diversification of animals. Annu. Rev. Ecol. Syst. 1997;28:593–619. doi:10.1146/annurev.ecolsys.28.1.593 [Google Scholar]
- Ellegren H. Microsatellites: simple sequences with complex evolution. Nat. Rev. Genet. 2004;5:435–445. doi: 10.1038/nrg1348. doi:10.1038/nrg1348 [DOI] [PubMed] [Google Scholar]
- Eswaran V, Harpending H, Rogers A.R. Genomics refutes an exclusively African origin of humans. J. Hum. Evol. 2005;49:1–18. doi: 10.1016/j.jhevol.2005.02.006. doi:10.1016/j.jhevol.2005.02.006 [DOI] [PubMed] [Google Scholar]
- Excoffier L, Novembre J, Schneider S. Simcoal: a general coalescent program for the simulation of molecular data in interconnected populations with arbitrary demography. J. Hered. 2000;91:506–509. doi: 10.1093/jhered/91.6.506. doi:10.1093/jhered/91.6.506 [DOI] [PubMed] [Google Scholar]
- Excoffier L, Laval G, Schneider S. Arlequin (version 3.0): an integrated software package for population genetics data analysis. Evol. Bioinform. Online. 2005;1:47–50. [PMC free article] [PubMed] [Google Scholar]
- Grant P.R, Grant B.R. Hybridization of bird species. Science. 1992;256:193–197. doi: 10.1126/science.256.5054.193. [DOI] [PubMed] [Google Scholar]
- Hebert P.D.N, Ratnasingham S, deWaard J.R. Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proc. R. Soc. B. 2003;270:S96–S99. doi: 10.1098/rsbl.2003.0025. doi:10.1098/rsbl.2003.0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd R.M, Fenton M.B. An electrophoretic, morphological, and ecological investigation of a putative hybrid zone between Myotis lucifugus and Myotis yumanensis (Chiroptera: Vespertilionidae) Can. J. Zool. 1983;61:2029–2050. [Google Scholar]
- Hoffmann F.G, Owen J.G, Baker R.J. mtDNA perspective of chromosomal diversification and hybridization in Peters' tent-making bat (Uroderma bilobatum: Phyllostomidae) Mol. Ecol. 2003;12:2981–2993. doi: 10.1046/j.1365-294x.2003.01959.x. doi:10.1046/j.1365-294X.2003.01959.x [DOI] [PubMed] [Google Scholar]
- Hubbs C.L. Hybridization between fish species in nature. Syst. Zool. 1955;4:1–20. doi:10.2307/2411933 [Google Scholar]
- Mayer F, Helversen v.O. Cryptic diversity in European bats. Proc. R. Soc. B. 2001;268:1825–1832. doi: 10.1098/rspb.2001.1744. doi:10.1098/rspb.2001.1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo-Ferreira J, Boursot P, Suchentrunk F, Ferrand N, Alves P.C. Invasion from the cold past: extensive introgression of mountain hare (Lepus timidus) mitochondrial DNA into three other hare species in northern Iberia. Mol. Ecol. 2005;14:2459–2464. doi: 10.1111/j.1365-294X.2005.02599.x. doi:10.1111/j.1365-294X.2005.02599.x [DOI] [PubMed] [Google Scholar]
- Morjan C.L, Rieseberg L.H. How species evolve collectively: implications of gene flow and selection for the spread of advantageous alleles. Mol. Ecol. 2004;13:1341–1356. doi: 10.1111/j.1365-294X.2004.02164.x. doi:10.1111/j.1365-294X.2004.02164.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit R.J, Bodenes C, Ducousso A, Roussel G, Kremer A. Hybridization as a mechanism of invasion in oaks. New Phytol. 2004;161:151–164. doi:10.1046/j.1469-8137.2003.00944.x [Google Scholar]
- Piksa K, Woloszyn B.W. Postglacial bat remains from the Polish Tatra caves. Lynx. 2001;32:301–311. [Google Scholar]
- Powell J.R. Interspecific cytoplasmic gene flow in the absence of nuclear gene flow: evidence from Drosophila. Proc. Natl Acad. Sci. USA. 1983;80:492–495. doi: 10.1073/pnas.80.2.492. doi:10.1073/pnas.80.2.492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randler C. Avian hybridization, mixed pairing and female choice. Anim. Behav. 2002;63:103–119. doi:10.1006/anbe.2001.1884 [Google Scholar]
- Rosenberg N.A. Distruct: a program for the graphical display of population structure. Mol. Ecol. Notes. 2004;4:137–138. doi:10.1046/j.1471-8286.2003.00566.x [Google Scholar]
- Ruedi M, Castella V. Genetic consequences of the ice ages on nurseries of the bat Myotis myotis: a mitochondrial and nuclear survey. Mol. Ecol. 2003;12:1527–1540. doi: 10.1046/j.1365-294x.2003.01828.x. doi:10.1046/j.1365-294X.2003.01828.x [DOI] [PubMed] [Google Scholar]
- Ruedi M, Arlettaz R, Maddalena T. Distinction morphologique et biochimique de deux espèces jumelles de chauves-souris: Myotis myotis (Bork.) et Myotis blythi (Tomes) (Mammalia; Vespertilionidae) Mammalia. 1990;54:415–429. [Google Scholar]
- Seehausen O. Hybridization and adaptive radiation. Trends Ecol. Evol. 2004;19:198–207. doi: 10.1016/j.tree.2004.01.003. doi:10.1016/j.tree.2004.01.003 [DOI] [PubMed] [Google Scholar]
- Simmons N.B. Order Chiroptera. In: Wilson D.E, Reeder D.M.e, editors. Mammal species of the World. A taxonomic and geographic reference. vol. 1. Johns Hopkins University Press; Washington, DC: 2005. pp. 312–529. [Google Scholar]
- Tegelström H. Transfer of mitochondrial DNA from the northern red-backed vole (Clethrionomys rutilus) to the Bank Vole (C. glareolus) J. Mol. Evol. 1987;24:218–227. doi: 10.1007/BF02111235. [DOI] [PubMed] [Google Scholar]
- Vähä J.-P, Primmer C.R. Efficiency of model-based Bayesian methods for detecting hybrid individuals under different hybridization scenarios and with different numbers of loci. Mol. Ecol. 2006;15:63–72. doi: 10.1111/j.1365-294X.2005.02773.x. doi:10.1111/j.1365-294X.2005.02773.x [DOI] [PubMed] [Google Scholar]
- Webb N.J, Tidemann C.R. Hybridisation between black (Pteropus alecto) and grey-headed (P. poliocephalus) flying-foxes (Megachiroptera: Pteropodidae) Aust. Mammal. 1995;18:19–26. [Google Scholar]
- Worthington Wilmer J, Barratt E. A non-lethal method of tissue sampling for genetic studies of Chiropterans. Bat Res. News. 1996;37:1–3. [Google Scholar]
- Wu C.I. The genetic view of speciation. J. Evol. Biol. 2001;14:851–865. doi:10.1046/j.1420-9101.2001.00335.x [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequence variation and lineage identification of the 32 M. myotis and M. blythii analysed for 308 bp of mtDNA HVII. Haplotypes H1 to H43 have already been reported in M. myotis (see Ruedi and Castella, 2003), while H58 to H75 are new variants. Dots represent the same nucleotide as in H1, and hyphens represent indels.