Abstract
We provide a revision to the calculation of effect sizes and heterogeneity statistics in our original article, ‘Facultative primary sex ratio variation: a lack of evidence in birds’ (Ewen et al. 2004). Our revision shows that significant heterogeneity in sex ratio study effect sizes does indeed exist and that for a series of key traits the average effect sizes (while still weak) are in fact significantly different from zero.
1. Introduction
In our earlier analysis of facultative primary sex ratio adjustment we concluded that facultative control of offspring sex is not a characteristic biological phenomenon in breeding birds (Ewen et al. 2004). It has recently been communicated to us that our spreadsheet calculation of Fisher's Z-transformed effect size was based on an erroneous function. We have corrected this calculation and re-analysed the dataset using meta-analysis code in SAS v. 8.02 (see White et al. in press). Note that the data, methods and formulae given in Ewen et al. (2004) remain correct and the only misleading result is the quantification and interpretation of heterogeneity across the Z-transformed effect sizes. We hope that by highlighting our own unfortunate mistake, results from the first analysis will not be over-interpreted or hinder the findings of recent studies, which continue to expand this exciting field of research. Importantly, we do not want future publications to erroneously highlight their own effect sizes as outliers based on our previous results. We reiterate our wish that biologists continue to provide empirical evidence to further develop the revised patterns we present. Our methods follow Hedges & Olkin (1985) and Ewen et al. (2004).
2. Results
The common weighted averages (overall mean effect size (95% CI)) in relation to both biological traits (0.01 (−0.01, 0.03)) and temporal traits (−0.01 (−0.04, 0.02)) did not differ significantly from zero (ZSTAT(BIOLOGICAL)=1.3; ZSTAT(TEMPORAL)=−0.6). However, heterogeneity in effect sizes across both biological traits (n=139, Q=350.5) and temporal traits (n=75, Q=270.9) were highly significant (cf. Ewen et al. 2004) and we therefore proceed to partition this heterogeneity by the individual traits given in Ewen et al. (2004), using a categorical model fitting procedure (sensu Hedges & Olkin 1985). Based on this partitioning we identified three variables (male quality (n=17) Z+=0.08 (0.03, 0.14); laying sequence (n=32) Z+=−0.15 (−0.21, −0.09); and season (n=33) Z+=0.04 (0.01, 0.08)) with common weighted averages significantly different from zero (figure 1). Significant heterogeneity in these traits was unexplained (male quality, Q=39.1; laying sequence, Q=150.4; season, Q=70.8). In all other traits common weighted average effect sizes were not significantly different from zero and unexplained heterogeneity was only significant in the biological trait territory quality (n=18, Q=139.5).
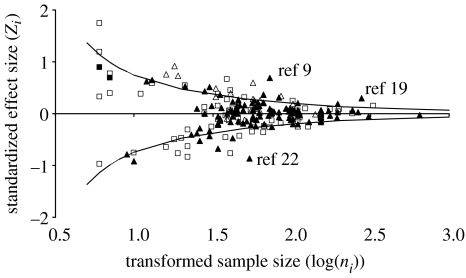
Figure 1.
Bivariate funnel plot of the relationship between sex ratio adjustment and biological (triangles; n=139) or temporal (squares; n=75) traits sensu Ewen et al. (2004). Overlaid significance lines are calculated for α=0.05 following Sutton (1990). Open symbols indicate the three study parameters with common weighted average effect sizes significantly different from zero (male quality a biological trait ZSTAT=2.9, n=17, p<0.005; laying sequence a temporal trait ZSTAT=−4.9, n=32, p<0.001; and season a temporal trait ZSTAT=2.3, n=33, p<0.05). The three marked observations refer to outliers for territory quality (a biological trait) previously identified in Ewen et al. (2004). Reference numbers indicate the original citations (see the electronic supplementary material).
3. Discussion
Our revised results confirm that, in the majority of cases, the overall trend for facultative control of offspring sex in birds is noticeably weak (figure 1). However, contrary to our previous finding we acknowledge that significant heterogeneity across studies does indeed exist. Importantly, as well as the previously highlighted significant heterogeneity among studies of territory quality (figure 1), common weighted average effect sizes for the traits male quality, laying sequence and season were individually all significantly different from zero. These results will be of particular interest to the authors of previous verbal reviews (Krackow 1999; Hasselquist & Kempenaers 2002; Komdeur & Pen 2002; Cockburn et al. 2002) and a recent quantitative review (West & Sheldon 2002) proposing that facultative female sex ratio adjustment was a consistent finding of empirical studies. In addition, our revision provides confidence, as well as important future research directions, for authors challenged by the overall lack of trends previously reported (e.g. see Dowling & Mulder 2006). Although we do not promote a conclusion of widespread facultative control of primary sex ratio by females, we do now support a weak pattern under specific biological and temporal traits. We trust that the importance of our revised findings are not over-shadowed by the previous error.
Acknowledgments
The authors are exceedingly grateful to their colleagues and the journal referees and editors who supported the publication of this revision. Communication with D. Dowling brought the original calculation error to P.C.'s attention.
Footnotes
The original article can be viewed online at http://dx.doi.org/10.1098/rspb.2004.2735.
The electronic supplementary material is available at http://dx.doi.org/10.1098/rspb.2006.3628 or via http://www.journals.royalsoc.ac.uk.
Supplementary Material
Highlighted (yellow) references correspond with citations in Figure 1 of the current manuscript.
References
- Cockburn A, Legge S, Double M.C. Sex ratios in birds and mammals: can the hypotheses be disentangled? In: Hardy I.C.W, editor. Sex ratios: concepts and research methods. Cambridge University Press; Cambridge, UK: 2002. pp. 266–268. [Google Scholar]
- Dowling D.K, Mulder R.A. Combined influence of maternal and paternal quality on sex allocation in red-capped robins. J. Evol. Biol. 2006;19:440–449. doi: 10.1111/j.1420-9101.2005.01017.x. [DOI] [PubMed] [Google Scholar]
- Ewen J.G, Cassey P, Møller A.P. Facultative primary sex ratio variation: a lack of evidence in birds. Proc. R. Soc. B. 2004;271:1277–1282. doi: 10.1098/rspb.2004.2735. doi:10.1098/rspb.2004.2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselquist D, Kempenaers B. Parental care and adaptive brood sex ratio manipulation in birds. Phil. Trans. R. Soc. B. 2002;357:363–372. doi: 10.1098/rstb.2001.0924. doi:10.1098/rstb.2001.0924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges L.V, Olkin I. Academic Press; San Diego, CA: 1985. Statistical methods for meta-analysis. [Google Scholar]
- Komdeur J, Pen I. Adaptive sex allocation in birds: the complexities of linking theory and practice. Phil. Trans. R. Soc. B. 2002;357:373–380. doi: 10.1098/rstb.2001.0927. doi:10.1098/rstb.2001.0927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krackow S. Avian sex ratio distortions: the myth of maternal control. In: Adams N.J, Slotow R.H, editors. Proc. 22 Int. Ornithol. Cong., Durbon. Birdlife South Africa; Johannesburg, South Africa: 1999. pp. 425–433. [Google Scholar]
- Sutton J.B. Values of the index of determination at the 5% significance level. Statistician. 1990;39:461–463. [Google Scholar]
- West S.A, Sheldon B.C. Constraints in the evolution of sex ratio adjustment. Science. 2002;295:1685–1688. doi: 10.1126/science.1069043. doi:10.1126/science.1069043 [DOI] [PubMed] [Google Scholar]
- White, C. R., Blackburn, T. M. & Cassey, P. In press. Allometric exponents do not support a universal metabolic allometry. Ecology [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Highlighted (yellow) references correspond with citations in Figure 1 of the current manuscript.