Abstract
High levels of binding sites for melanocortin peptides exist within the arcuate nucleus, and a functional response to melanocortin peptides has been demonstrated in arcuate POMC neurons. Because the MC3R is thought to function as an inhibitory autoreceptor on POMC neurons, we reasoned that peripheral injections of MC3R-specific agonists would act within the arcuate nucleus to inhibit POMC neurons and thereby stimulate feeding. We demonstrate that the peptidergic MC3R agonist, d-Trp8-γ-MSH, stimulates feeding via the MC3R when injected peripherally. These data provide the first evidence that feeding can be stimulated by peripheral injection of MC3R-specific agonists.
Keywords: Cachexia, Feeding behavior, Food intake, Melanocortin, Arcuate nucleus
1. Introduction
1.1. The melanocortin system and MC3R
Numerous studies have demonstrated that the hypothalamic melanocortin system is critical for the maintenance of body weight and energy homeostasis [6,12]. The available data strongly support a model wherein hypothalamic pro-opiomelanocortin (POMC) neurons in the hypothalamus and brainstem regulate body weight via their release of α-MSH that in turn activates the type 4 melanocortin receptor (MC4R) in many parts of the brain. Genetic deletion of POMC or the MC4R in mice (MC4RKO) and mutations in the MC4R in humans leads to a well-defined phenotype consisting of increased lean body mass and fat mass, relative hyperphagia, and disturbance in the metabolic response to overnutrition [12]. This has in turn led to the proposal that melanocortin agonists may be useful in the treatment of obesity, and that melanocortin antagonists may be useful to treat cachexia in chronic diseases [27]. While the importance of the MC4R in maintaining appropriate metabolic homeostasis is clear, the role of the melanocortin-3 receptor (MC3R) remains relatively obscure. MC3RKO mice have reduced lean body mass and increased fat mass and are slightly hypophagic relative to WT controls [2,4]. The effect of MC3R deficiency on feeding and resting oxygen consumption is complex. Remarkably, despite the increased adiposity seen in the MC3RKO mice, the animals do not exhibit increased food intake or weight gain, even on a high fat chow that is known to induce hyperphagia in MC4RKO animals [2,3]. It has also not been possible to demonstrate differences in resting basal or total metabolic rate in this model [2,4]. At this point, it appears that the obese phenotype of the MC3RKO mouse may be explained by defects in fatty acid oxidation and decreases in home-cage activity[2,4].
1.2. The MC3R as an inhibitory autoreceptor
Many of features of the MC3R KO phenotype are consistent with the putative role for the MC3R as an inhibitory autoreceptor on POMC neurons [1,10,23]. In this capacity, deletion of the MC3R might be expected to enhance POMC neuronal function, thereby leading to the observed decrease in lean body mass and relative hypophagia observed in this animal model. Obviously, the fact that the MC3R is also expressed in the ventromedial hypothalamus (VMH) and more than 30 other brain nuclei in the rat [29] as well as in multiple in peripheral tissues [5,16,17] indicates that this is unlikely to be the only role for this receptor. Nonetheless, our previous studies demonstrated that MC3RKO mice were highly susceptible to cachexia during acute and chronic disease, whereas the MC4RKO mice were resistant to cachexia [26,28]. Therefore, we proposed that peripheral signals produced during illness activate POMC neurons and thereby increase signaling at MC4 receptors. This predicts that the loss of an auto-inhibitory ‘brake’ on these neurons, as provided by the MC3R, would enhance cachexia in our models, and this what was found [26]. This model also predicts that MC3R agonists should stimulate feeding, and therefore provide a potential therapeutic target to dampen the effects of cachexia.
1.3. Specific peptide MC3R agonists
In a search for potent and receptor-selective agonists and antagonists of melanocortin receptors, Grieco et al. screened a series of peptide compounds created by substituting d-amino acids at each position of the endogenous MC3R selective melanocortin peptide, γ-MSH [18]. They found that the d-Trp8 analogue had both high affinity for the MC3R (IC50 approximately 6 nM), and 100-fold selectivity for the MC3R relative to the MC4R. In previous studies, we have demonstrated that this MC3 agonist limits POMC neuronal activation in slice culture by increasing the inhibitory GABA tone on these neurons, presumably by activating arcuate NPY/GABA neurons [10]. This area of the brain is also known to express the MC3R and to have a very high binding capacity for γ-MSH [29,31]. Furthermore, the arcuate nucleus is known to respond to a variety of circulating peptide compounds, and is thought to have a relatively permeable blood-brain barrier [7]. Thus, we hypothesized that peripheral injections of d-Trp8-γ-MSH would act on the MC3R within the arcuate nucleus to inhibit POMC neuronal activity, and thereby stimulate feeding. Presumably this peptidergic compound would have limited access to other brain regions, and therefore, provide us insight into the role of the arcuate MC3R in the absence of its effects elsewhere in the brain. Here, we have demonstrated that peripheral injection of this potent MC3R agonist can stimulate feeding by acting at the MC3R, even in satiated animals.
2. Materials and methods
2.1. Animals
WT C57BL/6J mice were obtained from Jackson Laboratories (Bar Harbor, Maine). MC3RKO mice, and their wild-type (WT) controls were derived from the original C57BL/6J × 129 colonies [2,21] maintained within the Vollum Institute that had been bred seven generations into the C57BL/6J strain and maintained as homozygous lines. MC4RKO mice, described previously [21], were bred 10 generations into the same C57BL/6J strain. All mice were raised group housed in a 12 h light/dark cycle. For studies measuring food intake, mice were housed individually and food intake estimated by measuring the weight of powdered food remaining in feeding chambers designed to maximize spill capture (Nalgene mouse food chambers). Mice were weaned at 21 days and allowed ad libitum access to powdered Laboratory Rodent Diet (Purina) that was weighed and replaced daily. In all studies, male animals aged 6–10 weeks were used. All studies were conducted according to the NIH Guide for the Care and Use of Laboratory Animal and approved by the Animal Care and Use Committee of the Oregon Health Sciences University.
2.2. Compound administration
Each animal was handled daily for a minimum of 5 consecutive days prior to the initiation of each experiment, simulating the restraint used during the injection of the compounds. d-Trp8-γ-MSH dissolved in normal saline and administered i.p., with a fresh batch of compound being prepared from lyophilized stock immediately prior to injection. Knockout mice and littermate controls had basal feeding monitored for 2 days, and then during each 12 h period following an i.p. saline injection to demonstrate that the observed effects were not due to differential stress responses observed in other models of melanocortin blockade [11]. All animals were weighed prior to compound injection, and the doses were normalized to individual animal body weight.
2.3. Feeding studies
Animals were individually housed for a minimum of 1 week prior to starting each experiment. Animals were habituated to eating powdered mouse chow from containers designed to minimize spill and contamination of the remaining food. For each of the daytime studies, the animals were first habituated to the handling and injection protocol by giving saline injections daily 1 h after lights on (0700 or 0800 h, depending on daylight savings time status) for 3 consecutive days. In each case, we confirmed that the food intake of the WT animals and the knockout models was not different after the third sham injection, indicating that the differential response to stress observed in other models of melanocortin blockade had been attenuated [11]. On the fourth experimental day, the animals were injected with d-Trp8-γ-MSH or saline control 1 h after lights on, and food intake was measured at 2, 4, 6, 12 and 24 h. For the nocturnal feeding study, an identical paradigm was used, except that the habituation and experimental injections were given 1 h prior to lights off (1800 h). In the initial dose–response study, animals were injected with doses ranging from 0.005 to 500 mcg i.p. (n = 10 per group) and food consumption at 2, 4, 6, 12 and 24 h was measured. The experiment was repeated on the following day with all groups re-randomized to treatment. For each of the experiments involving knockout models, half of the WT and half of the receptor-knockout animals received saline, and the other half received d-Trp8-γ-MSH.
2.4. Statistical methods
In the dose–response study, differences between groups were measured by one-way ANOVA to compare all groups. Differences between feeding and activity curves in all other experiments were analyzed by two-way, repeated measures ANOVA with time and treatment as the measured variables. Post hoc Bonferroni testing was used to determine differences between groups within an experiment. Data sets were analyzed for statistical significance using either the PRISM software package (GraphPad) for ANOVA with repeated measures.
3. Results
3.1. Daytime food intake, dose–response
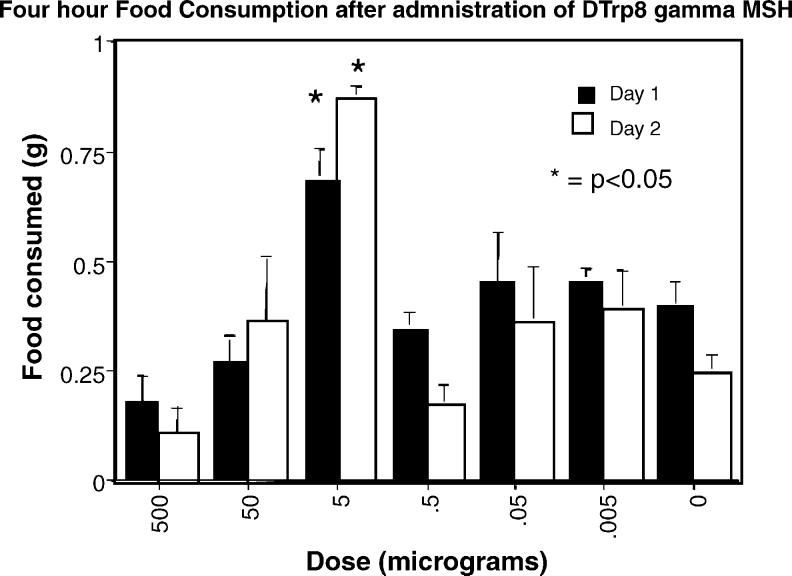
The MC3R is expressed in a limited number of hypothalamic nuclei, and is thought to function, in part, as an autoinhibitory receptor on POMC neurons [1]. We reasoned that a peptide agonist, injected peripherally, would have access to sites within the arcuate nucleus (which has a minimal blood-brain barrier), but not to sites further into the brain parenchyma. Therefore, the expected outcome of peripheral MC3R agonist injection would be an inhibition of tonic POMC neuronal activity that in turn would lead to an increase in food intake. In collaboration with Dr. Victor Hruby, we were able to obtain a very specific and potent MC3R agonist, d-Trp8-γ-MSH [18]. Animals were individually housed for 2 weeks prior to the start of the experiment. For 3 consecutive days prior to injecting the test compound, animals were injected i.p. with saline at 1 h after lights on. On the experimental day, animals were injected with doses ranging from 0.005 to 500 mcg i.p. (n = 10 per group) and food consumption at 2, 4, 6, 12 and 24 h was measured. The experiment was repeated on the following day with all groups re-randomized to treatment. The difference between groups was significant when the 5 mcg group was compared with all other groups at 2 and 4 h. The data for cumulative food intake at 4 h is shown in Fig. 1. No differences were observed in the consumption of water (ANOVA p = 0.8, not shown).
Fig. 1.
Feeding response to peripheral injection of d-Trp8-γ-MSH. Animals were injected with doses ranging from 0.005–500 mcg i.p. (n = 10 per group) and food consumption at 2, 4, 6, 12 and 24 h was measured (dark bars). The experiment was repeated on the following day with all groups re-randomized to treatment (open bars). The difference between groups was significant when the 5 mcg group was compared with all other groups at 2 and 4 h. The data for cumulative food intake at 4 h is shown (*p < 0.05).
3.2. Repeated daily injections
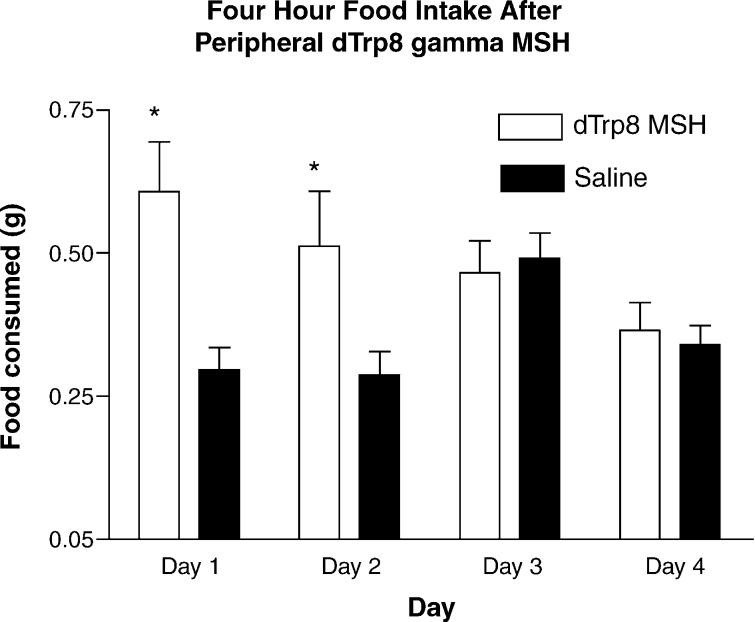
To confirm and extend these findings, groups of WT animals (n = 15) were injected with saline for 3 days (1 h after lights on), followed by an injection of 5 mcg d-Trp8-γ-MSH or saline control with food intake measured at noon on days 4–6. These data show that the effect of peripheral injection of MC3R agonists reproducibly stimulate food intake in satiated animals, and that the repeated injections of this compound may produce some tolerance effects. The results of this experiment are shown in Fig. 2. We were also able to demonstrate increased feed intake in animals injected immediately prior to lights-out (i.e. nocturnal food intake, Fig. 3).
Fig. 2.
Feeding response to peripheral injection of 5 mcg of d-Trp8-γ-MSH. Groups of WT animals (n = 15) were injected with saline for 3 days, followed by an injection of 5mcg d-Trp8-γ-MSH or saline control with food intake measured 4 h later (days 4–6 (*p < 0.05)).
Fig. 3.
Nocturnal feeding response to peripheral injection of 5 mcg of d-Trp8-γ-MSH at 1800 h. Groups of WT animals (n = 15) were injected with saline for 3 days (1800 h), followed on the experimental day by an injection of 5 mcg d-Trp8-γ-MSH or saline control at 1800 h (*p < 0.05).
3.3. Studies in MC4RKO mice
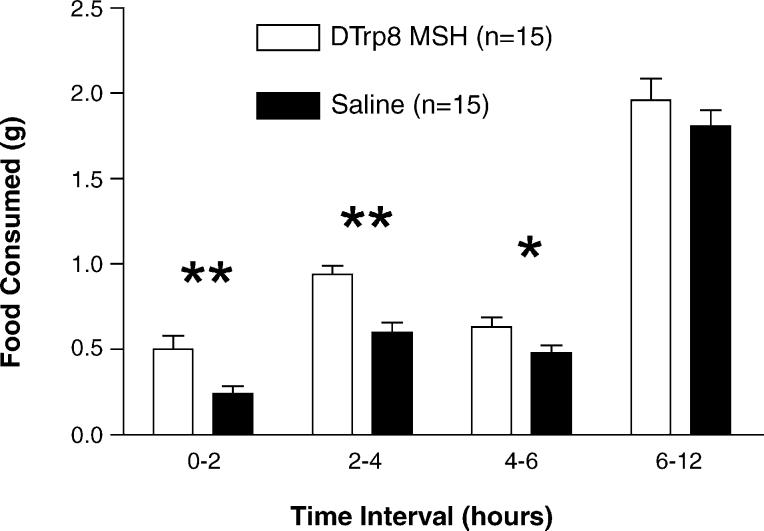
In our early dose–response studies, we found that animals treated with 50 or 500 mcg of d-Trp8-γ-MSH had lower food intake that saline-treated controls. We hypothesized that this effect was due to “spillover” stimulation of the hypothalamic MC4R. To test this hypothesis, and to demonstrate the specificity of the effect of this compound on the MC3R, we injected groups of MC4RKO mice (saline n = 14, d-Trp8 n = 14) or WT controls (saline n = 20, d-Trp8-γ-MSH n = 20) with saline on days 1–3, followed by test compound on day 4. Food intake was measured at 2, 4 and 6 h after injection. These data show that the inhibitory effect of 50 mcg of d-Trp8-γ-MSH on feeding are due to stimulation of the MC4R, as the MC4RKO animals showed stimulation of feeding with the 50 mcg dose, while WT animals showed a relative inhibition of feeding (Two-way repeated measures ANOVA p < 0.005 for group, p = ns time; Fig. 4). As with previous studies, this effect was of relatively short duration, with no effect seen after the 4 h time point (ANOVA 0–2 h p < 0.001, 2–4 h p < 0.01, 4–6 h p = 0.5; 6–12 h p = 0.6). The cumulative food intake over the first 4 h of this study is shown in Fig. 5.
Fig. 4.
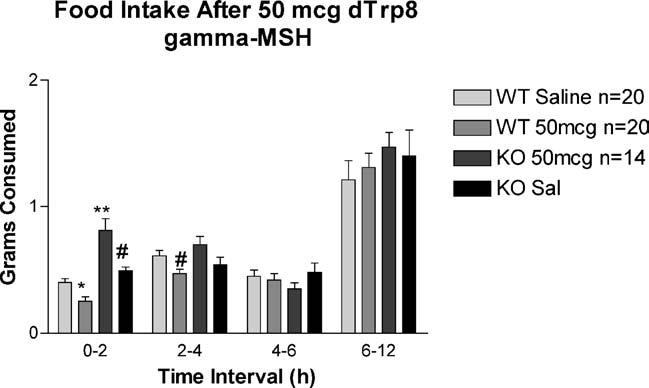
Feeding response to peripheral injection of high dose d-Trp8-γ-MSH in WT and MC4RKO animals. Groups of MC4RKO mice (n = 14) or WT controls (saline n = 20, d-Trp8-γ-MSH n = 20) were injected with saline on at 0800 days 1–3, followed by test compound on day 4. Food intake was measured at 2, 4, 6 and 12 h after injection (ANOVA p < 0.01 at 0–2 and 2–4 h intervals; *p < 0.05, **p < 0.001 vs. WT saline; #p < 0.01 vs. KO 50 mcg d-Trp8).
Fig. 5.
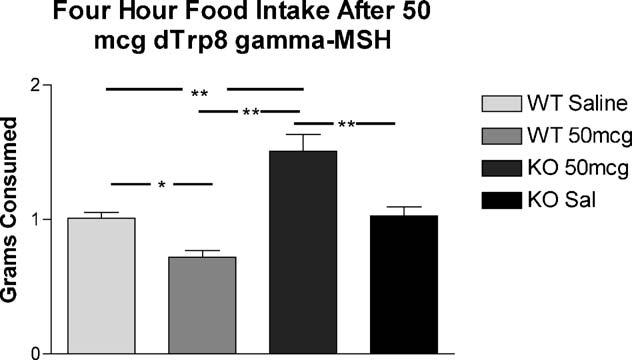
Four-hour cumulative feeding response to peripheral injection of d-Trp8-γ-MSH in WT and MC4RKO animals. (ANOVA p < 0.001 for treatment, post hoc *p < 0.05, **p < 0.001).
3.4. Studies in MC3RKO mice
In a final experiment, we tested the effect of d-Trp8-γ-MSH on feeding in MC3R knockout mice. In this case, we hypothesized that there would be no stimulation of feeding in the receptor-knockout mouse. Groups of MC3RKO mice and WT controls (n = 10) were individually housed for 2 weeks. On days 1–3, the animals were injected i.p. with saline at 0700 h. On the fourth day, animals were injected with 5 mcg of d-Trp8-γ-MSH (n = 5 per genotype) or saline (n = 5 per genotype) at 0700 h, and food intake was measured 2, 4 and 6 h later. In this case, significant stimulation of feeding was observed only in the WT animals, whereas no stimulation of feeding was observed in the MC3RKO (ANOVA p < 0.05 for group, p = 0.88 for time). As with previous experiments, this result was significant at 2 and 4h (Fig. 6).
Fig. 6.
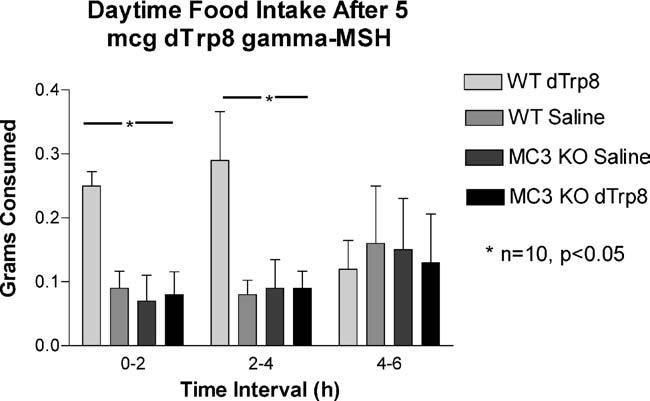
Feeding response to peripheral injection of d-Trp8-γ-MSH in WT and MC3RKO animals. Groups of MC3RKO mice and WT controls (n = 10) were injected i.p. with saline at 0700 h on days 1–3. On the fourth day, all animals were injected with 5 mcg if d-Trp8-γ-MSH at 0700 h, and food intake was measured 2, 4 and 6 h later. In this case, significant stimulation of feeding was observed only in the WT animal, whereas no stimulation of feeding was observed in the MC3RKO (ANOVA p < 0.01).
4. Discussion
4.1. Stimulation of food intake with MC3R agonists
Several lines of evidence suggest that γ-MSH acts on the MC3R within the hypothalamic arcuate nucleus to dampen the activity of arcuate POMC neurons via direct and indirect neural mechanisms [1,10,29,31]. Because hypothalamic POMC neurons provide a tonic inhibition of food intake, we reasoned that stimulation of the MC3R within the arcuate should lead to an increase in food intake [13]. We also proposed that peripheral injections of peptidergic compounds would be expected to have the most potent effects in brain areas with an attenuated blood-brain barrier, such as the arcuate nucleus. To test this hypothesis, we performed peripheral injections with a potent and receptor-specific MC3R agonist (d-Trp8-γ-MSH) in groups of wildtype and melanocortin receptor-knockout mice. In our first study, we established a general dose–response relationship and demonstrated that a dose of 5 mcg of this compound would reproducibly stimulate food intake both when given to satiated animals (daytime feeding) and when given to animals at the start of their normal feeding cycle. This stimulation of food intake was of short duration (4–6 h), and this compound was still effective in MC4RKO animals. These results are consistent with the findings of Cowley et al. who demonstrated that this compound produces an inhibition of POMC neuronal activity that is due to an increase in inhibitory synaptic transmission (due to GABA release onto POMC neurons), presumably due to the activation of GABAergic NPY neurons in the arcuate nucleus [8,10]. Thus, our observations may be due to the release and action of NPY, a peptide that is known to have orexigenic activity that is of short duration [24]. With repeated daily injections, an attenuation of the stimulatory effect on food intake was observed. Tolerance to the orexigenic effect of NPY is not observed with repeated injections [30]. Thus, we speculate that the decrease in efficacy of d-Trp8-γ-MSH over several days is due to tolerance to the drug itself, perhaps due to receptor desensitization.
4.2. Potential peripheral effects of MC3R agonists
The MC3R is expressed in multiple peripheral tissues, and MC3R agonists are known to limit the inflammatory response of macrophages [15,25], and produce sodium excretion by the kidney [20]. It is possible that our results are due to one or more peripheral effects of d-Trp8-γ-MSH. For example, sodium loss and diuresis could produce both thirst and hunger in an attempt to replace lost sodium. However, we did not observe any changes in water consumption with this compound, making this an unlikely explanation. Given that our animals were healthy and housed in a pathogen-free environment, it is also unlikely that the stimulation of feeding is due to an anti-inflammatory effect. The possibility that there were important effects on other tissues known to express the MC3R including adipose tissue, skeletal muscle, kidney, stomach, and pancreas, will require further studies [5,14].
4.3. Potential stimulation of the MC4R
We observed a decrease in feeding in animals given a high dose of d-Trp8-γ-MSH, relative to saline-treated controls. It is possible that this was due to either a non-specific illness effect of the compound, or due to one or more effects of peripheral stimulation of the MC3R (e.g. hypotension due to natriuresis). However, we did not observe this decrease in MC4RKO animals. Indeed, these animals responded to a dose of d-Trp8-γ-MSH that produced relative anorexia in WT animals with a further stimulation of food intake. Thus, we propose that this inhibition of food intake may be due to activation of the MC4R, which has moderate affinity for this compound (EC50 = 100 nM). Central and peripheral injections of MTII, a non-selective agonist of the MC3R and MC4R, inhibits food intake in both WT and MC3RKO mice [4,13,19]. This indicates that significant stimulation of the MC4R eliminates any potential orexigenic effects of stimulating the MC3R in the arcuate nucleus. This is consistent with the idea that the MC4R exerts its effects downstream of the arcuate POMC neurons, and that the cellular response to cells innervated by arcuate neurons depends on an integration of both orexigenic and anorexigenic neural inputs [9].
4.4. Conclusions
We have shown that peripheral injections of d-Trp8-γ-MSH, a potent and selective melanocortin-3 agonist, can stimulate food intake in mice. These data provide physiological support for the hypothesis that one role of the MC3R is to act as an inhibitory autoreceptor in POMC neurons. Compounds with orexigenic activity are known to improve the quality of life of individuals affected with involuntary weight loss due to chronic disease [22]. Our own studies have demonstrated that many features of experimental cachexia can be attenuated by blocking melanocortin signaling [28]. Thus, these data also provide a potential therapeutic target for this debilitating disorder of energy homeostasis.
REFERENCES
- 1.Bagnol D, Lu XY, Kaelin CB, Day HE, Ollmann M, Gantz I, et al. Anatomy of an endogenous antagonist: relationship between agouti-related protein and proopiomelanocortin in brain. J Neurosci. 1999;19(18):RC26. doi: 10.1523/JNEUROSCI.19-18-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butler AA, Kesterson RA, Khong K, Cullen MJ, Pellymounter MA, Dekoning J, et al. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141(9):3518–21. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- 3.Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD. Melanocortin-4 receptor is required for adaptive thermogenesis and behavioral responses to increased dietary fat. Nature Neurosci. 2001;4(6):605–11. doi: 10.1038/88423. [DOI] [PubMed] [Google Scholar]
- 4.Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26(1):97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 5.Chhajlani V. Distribution of cdna for melanocortin receptor subtypes in human tissues. Biochem Mol Biol Int. 1996;38:73–80. [PubMed] [Google Scholar]
- 6.Coll AP, Farooqi IS, Challis BG, Yeo GS, O'Rahilly S. Proopiomelanocortin and energy balance: insights from human and murine genetics. J Clin Endocrinol Metab. 2004;89(6):2557–62. doi: 10.1210/jc.2004-0428. [DOI] [PubMed] [Google Scholar]
- 7.Cone RD, Cowley MA, Butler AA, Fan W, Marks DL, Low MJ. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int J Obes Relat Metab Disord. 2001;25(Suppl 5):S63–7. doi: 10.1038/sj.ijo.0801913. [DOI] [PubMed] [Google Scholar]
- 8.Cowley MA, Cone RD, Enriori P, Louiselle I, Williams SM, Evans AE. Electrophysiological actions of peripheral hormones on melanocortin neurons. Ann NY Acad Sci. 2003;994:175–86. doi: 10.1111/j.1749-6632.2003.tb03178.x. [DOI] [PubMed] [Google Scholar]
- 9.Cowley MA, Fan W, Pronchuk N, Dinulescu DM, Colmers WF, Cone RD. Integration of npy, agrp, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999;24:155–63. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- 10.Cowley MA, Smart JL, Cerdan M, Rubinstein M, Diano C, Horvath TL, et al. Leptin activates anorexigenic pomc neurons through a neural network in the arcuate nucleus. Nature. 2001;24(1):155–63. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 11.De Souza J, Butler AA, Cone RD. Disproportionate inhibition of feeding in a mice by certain stressors: a cautionary note. Neuroendocrinology. 2000;72(2):126–32. doi: 10.1159/000054579. [DOI] [PubMed] [Google Scholar]
- 12.Ellacott KL, Cone RD. The central melanocortin system and the integration of short- and long-term regulators of energy homeostasis. Recent Prog Horm Res. 2004;59:395–408. doi: 10.1210/rp.59.1.395. [DOI] [PubMed] [Google Scholar]
- 13.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–8. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 14.Gantz I, Konda Y, Tashiro T, Shimoto Y, Miwa H, Munzert G, et al. Molecular cloning of a novel melanocortin receptor. J Biol Chem. 1993;268:8246–50. [PubMed] [Google Scholar]
- 15.Getting SJ, Allcock GH, Flower R, Perretti M. Natural and synthetic agonists of the melanocortin receptor type 3 possess anti-inflammatory properties. J Leukoc Biol. 2001;69(1):98–104. [PubMed] [Google Scholar]
- 16.Getting SJ, Flower RJ, Perretti M. Agonism at melanocortin receptor type 3 on macrophages inhibits neutrophil influx. Inflamm Res. 1999;48(Suppl 2):S140–1. doi: 10.1007/s000110050557. [DOI] [PubMed] [Google Scholar]
- 17.Getting SJ, Gibbs L, Clark AJ, Flower RJ, Perretti M. Pomc gene-derived peptides activate melanocortin type 3 receptor on murine macrophages, suppress cytokine release, and inhibit neutrophil migration in acute experimental inflammation. J Immunol. 1999;162(12):7446–53. [PubMed] [Google Scholar]
- 18.Grieco P, Balse PM, Weinberg D, MacNeil T, Hruby VJ. d-amino acid scan of gamma-melanocyte-stimulating hormone: importance of trp(8) on human mc3 receptor selectivity. J Med Chem. 2000;43(26):4998–5002. doi: 10.1021/jm000211e. [DOI] [PubMed] [Google Scholar]
- 19.Hruby VJ, Lu D, Sharma SD, Castrucci AL, Kesterson RA, AlObeidi FA, et al. Cyclic lactam alpha-melanotropin analogues of ac-nle4-cyclo [asp5, d-phe7, lys10] alpha-melanocyte-stimulating hormone-(4-10)-nh2 with bulky aromatic amino acids at position 7 show high antagonist potency and selectivity at specific melanocortin receptors. J Med Chem. 1995;38(18):3454–61. doi: 10.1021/jm00018a005. [DOI] [PubMed] [Google Scholar]
- 20.Humphreys MH, Wiedemann E, Valentin JP, Chen XW, Ying WZ. Natriuretic actions of gamma-melanocyte-stimulating hormone. Ann NY Acad Sci. 1993;680:545–8. doi: 10.1111/j.1749-6632.1993.tb19734.x. [DOI] [PubMed] [Google Scholar]
- 21.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–41. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 22.Jatoi A, Windschitl HE, Loprinzi CL, Sloan JA, Dakhil SR, Mailliard JA, et al. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: a north central cancer treatment group study. J Clin Oncol. 2002;20(2):567–73. doi: 10.1200/JCO.2002.20.2.567. [DOI] [PubMed] [Google Scholar]
- 23.Jegou S, Boutelet I, Vaudry H, Articles R. Melanocortin-3 receptor mrna expression in pro-opiomelanocortin neurones of the rat arcuate nucleus. J Neuroendocrinol. 2000;12(6):501–5. doi: 10.1046/j.1365-2826.2000.00477.x. [DOI] [PubMed] [Google Scholar]
- 24.Levine AS, Morley JE. Neuropeptide g: a potent inducer of consummatory behavior in rats. Peptides. 1984;5(6):1025–9. doi: 10.1016/0196-9781(84)90165-7. [DOI] [PubMed] [Google Scholar]
- 25.Mandrika I, Muceniece R, Wikberg JE. Effects of melanocortin peptides on lipopolysaccharide/interferon-gamma-induced nf-kappab DNA binding and nitric oxide production in macrophage-like raw 264.7 cells: evidence for dual mechanisms of action. Biochem Pharmacol. 2001;61(5):613–21. doi: 10.1016/s0006-2952(00)00583-9. [DOI] [PubMed] [Google Scholar]
- 26.Marks DL, Butler AA, Turner R, Brookhart GB, Cone RD. Differential role of melanocortin receptor subtypes in cachexia. Endocrinology. 2003;144(4):1513–23. doi: 10.1210/en.2002-221099. [DOI] [PubMed] [Google Scholar]
- 27.Marks DL, Cone RD. Central melanocortins and the regulation of weight during acute and chronic disease. Recent Prog Horm Res. 2001;56:359–75. doi: 10.1210/rp.56.1.359. [DOI] [PubMed] [Google Scholar]
- 28.Marks DL, Ling N, Cone RD. Role of the central melanocortin system in cachexia. Cancer Res. 2001;61(4):1432–8. [PubMed] [Google Scholar]
- 29.Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB, et al. Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc Natl Acad Sci USA. 1993;90(19):8856–60. doi: 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanley BG, Kyrkouli SE, Lampert S, Leibowitz SF. Neuropeptide γ chronically injected into the hypothalamus: a powerful neurochemical inducer of hyperphagia and obesity. Peptides. 1986;7(6):1189–92. doi: 10.1016/0196-9781(86)90149-x. [DOI] [PubMed] [Google Scholar]
- 31.Tatro JB, Entwistle ML. Heterogeneity of brain melanocortin receptors suggested by differential ligand binding in situ. Brain Res. 1994;635(1–2):148–58. doi: 10.1016/0006-8993(94)91434-6. [DOI] [PubMed] [Google Scholar]