Abstract
The TGF-β signaling pathway has a complex role in regulating mammary carcinogenesis. Here we demonstrate that the type III TGF-β receptor (TβRIII, or betaglycan), a ubiquitously expressed TGF-β coreceptor, regulated breast cancer progression and metastasis. Most human breast cancers lost TβRIII expression, with loss of heterozygosity of the TGFBR3 gene locus correlating with decreased TβRIII expression. TβRIII expression decreased during breast cancer progression, and low TβRIII levels predicted decreased recurrence-free survival in breast cancer patients. Restoring TβRIII expression in breast cancer cells dramatically inhibited tumor invasiveness in vitro and tumor invasion, angiogenesis, and metastasis in vivo. TβRIII appeared to inhibit tumor invasion by undergoing ectodomain shedding and producing soluble TβRIII, which binds and sequesters TGF-β to decrease TGF-β signaling and reduce breast cancer cell invasion and tumor-induced angiogenesis. Our results indicate that loss of TβRIII through allelic imbalance is a frequent genetic event during human breast cancer development that increases metastatic potential.
Introduction
TGF-β is a member of a superfamily of functionally diverse, but structurally conserved, cytokines that regulate cell proliferation, differentiation, apoptosis, and motility in a cell- and context-specific manner (1). TGF-β exerts these biological effects by binding to 2 high-affinity cell surface receptors, the type II TGF-β receptor (TβRII) and the type III TGF-β receptor (TβRIII, or betaglycan); TβRIII functions as a coreceptor to increase ligand binding to TβRII. Once bound to TGF-β, TβRII recruits, binds, and transphosphorylates the type I TGF-β receptor (TβRI), thereby stimulating its protein kinase activity. The activated TβRI phosphorylates transcription factors Smad2 or Smad3, which then binds to Smad4. The resulting Smad complex translocates into the nucleus and interacts with other transcription factors to specifically regulate the transcription of a multitude of TGF-β–responsive genes.
TGF-β has an important role in normal mammary biology as a potent inhibitor of mammary epithelial proliferation and regulator of mammary ductal and alveolar development (2, 3). Early in mammary carcinogenesis the TGF-β signaling pathway functions as a tumor suppressor, with most human breast cancers developing resistance to the growth-inhibitory effects of TGF-β and with elevated levels of TGF-β associated with decreased incidence of mammary cancer in mouse models (4) and decreased breast cancer incidence in humans (5, 6). However, at later stages of mammary carcinogenesis, levels of TGF-β increase with tumor progression (7–9) and confer a poorer prognosis for human breast cancer patients (10).
Although the TGF-β signaling pathway has an important role in regulating mammary carcinogenesis, alterations in the main components of the pathway, including TβRII, TβRI, Smad2, Smad3, and Smad4, are infrequent in human breast cancers (6, 11). A role for the TGF-β coreceptor TβRIII as a mediator and regulator of TGF-β signaling has emerged as a result of recent studies, with essential roles in chick heart and mouse development (12, 13) and in regulating TβRII and TβRI cell surface expression and internalization as well as TGF-β signaling (14, 15). TβRIII has been reported to be expressed at low levels in the MCF-7 human breast cancer cell line (16), and restoring TβRIII expression in these cells suppresses their anchorage-independent growth in vitro as assayed by colony formation in soft agarose (16), while increasing TβRIII expression in MDA-MB231 breast cancer cells suppresses their tumorigenicity in vivo as assessed by tumor formation in athymic nude mice (17). These results suggest that decreased TβRIII expression may be a mechanism for altering TGF-β responsiveness during mammary carcinogenesis. Here we demonstrate that TβRIII is a suppressor of breast cancer progression and that, when TβRIII expression is restored in human breast cancer cells, breast tumor invasion, angiogenesis, and metastasis are inhibited in vivo.
Results
Decreased TβRIII expression in human breast cancer.
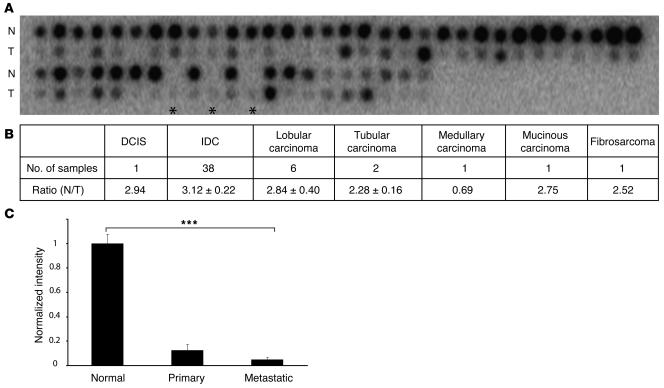
As evidence supporting roles for TβRIII in regulating TGF-β signaling have emerged (12–15), and a low level of TβRIII expression has been reported in the MCF-7 human breast cancer cell line (16), we investigated the expression status of TβRIII in human breast cancer. Breast cancers are classified into different histologic subtypes, with invasive ductal carcinoma (IDC) being the most common (~70%), followed by lobular carcinoma (~8%). The development of IDC has been proposed to follow a stepwise process — including ductal carcinoma in situ (DCIS) — culminating in the potentially lethal stage of IDC. We initially analyzed a cDNA array containing 50 human breast cancer samples with matched normal controls (Figure 1A). TβRIII mRNA levels were reduced in 60% of the lymph node–negative IDCs (2.64 ± 0.49–fold), 64.7% of the lymph node–positive IDCs (2.47 ± 0.29–fold) and in 100% of the IDCs with distant metastasis (3.98 ± 0.79–fold) as well as in all histological subtypes represented in Figure 1B, suggesting an increased frequency of loss with disease progression. TβRIII mRNA levels were also significantly reduced in 83.3% of lobular carcinomas (2.84 ± 0.40–fold). We also examined 3 sets of specimens on the cDNA array with matched normal breast, primary breast cancer, and metastatic breast cancer tissue from the same patient. In all 3 cases, TβRIII expression decreased from normal breast to primary breast cancer to metastatic breast cancer, with an average 88% decrease in expression from normal breast to primary breast cancer and a further 61% decrease from primary breast cancer to metastatic breast cancer (Figure 1C; P < 0.0001), suggesting progressive loss of TβRIII expression with cancer progression.
Figure 1. Loss of TβRIII mRNA expression during mammary carcinogenesis.
(A) TβRIII mRNA levels were detected by hybridizing [32P]-labeled human TβRIII cDNA probe to the Clontech Cancer Profiling Array I. The portion of the array containing breast samples is shown, with tumor specimens (T) and matched normal breast tissue (N). Asterisks indicate metastatic specimens corresponding to the normal and tumor samples spotted on the immediate left. (B) Quantitative data were obtained by analyzing the array with NIH ImageJ software, summarized as the ratio relative to normal breast, and expressed as mean ± SEM. (C) Quantitative data from matched normal, primary breast tumor, and metastatic breast tumor tissue expressed as mean ± SEM. ***P < 0.0001, ANOVA.
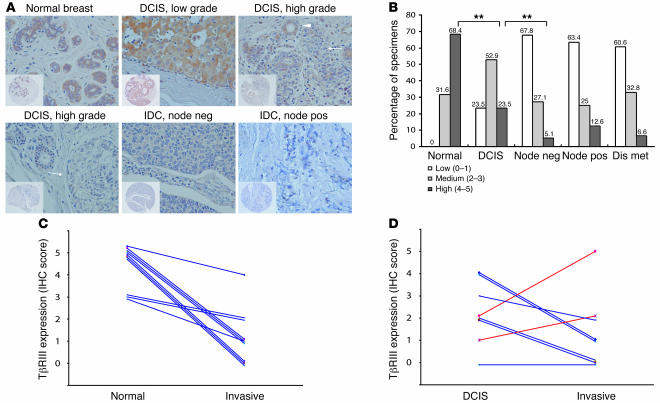
To confirm decreased expression of TβRIII and establish its association with breast cancer progression, we performed immunohistochemical (IHC) analysis for TβRIII expression on a breast cancer tissue array containing 252 breast cancers of different stages (20 DCIS, 64 lymph node–negative, 64 lymph node–positive, and 64 distant metastatic) and 40 normal breast specimens with available pathologic information including tumor size, TNM stage, number of nodes positive, invasive grade, and estrogen receptor (ER) and progesterone receptor status. TβRIII expression progressively decreased from normal breast specimens (Figure 2, A and B) to DCIS to lymph node–negative breast cancer. The proportion with abundant TβRIII expression decreased from 68.4% in normal breast specimens to 23.5% in DCIS specimens to 5.1% in lymph node–negative breast cancer specimens (P < 0.01, 2-tailed Fisher’s exact probability). At the same time, the proportion with no TβRIII expression increased from 0% in normal breast specimens to 23.5% in DCIS specimens to 67.8% in lymph node–negative breast cancer specimens (P < 0.01, 2-tailed Fisher’s exact probability). In DCIS specimens with loss of TβRIII expression (Figure 2A, arrow), TβRIII was present in adjacent normal-appearing breast ducts (Figure 2A, arrowhead), which served as a useful internal control. To directly address the role of loss of TβRIII expression in breast cancer progression, we assessed matched tissue sets for which either matching normal breast and invasive breast cancer specimens (Figure 2C) or matching DCIS and invasive breast cancer specimens (Figure 2D) were available for analysis. In addition, one of these samples had matching normal breast, DCIS, and invasive breast cancer specimens available for analysis. When examining TβRIII expression in matched normal breast and invasive breast cancer specimens, TβRIII expression decreased in every case (10 of 10), with 6 cases decreasing from high expression (IHC score of 5) in normal breast tissue to low expression (IHC score of 0–1) in the matching invasive breast cancer tissue (Figure 2C). When examining TβRIII expression in matched DCIS and invasive breast cancer specimens, TβRIII expression decreased in 63% of the cases (5 of 8), with 1 additional case where expression was already absent at the DCIS stage (Figure 2D). In the sample with matching normal breast, DCIS, and invasive breast cancer specimens, TβRIII expression decreased from an IHC score of 5 in the normal breast specimen to 2 in the DCIS specimen to 0 in the invasive breast cancer specimen. These data indicate that TβRIII expression is significantly decreased in breast cancer, with loss of TβRIII expression correlating with breast cancer progression.
Figure 2. Progressive loss of TβRIII protein expression during mammary carcinogenesis.
(A) Representative IHC analysis of TβRIII expression (original magnification, ×40) in normal breast ductal cells, in different grades of DCIS, and in lymph node–negative (node neg) and –positive (node pos) IDC. Insets depict staining of entire tissue core (original magnification, ×10). Immunoreactivity for TβRIII was scored as 0–5 and categorized as low (0–1), medium (2–3), or high (4–5). Note the absence of TβRIII staining in IDC and high-grade DCIS (arrows) versus presence of staining in normal ducts and normal-appearing ducts adjacent to the DCIS lesion (arrowhead). (B) Summary of IHC results, with percentages shown. Dis met, distant metastasis. **P < 0.01, 2-tailed Fisher’s exact probability. (C) Patient-matched normal and invasive breast cancer IHC TβRIII scores. (D) Patient-matched DCIS and invasive breast cancer IHC TβRIII scores.
Loss of heterozygosity and transcriptional downregulation of the TβRIII gene in human breast cancer.
Members of the TGF-β signaling pathway, including TβRII and Smad4, frequently have inactivating mutations in human cancers (18, 19). To investigate whether there are mutations in the TβRIII gene, TGFBR3 (216 kb of genomic DNA composed of 17 exons), that could abrogate TβRIII function in breast cancer, sequence analysis of the 16 coding exons (exon 1 is untranslated) was carried out on 20 primary breast cancer DNA samples. Although several polymorphisms were detected (data not shown), no mutations were found. Thus, TGFBR3 does not appear to be a target for mutational inactivation in breast cancer.
TGFBR3 maps to chromosome 1p32, a region that has been reported to exhibit loss of heterozygosity (LOH) in a variety of human cancers, including breast cancer (20–22).
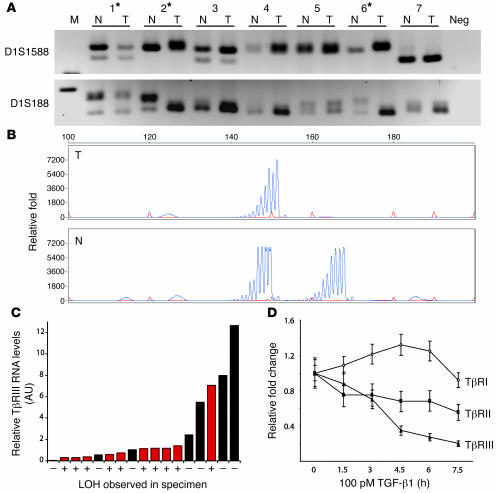
Therefore, to investigate the mechanism for loss of TβRIII expression during breast tumorigenesis, we examined LOH at the TGFBR3 locus using microsatellite markers on DNA samples extracted from 26 human breast cancer specimens and the matching normal peripheral lymphocytes. With 4 microsatellite markers immediately adjacent to and within the TGFBR3 locus, we were able to establish that 50% (13 of 26) of these samples exhibited LOH at the TGFBR3 locus (Figure 3, A and B), closely matching the 43%–61% LOH reported for the 1p region and the 58% reported for 1p32 in human breast cancers (20–22). LOH at the TGFBR3 locus correlated with loss of TβRIII expression, with 75% (9 of 12) of those with the lowest TβRIII expression exhibiting LOH at the TGFBR3 locus and only 20% (1 of 5) with the highest TβRIII expression exhibiting LOH at the TGFBR3 locus (Figure 3C). Taken together, these data support LOH as a mechanism for loss of TβRIII expression in breast cancer.
Figure 3. Frequent LOH of the TGFBR3 gene locus in human breast cancers correlates with loss of TβRIII mRNA expression.
LOH analysis was performed on DNA extracted from 26 human breast cancer specimens and matching normal lymphocytes. (A) Representative results showing allelic loss in tumors 1, 2, and 6 (denoted by asterisks) when PCR products were separated on a MetaPhor agarose gel. Microsatellite markers D1S1588 and D1S188 are described in Methods. (B) LOH was confirmed using an ABI sequencer and quantified using GeneScan software. A representative sample with LOH is shown. (C) Quantitative real-time PCR analysis of TβRIII mRNA levels in breast cancer specimens with (red bars) and without (black bars) LOH. (D) Quantitative real-time PCR analysis of mRNA levels of TβRI, TβRII, and TβRIII in MDA-MB231 cells in response to TGF-β1 (100 pM) stimulation for the indicated times.
During later stages of mammary carcinogenesis, levels of TGF-β increase with tumor progression (7–9) and confer a poorer prognosis for human breast cancer patients (10). As TGF-β isoforms have previously been demonstrated to decrease TβRIII promoter activity (23), we assessed whether the elevated levels of TGF-β could repress TβRIII expression at the transcriptional level in breast cancer cells. In MDA-MB231 breast cancer cells, which exhibit basal TβRIII expression, TGF-β1 treatment resulted in a significant (up to 80%) reduction in the TβRIII mRNA level (Figure 3D). This effect was relatively specific for TβRIII, as TGF-β1 treatment slightly increased TβRI mRNA levels and decreased TβRII mRNA levels by less than 50% (Figure 3D). These results suggest that, apart from LOH, transcriptional downregulation due to increased TGF-β in the breast cancer microenvironment could be another mechanism leading to decreased TβRIII expression during mammary carcinogenesis.
TβRIII delays and decreases metastatic potential of breast cancer cells in vivo.
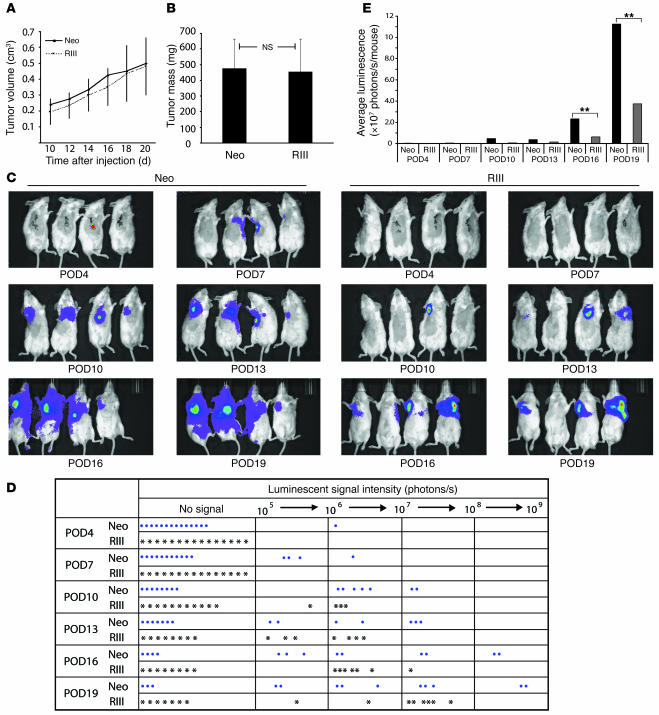
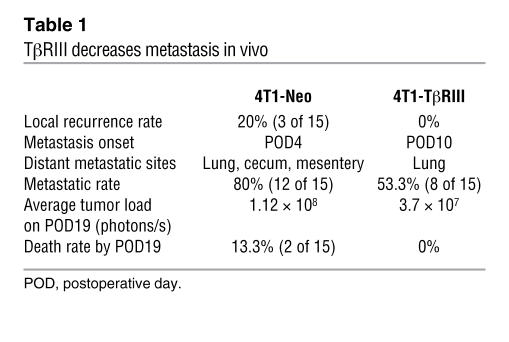
The frequent loss of TβRIII expression observed during progression to invasive disease suggested that TβRIII loss during mammary carcinogenesis may specifically promote tumor invasion and metastasis in vivo. To investigate a causal role for decreased TβRIII expression in breast cancer progression, we examined the effect of TβRIII on in vivo tumor growth and metastasis using a murine model for mammary carcinogenesis. Murine 4T1 mammary cancer cells, which are derived from a BALB/c murine mammary tumor, share many characteristics with human mammary cancers including spontaneous lung metastasis in immunocompetent mice and have been widely used as a model of breast cancer (24, 25). The 4T1 cells were genetically engineered to express the firefly luciferase gene so that by periodically injecting the substrate luciferin into mice carrying these cells and taking bioluminescent images, we were able to closely and quantitatively follow their in vivo growth and metastatic potential. The 4T1 cells were stably transfected with TβRIII (4T1-TβRIII cells, see Supplemental Figure 1; supplemental material available online with this article; doi:10.1172/JCI29293DS1), resulting in 4T1 cells with increased TβRIII expression. The 4T1-TβRIII cells and control 4T1 cells stably expressing the pcDNA-Neo expression vector (4T1-Neo cells) were injected into the axillary mammary fat pads of BALB/c mice. The primary tumor was measured every 2 days starting from day 10 after injection and removed on day 20. Tumor metastases were then followed by bioluminescent imaging every 3 days over a period of 19 days. No significant difference was observed in the growth of the primary tumors from 4T1-TβRIII and 4T1-Neo cells as shown by the growth curve (Figure 4A) and tumor mass at the time of resection (Figure 4B), establishing that TβRIII had no effect on tumorigenicity in vivo. However, mice injected with 4T1-TβRIII cells demonstrated a significantly delayed onset of tumor metastasis as well as a significant reduction in both the size and number of lung metastases compared with the mice injected with control 4T1-Neo cells (Figure 4, C–E). In addition, while no tumor recurrence at the primary site or animal death was observed in mice injected with 4T1-TβRIII cells, the control mice with the 4T1-Neo cells had a 20% local recurrence rate and a 13.3% death rate during the study (Table 1).
Figure 4. TβRIII delayed and decreased metastatic potential of breast cancer cells in vivo.
Either 4T1-Neo (Neo) or 4T1-TβRIII (RIII) cells (75,000 cells/mouse) were implanted into the axillary mammary fat pads of BALB/c mice. (A) Primary tumor growth was recorded by measuring tumor size every 2 days beginning at 10 days after injection and presented as mean ± SEM. (B) Weight of the primary tumors upon surgical removal on day 20 after injection. Data are mean ± SEM (n = 16). (C) Bioluminescence imaging was performed every 3 postoperative days (POD). Representative images are shown. Red and violet signals correspond to the maximum and minimum intensity values, respectively, with other colors representing the values in between. (D) Record of luminescent signals for every mouse in each group at the indicated time points. (E) Average luminescent signal in each group at the indicated time points. **P < 0.01.
Table 1 .
TβRIII decreases metastasis in vivo
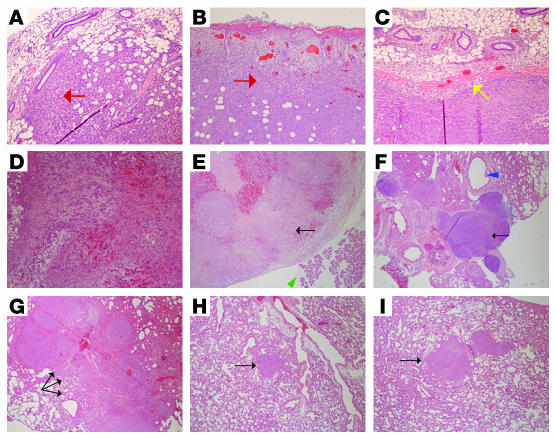
Further pathologic examination of the primary tumors demonstrated that the 4T1-Neo tumors exhibited increased invasion of the surrounding normal mammary tissue (Figure 5A) and skin (Figure 5B), while the 4T1-TβRIII tumors exhibited little to no invasion and instead maintained a distinct margin with the adjacent normal tissue (Figure 5C). In addition, primary recurrences in the 4T1-Neo mice exhibited invasion of tumor cells into the blood vessels, resulting in internal hemorrhage (Figure 5D). Pathologic examination of tumor metastasis revealed distant metastasis to the mesentery (Figure 5E), the paratracheal lymph nodes (Figure 5F), and the cecum in addition to the lung in control 4T1-Neo mice, while 4T1-TβRIII exhibited only lung metastases. In addition, when lung metastases were observed in 4T1-TβRIII mice, these metastatic lesions were always small, well circumscribed, and isolated (Figure 5, H and I) compared with the large, locally invasive lung metastases observed in 4T1-Neo mice (Figure 5G). These studies support a specific suppressor effect of TβRIII on cellular invasiveness and metastasis, but not on primary tumorigenesis.
Figure 5. TβRIII decreased tumor cell invasiveness and metastasis in vivo.
Representative H&E staining (original magnification, ×10) of (A and B) primary tumors from mice implanted with 4T1-Neo cells exhibiting local invasion (red arrows) of tumor cells into the adjacent normal mammary tissue (A) and skin (B); (C) a representative primary tumor from mice implanted with 4T1-TβRIII cells demonstrating the absence of local invasion, as indicated by the clear margin between the tumor and the adjacent normal mammary tissue (yellow arrow); (D) a recurring tumor in a mouse at the primary injection site of 4T1-Neo cells exhibiting internal bleeding due to invasion of tumor cells into the blood vessels; (E) a metastatic tumor (black arrow) adjacent to the pancreas (green arrowhead) found on the mesentery of a mouse implanted with 4T1-Neo cells; (F) a significantly enlarged paratracheal lymph node adjacent to the trachea (blue arrowhead) containing metastatic tumor cells (black arrow) in a mouse with 4T1-Neo cells, indicating the presence of lymphatic metastasis; (G) multiple large metastatic tumor nodules (black arrows) in the lung of a mouse implanted with 4T1-Neo cells; and (H and I) representative lung metastases in mice implanted with 4T1-TβRIII cells (black arrows).
TβRIII decreases angiogenesis in vivo.
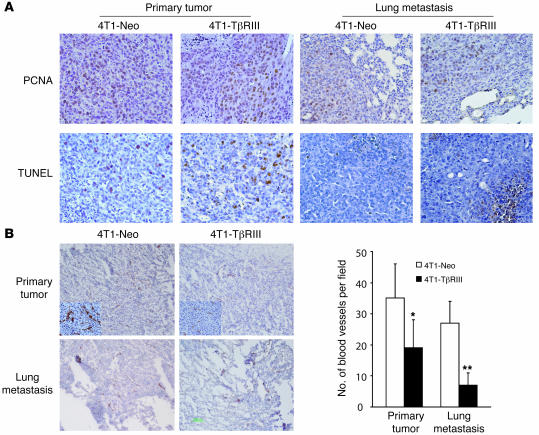
Cancer metastasis is a multi-step process requiring the cells growing at the primary site to invade through the basement membrane, enter lymph or blood vessels, extravasate from the vessel, and then grow at the distant site. Many of the processes involved in primary tumorigenesis and growth of metastases are similar, including increased proliferation, decreased apoptosis, and increased angiogenesis. To further establish the mechanism of TβRIII on decreasing metastasis in vivo, we performed immunohistochemistry for the proliferation marker proliferating cell nuclear antigen (PCNA), TUNEL staining as a marker for apoptosis, and immunohistochemistry for CD31 as an endothelial surface marker on primary tumors and metastatic lesions. There were no significant differences observed in PCNA or TUNEL staining in 4T1-Neo and 4T1-TβRIII primary tumors or lung metastases (Figure 6A), suggesting that differences in proliferation or apoptosis did not account for the differential metastatic behavior of 4T1-Neo and 4T1-TβRIII cells. However, CD31 staining revealed a decrease in the number of tumor-associated blood vessels per field, smaller vessel diameters, and less staining intensity in 4T1-TβRIII tumors (Figure 6B), which supported an inhibitory effect of TβRIII on tumor angiogenesis. Taken together, these data indicate that loss of TβRIII expression facilitates tumor metastasis in vivo not only through an increase in tumor cell invasiveness but also through enhanced tumor angiogenesis.
Figure 6. TβRIII inhibits tumor angiogenesis without altering cancer cell proliferation and apoptosis in vivo.
(A) Tissue sections of primary tumors and lung metastases from mice implanted with 4T1-Neo and 4T1-TβRIII cells were immunostained for PCNA and TUNEL to evaluate cell proliferation and apoptosis, respectively. Representative staining frequency and intensity is shown (original magnification, ×40). (B) Immunostaining of CD31 (original magnification, ×10) was performed as a marker to evaluate angiogenesis. Note the decreased number and size of tumor-associated blood vessels as well as decreased staining intensity (insets; original magnification, ×100) in 4T1-TβRIII primary tumors and lung metastases. Values are the averages from 6 mice and expressed as mean ± SD. *P < 0.05; **P < 0.01.
TβRIII inhibits the invasiveness of breast cancer cells through the generation of soluble TβRIII.
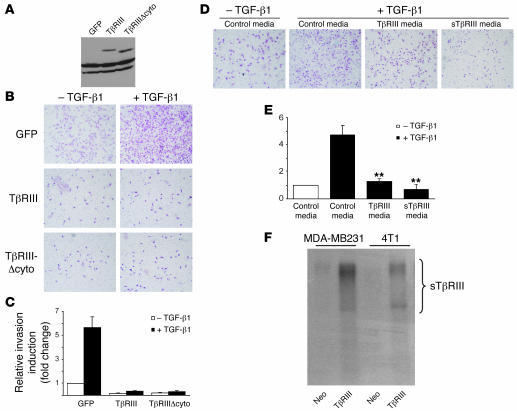
To further define the mechanisms by which TβRIII regulated breast cancer invasiveness and metastasis in vivo, we examined the effect of increasing TβRIII expression on the invasiveness of breast cancer cell lines in vitro. We initially assessed the 4T1-Neo and 4T1-TβRIII cell lines; however, these cell lines both tended to aggregate and were not significantly invasive in vitro (data not shown). Therefore, we used the tumorigenic, invasive, and metastatic MDA-MB231 cell line. Overexpression of TβRIII had no significant effect on the rate of cell division, nor did it restore cell responsiveness to TGF-β–induced growth inhibition (Supplemental Figure 2). However, it dramatically repressed the ability of MDA-MB231 cells to invade through Matrigel and significantly attenuated the responsiveness of the MDA-MB231 cells to TGF-β–induced invasion (Figure 7, A–C). These results confirm a direct effect of TβRIII on inhibiting breast cancer cell invasiveness.
Figure 7. Restoration of TβRIII expression inhibits Matrigel invasiveness of MDA-MB231 breast cancer cells.
(A) MDA-MB231 cells were infected with equivalent amounts of adenoviral constructs carrying GFP, HA-tagged TβRIII, and a TβRIII mutant lacking the entire cytoplasmic domain (TβRIIIΔcyto). Expression of the transgenes was confirmed by Western blotting of cell lysate using anti-HA antibody. (B and C) Matrigel invasion assay. Adenovirally infected MDA-MB231 cells (75,000 cells) were seeded in a Matrigel-coated upper chamber and treated with TGF-β1 (15 pM) 2 hours later. Cell invasion through the Matrigel after 24 hours’ incubation was detected by H&E staining and quantitated. (D and E) Matrigel invasion assay was performed after resuspending MDA-MB231 cells in the conditioned media collected from pcDNA3.1-Neo–, TβRIII-, and sTβRIII-transfected COS-7 cells. Data are mean ± SEM, n = 3 in triplicate. **P < 0.01. (F) Detection of sTβRIII in media of MDA-MB231–TβRIII and 4T1-TβRIII cells by [125I]TGF-β1–binding crosslinking followed by immunoprecipitation.
We next assessed the ability of specific TβRIII mutants to mediate this function. Interestingly, a TβRIII mutant lacking the entire cytoplasmic domain inhibited breast cancer cell invasiveness to an extent similar to that of full-length TβRIII (Figure 7, A–C), suggesting that the effect of TβRIII on regulating invasion is independent of functions mediated by the cytoplasmic domain of TβRIII, including binding Gα-interacting protein–interacting protein, C terminus (GIPC) (26) and β-arrestin2 (15) and mediating TGF-β signaling (14).
The extracellular domain of TβRIII can be proteolytically cleaved in the juxtamembrane region (27), and the resulting soluble TβRIII (sTβRIII) has been demonstrated to suppress tumor growth and angiogenesis, potentially through binding and sequestering TGF-β and preventing signaling through the membrane-bound receptors (28). To assess whether the effects of TβRIII could be mediated by the production of sTβRIII, we first examined whether the 4T1-TβRIII and MDA-MB231–TβRIII cells lines produced sTβRIII. We collected conditioned media from each cell line, crosslinked iodinated TGF-β1, and specifically immunoprecipitated sTβRIII with an antibody to the extracellular domain. These studies confirmed that both the 4T1-TβRIII and the MDA-MB231–TβRIII cell lines produced a significant amount of sTβRIII (Figure 7F). Accordingly, we examined the effect of sTβRIII on MDA-MB231 breast cancer cell invasion in vitro. Conditioned media collected from COS-7 cells transiently transfected with full-length TβRIII or sTβRIII potently decreased TGF-β–induced invasion of MDA-MB231 breast cancer cells through Matrigel (Figure 7, D and E).
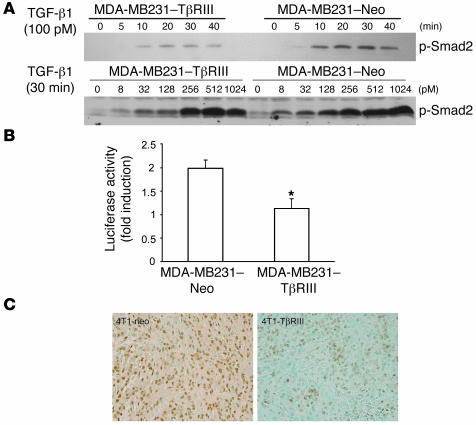
As sTβRIII mediated the effects of TβRIII expression on breast cancer invasiveness in vitro and in vivo, we reasoned that TβRIII would attenuate TGF-β signaling in the MDA-MB231–TβRIII cells in vitro and in the 4T1-TβRIII tumors in vivo. To examine the effect of TβRIII expression on activation of the Smad pathway in response to TGF-β stimulation, MDA-MB231–TβRIII and MDA-MB231–Neo breast cancer cells were treated with TGF-β, and phosphorylation levels of Smad2 were quantified. As shown in Figure 8A, TβRIII expression in the MDA-MB231 cells resulted in reduced TGF-β–stimulated Smad2 phosphorylation compared with the MDA-MB231–Neo cells. In addition, TGF-β1–mediated activation of TGF-β1–responsive, Smad-dependent promoter pE2.1 was also reduced in the MDA-MB231–TβRIII cells (Figure 8B). Consistent with this in vitro result, immunohistochemistry of the mouse mammary tumors revealed decreased frequency and intensity of phosphorylated Smad2 nuclear staining in the 4T1-TβRIII tumors compared with the 4T1-Neo tumors (Figure 8C). Further support for a significant role for sTβRIII in mediating the effects of TβRIII was provided by the decreased angiogenesis demonstrated in the 4T1-TβRIII tumors in vivo (Figure 6B), as sTβRIII has been demonstrated to decrease angiogenesis in vivo (28, 29).
Figure 8. TβRIII attenuates Smad2 phosphorylation in vitro and in vivo.
(A) TβRIII-overexpressing and control MDA-MB231 cells were treated with TGF-β1 under the indicated conditions, and cell lysates were analyzed with a phospho-Smad2 (p-Smad2) antibody. (B) Cells were transfected with pE2.1 and pSVβ vector. Luciferase activity was determined after 24 hours of TGF-β1 treatment (100 pM) and is expressed as the fold induction over no TGF-β treatment after adjusting for β-galactosidase expression. This assay was performed in triplicate at least 3 times. *P < 0.05. (C) Phosphorylated Smad2 immunostaining of tissue sections from 4T1-Neo and 4T1-TβRIII primary tumors. Representative results are shown. Note the significant decrease in staining intensity in the 4T1-TβRIII tumor. Original magnification, ×40.
sTβRIII is produced from cells and tissues from 7 different mammalian species, including humans (30, 31), and has also been detected in serum (30) and human milk (32). In addition, the expression of sTβRIII has been demonstrated to closely correlate with the cell surface expression of TβRIII (30), suggesting that it is released constitutively. To support a physiological role for sTβRIII in mediating the effects of TβRIII on breast cancer invasiveness, we examined expression of sTβRIII in a panel of human breast epithelial and breast cancer cell lines. sTβRIII was expressed in all human breast cell lines tested, including the human mammary epithelial cell line MCF-10A and the human breast cancer cell lines MCF-7, T47D, and MDA-MB231 (Supplemental Figure 3A). As previously reported, the level of sTβRIII usually correlated with cell surface expression of TβRIII. Finally, we examined expression of sTβRIII in plasma from normal human volunteers as well as from patients with breast cancer. While we detected expression of sTβRIII (a heterogeneous product from approximately 65–250 kDa) in plasma in all (5 of 5) of the normal human volunteers, we did not detect sTβRIII in the plasma of any breast cancer patients (0 of 13; Supplemental Figure 3B). Taken together, these data support a model in which ectodomain shedding of TβRIII produces sTβRIII, which then functions to attenuate TGF-β–mediated invasiveness of breast cancer cells and tumor-induced angiogenesis in vitro and in vivo.
Decreased TβRIII expression correlates with decreased recurrence-free survival in breast cancer patients.
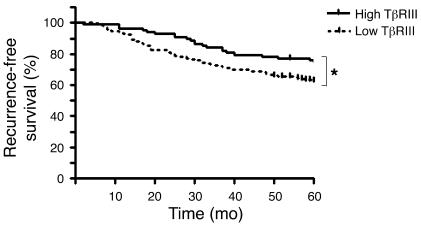
As decreased TβRIII expression is frequently observed in human breast cancers and restoring TβRIII expression decreased invasiveness and metastasis in vivo, we explored whether TβRIII expression could be a useful prognostic marker for breast cancer patients. We examined publicly available microarray data sets in which both TβRIII expression and recurrence-free survival data were available (33–36). We set TβRIII expression as a dichotomous variable, with high expression as above the mean and low expression as below the mean. In the largest data set (that of Wang et al., ref. 36), composed of 286 patients with lymph node–negative breast cancers, low expression of TβRIII was significantly associated with a decrease in recurrence-free survival (Figure 9; P = 0.043), with recurrence defined as a distant metastatic event. The hazard ratio (HR) for recurrence based on TβRIII expression (HR, 1.569) was higher than that for ER status (HR, 1.18) or for Her2/Neu status (HR, 1.06) (37). In addition, we examined whether the predictive value of TβRIII was independent of other known prognostic factors. As all samples in the Wang et al. data set (36) came from lymph node–negative patients, we analyzed the only other available prognostic factor within the data set, ER status. A Pearson correlation coefficient of –0.08 (95% confidence interval, –0.19 to 0.036) supported little correlation between TβRIII expression and ER status, although the data set was not large enough to power the analysis (P = 0.177). In 3 other completely independent data sets (Sorlie et al., ref. 34, containing 74 locally advanced ER-positive and -negative primary breast cancers; van’t Veer et al., ref. 33, containing 97 ER-positive and -negative lymph node–negative breast cancers; and Ma et al., ref. 35, containing 60 hormone receptor–positive breast cancers), there was a trend toward decreased recurrence-free survival associating with low TβRIII expression, although in each case the number of patients was not large enough to reach statistical significance (data not shown). Taken together, these data suggest that TβRIII expression is predictive of recurrence-free survival in breast cancer patients.
Figure 9. Low levels of TβRIII predict decreased recurrence-free survival in women with breast cancer.
Five-year recurrence-free survival for breast cancer with high or low TβRIII expression was analyzed based on a microarray data set containing 286 patients. *P < 0.05.
Discussion
Breast cancer is the leading cause of cancer death in women in the world, with most breast cancer morbidity and mortality resulting from metastatic disease (38). Although the TGF-β signaling pathway has an important role in mammary carcinogenesis, the major components of the pathway, including the signaling receptors, TβRII and TβRI, and the predominant signaling pathway downstream of these receptors, Smad2, Smad3, and Smad4, are usually intact in human breast cancers (6, 11). In the present study, we demonstrate that expression of the TGF-β coreceptor TβRIII was frequently decreased at the mRNA and protein levels in human breast cancer, with approximately 90% of specimens demonstrating decrease or loss at the mRNA level and approximately 70% demonstrating decrease or loss at the protein level. Thus, we believe loss of TβRIII expression to be the most common alteration in the TGF-β signaling pathway described in human breast cancer to date. We have further demonstrated that loss of TβRIII expression was an early event, occurring initially in the preinvasive state, DCIS, with degree of loss correlating with breast cancer progression and corresponding to a decrease in patient survival. Mechanisms for decreased expression include LOH at the TGFBR3 gene locus and potential transcriptional downregulation of TβRIII by elevated TGF-β levels in the breast tumor microenvironment. Finally, we established a functional role for loss of TβRIII expression, as restoring TβRIII expression dramatically inhibited tumor invasiveness in vitro and tumor invasion, angiogenesis, and metastasis in vivo. Mechanistically, TβRIII appeared to function by undergoing ectodomain shedding, with sTβRIII antagonizing TGF-β signaling and reducing invasiveness and angiogenesis in vivo. Taken together, these results support loss of TβRIII expression as a frequent and important step in breast cancer progression, directly promoting breast cancer invasion and metastasis.
The dichotomous role of TGF-β signaling in breast cancer development has been experimentally verified in several murine models. Specifically, blocking TGF-β signaling in a series of human breast-derived cell lines representing different stages in breast cancer progression rendered premalignant cells tumorigenic, and low-grade tumorigenic cells more invasive, while making high-grade tumorigenic cells less metastatic (39). In addition, introduction of constitutively active TβRI delayed oncogenic Neu-induced breast tumor onset but enhanced the frequency of lung metastasis in transgenic mice, whereas dominant-negative TβRII enhanced Neu-induced tumor onset but decreased subsequent lung metastasis (40). Furthermore, inducing expression of active TGF-β1 after primary breast tumor formation dramatically enhanced lung metastasis in a murine breast cancer model without a detectable effect on primary tumor size (41). Taken together, the results of these studies suggest that TGF-β suppresses breast cancer progression in the early stages, but enhances tumor progression and metastasis in the later stages. Different explanations for this dichotomous function have been proposed, including TGF-β exerting tumor-suppressing effects on epithelial-derived tumor cells and tumor-promoting effects on stromal cells (increased angiogenesis and immunosuppression, altered tumor cell–extracellular matrix interactions to enhance invasion and metastasis) (6). However, emerging evidence suggests that TGF-β may exert its dichotomous effects during carcinogenesis at least in part through biphasic effects on the epithelial derived cancer cells themselves, as the cells alter their molecular profiles to differentially respond to TGF-β (6). Thus, even though resistant to the tumor suppressor effects of TGF-β during tumorigenesis (growth inhibition, apoptosis, and differentiation), the cancer cells may respond to TGF-β with increased motility and invasiveness. Based on the present findings, we propose that loss of TβRIII expression may be a mechanism for this differential response to TGF-β during mammary carcinogenesis.
How might loss of TβRIII expression alter cellular responses to TGF-β during mammary carcinogenesis? Although TβRIII was the first TGF-β receptor cloned, as it has a short cytoplasmic domain with no intrinsic kinase activity, its role in TGF-β signaling has not been well characterized. TβRIII has classically been thought to act as a TGF-β coreceptor, concentrating ligand on the cell surface and enhancing ligand binding to the signaling TGF-β receptor TβRII (42). However, emerging evidence supports a more substantial role for TβRIII in regulating and mediating TGF-β signaling. TβRIII has essential roles in chick (12) and murine development, with the TβRIII knockout mouse having an embryonic lethal phenotype (13). In addition, we have previously established that regulating TβRIII expression is sufficient to alter TGF-β signaling (26), that the short cytoplasmic domain of TβRIII is phosphorylated by TβRII (14) and interacts with the PDZ domain–containing protein GIPC to stabilize TβRIII expression on the cell surface and increasing TGF-β signaling (26) as well as with the scaffolding protein β-arrestin2 to mediate internalization of TβRIII and TβRII and downregulation of TGF-β signaling (15). In addition, TβRIII undergoes ectodomain shedding that releases the soluble extracellular domain (sTβRIII), which has been demonstrated to effectively neutralize TGF-β and antagonize autocrine TGF-β signaling. In breast cancer models, expressing sTβRIII has been demonstrated to decrease tumorigenicity and spontaneous lung metastasis in immunocompromised mice through effects on both the tumor cells (decreasing cell growth and increasing apoptosis) (43) and the stroma (decreasing angiogenesis) (28, 29). While our results in the immunocompetent 4T1 model confirm the effects of TβRIII on angiogenesis, we found no significant effect of TβRIII expression on cellular proliferation or apoptosis in either primary tumor or distant tumor metastastic lesions in vivo. Instead, in addition to decreased angiogenesis, the major effect of TβRIII in vitro and in vivo was to decrease cellular invasiveness, with this effect mediated at least in part through the production of sTβRIII. Therefore, we propose a model in which loss of TβRIII expression results in alterations in TGF-β responsiveness in both a cell-autonomous fashion (resulting in relative resistance of breast cancer cells to TGF-β) and a non–cell-autonomous fashion (by decreasing production of sTβRIII), effectively increasing TGF-β signaling in both the cancer cells and the stromal elements. Our in vitro and in vivo results demonstrating decreased Smad2 phosphorylation and decreased TGF-β responsiveness in the presence of TβRIII suggest that non–cell-autonomous regulation by sTβRIII may have a dominant role in both tumor and stromal compartments. The contribution of TβRIII and sTβRIII on the balance of TGF-β signaling and responsiveness in epithelial and stromal compartments remains an area of active investigation.
TβRIII is located on chromosome 1p32, a region that frequently exhibits LOH in a wide variety of human cancers, including breast, colon, endometrial, gastric, kidney, lung, ovarian, and testicular cancer (20–22). For breast cancer, LOH at 1p32 is associated with a poorer prognosis (20, 21). Previous studies have examined several potential tumor suppressor genes in this region, including mammary-derived growth inhibitor (44) and TP73 (45); however, expression and functional studies did not provide sufficient evidence supporting their role as tumor suppressor genes in breast cancer. In the present study, LOH analysis revealed allelic imbalance at the TβRIII loci in 50% of the patients, with LOH correlating with loss of TβRIII expression. The observed decrease in TβRIII mRNA and protein expression could result from haploid insufficiency, as previously reported for TGF-β1 (46), or from transcriptional downregulation or promoter hypermethylation of the remaining allele. The current data strongly support TβRIII as a suppressor of breast cancer progression. TβRIII has also been reported to be lost at an early stage in renal cell carcinogenesis (47). Whether TβRIII functions as a suppressor of cancer progression in renal cell and other human cancers remains to be discerned.
Although breast cancer is thought to progress from a preinvasive state (DCIS) to invasive disease, we currently cannot determine which DCIS lesions are likely to remain indolent, and thus may be treated by local resection only, versus those DCIS lesions that will progress to invasive disease and/or recur, necessitating more aggressive treatment (i.e., postresection radiation, mastectomy, or adjuvant hormonal or chemotherapy). Clearly, understanding the molecular mechanisms by which DCIS becomes invasive and ultimately metastatic will allow identification of patients at low or high risk of recurrence and invasion/metastasis and guide these treatment options. In the present study, our data support loss of TβRIII expression in DCIS as a common event potentially resulting in invasive and metastatic disease. Thus, as would be predicted, later-stage invasive cancers have a significantly higher frequency of TβRIII loss, and lower TβRIII expression correlates with a poorer prognosis for patients with invasive breast cancer. As this retrospective analysis was performed on patient tumor samples that were heterogeneous for both tumor and surrounding stromal tissue, we cannot be certain whether the loss in TβRIII expression was in the tumor, stroma, or both, although our own IHC analysis of a large tissue array support that loss was primarily in tumor cells. Whether TβRIII-negative DCIS lesions have a worse natural history and thus warrant more aggressive intervention than TβRIII-positive DCIS lesions requires prospective validation.
Methods
TβRIII gene expression analysis on cDNA filter array.
A filter array containing normalized cDNA from 50 breast cancers and corresponding normal tissues (Cancer Profiling Array; Clontech; Takara Bio Co.) was probed with [32P]-labeled cDNA probes for TβRIII following methods recommended by the manufacturer. In the 50 breast cancer samples, 33 were ductal carcinoma, 10 were lobular carcinoma, and 2 were tubular carcinoma; the remaining samples consisted of 1 each of mixed lobular/ductal carcinoma, medullary carcinoma, mucinous adenocarcinoma, fibrosarcoma, and DCIS. The TβRIII cDNA probe was PCR amplified using the forward primer GTAGTGGGTTGGCCAGATGGT and reverse primer CTGCTGTCTCCCCTGTGTG. Purified PCR products (25 ng) were labeled by random primed DNA labeling using [α-32P]dCTP following the manufacturer’s protocol (Roche Diagnostics). Labeled cDNA probe was purified on a BD CHROMA SPIN+STE-100 column (BD Biosciences — Clontech). Images were acquired using a phosphorimager, and subsequent data analysis was performed using NIH ImageJ software (http://rsb.info.nih.gov/ij/). A normal/tumor ratio of 2 or higher was considered to be significant.
Breast cancer tissue array.
A polyclonal antibody recognizing TβRIII protein was custom made by immunizing rabbits with a GST-fusion protein of the entire cytoplasmic domain of human TβRIII. The IgG fraction of pre-immune and immune rabbit serum was collected using ImmunoPure IgG Purification kit (Pierce Biotechnology), and the specificity of the antibody was established by comparing staining of the breast cancer tissue arrays (Cooperative Breast Cancer Tissue Resource; National Cancer Institute) with preimmune serum and with the immune serum under identical conditions, by the specific pattern of staining of cells known to express TβRIII (breast epithelial cells) and lack of staining of cells known not to express TβRIII (lymphocytes), and by Western blot using protein extract from cell lines overexpressing human TβRIII. Breast cancer tissue arrays (Cooperative Breast Cancer Tissue Resource) were deparaffinized, rehydrated, treated with 3% hydrogen peroxide, and then blocked with 10% goat serum. The arrays were incubated with anti-TβRIII antibody overnight at 4°C, washed in PBS, and further incubated with HRP-conjugated anti-rabbit IgG (Vector Laboratory). Counterstaining was performed using hematoxylin. As a negative control, duplicate sections were immunostained with IgG purified from prebleed rabbit serum. The immunoreactivity for TβRIII in breast epithelial and breast cancer cells was relatively uniform within a specimen and was thus semiquantitatively scored by staining intensity in a blinded manner with 0–1 defined as no or weak staining, 2–3 as moderate staining, and 4–5 as intense staining. Standards for each staining score were used to maintain consistent scoring across specimens.
LOH and sequence analysis.
Genomic DNA extracted from human breast cancer specimens and matching normal peripheral lymphocytes was kindly provided by the Breast Cancer Tissue Repository at Duke University. Microsatellite markers D1S1588, D1S188, D1S2804, and D1S435 were used in PCR reactions in which the forward primer was synthesized with a 5′ fluorescent tag (Integrated DNA Technologies). PCR products were visualized using an ABI PRISM 3100 Genetic Analyzer (Applied Biosystems), and data were analyzed using GeneScan software (version 3.1; Applied Biosystems). LOH was determined as at least a 50% reduction in the relative intensity of one allele compared with the normal control. All samples positive for LOH were independently analyzed twice. PCR products were also analyzed on 3% MetaPhor agarose gels (Cambrex). For sequence analysis, 16 coding exons of TβRIII were PCR amplified from the same DNA samples and subjected to sequence analysis (Supplemental Table 1).
Real-time PCR.
Cells were treated with 100 pM TGF-β1 for the times indicated in Figures 3 and 8 and the Figure 7 legend. Total RNA was isolated using RNeasy Mini Kit (Qiagen), and first-strand cDNA was synthesized by M-MLV reverse transcriptase (Invitrogen). Quantitative real-time PCR was performed in the presence of iQ SYBR Green Supermix (Bio-Rad) on a Bio-Rad iCycler. Primer sequences are provided in Supplemental Table 2. Relative levels were calculated using the comparative threshold cycle method, with data normalized to GAPDH and expressed relative to untreated controls. All experiments were carried out in triplicate.
In vivo tumorigenicity and metastasis.
Animal procedures were approved by the Institutional Animal Care and Use Committee of Duke University. The 4T1-Luc cell line stably expressing firefly luciferase gene under the selection of puromycin was generously provided by M.W. Dewhirst (Duke University Medical Center). These cells were further transfected with HA-tagged rat TβRIII under the selection of neomycin and expression confirmed by [125I]TGF-β1 binding and crosslinking. Cells were implanted (50,000 cells/mouse) into the right-side axillary mammary gland of 7-week-old virgin, female BALB/c mice (Charles River Laboratories). Starting from day 10 after the implantation, growth of the tumors was measured with a caliber in 2 dimensions on alternate days and expressed as (length × width2) × 0.5. On day 20 after implantation, surgical resection of the primary tumors was performed under sterile conditions. Four days after the surgery, tumor metastasis was recorded by bioluminescence imaging of the mice every 3 days for 19 days. Briefly, mice were intraperitoneally injected with D-luciferin (Xenogen) at 150 μg/g. Fifteen minutes following the injection, bioluminescence images were acquired using a IVIS camera (Xenogen). Bioluminescence for ROI was defined automatically, and data were expressed as photon flux (photons/s/cm2/steradian). Background photon flux was defined from a ROI drawn over a mouse that was not given luciferin. At the end of the study mice were sacrificed, and sites of metastasis were determined by visual inspection. Interested organs were harvested for further IHC analysis and RNA and protein extraction. We used 4T1-Luc cells, stably transfected with the vector pcDNA3.1-Neo, in parallel as controls.
TUNEL, PCNA, CD31, and phosphorylated Smad2 immunostaining.
TUNEL and PCNA immunostainings were performed on paraffin-embedded tissue sections according to the manufacturer’s instructions (TUNEL, In Situ Cell Death Detection kit, POD; Roche Diagnostics; PCNA, Santa Cruz Biotechnology Inc.). CD31 immunostaining was performed on frozen tissue sections as specified by the manufacturer (Cell Signaling Technology). For phosphorylated Smad2 staining, antigen retrieval was carried out by boiling slides in 10 mM citrate buffer (pH 6.0) for 5 minutes in a microwave after blocking in 3% hydrogen peroxide.
Matrigel invasion assay.
We seeded 75,000 cells in the Matrigel-coated upper chamber (BD Biosciences) of a 24-well transwell. Media containing 10% FBS was placed in the lower chamber as a chemoattractant. After 2 hours’ incubation, 15 pM TGF-β1 was added into the designated upper chambers. Twenty-four hours later, the cells on the upper surface of the filter were removed by gently scrubbing with a cotton swab. The cells that migrated to the underside of the filter were fixed and stained with H&E. Each filter was removed and examined microscopically, and 3 random images were acquired. Cells present in each image were counted. In some assays, conditioned serum-free medium collected from COS-7 cells transiently transfected with empty vector, full-length TβRIII, or sTβRIII construct was used to resuspend cells to be seeded in the upper chambers. These assays were performed in triplicate at least 3 times.
TGF-β binding and crosslinking assay.
We incubated 100 pM [125I]TGF-β1 with 500 μl of the cell medium in the presence of protease inhibitors for 3 hours at 4°C. The [125I]TGF-β1–sTβRIII complex was then crosslinked with 0.5 mg/ml disuccinimidyl suberate and immunoprecipitated with a polyclonal antibody recognizing the extracellular domain of TβRIII (R&D Systems). The final complex was visualized after SDS-PAGE and autoradiography.
Transcription reporter luciferase assays.
Cells were transfected with a pE2.1 vector that contains the luciferase gene under the regulation of a promoter based on the TGF-β–inducible promoter PAI-1 and the pSVβ vector encoding β-galactosidase as a control for transfection efficiency. After 24 hours, the cells were treated with TGF-β1 (100 pM) for an additional 24-hour period. The cells were lysed in luciferase lysis buffer (Promega). The luciferase activity was read after the addition of luciferin (Promega) using an automated luminometer. The luciferase activity was expressed as the fold induction over no TGF-β treatment after adjusting for β-galactosidase expression.
[3H]Thymidine incorporation assay.
Cells growing in 24-well plates were treated with 0–200 pm TGF-β1 for 48 hours when they reached 80% confluency and then incubated with 10 μCi of [3H]thymidine (Amersham Biosciences) for an additional 4 hours. Cells were washed with PBS and 5% trichloroacetic acid before being harvested with 0.1 N NaOH. The amount of incorporated [3H]thymidine was determined by scintillation counting. Growth inhibition was calculated as the ratio of radioactivity with TGF-β1 treatment to radioactivity without TGF-β1 treatment.
Statistics.
Statistical analysis was performed using the 2-tailed Student’s t test unless otherwise indicated. All data are presented as mean ± SEM. P values less than 0.05 were considered to be significant.
Supplementary Material
Acknowledgments
Tissue microarray slides were provided by the Cooperative Breast Cancer Tissue Resource, which is funded by the National Cancer Institute. We thank M.W. Dewhirst for the 4T1-Luc cell line. We thank N. Glover and J. Fuller for technical assistance. These studies were supported by National Institutes of Health/National Cancer Institute grant R01-CA106307 (to G.C. Blobe), by a postdoctoral fellowship from the Susan G. Komen Breast Cancer Foundation (to M. Dong), and by predoctoral fellowships from the Department of Defense Breast Cancer Research Program (to K.C. Kirkbride and J.D. Lee).
Footnotes
Nonstandard abbreviations used: DCIS, ductal carcinoma in situ; ER, estrogen receptor; IDC, invasive ductal carcinoma; IHC, immunohistochemical; LOH, loss of heterozygosity; PCNA, proliferating cell nuclear antigen; sTβRIII, soluble TβRIII; TβRI, type I TGF-β receptor; TβRII, type II TGF-β receptor; TβRIII, type III TGF-β receptor; 4T1-Neo cells, 4T1 cells stably expressing the pcDNA-Neo expression vector; 4T1-TβRIII cells, 4T1 cells stably expressing TβRIII.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 117:206–217 (2007). doi:10.1172/JCI29293
Tam How, Kellye C. Kirkbride, Kelly J. Gordon, Jason D. Lee, and Nadine Hempel contributed equally to this work.
References
- 1.Massague J. TGF-beta signal transduction. Annu. Rev. Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 2.Jhappan C., et al. Targeting expression of a transforming growth factor beta 1 transgene to the pregnant mammary gland inhibits alveolar development and lactation. Embo J. 1993;12:1835–1845. doi: 10.1002/j.1460-2075.1993.tb05832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pierce D.F., et al. Inhibition of mammary duct development but not alveolar outgrowth during pregnancy in transgenic mice expressing active TGF-beta 1. Genes Dev. 1993;7:2308–2317. doi: 10.1101/gad.7.12a.2308. [DOI] [PubMed] [Google Scholar]
- 4.Pierce D.F., et al. Mammary tumor suppression by transforming growth factor beta 1 transgene expression. Proc. Natl. Acad. Sci. U. S. A. 1995;92:4254–4258. doi: 10.1073/pnas.92.10.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Decensi A., et al. Correlation between plasma transforming growth factor-beta 1 and second primary breast cancer in a chemoprevention trial. Eur. J. Cancer. 1998;34:999–1003. doi: 10.1016/s0959-8049(97)10170-8. [DOI] [PubMed] [Google Scholar]
- 6.Elliott R.L., Blobe G.C. Role of transforming growth factor Beta in human cancer. J. Clin. Oncol. 2005;23:2078–2093. doi: 10.1200/JCO.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 7.Walker R.A., Dearing S.J. Transforming growth factor beta 1 in ductal carcinoma in situ and invasive carcinomas of the breast. Eur. J. Cancer. 1992;28:641–644. doi: 10.1016/s0959-8049(05)80116-9. [DOI] [PubMed] [Google Scholar]
- 8.Dalal B.I., Keown P.A., Greenberg A.H. Immunocytochemical localization of secreted transforming growth factor-beta 1 to the advancing edges of primary tumors and to lymph node metastases of human mammary carcinoma. Am. J. Pathol. 1993;143:381–389. [PMC free article] [PubMed] [Google Scholar]
- 9.Gorsch S.M., Memoli V.A., Stukel T.A., Gold L.I., Arrick B.A. Immunohistochemical staining for transforming growth factor beta 1 associates with disease progression in human breast cancer. Cancer Res. 1992;52:6949–6952. [PubMed] [Google Scholar]
- 10.Ghellal A., et al. Prognostic significance of TGF beta 1 and TGF beta 3 in human breast carcinoma. Anticancer Res. 2000;20:4413–4418. [PubMed] [Google Scholar]
- 11.Xie W., et al. Alterations of Smad signaling in human breast carcinoma are associated with poor outcome: a tissue microarray study. Cancer Res. 2002;62:497–505. [PubMed] [Google Scholar]
- 12.Brown C.B., Boyer A.S., Runyan R.B., Barnett J.V. Requirement of type III TGF-beta receptor for endocardial cell transformation in the heart. Science. 1999;283:2080–2082. doi: 10.1126/science.283.5410.2080. [DOI] [PubMed] [Google Scholar]
- 13.Stenvers K.L., et al. Heart and liver defects and reduced transforming growth factor beta2 sensitivity in transforming growth factor beta type III receptor-deficient embryos. Mol. Cell. Biol. 2003;23:4371–4385. doi: 10.1128/MCB.23.12.4371-4385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blobe G.C., et al. Functional roles for the cytoplasmic domain of the type III transforming growth factor beta receptor in regulating transforming growth factor beta signaling. J. Biol. Chem. 2001;276:24627–24637. doi: 10.1074/jbc.M100188200. [DOI] [PubMed] [Google Scholar]
- 15.Chen W., et al. Beta-arrestin 2 mediates endocytosis of type III TGF-beta receptor and down-regulation of its signaling. Science. 2003;301:1394–1397. doi: 10.1126/science.1083195. [DOI] [PubMed] [Google Scholar]
- 16.Chen C., Wang X.F., Sun L. Expression of transforming growth factor beta (TGFbeta) type III receptor restores autocrine TGFbeta1 activity in human breast cancer MCF-7 cells. J. Biol. Chem. 1997;272:12862–12867. doi: 10.1074/jbc.272.19.12862. [DOI] [PubMed] [Google Scholar]
- 17.Sun L., Chen C. Expression of transforming growth factor beta type III receptor suppresses tumorigenicity of human breast cancer MDA-MB-231 cells. J. Biol. Chem. 1997;272:25367–25372. doi: 10.1074/jbc.272.40.25367. [DOI] [PubMed] [Google Scholar]
- 18.Parsons R., et al. Microsatellite instability and mutations of the transforming growth factor beta type II receptor gene in colorectal cancer. Cancer Res. 1995;55:5548–5550. [PubMed] [Google Scholar]
- 19.Hahn S.A., et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271:350–353. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- 20.Ragnarsson G., et al. Loss of heterozygosity at chromosome 1p in different solid human tumours: association with survival. Br. J. Cancer. 1999;79:1468–1474. doi: 10.1038/sj.bjc.6690234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borg A., Zhang Q.X., Olsson H., Wenngren E. Chromosome 1 alterations in breast cancer: allelic loss on 1p and 1q is related to lymphogenic metastases and poor prognosis. Genes Chromosomes Cancer. 1992;5:311–320. doi: 10.1002/gcc.2870050406. [DOI] [PubMed] [Google Scholar]
- 22.Bieche I., Khodja A., Lidereau R. Deletion mapping of chromosomal region 1p32-pter in primary breast cancer. Genes Chromosomes Cancer. 1999;24:255–263. doi: 10.1002/(sici)1098-2264(199903)24:3<255::aid-gcc11>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 23.Ji C., Chen Y., McCarthy T.L., Centrella M. Cloning the promoter for transforming growth factor-beta type III receptor. Basal and conditional expression in fetal rat osteoblasts. J. Biol. Chem. 1999;274:30487–30494. doi: 10.1074/jbc.274.43.30487. [DOI] [PubMed] [Google Scholar]
- 24.Pulaski B.A., Ostrand-Rosenberg S. Reduction of established spontaneous mammary carcinoma metastases following immunotherapy with major histocompatibility complex class II and B7.1 cell-based tumor vaccines. Cancer Res. 1998;58:1486–1493. [PubMed] [Google Scholar]
- 25.Heppner G.H., Miller F.R., Shekhar P.M. Nontransgenic models of breast cancer. Breast Cancer Res. 2000;2:331–334. doi: 10.1186/bcr77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blobe G.C., Liu X., Fang S.J., How T., Lodish H.F. A novel mechanism for regulating transforming growth factor beta (TGF-beta) signaling. Functional modulation of type III TGF-beta receptor expression through interaction with the PDZ domain protein, GIPC. J. Biol. Chem. 2001;276:39608–39617. doi: 10.1074/jbc.M106831200. [DOI] [PubMed] [Google Scholar]
- 27.Lopez-Casillas F., Payne H.M., Andres J.L., Massague J. Betaglycan can act as a dual modulator of TGF-beta access to signaling receptors: mapping of ligand binding and GAG attachment sites. J. Cell Biol. 1994;124:557–568. doi: 10.1083/jcb.124.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bandyopadhyay A., et al. Extracellular domain of TGFbeta type III receptor inhibits angiogenesis and tumor growth in human cancer cells. Oncogene. 2002;21:3541–3551. doi: 10.1038/sj.onc.1205439. [DOI] [PubMed] [Google Scholar]
- 29.Bandyopadhyay A., et al. Antitumor activity of a recombinant soluble betaglycan in human breast cancer xenograft. Cancer Res. 2002;62:4690–4695. [PubMed] [Google Scholar]
- 30.Andres J.L., Stanley K., Cheifetz S., Massague J. Membrane-anchored and soluble forms of betaglycan, a polymorphic proteoglycan that binds transforming growth factor-beta. J. Cell Biol. 1989;109:3137–3145. doi: 10.1083/jcb.109.6.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Philip A., Hannah R., O’Connor-McCourt M. Ectodomain cleavage and shedding of the type III transforming growth factor-beta receptor in lung membranes effect of temperature, ligand binding and membrane solubilization. Eur. J. Biochem. 1999;261:618–628. doi: 10.1046/j.1432-1327.1999.00298.x. [DOI] [PubMed] [Google Scholar]
- 32.Cheung H.K., Mei J., Xu R.J. Quantification of soluble betaglycan in porcine milk. Asia Pac. J. Clin. Nutr. 2003;12(Suppl.):S61. [Google Scholar]
- 33.van’t Veer L.J., et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 34.Sorlie T., et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl. Acad. Sci. U. S. A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma X.J., et al. A two-gene expression ratio predicts clinical outcome in breast cancer patients treated with tamoxifen. Cancer Cell. 2004;5:607–616. doi: 10.1016/j.ccr.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y., et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365:671–679. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- 37.Moody S.E., et al. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005;8:197–209. doi: 10.1016/j.ccr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 38.Kamangar F., Dores G.M., Anderson W.F. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 39.Tang B., et al. TGF-β switches from tumor suppressor to prometastatic factor in a model of breast cancer progression. J. Clin. Invest. 2003;112:1116–1124. doi: 10.1172/JCI200318899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siegel P.M., Shu W., Cardiff R.D., Muller W.J., Massague J. Transforming growth factor beta signaling impairs Neu-induced mammary tumorigenesis while promoting pulmonary metastasis. Proc. Natl. Acad. Sci. U. S. A. 2003;100:8430–8435. doi: 10.1073/pnas.0932636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muraoka-Cook R.S., et al. Conditional overexpression of active transforming growth factor beta1 in vivo accelerates metastases of transgenic mammary tumors. Cancer Res. 2004;64:9002–9011. doi: 10.1158/0008-5472.CAN-04-2111. [DOI] [PubMed] [Google Scholar]
- 42.Lopez-Casillas F., Wrana J.L., Massague J. Betaglycan presents ligand to the TGF beta signaling receptor. Cell. 1993;73:1435–1444. doi: 10.1016/0092-8674(93)90368-z. [DOI] [PubMed] [Google Scholar]
- 43.Lei X., Bandyopadhyay A., Le T., Sun L. Autocrine TGFbeta supports growth and survival of human breast cancer MDA-MB-231 cells. Oncogene. 2002;21:7514–7523. doi: 10.1038/sj.onc.1205966. [DOI] [PubMed] [Google Scholar]
- 44.Huynh H., Alpert L., Pollak M. Silencing of the mammary-derived growth inhibitor (MDGI) gene in breast neoplasms is associated with epigenetic changes. Cancer Res. 1996;56:4865–4870. [PubMed] [Google Scholar]
- 45.Ahomadegbe J.C., et al. Loss of heterozygosity, allele silencing and decreased expression of p73 gene in breast cancers: prevalence of alterations in inflammatory breast cancers. Oncogene. 2000;19:5413–5418. doi: 10.1038/sj.onc.1203914. [DOI] [PubMed] [Google Scholar]
- 46.Tang B., et al. Transforming growth factor-beta1 is a new form of tumor suppressor with true haploid insufficiency. Nat. Med. 1998;4:802–807. doi: 10.1038/nm0798-802. [DOI] [PubMed] [Google Scholar]
- 47.Copland J.A., et al. Genomic profiling identifies alterations in TGFbeta signaling through loss of TGFbeta receptor expression in human renal cell carcinogenesis and progression. Oncogene. 2003;22:8053–8062. doi: 10.1038/sj.onc.1206835. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.