Abstract
In this paper, we demonstrate that the seasonal dynamics in the abiotic factors, without including seasonal changes in the biological relationships, can appropriately account for the seasonal dynamics of Chrysochromulina spp. This is through the analysis of data on the population dynamics of Chrysochromulina spp. off southern Norway that is evaluated in relation to environmental factors and season by the analyses of 12 year monthly time-series. Chrysochromulina spp. abundance, nutrient concentrations, hydrographical properties, as well as current and wind data were analysed on a monthly scale by means of autoregressive moving average models, principal component analyses (PCA), and linear and nonlinear regression models. Seasonal development of the Chrysochromulina assemblage was well predicted from regression models forced with two PCA components representing seasonal variation in nutrient and chlorophyll a levels and ratios, inflow of North Seawater to the Skagerrak and northeasterly wind along the Norwegian coast. Assuming these to be general results, we might hypothesis that marine algal communities are governed by seasonally varying abiotic factors to a large extent.
Keywords: Chrysochromulina, Skagerrak, blooms, population dynamics, season, timing
1. Introduction
The focal group of algae, Chrysochromulina spp., exhibited an extensively harmful bloom in the Skagerrak and Kattegat (figure 1) during the late spring and summer of 1988 (Dahl et al. 1989; Gjøsæter et al. 2000). The bloom killed a wide range of wild organisms as well as farmed fish. The reasons for the great intensity and toxicity of this bloom are still not understood, and no similar events of Chrysochromulina polylepis have occurred subsequently. The great toxicity experienced in 1988 has not been reproduced under laboratory conditions. The 1988 episode evoked interest in the population dynamics of Chrysochromulina spp. and led to the initiation of a surveillance programme for these species off southern Norway. High concentrations of C. polylepis were recorded in the same area during 1994 and 1995, but no harmful effects on feral biota were detected (Dahl et al. 1998).
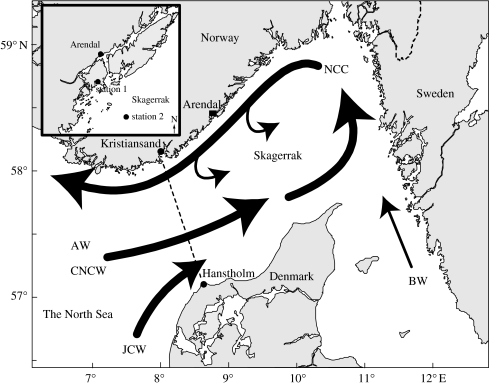
Figure 1.
Location of the study areas along the Norwegian Skagerrak coast (main map). A simplified picture of the general circulation pattern in the Skagerrak is indicated. AW, Atlantic Water; CNSW, Central North Sea Water; JCW, Jutland Coastal Water; BW, Baltic Water; NCC, Norwegian Coastal Current. Inset is a detailed map showing the sampling stations. Dashed line, the transect Kristiansand–Hantsholm.
2. Material and methods
(a) The study area
The Skagerrak and the southern coast of Norway are located downstream of the North Sea, the Baltic and the Kattegat. Water bodies of different origin enter and influence the Skagerrak, where shifting wind conditions may cause pronounced hydrophysical and chemical variability in the surface layer. The simplified and general current pattern in this area is shown in figure 1. In the Jutland Water Current (JWC), nitrogen : phosphorus (N : P) ratios above the Redfield ratio may occur due to elevated nitrogen (nitrate) values (Aure et al. 1998). The Norwegian Coastal Current (NCC) starts in the eastern Skagerrak and flows westwards as a ‘large, stratified river’. The upper 30 m of the NCC off Arendal is mainly influenced by water entering the Skagerrak along the JWC coast and Baltic Water, the latter entering via the Kattegat (Aure et al. 1998). Easterly winds accelerate the NCC and force it closer to the southern coast of Norway, while westerly winds have the opposite effect, thereby causing nearshore upwelling and an anticlockwise recirculation of upper layers in the Skagerrak.
The Skagerrak is further characterized by strong seasonal variations in temperature, light and nutrient conditions. A diatom-dominated spring bloom usually occurs during February–March along the Norwegian coast and up to one month later in southern Skagerrak along the Danish coast (Dahl & Danielssen 1981). In summer, surface waters are generally depleted of nutrients, and primary production is mainly dependent on regeneration of nutrients. At this time, the plankton community is typically dominated by auto-, mixo-, heterotrophic nano- and picoplankton.
(b) Data collection and preparation
Since 1989, Chrysochromulina spp. have been monitored three times per week in the 0–3 m stratum in Flødevigen Bay, Station 1 (figure 1) by the Institute of Marine Research (Dahl & Johannessen 1998; for the details of sampling and phytoplankton quantification, see Dahl et al. 2005). The phytoplankton assemblage sampled in Flødevigen Bay is considered to reflect the abundance in the NCC along the coast (Dahl et al. 1989; Dahl & Tangen 1993). The raw time-series of Chrysochromulina spp. is displayed in figure 2a. Since the monitoring dataset does not distinguish between the different Chrysochromulina spp. present in the Skagerrak, the genus is the entity in our analysis.
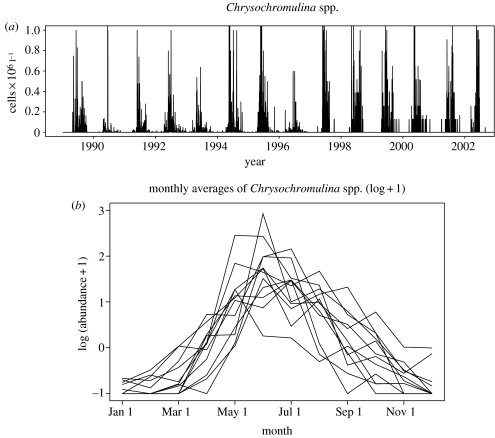
Figure 2.
Chrysochromulina spp. data. (a) The raw data; observations sampled about every second day from the bay of Flødevigen (station 1 in figure 1). (b) Monthly averages of Chrysochromulina spp. data after removing an AR(35) time-series from the raw data (see text).
Nutrient and chlorophyll a concentrations, along with temperature and salinity have generally been sampled every second to third week since 1990 at station 2 (figure 1). This station has a depth of about 100 m, and is situated about 3 km to the south of station 1 in Flødevigen Bay. Profiles of temperature and salinity from the surface to 75 m are recorded by a Neil Brown CTD , while water samples for analysing nutrients and chlorophyll a are sampled at standard depths (0, 5, 10, 20, 30, 50, 75 m). The chemical analyses are performed according to standard procedures (see Dahl et al. 2005).
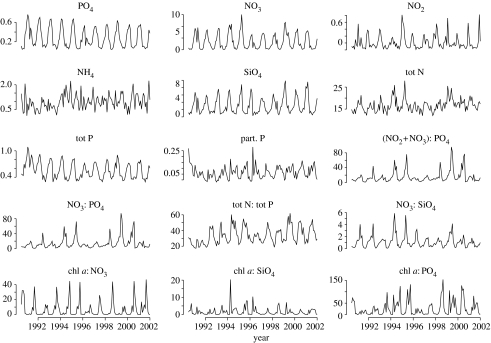
Monthly average concentrations of Chrysochromulina spp. were calculated from the three weekly samples (after correcting for autocorrelations—described later). For levels and ratios of nutrients and chlorophyll a, temperature salinity depth averages were calculated. We used the depth interval of 0–30 m, since this stratum should represent the habitat of phytoplankton in general, including Chrysochromulina (Dahl & Johannessen 1998). The depth averages for the various environmental variables at each sampling occasion, using the depths 0, 10, 20 and 30 m and weighting these equally, were then used to calculate monthly averages. No corrections were made here when the environmental samplings were undertaken in a given month. Fifteen different measures of nutrient and chlorophyll a levels and ratios in the water masses were available on a monthly scale from June 1990 (figure 3).
Figure 3.
Environmental data sampled from station 2 outside Flødevigen (see figure 1). The data are sampled every second to third week at standard depths from 1990 to 2002. The data are averaged over 0–30 m.
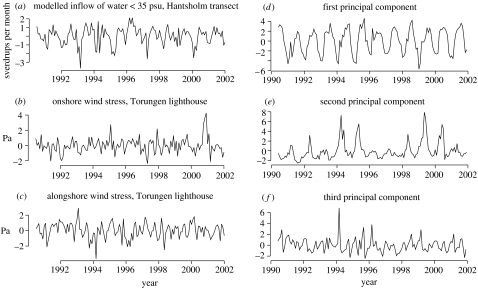
One current and two wind measures were included in the analyses. Simulated inflow data for water crossing the Kristiansand–Hantsholm transect north of Denmark (see figure 1) were used to characterize the strength of the currents flowing anticlockwise in the Skagerrak basin (Danielssen et al. 1996, 1997), a current seen in our another study to have biological importance (Stenseth et al. 2006a). These simulated inflow data stem from a three-dimensional wind and density driven ocean model, the Norwegian ecological model system (Skogen & Søiland 1998). The model is forced by 6-hourly wind and pressure data (obtained from The Norwegian Meteorological Institute; met. no), four tidal constituents and freshwater runoff, on an area covering the North Sea (including the Skagerrak and Kattegat) and the Atlantic inflowing area. The horizontal resolution is 20×20 km, and in the vertical 11 sigma layers following the bottom are used. Velocity fields from the model are stored as monthly means, and the monthly average inflows to the Skagerrak are calculated from these fields (figure 4a) The calculated inflows are restricted to the upper 50 m and salinity less than 35‰, so as to exclude the more saline Atlantic water masses. As the water masses take about three weeks to flow through the Skagerrak basin (Danielssen et al. 1997), we use a monthly net inflow at t−1 as the predictor in our analyses, i.e. the water inflow during the previous month.
Figure 4.
(a) Modelled inflow of water over the Hantsholm transect off northern Denmark (see text) from the Norwegian Ecological Model System model forced by wind, air pressure, tides and freshwater runoff, using a spatial resolution of 20 km2 for the upper 50 m and salinity less than 35‰. (b) Onshore wind stress, representing wind towards the shore. (c) Alongshore wind representing northeasterly winds blowing along the Norwegian Skagerrak coast. (d–f) The first three principal components obtained from the environmental data (displayed in figure 3). The three principal components are uncorrelated with each other.
In the Northern Hemisphere, the ‘Coriolis force’ sets up a net transport of water that is diverted 90° to the right of the wind direction (the ‘Ekman drift’; Mann & Lazier 1991). This phenomenon is due to the rotation of the Earth. Hence, persistent northeasterly winds (i.e. winds from the northeast blowing along the Norwegian Skagerrak coast) set up currents towards the shore. Such wind-induced currents may transport algae. Since the sampling of Chrysochromulina is undertaken close to shore, we include two related wind variables (i.e. ‘onshore’ representing wind towards the shore and ‘alongshore’ representing northeasterlies) to account for such possible sampling effects (figure 4b,c; see Ottersen & Sundby (1995) and Lekve et al. (2002) for details concerning the calculation of the wind variables).
In addition, temperature and salinity data from station 2 were included in the analysis, while indices for the North Atlantic Oscillation (NAO; Hurrell 1995; Hurrell et al. 2003; see also Stenseth et al. 2003) were included to evaluate potential large-scale patterns. These variables showed no associations with the Chrysochromulina spp. data in the early stages of the study and were therefore omitted in the following.
3. Analyses
In this study, short-term correlation structure on the scale of days is not of prime interest. The autoregressive structure in the Chrysochromulina data series was therefore removed before estimating monthly mean abundances. The Akaike Information Criterion (AIC Brockwell & Davies 1991) was used to identify the most appropriate autoregressive (AR) model of lag p (‘AR(p)’, where p is the lag in days; Brockwell & Davies 1991). Once the appropriate AR(p) model was identified, we fitted the model to the raw data, removed the fitted series, and used only the residuals in the subsequent regression analyses (described subsequently). The purpose of this approach was to avoid spurious correlations on the monthly scale between the time-series of Chrysochromulina and environmental data.
The nutrient measures were not mutually independent, partly several of them derived from the same underlying data. To create independent nutrient data, component analysis (PCA; Mardia et al. 1979) was applied. The PCA serves two purposes. First, the PCA reduces multidimensional data into fewer, uncorrelated dimensions of variability that can be analysed at a later stage. Instead of 15 dimensions of variability in the data, we ended up with three principal axes of variability. Second, the PCA can be used to reveal outliers in the data. The first three axes of variability extracted in the PCA, representing nutrient and chlorophyll a levels and ratios, were used as predictor variables in the subsequent regression analyses where Chrysochromulina spp. monthly abundance was used as response variable.
To test the importance of the potential forcing factors determining the timing and amplitude of the blooms of Chrysochromulina spp., we formulated a generalized additive model (GAM; Hastie & Tibshirani 1990) for the relationship between the environmental factors and the Chrysochromulina abundance (standardized residuals from the AR(p) model):
where gn(·) are generalized functions, xt is the log-transformed abundance of Chrysochromulina spp. at time t (natural logarithm of standardised residuals from the AR(p) model, adding a constant of one to avoid undefined data; Sen & Srivastava 1990), while the other variables should be self-explanatory. Such generalized functions allow nonlinearities in the relationships between the forcing factors and the response variable. Many different functional forms may be used—we chose natural splines to fit the nonlinear functions. This approach furthermore allows us to test the degree of nonlinearities in the relationships (e.g. setting the degrees of freedom in the natural spline smoother to 1 results in a linear relationship, degrees of freedom of 2 results in a square relationship, and so on up to 4 degrees of freedom). We used a stepwise search to find the best regression model, using AIC as the model selection criterion (function ‘step.gam’ software S-plus v. 4.5; Venables & Ripley 1997).
To assess the adequacy of the selected regression model (Chrysochromulina spp. as response variable and environmental variables as predictors), three approaches were made in addition to residual evaluation. First, we calculated the Pearson correlation coefficient for the observed data versus the fitted model. Second, we repeated the entire procedure for the first 10 years (1990–1999) and used the estimated model for these years to predict the three final years of the data series (using the environmental data as forcing factors). Third, we fitted an autoregressive moving average (ARMA) model to the residuals to search for the remaining time structure in the data.
Modelling the residuals from the estimated regression model (monthly scale) as an ARMA process (Brockwell & Davies 1991) may reveal biotic relationships on a monthly scale, which are not included in the model. This can be explained in the following way: potential biotic interactions in the system from which the data were derived (i.e. grazer–prey, competition for nutrients, etc.) will be indicated in the most appropriate model as some delayed autoregressive relationship, while environmental factors will lead to moving average terms in the model (see, e.g. Royama 1992).
The data used in this analysis are highly seasonal. By retaining the seasonal patterns in the data, relationships that are coordinated in time will be accentuated. An alternative to keep the seasonal patterns in the data could be to include irradiance as a threshold effect, thus only searching for abiotic forcing factors, when there is sufficient light to sustain a net algal production. To exemplify this further, the effects of nutrient levels on the Chrysochromulina dynamics would not be expected during mid-winter, when the water column lacks stratification. Thus, we find it biologically reasonable to retain the seasonal signals in the data used in this study. However, we did test for relationships unrelated to seasonal patterns by repeating all the analyses mentioned earlier after removing constant seasonal terms from both the Chrysochromulina spp. and the environmental data (using the function ‘stl’ in S-plus; Venables & Ripley 1997). However, no clear patterns were revealed, and these results are not included in the following.
4. Results
The empirical autocorrelation function revealed significant autocorrelation coefficients all over the spectre for as long as 37 days for the Chrysochromulina spp. abundance. An autoregressive model with lag of 35 days, AR(35), was found to be the most appropriate model for the Chrysochromulina spp. log-transformed data (using the natural logarithm adding a constant of 1 for zero measurements). Monthly Chrysochromulina abundance values (corrected for autocorrelations) were calculated simply by averaging over the month after the fitted AR(35) model had been subtracted from the raw data (i.e. using only the model residuals). As the sampling intensity was the same during the whole month (three times weekly), no bias should be introduced. The time of the population(s) peaks varies from May to July, some years including a secondary autumn peak as well (figure 2b).
In our data, there was an extreme measurement of chlorophyll a in March 1996, which was more than twice that of the second largest value. As chlorophyll a is part of several of the other measures (the ratios of chlorophyll a to nitrate, silicate and phosphate), this leads to a strong outlier in the data (not shown). To alleviate this, the values of chlorophyll a and all its derived measures for March 1996 were set to the mean of the two previous and the two subsequent March months. The subsequent PCA revealed no outliers.
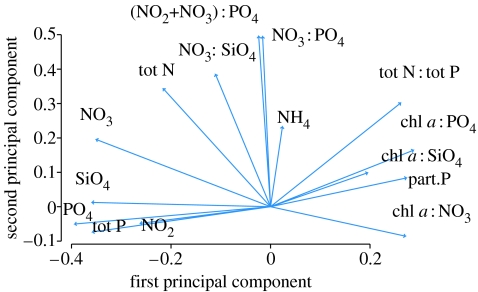
Three axes of variability extracted by the PCA procedure are displayed in figure 4d–f, while the scores of the individual variables on the first and second principal axes of variation are displayed in figure 5.
Figure 5.
The scores of each individual environmental variable on the first and second principal axes of variability (as displayed in figure 4d–f).
The model selection procedure using AIC as a selection criterion suggested a regression model (with the natural logarithm of Chrysochromulina spp. abundance as the response variable) including the first two principal components from the PCA analysis (representing nutrient and chlorophyll a levels and ratios): water inflow from the North Sea and alongshore wind stress as predictor variables. All these are in linear form:
where c0 is the constant term and c1, …, c4 are the coefficients of the variables. The parametric result for the linear model is:
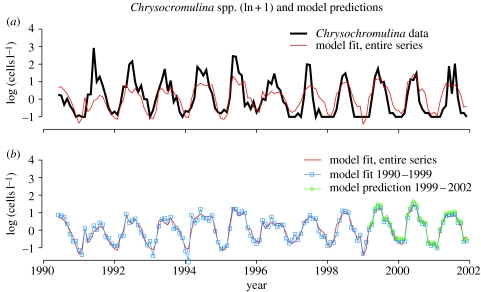
The residuals are reasonably well behaved (not shown). The correlation between the data and this fitted model was 0.72, which is also reflected in the plot (figure 6a). As no nonlinearities were included in the GAM, we refitted the model as an ordinary linear model (using function ‘lm’ in S-plus; Venables & Ripley 1997) (equation presented earlier). All predictor variables were significant at the 0.05 level, with an R2 of 0.51. We observe that the model fits the data very well regarding the timing (figure 6a). The model predicts blooms both earlier and later than the average peak (not shown).
Figure 6.
(a) Chrysochromulina spp. data (solid thick black line) and model predictions based the entire time-series of monthly data 1990–2002 (solid thin red line). (b) The model predictions as displayed in (a) along with the model fit based on the first 10 years of the time-series (solid thin blue line with open squares), as well as the model predictions of the three final years of the time-series. The latter is based on the model fitted from the first 10 years and forced with the first and second principal components, alongshore wind stress and inflow of water to the Skagerrak basin (solid thin green line with open diamonds). See text for details.
Chrysochromulina spp. was positively related to PCA1 and PCA2, as well as with alongshore northeasterly wind stress, but negatively related to inflow data. Referring back to figure 5, this implies that Chrysochromulina spp. abundance is positively correlated to the chlorophyll a-derived measures (i.e. chlorophyll a/nutrient ratios), while negatively related to the concentrations of NO3, NO2, SiO4, PO4 and total P (the effects of PCA1). Moreover, both the dissolved N : P and NO3 : SiO4 ratios, as well as the NH4 concentration, are positively related to the size of the Chrysochromulina assemblage (the effects of PCA2). The abundance of Chrysochromulina spp. increases with alongshore wind stress (i.e. as the northeasterlies sets up advection towards the coast). Finally, the lower the flux of North Seawater into the Skagerrak during the previous month, and hence the slower the NCC and longer water residence time during that period, the higher the abundance of Chrysochromulina.
When the first 10 years were used for model selection (using AIC as the selection criterion), the same model structure was chosen, and when using this model for predicting the three final years of the dataset, the fit is very good (figure 6b).
(a) Autoregressive moving average-modelling of the residuals
There is, however, still temporal structure left in the residuals from the regression model due to the remaining seasonal variation. Thus, the residuals from the fitted regression model described earlier were analysed through ARMA modelling (Chatfield 1996). By using the corrected AIC criterion for model selection, two models were selected as the best (i.e. a difference in AICC values of less than 2): a moving average model with two lags (i.e. two months; MA(2)) and an autoregressive model with two lags; AR(2). By inspecting simulated data using coefficients from the two models, we find that the MA(2) model represents the residuals slightly better. The resulting MA(2) model for the residuals can thus be estimated as the following:
5. Discussion
The unique demonstration of the present study is the predictive power of the abiotic factors for both timing and amplitude of the Chrysochromulina assemblage. Long time-series are very useful for testing hypotheses regarding the dynamics of biological populations (Royama 1992), as demonstrated here. This main conclusion of this study corresponds well with another study that we have made on a different plankton system (Stenseth et al. 2006b).
The variability in timing and concentrations of algal blooms in marine ecosystems is known to be very high (see, e.g. Russel 1973; Cushing 1982; Southward et al. 1995). In this context, an explanatory power of 50%, as obtained by our selected regression model for the relationship between the environmental variables and Chrysochromulina abundance, must be considered strong (see subsequently).
We found the MA(2) model to be slightly better than the AR(2) model for the residuals of the regression model. This emphasises the importance of environmental factors to the Chrysochromulina spp. dynamics. The MA(2) model may be interpreted in the following way: the short-time variation (i.e. 1–2 months) of Chrysochromulina spp. is influenced by short-time variation in exogenous factors. It is reasonable to infer that factors other than the ones included in the data are important. For instance, for plankton data, it is obvious that advective processes are important, and this could serve as one of the several candidates for such exogenic factors.
We suggest that abiotic environmental factors are equally or more important than biological interactions (e.g. competition from conspecifics and other species, and possibly predation) for the Chrysochromulina dynamics (see §3) based on the explanatory power of the regression model, and the slightly better performance of the MA(2) than the AR(2) model for its residuals. From an evolutionary perspective, this is in accordance with the observation that toxicity of C. polylepis in culture makes it less attractive as food for grazers (John et al. 2002). However, further studies would be required to evaluate the relative importance of bottom-up versus top-down control of this genus in the Skagerrak.
The timing of the annual Chrysochromulina peak varied within the three-month period, May–July (see figure 2b), during the years studied here and were not concurrent with the annual chlorophyll a peaks. The selected regression model adequately predicted the time for the observed Chrysochromulina highs (cf. figure 6), demonstrating that we have picked up the signals specific for Chrysochromulina assemblage and not the algal community as a whole.
The population dynamics of the Chrysochromulina assemblage was well predicted by the abiotic environmental forcing factors included in the selected regression model. Nutrient levels and ratios, as represented by PCA1 and PCA2, were indicated to be of prime importance for the Chrysochromulina blooms. This supports the hypotheses forwarded soon after the extreme bloom of C. polylepis in 1988 (Aksnes et al. 1989; Dahl et al. 1989; Maestrini & Granéli 1991), as well as the results from the more preliminary evaluation of a subset of the same data as analysed in depth in the present paper (see Dahl et al. 2005). In addition to the effect of the nutrient status of the system, our present study shows that the flux of North Seawater into the Skagerrak (simulated inflow data), as well as alongshore northeasterly wind stress (setting up shoreward water transport) play important roles in the Chrysochromulina concentration at our coastal sampling station.
Even if potential nonlinear relations were evaluated, none of the kind was suggested from our analysis. On the contrary, our selected model that included only linear effects of the environmental variables on Chrysochromulina abundance performed the best.
(a) Abiotic forcing factors
The strength of the PCA in this study is that the loadings of the individual nutrients grouped into reasonably interpretable assemblages. While all the chlorophyll a: nutrient ratios loaded positively on the first principal axis of the PCA, most of the dissolved nutrients loaded negatively on the same axis. On the other hand, the N : P ratios loaded positively on the second axis of variability of the PCA, but generally correlated little with the first PCA axis. The crux of PCA is that the axes are defined to be uncorrelated, which is an advantage as we use the extracted components as forcing variables in the subsequent regression analyses. In this way, the problem of many strongly interrelated environmental variables can be dealt with, while also reducing the number of dimensions of variation. However, using composite variables makes it somewhat more difficult to pinpoint the variables of strongest influence.
The monitoring dataset does not distinguish between the different Chrysochromulina spp. present in the Skagerrak, as this would require identification and quantification by electron microscopy and/or molecular biological methods. For this reason, we recognize some limitations regarding the inferences that can be drawn from our study. Most importantly, the species composition of the Chrysochromulina assemblage is unknown, since more than 40 species of this genus have been recorded in the Skagerrak and Kattegat (Jensen 1998). Hence, the relative proportion of potentially toxic species and to which degree these were actually producing toxins during the study period are unclear. On the other hand, it seems fair to assume that the different species of the genus Chrysochromulina have somewhat similar although not identical responses to the environment due to the similarities in morphology (unicellular flagellates with haptonema), cell size (3–20 μm), nutrition (phototrophic, auxotrophic and some also phagotrophic) and maximum growth rate (about 1 division per day; reviews by Edvardsen & Paasche 1998; Moestrup & Thomsen 2003 and references therein). Thus, the objective of this exercise is to evaluate the environmental forcing of the population dynamics of the genus and not to predict toxic incidents.
Our model adequately simulates the timing of the observed Chrysochromulina spp. blooms. The model suggests that the size of the Chrysochromulina assemblage is inversely related to the amount of several nutrients in upper waters and positively related to the nutrient ratios included (N : P, N : SiO4). The levels of inorganic dissolved nutrients are lowest, while the ratios of inorganic dissolved nitrogen to both phosphorous and silicate are highest during summer. At this time of year, the algal community is dominated by small flagellates (i.e. a ‘mature’ algal community structure). Our positive correlation between Chrysochromulina abundance and PCA2, on which the N : P ratio loaded strongly, supports the inference made shortly after the bloom in 1988 that unusual weather conditions creating high N : P ratios may be favourable to C. polylepis. The Chrysochromulina spp. bloom in late spring or summer in our sampling area. Like other small algal flagellates, Chrysochromulina is efficient in utilizing the low levels of dissolved nutrients typical during summer, which may provide this genus with a competitive advantage compared to larger algal groups. Moreover, Chrysochromulina is capable of obtaining phosphorus from other algae or bacteria by phagotrophy (review by Jones et al. 1994) and also possibly by dasmotrophy (Estep & Macintyre 1989), in this way rather acting as a grazer. It is motile and might be able to move actively towards depths with higher nutrient levels, and be also able to make use of the available light (Kaas et al. 1991). We suggest that low levels of NO3 and NO2 are not advantageous to Chrysochromulina by itself, but may be associated with favourable conditions for growth, such as high light availability, strong stratification, low turbulence and availability of regenerated NH4 and possibly micronutrients. Limiting PO4 concentrations may also promote toxin production and reduced grazing (review by Edvardsen & Paasche 1998). The fact that the Chrysochromulina species bloom when most of the dissolved nutrients are already consumed by diatoms and other algae results in a negative relationship between levels of dissolved nutrients and abundance of Chrysochromulina spp. The positive relationship with chlorophyll a may simply imply that there are high abundances of other algae as well, since all algae contain chlorophyll a. As a control, we repeated the analyses without including chlorophyll a and its derived measures, but this had little effect on the results.
(b) Currents and wind: sampling effects and large-scale variability
In the raw data, increases in abundance on the two-day scale could not be explained from experimental growth rates of C. polylepis. Aggregation created by Ekman transport is a plausible explanation for such observed jumps in algal concentration in the samples. We believe that the alongshore wind stress represents this effect well (Lekve et al. 2002) and emphasizes the importance of including variables that capture sampling effects.
The effect of inflow of North Seawater to the Skagerrak may also be interpreted as a more indirect and large-scale effect. We found that the lower the flux of North Seawater into the Skagerrak during the previous month, and hence the slower the NCC during that period, the higher the abundance of Chrysochromulina. This may be explained by relatively calm weather conditions and low turbulence creating favourable conditions for Chrysochromulina combined with a longer residence time and thereby a longer period to build up phytoplankton biomass. We speculate that the inflow variable represents the availability of nutrients, stratification of water masses, as well as the strength of the NCCs and hence the residence time of its water. These factors may be of importance to Chrysochromulina spp. The fact that NAO, temperature and salinity were redundant to the effect of inflow (see e.g. Stenseth et al. 2003) further supports such a hypothesis. However, variables similar to NAO (e.g. pressure fields) are included in the inflow variable, and thus capture some of the variability that might otherwise be captured by the NAO (Reid et al. 2003). To sort out this effect is beyond the scope of our investigation.
(c) Bottom-up versus top-down control
Bottom-up control of a community arises, when direct or indirect dependence of community structure on factors producing variation at lower trophic levels or in their resources is present (Menge 1992). The strong predictive power displayed in this study (a correlation of 0.72 between the observed and predicted time-series of Chrysochromulina spp.) emphasizes the importance of abiotic factors for the dynamics of this algal genus. We do not believe that there is an either–or relationship between bottom-up and top-down control. However, from our analysis of the Skagerrak dataset, we suggest that environmental abiotic factors are the main determinants of the bloom dynamics for Chrysochromulina spp. in this area.
6. Conclusion
The overall conclusion of this study is that the seasonal dynamics of Chrysochromulina were very well accounted for by abiotic factors, such as nutrient conditions, currents and wind, without including seasonal biological relationships. This furthermore implies, as found through our analysis, that the between-year variation in the timing and abundance is very well accounted for by between-year variation in the abiotic and environmental conditions.
Acknowledgments
Financial support from The Research Council of Norway (NFR; Biodiversity programme) to K.L. and N.C.S. made the reported analyses possible. E.B. is grateful to The Research Council of Norway for funding (the EcoClim-project), as well as a Marie Curie post doctoral fellowship (MCFI-2002-01148) from the EU. Jon-Olav Vik is kindly thanked for nasty comments and constructive suggestions.
References
- Aksnes, D., Aure, J. Furnes, G. K. Skjoldal, H. R. & Sætre, R. 1989 Analysis of the Chrysochromulina polylepis bloom in the Skagerrak, May 1988. Environmental conditions and possible causes, pp. 1–38, 28 figs. Bergen, Norway: Bergen Scientific Centre.
- Aure J, Danielssen D, Svendsen E. The origin of Skagerrak coastal water off Arendal in relation to variation in nutrient concentrations. ICES J. Mar. Sci. 1998;55:610–619. doi:10.1006/jmsc.1998.0395 [Google Scholar]
- Brockwell P.J, Davies R.A. Springer series in statistics. vol. 2. Springer; New York, NY: 1991. Time series: theory and methods. [Google Scholar]
- Chatfield C. Chapman & Hall; London, UK: 1996. The analysis of time series: an introduction. [Google Scholar]
- Cushing D.H. Academic Press; London, UK: 1982. Climate and fisheries. [Google Scholar]
- Dahl E, Danielssen D.S. Hydrography, nutrients and phytoplankton in the Skagerrak along the section Torungen–Hirtshals, January–June 1980. In: Sætre R, Mork M, editors. The Norwegian coastal current. University of Bergen; Bergen, Norway: 1981. pp. 294–310. [Google Scholar]
- Dahl E, Johannessen T. Temporal and spatial variability of phytoplankton and chlorophyll a: lessons from the south coast of Norway and the Skagerrak. ICES J. Mar. Sci. 1998;55:680–687. doi:10.1006/jmsc.1998.0401 [Google Scholar]
- Dahl E, Tangen K. 25 years experience with Gyrodinium aureolum in Norwegian waters. In: Smayda T.J, Shimizu Y, editors. Toxic phytoplantkon blooms in the sea. Proc. 5th Int. Conf. on Toxic Phytoplankton, Newport, R.I., 28 October–1 November, 1991. Elsevier; Amsterdam, The Netherlands: 1993. pp. 15–21. [Google Scholar]
- Dahl E, Lindahl O, Paasche E, Throndsen J. The Chrysochromulina polylepis bloom in Scandinavian waters during spring 1988. In: Cosper E.M, Bricelj V.M, Carpenter E.J, editors. Novel phytoplankton blooms. vol. 35. Springer; New York, NY: 1989. pp. 383–405. [Google Scholar]
- Dahl E, Edvardsen B, Eikrem W. Chrysochromulina blooms in the Skagerrak after 1988. In: Reguera B, Blanco J, Fernández M.L, Wyatt T, editors. Harmful microalgae. UNESCO; Paris, France: 1998. pp. 104–105. [Google Scholar]
- Dahl E, Bagøien E, Edvardsen B, Stenseth N.C. The dynamics of Chrysochromulina species in the Skagerrak in relation to environmental conditions. J. Sea Res. 2005;54:15–24. doi:10.1016/j.seares.2005.02.004 [Google Scholar]
- Danielssen D.S, Svendsen E, Ostrowski M. Long-term hydrographic variation in the Skagerrak based on the section Torungen–Hirtshals. ICES J. Mar. Sci. 1996;53:917–925. doi:10.1006/jmsc.1996.0113 [Google Scholar]
- Danielssen D.S, Edler L, Fonselius S, Hernroth L, Ostrowski M, Svendsen E, Talpsepp L. Oceanographic variability in the Skagerrak and northern Kattegat, May–June, 1990. ICES J. Mar. Sci. 1997;54:753–773. doi:10.1006/jmsc.1996.0210 [Google Scholar]
- Edvardsen B, Paasche E. Bloom dynamics and physiology of Prymnesium and Chrysochromulina. In: Anderson D.M, Cembella A.D, Hallegraeff G.M, editors. Physiological ecology of harmful algal blooms. vol. 41. Springer; Heidelberg, Germany: 1998. pp. 193–208. [Google Scholar]
- Estep K.W, Macintyre F. Taxonomy, life cycle, distribution and dasmotrophy of Chrysochromulina: a theory accounting for scales, haptonema, muciferous bodies and toxicity. Mar. Ecol. Prog. Ser. 1989;57:11–21. [Google Scholar]
- Gjøsæter J, et al. A long-term perspective on the Chrysochromulina bloom on the Norwegian Skagerrak coast 1988: a catastrophe or an innocent incident? Mar. Ecol. Prog. Ser. 2000;207:201–218. [Google Scholar]
- Hastie T.J, Tibshirani R.J. Chapman & Hall; London, UK: 1990. Generalized additive models. [DOI] [PubMed] [Google Scholar]
- Hurrell J.W. Decadal trends in the North Atlantic Oscillation: regional temperatures and precipitation. Science. 1995;269:676–679. doi: 10.1126/science.269.5224.676. [DOI] [PubMed] [Google Scholar]
- Hurrell J.W, Kushnir Y, Ottersen G, Visbeck M. Geophysical Monograph Series. vol. 134. American Geophysical Union; Washington, DC: 2003. The North Atlantic Oscillation: climatic significance and environmental impact. pp. 1–35. [Google Scholar]
- Jensen, M. Ø. 1998 The genus Chrysochromulina (Prymnesiophyceae) in Scandinavian coastal waters. Ph.D. thesis, University of Copenhagen, Copenhagen.
- John U, Tillmann U, Medlin L.K. A comparative approach to study inhibition of grazing and lipid composition of a toxic and non-toxic clone of Chrysochromulina polylepis (Prymnesiophyceae) Harmful Algae. 2002;1:45–57. doi:10.1016/S1568-9883(02)00005-7 [Google Scholar]
- Jones H.L.J, Leadbeater B.S.C, Green J.C. Mixotrophy in haptophytes. In: Green J.C, Leadbeater B.S.C, editors. The haptophyte algae. Clarendon Press; Oxford, UK: 1994. pp. 247–263. [Google Scholar]
- Kaas H, Larsen J, Mohlenberg F, Richardson K. The Chrysochromulina polylepis bloom in the Kattegat (Scandinavia) May–June 1988. Distribution, primary production and nutrient dynamics in the late stage of the bloom. Mar. Ecol. Prog. Ser. 1991;79:151–161. [Google Scholar]
- Lekve K, Ottersen G, Stenseth N.C, Gjøsæter J. Length dynamics in juvenile coastal Skagerrak cod: effects of biotic and abiotic processes. Ecology. 2002;83:1676–1688. [Google Scholar]
- Maestrini S.Y, Granéli E. Environmental conditions and ecophysiological mechanisms which led to the 1988 Chrysochromulina bloom: a hypthothesis. Oecologia Acta. 1991;14:397–413. [Google Scholar]
- Mann K.H, Lazier J.R.N. Blackwell; Cambridge, UK: 1991. Dynamics of marine ecosystems. [Google Scholar]
- Mardia K.V, Kent J.T, Bibby J.M. Academic Press; London, UK: 1979. Multivariate analysis. [Google Scholar]
- Menge B.A. Community regulation: under what conditions are bottom-up factors important on rocky shores. Ecology. 1992;73:755–765. doi:10.2307/1940155 [Google Scholar]
- Moestrup Ø, Thomsen H.A. Taxonomy of toxic haptophytes (prymnesiophytes) In: Hallegraeff G.M, Anderson D.M, Cembella A.D, editors. Manual on harmful marine microalgae. UNESCO Publishing; Paris, France: 2003. pp. 433–463. [Google Scholar]
- Ottersen G, Sundby S. Effects of temperature, wind and spawning stock biomass on recruitment of Arcto-Norwegian cod. Fish. Oceanogr. 1995;4:278–292. [Google Scholar]
- Reid P.C, Edwards M, Beaugrand G, Skogen M.D, Stevens D. Periodic changes in the zooplankton of the North Sea during the 20th century linked to oceanic inflow. Fish. Oceanogr. 2003;12:260–269. doi:10.1046/j.1365-2419.2003.00252.x [Google Scholar]
- Royama T. Chapman & Hall; London, UK: 1992. Analytic population dynamics. [Google Scholar]
- Russel F.S. A summary of the observations on the occurrence of planktonic stages of fish off Plymouth 1924–1972. J. Mar. Biol. Assoc. UK. 1973;53:347–355. [Google Scholar]
- Sen A, Srivastava M. Springer; New York, NY: 1990. Regression analysis: theory, methods, and applications. [Google Scholar]
- Skogen, M. D. & Søiland, H. 1998 A User's guide to NORWECOM v2.0. The NORWegian ECOlogical Model system. Technical Report Fisken og Havet, vol. 18, 42 pp. Bergen, Norway: Institute of Marine Research.
- Southward A.J, Hawkins S.J, Burrows M.T. Seventy years' observations of changes in distribution and abundance of zooplankton and intertidal organisms in the western English Channel in relation to rising sea temperature. J. Therm. Biol. 1995;20:127–155. doi:10.1016/0306-4565(94)00043-I [Google Scholar]
- Stenseth N.C, Ottersen G, Hurrell J.W, Mysterud A, Lima M, Chan K.S, Yoccoz N.G, Adlandsvik B. Studying climate effects on ecology through the use of climate indices: the North Atlantic Oscillation, El Niño Southern Oscillation and beyond. Proc. R. Soc. B. 2003;270:2087–2096. doi: 10.1098/rspb.2003.2415. doi:10.1098/rspb.2003.2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenseth N.C, Jorde P.E, Chan K.-S, Hansen E, Knutsen H, André C, Skogen M.D, Lekve K. Ecological and genetic impact of Atlantic cod larval drift in the Skagerrak. Proc. R. Soc. B. 2006a;273:1085–1092. doi: 10.1098/rspb.2005.3290. doi:10.1098/rspb.2005.3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenseth N.C, Llope M, Anadón R, Ciannelli L, Chan K.-S, Hjermann D.Ø, Bagøien E, Ottersen G. Seasonal plankton dynamics along a cross-shelf gradient. Proc. R. Soc. B. 2006b;273:2831–2838. doi: 10.1098/rspb.2006.3658. doi:10.1098/rspb.2006.3658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables W.N, Ripley B.D. Springer; New York, NY: 1997. Modern applied statistics with S-PLUS. [Google Scholar]