Abstract
Bone loss occuring with unloading is associated with decreased osteoblastogenesis and increased bone marrow adipogenesis, resulting in bone loss and decreased bone formation. Here, we review the present knowledge on the role of PPARγ in the control of osteoblastogenesis and bone mass in skeletal unloading. We showed that PPARγ positively promotes adipogenesis and negatively regulates osteoblast differentiation of bone marrow stromal cells in unloading, resulting in bone loss. Manipulation of PPARγ2 expression by exogenous TGF-β2 inhibits the exaggerated adipogenesis and corrects the balance between osteoblastogenesis and adipogenesis induced by unloading, leading to prevention of bone loss. This shows that PPARγ plays an important role in the control of bone mass in unloaded bone. Moreover, this opens the possibility that manipulation of PPARγ may correct the balance between osteoblastogenesis and adipogenesis and prevent bone loss, which may have potential implications in the treatment of bone loss in clinical conditions.
INTRODUCTION
The maintainance of both bone mass and bone microarchitecture is controlled by the balance between bone resorption and formation. At the cellular level, this balance is largely dependent on the number and activity of bone forming and resorbing cells. Any alteration in the number or activity of bone cells will result in an imbalance between resorption and formation, resulting in microarchitecture deterioration and altered bone mass and strength.
The control of bone forming cells is largely influenced by weight bearing and exercise that induce mechanical forces on the skeleton. Mechanical forces induce anabolic effects by promoting bone formation at multiple levels [1–3]. Bone formation is a complex process that is dependent on the recruitment, differentiation, and function of osteoblasts. The osteogenic process starts by the commitment of osteoprogenitor cells into osteoblasts under the control of transcription factors, followed by their progressive differentiation into mature osteoblasts [4, 5]. In the recent years, the development of cellular, molecular, and genetic studies has led to the identification of a number of important transcription factors that are essential in the control of bone formation. Specifically, several studies have provided evidence for a role of PPARγ in the control of bone formation and bone mass through modulation of bone marrow stromal cell differentiation. In this brief review, we summarize the present knowledge on the role of PPARγ in the control of osteoblastogenesis and bone mass, with a particular reference to skeletal unloading.
Reciprocal relationship between osteoblastogenesis and adipogenesis in the bone marrow
Several conditions associated with bone loss such as aging [6], glucocorticoid treatment [7], estrogen deficiency [8], or immobilization [9] are characterized by decreased osteoblastogenesis associated with increased adipogenesis in the bone marrow. This supports the concept that there is a reciprocal relationship between adipocyte and osteoblast differentiation [10]. Early studies showed that bone marrow stromal cells can be differentiated into several lineages in vitro [11–13], and that differentiation towards one lineage is dependent on local or hormonal factors [14]. Further studies showed that clonal marrow stromal cells can be differentiated into adipocytes, osteoblasts, or chondrocytes in different species including humans [15–17]. Notably, a single marrow stromal cell may have multipotential competence in vitro and differentiation towards one pathway restricts expression of other lineage-specific genes [18]. This provides evidence that adipocytes and osteoblasts are derived from a common mesenchymal stromal cell and that a reciprocal relationship exists between osteoblastogenesis and adipogenesis in the bone marrow [10].
PPARγ2 is a positive promoter of adipogenesis and a negative regulator of osteoblastogenesis
The mechanisms involved in adipogenesis have been studied extensively in adipose tissue. The differentiation of preadipocytes into mature adipocytes is primarily controlled by peroxisome proliferator-activated receptor γ (PPARγ) which is a key transcription factor involved in adipocyte differentiation [19]. PPARγ exists in two isoforms PPARγ1 and PPARγ2 as a result of alternative splicing. PPARγ2 is expressed at high levels in fat tissue and is essential for adipogenesis in vitro and in vivo. CCAAT/enhancer binding proteins (C/EBP) are other important transcription factors that control the expression of adipocyte genes by acting synergistically with PPARγ to activate adipocyte gene expression [20]. In vitro, C/EBPs activate the expression of PPARγ and C/EBPα and promote PPARγ2 activity in preadipocyte cultures, which contributes to the expression of genes that characterize the adipocyte phenotype [21].
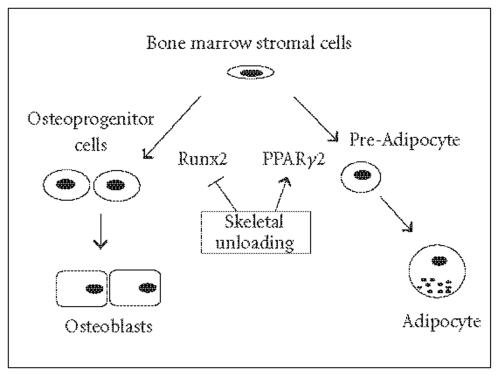
In bone, recent advances have been made in the role of PPARγ in the interconversion of marrow stromal cells into osteoblasts or adipocytes in vitro (Figure 1). In cultured murine and human cells, PPARγ agonists and overexpression of PPARγ2 induce the differentiation of bone marrow stromal cells into the adipocyte lineage and negatively regulate osteoblast differentiation by repressing the osteoblast specific transcription factor Runx2 [22–24]. There is also evidence that PPARγ negatively regulates osteoblast differentiation. For example, activation of PPARγ with a thiazolidinediones with high affinity for PPARγ increases adipogenesis and decreases osteoblastogenesis in vitro [25–27]. Additionally, activation of PPARγ with rosiglitazone in mice or ovariectomized rats decreases Runx2 expression and bone formation, and increases adipogenesis in the bone marrow, resulting in decreased bone mass [28, 29]. Consistently, PPARγ haploinsufficiency in mice was shown to decrease adipogenesis and to increase Runx2 expression and bone formation, resulting in increased bone mass [30]. These findings indicate that PPARγ positively promotes adipogenesis and negatively regulates osteoblast differentiation of bone marrow stromal cells in vivo, suggesting that PPARγ is a negative regulator of bone mass.
Figure 1.
The in vivo differentiation of bone marrow stromal cells towards adipocytes and osteoblasts is governed by the balance between PPARγ2 and Runx2 expression. In unloaded bone, decreased Runx2 and increased PPARγ2 expression result in decreased osteoblastogenesis, increased adipogenesis, and bone loss.
Skeletal unloading decreases osteoblast differentiation and induces bone loss
A representative model of bone loss resulting from alterations in osteoblasts is skeletal unloading [31]. Skeletal unloading induced by hind limb suspension rapidly causes a marked trabecular bone loss in the long bone metaphysis, resulting mainly from reduced trabecular thickness and number associated with inhibition of endosteal bone formation [32]. Although both the number and activity of osteoblasts are decreased in the unloaded metaphyseal bone [32, 33], the number of osteoblasts is more affected than their activity [34]. Although the mechanisms underlying bone loss induced by unloading in rats are not fully understood, bone loss does not appear to result from changes in serum corticosteroid, 25-hydroxyvitamin D or PTH levels [31]. However, there is some evidence that skeletal unloading may result in part from to decreased expression [34] or response [35] to local growth factors.
The cellular mechanisms underlying the alterations of bone formation induced by skeletal unloading in rats have been partly identified [36]. We initially showed that the decreased bone formation in unloaded rat bone results from an impaired recruitment of osteoblast precursor cells in the bone marrow stroma and in the metaphysis [33]. In addition to affect osteoblast recruitment, skeletal unloading in this model alters the function of differentiated osteoblasts. This is reflected by the decreased expression of bone matrix type-1 collagen and osteocalcin and osteopontin mRNA levels [37–40], which correlates well with the decreased bone matrix synthesis measured at the tissue level [32, 33]. These findings indicate that removal of mechanical forces on the skeleton rapidly alters both the recruitment of osteoblast progenitor cell and the function of differentiated osteoblasts, resulting in a marked reduction of bone formation. Such alterations are consistent with the effects of unloading in other rat models in which there is a reduction of the osteogenic capacity of bone marrow osteoblast precursor cells and a decreased expression of bone matrix proteins in rat long bones [41, 42].
PPARγ controls the osteoblast/adipocyte relationship in unloaded bone
The altered bone metabolism induced by skeletal unloading is asociated with alterations in transcription factor expression. Specifically, the decreased osteoblastogenesis and bone formation induced by skeletal unloading in rats are associated with reduced Runx2 expression [34]. Additionally, we showed that skeletal unloading is associated with increased adipocyte differentiation in the bone marrow stroma [43], suggesting that unloading not only impairs osteoprogenitor cell differentiation into osteoblasts but also promotes adipocyte differentiation. The exagerated reciprocal relationship between osteoblastogenesis and adipogenesis may account for the decreased bone formation associated with the increased bone marrow adipogenesis in unloaded rats (Figure 1).
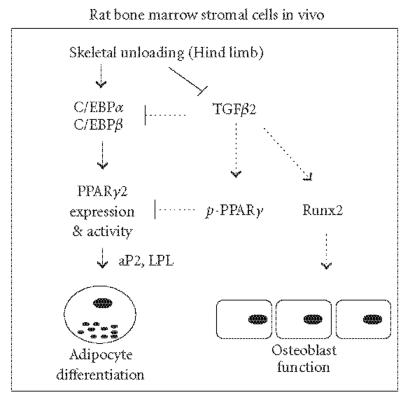
Interestingly, the adipogenic differentiation of bone marrow stromal cells in unloaded bone is consistent with the temporal gene expression observed during adipocyte differentiation in vitro. Specifically, skeletal unloading in rats increases C/EBPα and C/EBPβ expression followed by increased expression of PPARγ, resulting in activation of adipocyte gene expression such as adipocytic differentiation-related genes adipocyte binding protein (aP2) and lipoprotein lipase (LPL) in bone marrow stromal cells [44] (Figure 2). Thus, PPARγ with other transcription factors are involved in adipogenic conversion of bone marrow stromal cells in vivo, indicating that PPARγ is a negative regulator of bone mass in unloaded rats.
Figure 2.
Skeletal unloading decreases TGF-β expression and activates the expression of C/EBPα, C/EBPβ, and PPARγ2, resulting in activation of adipocyte gene expression such as adipocytic differentiation-related genes adipocyte binding protein (aP2) and lipoprotein lipase (LPL) in bone marrow stromal cells. Exogenous TGF-β2 (dotted lines) reduces C/EBPα, C/EBPβ, and PPARγ expressions, induces PPARγ phosphorylation (p-PPARγ), and increases Runx2 expression, resulting in decreased adipogenesis, increased osteoblast function, and prevention of bone loss.
The mechanisms underlying the expression of Runx2 and PPARγ in unloaded bone may involve decreased signaling pathways that are normally transmitted by loading. Mechanical forces are believed to transduce signals through cell-matrix interactions [45–48]. Part of the communication between the matrix and cells is ensured by integrins which interact with bone matrix proteins [49]. In bone, integrin-matrix interactions are important modulators of osteoblast differentiation in vitro [50, 51]. It is thus possible that the lack of mechanical strain is induced by unloading results in decreased integrin-matrix interactions and signaling, and consequently decreased osteoblast differentiation. This is supported by the finding that mechanical forces increase Runx2 expression in cultured preosteoblastic cells [52]. One recent study indicates that stretching induces downregulation of PPARγ2 and adipocyte differentiation in mouse preadipocytes [53], suggesting that mechanical forces may play a dual role in the control of Runx2 and PPARγ expression in preosteoblasts.
How mechanical signals may modulate PPARγ expression or activity and thereby induce adipogenesis rather than osteoblastogenesis in bone marrow stromal cells is not fully understood. One interesting hypothesis is that specific pathways controlling osteoblastogenesis/adipogenesis may be sensitive to biomechanical forces. For example, changes in cell shape or modulation of the cytoskeletal-related GTPase RhoA were recently found to induce stem cell adipogenic or osteoblast differentiation [54]. Additionally, multiple signal pathways, including ERK and Wnt signaling, may control the balance between adipogenesis and osteoblastogenesis in vitro [53, 55]. It remains however to determine which pathway may be involved in the altered balance between osteoblastogenesis and adipogenesis in vivo.
TGF beta is a negative regulator of PPARγ and adipogenesis in unloaded rats
Transforming growth factor beta (TGF-β) is an important regulator of bone formation by modulating osteoblastic cell proliferation and differentiation [56]. Additionally, TGF-β is also an important modulator of adipocyte differentiation. TGF-β inhibits adipogenesis in preadipocyte cell lines and reduces adipocyte differentiation in vitro [57, 58]. In vivo, we found that skeletal unloading results in a rapid reduction in TGF-β1 and TGF-β receptor II mRNA expression in bone marrow stromal cells [34]. Others found reduced TGF-β2 mRNA levels in bone marrow stromal cells in this model [37], suggesting that TGF-β signaling may mediate part of the altered bone formation induced by unloading. Although diminished, TGF-β receptors can still be activated by TGF-β since we showed that exogenous TGF-β2 in unloaded rats increased Runx2 expression and osteoblastogenesis, resulting in prevention of trabecular bone loss [59]. Beside this positive effect on osteoblastogenesis, TGF-β2 administration downregulated the expression of C/EBPα, C/EBPβ, and PPARγ in bone marrow stromal cells, and reduced the expression of adipocyte genes such as aP2 and LPL in bone marrow stromal cells, thus preventing the adipocyte conversion of bone marrow stromal cells induced by unloading [43, 44]. This indicates that TGF-β is a negative regulator of PPARγ and adipogenesis in unloaded rats (Figure 2).
One mechanism by which TGF-β may negatively regulate adipogenesis in unloaded rats is through MAPK activation. TGF-β is known to induce phosphorylation of PPARγ in adipocyte cells, and MAPK-dependent PPARγ phosphorylation results in the reduction of PPARγ transcriptional activity and repression of adipocyte differentiation [60–62]. In vitro, ERK activation was found to induce osteogenic differentiation of human mesenchymal stem cells, whereas its inhibition induces adipogenic differentiation [63]. In unloaded bone, we showed that TGF-β2 increased PPARγ phosphorylation and inhibited adipocyte differentiation of bone marrow stromal cells through MAPK phosphorylation [44]. Thus, exogenous TGF-β can inhibit the excessive adipogenic differentiation induced by skeletal unloading by reducing PPARγ2 expression, resulting in the inhibition of adipogenesis. This effect, combined with the upregulation of Runx2 expression and osteoblast differentiation induced by exogenous TGF-β on bone marrow stromal cells, leads to correcting the imbalance between osteoblastogenesis and adipogenesis and results in a positive effect on bone mass (Figure 2). This demonstrates that appropriate manipulation of PPARγ2 expression in vivo can lead to prevent bone loss in unloaded bone.
CONCLUSION
There is now clear evidence that PPARγ plays an important role in the control of marrow stromal cell differentiation to osteoblasts or adipocytes in unloaded bone. In this model, PPARγ positively promotes adipogenesis and negatively regulates osteoblast differentiation of bone marrow stromal cells, indicating that PPARγ is a negative regulator of bone mass. This concept provides a possible target for therapeutic intervention in osteopenic disorders characterized by altered osteoblast and adipocyte differentiation of bone marrow stromal cells [64]. As an example, we showed that exogenous manipulation of PPARγ expression by TGF-β can inhibit adipogenesis induced by skeletal unloading and correct the balance between osteoblastogenesis and adipogenesis, resulting in prevention of bone loss. This opens the possibility that manipulation of PPARγ may have potential implications in the treatment of bone loss associated with immobilization [65].
ACKNOWLEDGMENTS
The authors thank the contributors in P. Marie's group at Inserm U606 and the collaborators at IMASSA-CERMA (Département de Physiologie Aérospatiale, Brétigny sur Orge, France) for their contribution. The work presented was supported in part by grants from the Centre National d'Etudes Spatiales (CNES, Paris, France).
References
- 1.Marie PJ. The effects of microgravity on skeletal remodeling and bone cells. In: Massaro EJ, Rogers JM, editors. The Skeleton: Biochemical Genetic, and Molecular Interactions in Development an Homeostasis. Vol 18. New Jersey: Humana Press; 2004. pp. 263–276. [Google Scholar]
- 2.Marie PJ, Jones D, Vico L, Zallone A, Hinsenkamp M, Cancedda R. Osteobiology, strain, and microgravity. Part I: studies at the cellular level. Calcified Tissue International. 2000;67(1):2–9. doi: 10.1007/s00223001088. [DOI] [PubMed] [Google Scholar]
- 3.Vico L, Hinsenkamp M, Jones D, Marie PJ, Zallone A, Cancedda R. Osteobiology, Strain, and Microgravity. Part II: studies at the Tissue Level. Calcified Tissue International. 2001;68(1):1–10. doi: 10.1007/BF02684996. [DOI] [PubMed] [Google Scholar]
- 4.Aubin JE. Regulation of osteoblast formation and function. Reviews in Endocrine and Metabolic Disorders. 2001;2(1):81–94. doi: 10.1023/a:1010011209064. [DOI] [PubMed] [Google Scholar]
- 5.Wagner EF, Karsenty G. Genetic control of skeletal development. Current Opinion in Genetics and Development. 2001;11(5):527–532. doi: 10.1016/s0959-437x(00)00228-8. [DOI] [PubMed] [Google Scholar]
- 6.Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clinical Orthopaedics and Related Research. 1971;80:147–154. doi: 10.1097/00003086-197110000-00021. [DOI] [PubMed] [Google Scholar]
- 7.Wang GJ, Sweet DE, Reger SI. Fat-cell changes as a mechanism of avascular necrosis of the femoral head in cortisone-treated rabbits. Journal of Bone and Joint Surgery - Series A. 1977;59(6):729–735. [PubMed] [Google Scholar]
- 8.Martin RB, Zissimos SL. Relationships between marrow fat and bone turnover in ovariectomized and intact rats. Bone. 1991;12(2):123–131. doi: 10.1016/8756-3282(91)90011-7. [DOI] [PubMed] [Google Scholar]
- 9.Minaire P, Edouard C, Arlot M, Meunier PJ. Marrow changes in paraplegic patients. Calcified Tissue International. 1984;36(3):338–340. doi: 10.1007/BF02405340. [DOI] [PubMed] [Google Scholar]
- 10.Nuttall ME, Gimble JM. Controlling the balance between osteoblastogenesis and adipogenesis and the consequent therapeutic implications. Current Opinion in Pharmacology. 2004;4(3):290–294. doi: 10.1016/j.coph.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Friedenstein AJ. Osteogenic stem cells in the bone marrow. Journal of Bone and Mineral Research. 1990;7:243–272. [Google Scholar]
- 12.Bennett JH, Joyner CJ, Triffitt JT, Owen ME. Adipocytic cells cultured from marrow have osteogenic potential. Journal of Cell Science. 1991;99(pt 1):131–139. doi: 10.1242/jcs.99.1.131. [DOI] [PubMed] [Google Scholar]
- 13.Beresford JN, Bennett JH, Devlin C, Leboy PS, Owen ME. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. Journal of Cell Science. 1992;102(pt 2):341–351. doi: 10.1242/jcs.102.2.341. [DOI] [PubMed] [Google Scholar]
- 14.Gimble JM, Robinson CE, Wu X, Kelly KA. The function of adipocytes in the bone marrow stroma: an update. Bone. 1996;19(5):421–428. doi: 10.1016/s8756-3282(96)00258-x. [DOI] [PubMed] [Google Scholar]
- 15.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 16.Oyajobi BO, Lomri A, Hott M, Marie PJ. Isolation and characterization of human clonogenic osteoblast progenitors immunoselected from fetal bone marrow stroma using STRO-1 monoclonal antibody. Journal of Bone and Mineral Research. 1999;14(3):351–361. doi: 10.1359/jbmr.1999.14.3.351. [DOI] [PubMed] [Google Scholar]
- 17.Dennis JE, Merriam A, Awadallah A, Yoo JU, Johnstone B, Caplan AI. A quadripotential mesenchymal progenitor cell isolated from the marrow of an adult mouse. Journal of Bone and Mineral Research. 1999;14(5):700–709. doi: 10.1359/jbmr.1999.14.5.700. [DOI] [PubMed] [Google Scholar]
- 18.Ahdjoudj S, Lasmoles F, Oyajobi BO, Lomri A, Delannoy Ph, Marie PJ. Reciprocal control of osteoblast/chondroblast and osteoblast/adipocyte differentiation of multipotential clonal human marrow stromal F/STRO-1+ cells. Journal of Cellular Biochemistry. 2001;81(1):23–38. doi: 10.1002/1097-4644(20010401)81:1<23::aid-jcb1021>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 19.Tontonoz P, Hu E, Spiegelman BM. Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor γ. Current Opinion in Genetics and Development. 1995;5(5):571–576. doi: 10.1016/0959-437x(95)80025-5. [DOI] [PubMed] [Google Scholar]
- 20.Yeh W-C, Cao Z, Classon M, McKnight SL. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes and Development. 1995;9(2):168–181. doi: 10.1101/gad.9.2.168. [DOI] [PubMed] [Google Scholar]
- 21.Rosen ED, Hsu C-H, Wang X, et al. C/EBPα induces adipogenesis through PPARγ: a unified pathway. Genes and Development. 2002;16(1):22–26. doi: 10.1101/gad.948702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gori F, Thomas T, Hicok KC, Spelsberg TC, Riggs BL. Differentiation of human marrow stromal precursor cells: bone morphogenetic protein-2 increases OSF2/CBFA1, enhances osteoblast commitment, and inhibits late adipocyte maturation. Journal of Bone and Mineral Research. 1999;14(9):1522–1535. doi: 10.1359/jbmr.1999.14.9.1522. [DOI] [PubMed] [Google Scholar]
- 23.Lecka-Czernik B, Gubrij I, Moerman EJ, et al. Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARγ2. Journal of Cellular Biochemistry. 1999;74(3):357–371. [PubMed] [Google Scholar]
- 24.Lecka-Czernik B, Moerman EJ, Grant DF, Lehmann JM, Manolagas SC, Jilka RL. Divergent effects of selective peroxisome proliferator-activated receptor-γ2 ligands on adipocyte versus osteoblast differentiation. Endocrinology. 2002;143(6):2376–2384. doi: 10.1210/endo.143.6.8834. [DOI] [PubMed] [Google Scholar]
- 25.Gimble JM, Robinson CE, Wu X, et al. Peroxisome proliferator-activated aeceptor-γ activation by thiazolidinediones induces adipogenesis in bone marrow stromal cells. Molecular Pharmacology. 1996;50(5):1087–1094. [PubMed] [Google Scholar]
- 26.Rzonca SO, Suva LJ, Gaddy D, Montague DC, Lecka-Czernik B. Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology. 2004;145(1):401–406. doi: 10.1210/en.2003-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazarenko OP, Rzonca SO, Suva LJ, Lecka-Czernik B. Netoglitazone is a PPAR-gamma ligand with selective effects on bone and fat. Bone. 2006;38(1):74–84. doi: 10.1016/j.bone.2005.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ali AA, Weinstein RS, Stewart SA, Parfitt AM, Manolagas SC, Jilka RL. Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology. 2005;146(3):1226–1235. doi: 10.1210/en.2004-0735. [DOI] [PubMed] [Google Scholar]
- 29.Sottile V, Seuwen K, Kneissel M. Enhanced marrow adipogenesis and bone resorption in estrogen-deprived rats treated with the PPARgamma agonist BRL49653 (rosiglitazone) Calcified Tissue International. 2004;75(4):329–337. doi: 10.1007/s00223-004-0224-8. [DOI] [PubMed] [Google Scholar]
- 30.Akune T, Ohba S, Kamekura S, et al. PPARγ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. Journal of Clinical Investigation. 2004;113(6):846–855. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morey-Holton ER, Globus RK. Hindlimb unloading of growing rats: a model for predicting skeletal changes during space flight. Bone. 1998;22(suppl 5):83S–88S. doi: 10.1016/s8756-3282(98)00019-2. [DOI] [PubMed] [Google Scholar]
- 32.Wronski TJ, Morey ER. Skeletal abnormalities in rats induced by simulated weightlessness. Metabolic Bone Disease and Related Research. 1982;4(1):69–75. doi: 10.1016/0221-8747(82)90011-x. [DOI] [PubMed] [Google Scholar]
- 33.Machwate M, Zerath E, Holy X, et al. Skeletal unloading in rat decreases proliferation of rat bone and marrow-derived osteoblastic cells. American Journal of Physiology - Endocrinology and Metabolism. 1993;264(5):E790–E799. doi: 10.1152/ajpendo.1993.264.5.E790. [DOI] [PubMed] [Google Scholar]
- 34.Drissi H, Lomri A, Lasmoles F, Holy X, Zerath E, Marie PJ. Skeletal unloading induces biphasic changes in insulin-like growth factor-I mRNA levels and osteoblast activity. Experimental Cell Research. 1999;251(2):275–284. doi: 10.1006/excr.1999.4539. [DOI] [PubMed] [Google Scholar]
- 35.Bikle DD, Sakata T, Halloran BP. The impact of skeletal unloading on bone formation. Gravitational and Space Biology Bulletin. 2003;16(2):45–54. [PubMed] [Google Scholar]
- 36.Marie PJ, Zerath E. Role of growth factors in osteoblast alterations induced by skeletal unloading in rats. Growth Factors. 2000;18(1):1–10. doi: 10.3109/08977190009003230. [DOI] [PubMed] [Google Scholar]
- 37.Zhang R, Supowit SC, Klein GL, et al. Rat tail suspension reduces messenger RNA level for growth factors and osteopontin and decreases the osteoblastic differentiation of bone marrow stromal cells. Journal of Bone and Mineral Research. 1995;10(3):415–423. doi: 10.1002/jbmr.5650100312. [DOI] [PubMed] [Google Scholar]
- 38.Bikle DD, Harris J, Halloran BP, Morey-Holton E. Altered skeletal pattern of gene expression in response to spaceflight and hindlimb elevation. The American Journal of Physiology. 1994;267(6 pt 1):E822–E827. doi: 10.1152/ajpendo.1994.267.6.E822. [DOI] [PubMed] [Google Scholar]
- 39.Machwate M, Zerath E, Holy X, Pastoureau P, Marie PJ. Insulin-like growth factor-I increases trabecular bone formation and osteoblastic cell proliferation in unloaded rats. Endocrinology. 1994;134(3):1031–1038. doi: 10.1210/endo.134.3.8119139. [DOI] [PubMed] [Google Scholar]
- 40.Shiiba M, Arnaud SB, Tanzawa H, Uzawa K, Yamauchi M. Alterations of collagen matrix in weight-bearing bones during skeletal unloading. Connective Tissue Research. 2001;42(4):303–311. doi: 10.3109/03008200109016844. [DOI] [PubMed] [Google Scholar]
- 41.Wakley GK, Portwood JS, Turner RT. Disuse osteopenia is accompanied by downregulation of gene expression for bone proteins in growing rats. The American Journal of Physiology. 1992;263(6 pt 1):E1029–E1034. doi: 10.1152/ajpendo.2006.263.6.E1029. [DOI] [PubMed] [Google Scholar]
- 42.Keila S, Pitaru S, Grosskopf A, Weinreb M. Bone marrow from mechanically unloaded rat bones expresses reduced osteogenic capacity in vitro. Journal of Bone and Mineral Research. 1994;9(3):321–327. doi: 10.1002/jbmr.5650090306. [DOI] [PubMed] [Google Scholar]
- 43.Ahdjoudj S, Lasmoles F, Holy X, Zerath E, Marie PJ. Transforming growth factor β2 inhibits adipocyte differentiation induced by skeletal unloading in rat bone marrow stroma. Journal of Bone and Mineral Research. 2002;17(4):668–677. doi: 10.1359/jbmr.2002.17.4.668. [DOI] [PubMed] [Google Scholar]
- 44.Ahdjoudj S, Kaabeche K, Holy X, et al. Transforming growth factor-β inhibits CCAAT/enhancer-binding protein expression and PPARγ activity in unloaded bone marrow stromal cells. Experimental Cell Research. 2005;303(1):138–147. doi: 10.1016/j.yexcr.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 45.Duncan RL, Turner CH. Mechanotransduction and the functional response of bone to mechanical strain. Calcified Tissue International. 1995;57(5):344–358. doi: 10.1007/BF00302070. [DOI] [PubMed] [Google Scholar]
- 46.El Haj AJ, Minter SL, Rawlinson SCF, Suswillo R, Lanyon LE. Cellular responses to mechanical loading in vitro. Journal of Bone and Mineral Research. 1990;5(9):923–932. doi: 10.1002/jbmr.5650050905. [DOI] [PubMed] [Google Scholar]
- 47.Jones DB, Nolte H, Scholubbers J-G, Turner E, Veltel D. Biochemical signal transduction of mechanical strain in osteoblast-like cells. Biomaterials. 1991;12(2):101–110. doi: 10.1016/0142-9612(91)90186-e. [DOI] [PubMed] [Google Scholar]
- 48.Duncan RL. Transduction of mechanical strain in bone. Gravitational and Space Biology Bulletin. 1995;8(2):49–62. [PubMed] [Google Scholar]
- 49.Shyy JY-J, Chien S. Role of integrins in cellular responses to mechanical stress and adhesion. Current Opinion in Cell Biology. 1997;9(5):707–713. doi: 10.1016/s0955-0674(97)80125-1. [DOI] [PubMed] [Google Scholar]
- 50.Moursi AM, Globus RK, Damsky CH. Interactions between integrin receptors and fibronectin are required for calvarial osteoblast differentiation in vitro. Journal of Cell Science. 1997;110(18):2187–2196. doi: 10.1242/jcs.110.18.2187. [DOI] [PubMed] [Google Scholar]
- 51.Xiao G, Wang D, Benson MD, Karsenty G, Franceschi RT. Role of the α2-integrin in osteoblast-specific gene expression and activation of the Osf2 transcription factor. Journal of Biological Chemistry. 1998;273(49):32988–32994. doi: 10.1074/jbc.273.49.32988. [DOI] [PubMed] [Google Scholar]
- 52.Yuge L, Okubo A, Miyashita T, et al. Physical stress by magnetic force accelerates differentiation of human osteoblasts. Biochemical and Biophysical Research Communications. 2003;311(1):32–38. doi: 10.1016/j.bbrc.2003.09.156. [DOI] [PubMed] [Google Scholar]
- 53.Tanabe Y, Koga M, Saito M, Matsunaga Y, Nakayama K. Inhibition of adipocyte differentiation by mechanical stretching through ERK-mediated downregulation of PPARγ2. Journal of Cell Science. 2004;117(16):3605–3614. doi: 10.1242/jcs.01207. [DOI] [PubMed] [Google Scholar]
- 54.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Developmental Cell. 2004;6(4):483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 55.Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME. Playing with bone and fat. Journal of Cellular Biochemistry. 2006;98(2):251–266. doi: 10.1002/jcb.20777. [DOI] [PubMed] [Google Scholar]
- 56.Centrella M, McCarthy TL, Canalis E. Transforming growth factor-beta and remodeling of bone. Journal of Bone and Joint Surgery - Series A. 1991;73(9):1418–1428. [PubMed] [Google Scholar]
- 57.Torti FM, Torti SV, Larrick JW, Ringold GM. Modulation of adipocyte differentiation by tumor necrosis factor and transforming growth factor beta. Journal of Cell Biology. 1989;108(3):1105–1113. doi: 10.1083/jcb.108.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choy L, Skillington J, Derynck R. Roles of autocrine TGF-β receptor and Smad signaling in adipocyte differentiation. Journal of Cell Biology. 2000;149(3):667–682. doi: 10.1083/jcb.149.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Machwate M, Zerath E, Holy X, et al. Systemic administration of transforming growth factor-β2 prevents the impaired bone formation and osteopenia induced by unloading in rats. Journal of Clinical Investigation. 1995;96(3):1245–1253. doi: 10.1172/JCI118158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARγ. Science. 1996;274(5295):2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- 61.Adams M, Reginato MJ, Shao D, Lazar MA, Chatterjee VK. Transcriptional activation by peroxisome proliferator-activated receptor γ is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. Journal of Biological Chemistry. 1997;272(8):5128–5132. doi: 10.1074/jbc.272.8.5128. [DOI] [PubMed] [Google Scholar]
- 62.Camp HS, Tafuri SR. Regulation of peroxisome proliferator-activated receptor γ activity by mitogen-activated protein kinase. Journal of Biological Chemistry. 1997;272(16):10811–10816. doi: 10.1074/jbc.272.16.10811. [DOI] [PubMed] [Google Scholar]
- 63.Jaiswal RK, Jaiswal N, Bruder SP, Mbalaviele G, Marshak DR, Pittenger MF. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. Journal of Biological Chemistry. 2000;275(13):9645–9652. doi: 10.1074/jbc.275.13.9645. [DOI] [PubMed] [Google Scholar]
- 64.Nuttall ME, Gimble JM. Controlling the balance between osteoblastogenesis and adipogenesis and the consequent therapeutic implications. Current Opinion in Pharmacology. 2004;4(3):290–294. doi: 10.1016/j.coph.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 65.Ahdjoudj S, Fromigué O, Marie PJ. Plasticity and regulation of human bone marrow stromal osteoprogenitor cells: potential implication in the treatment of age-related bone loss. Histology and Histopathology, Cellular and Molecular Biology. 2004;19(1):151–158. doi: 10.14670/HH-19.151. [DOI] [PubMed] [Google Scholar]