Abstract
This experiment assessed the effects of d-amphetamine and ethanol on reinforced variable and repetitive key-peck sequences in pigeons. Pigeons responded on two keys under a multiple schedule of Repeat and Vary components. In the Repeat component, completion of a target sequence of right, right, left, left resulted in food. In the Vary component, 4-peck sequences differing from the previous 10 produced food. d-Amphetamine (0.1–3.0 mg/kg, i.m.) was administered in two separate phases, separated by ethanol administration (1.0–2.0 g/kg, i.g.). Under control conditions, measures of variability were high in the Vary component, and lower in the Repeat component. Following administration of the highest dose of d-amphetamine, but not ethanol, response rates decreased in both components. d-Amphetamine and ethanol tended to increase overall sequence variability in the Repeat component, and had less of an effect in the Vary component. Performance in the Repeat component during Phase 2 of d-amphetamine administration was more disrupted than during Phase 1. Measures of variability and repetition based on shifts in the relative frequency distributions of the 16 possible key-peck sequences differed from those based on the overall measure of variability, highlighting the importance of considering both molar and molecular measures when assessing the effects of drugs on reinforced variability and repetition. In addition, the shifts in the relative frequency distribution of response sequences suggest that d-amphetamine produced decrements in repeat performance by decreasing discriminative control within response sequences, whereas ethanol decreased repeat performance by decreasing discriminability between components as well as discriminative control within response sequences.
Keywords: behavioral variability, repetition, response sequences, stereotypy, d-amphetamine, ethanol, key peck, pigeons
A large body of evidence suggests that behavioral variability can be controlled by its consequences similarly to other aspects of responding (e.g., rate, topography, force) and may therefore be an operant dimension of behavior (see Neuringer, 2002, 2004 for review). Control of variability by reinforcement contingencies has been demonstrated in rats (e.g., Bryant & Church, 1974), pigeons (e.g., Blough, 1966; Page & Neuringer, 1985), dolphins (Pryor, Haag, & O'Reilly, 1969), and humans (e.g., Neuringer, 1986). Furthermore, the level of variability depends on the reinforcement contingencies; when a high level of variability is required for reinforcement, a high level is emitted, and vice versa (e.g., Machado, 1989). The finding that variable and repetitive behavior can come under the control of discriminative stimuli further supports the suggestion that variability is an operant dimension of behavior (e.g., Denney & Neuringer, 1998; Page & Neuringer, 1985).
One area of research interest is the effect of various drugs on variable and repetitive behavior. For example, McElroy and Neuringer (1990) assessed the effects of alcohol on repetitive and variable behavior in two groups of rats emitting response sequences on left (L) and right (R) levers. For one group (Repeat), emitting a target sequence of LLRR resulted in food. For the other group (Vary), sequences of lever presses only were reinforced if they differed from the previous five sequences (lag 5). Ethanol (0.75 g/kg and 2.0 g/kg, i.p.) profoundly impaired performance in the Repeat group by increasing variability, but had little effect on the quality of performance in the Vary group. Cohen, Neuringer, and Rhodes (1990) replicated this result with rats in a within-subject design.
The present study assessed the effects of d-amphetamine on reinforced variation and repetition of key-peck sequences in pigeons. Few studies have assessed the effects of d-amphetamine on this type of behavior. In two experiments, Mook and Neuringer (1994) assessed the effects of d-amphetamine on variable and repetitive behavior under different contingencies in Spontaneously Hypertensive and Wistar-Kyoto rats. Across experiments, d-amphetamine (0.75 mg/kg, i.p., for 10 consecutive days) had no systematic effects on the measure of variability. These results, combined with the fact that the effects of only one dose of d-amphetamine were examined, make characterization of the effects of d-amphetamine on behavior maintained by variable and repetitive contingencies difficult. Accordingly, one goal of the present study was to examine the full dose-effect curve of the effects of d-amphetamine on reinforced variability and repetition.
Although the effects of d-amphetamine on reinforced variability and repetition are not well established, d-amphetamine has been shown to have certain characteristic behavioral effects. One common effect following administration of relatively high doses of d-amphetamine is the development of robust locomotor stereotypies. d-Amphetamine-induced stereotypies have been reported in rats, mice, guinea pigs, cats, and monkeys (Randrup & Munkvad, 1967). The form of the stereotypy varies between species and subjects, but often includes biting, sniffing, and licking the cage floor or wall, repetitive head movements, and pacing. In the current experiment, stereotypy is a measured dimension of the emitted key-peck sequences. Aside from this difference in the measure of stereotypy, another difference between traditional preparations measuring behavioral stereotypy and the current procedure is that in contrast to other experimental preparations, in which there are no experimenter-defined consequences for variation or repetition, behaving variably or repetitively under designated stimulus conditions in the current procedure was required for reinforcement. This contingency may affect the way in which d-amphetamine-induced changes in behavior are manifest (see also Lyon & Randrup, 1972). If d-amphetamine produces systematic repetition in the current procedure, this result might indicate similarities in the underlying mechanisms of drug-induced stereotypies under different experimental preparations. Accordingly, another goal of the present experiment was to assess whether d-amphetamine would produce increased repetition of specific key-peck sequences.
To conduct the assessment, we trained pigeons on a multiple schedule of Repeat and Vary components. In one component (Vary) four-peck sequences resulted in food if the present sequence differed from the last ten (lag 10). In the other component (Repeat), food was presented for a four-peck target sequence, RRLL. In this preparation, increased stereotypy could be evidenced by performance decrements in the Vary component following d-amphetamine administration, whereas performance in the Repeat component would be unaffected or possibly improved if the target sequence became more frequent. If d-amphetamine produced increases in the frequency of another sequence, however, performance in the Repeat component could be negatively affected.
After the initial d-amphetamine administration, in a separate later condition we examined the effects of ethanol on the behavior maintained by the multiple Vary–Repeat schedule. This assessment was conducted to determine whether the effects of ethanol reported by Cohen et al. (1990) could be replicated across species. Following the ethanol condition, d-amphetamine administration was repeated.
Method
Subjects
Four White Carneau pigeons that had prior experimental histories with a variety of related procedures served as subjects. All pigeons previously had been exposed to d-amphetamine 7 months prior. Pigeons were maintained at 80% ± 15 g of their free-feeding weight by postsession feeding as needed. The pigeons' 80% of free-feeding weights ranged from 368 g to 478 g. Between sessions, pigeons were individually housed in a temperature-controlled colony under a 12∶12 hr light/dark cycle and had free access to water. This research was approved by the Utah State University Institutional Animal Care and Use Committee.
Apparatus
Four Lehigh Valley Electronics sound-attenuating chambers were used. Chambers were constructed of painted metal with aluminum front panels. The chambers measured 30 cm across, 35 cm deep, and 35 cm high. Each front panel had three translucent plastic keys that could be lit from behind with white or red light and required a force of at least 0.10 N to record a response. Keys were 2.5 cm in diameter and 24 cm from the floor. The left and right side keys were located 6.7 cm (to key center) from their respective side walls. A lamp (28 V, 1.1 W) mounted 4.5 cm above the center key served as a houselight. A rectangular opening 8.5 cm below the center key provided access to a solenoid-operated hopper filled with pelleted pigeon chow. During hopper presentations, the opening was lit with white light and the houselight and keylights were extinguished. White noise and chamber ventilation fans masked extraneous noise. Contingencies were programmed and data collected by a microcomputer using Med Associates® interfacing and software.
Procedure
Experimental sessions were conducted daily at approximately the same time. Due to the subjects' experimental history, no hopper or key-peck training was necessary. To allow time for drug absorption prior to selected sessions, each session was preceded by a 10-min blackout. Following the blackout, the side keys were lit to begin the session.
Pretraining
During initial training, only the repeat component was presented (cf. Cohen et al., 1990). During this component, the side keys were lit red and the two-response sequence RR produced 3-s access to food. In this and the following two pretraining steps, the first incorrect response in the sequence resulted in a 3-s blackout, after which the pigeon was required to make the target sequence from the beginning. In pretraining step two, the three-response sequence RRL produced food. Next, only the four-response sequence RRLL produced food. In the final Repeat pretraining step, the sequence RRLL produced food and all other sequences resulted in the houselight flashing for 3 s. In this phase, unlike the first three phases of initial training, incorrect sequences were not terminated at the point of error, but were consequated after the four-response sequence was completed. The consequence for incorrect sequences in this final training step was a flashing houselight rather than a blackout to distinguish it from the intercomponent interval (ICI) blackout in the final multiple schedule (described below). In all phases of Repeat pretraining, the next step was implemented when percent reinforced sequences reached at least 80%. In this and all subsequent conditions of the experiment, each response in a sequence was required to be at least 0.5-s after the previous response. Specifically, each key peck in a sequence resulted in the two side keys being darkened for 0.5 s. Pecks to the keys during this period reset the interval.
Multiple schedule
When performance on this final training procedure was judged stable by visual inspection (24–27 sessions, across pigeons), the Vary component was added so that a two-component multiple schedule was in effect. The components were separated by a 10-s ICI during which the chamber was dark and responses had no programmed consequences. In the Vary component, the side keys were lit white and a four-response sequence that differed from the previous six (Lag 6) was required for food. All other response sequences resulted in the houselight flashing for 3 s. The last six four-response sequences from the previous session were used for comparison at the beginning of each session in the Vary component. The contingencies in the Repeat component were the same as in the final step of pretraining.
The final procedure consisted of a multiple schedule with alternating Vary and Repeat components as described above with the exception that a lag 10 requirement was in effect in the Vary component. The last ten four-response sequences from the previous session were used for comparison at the beginning of each session. Prior to the change in the lag requirement, the percentage of reinforced sequences was higher in the Vary than the Repeat component. This difference could have contributed to greater resistance to d-amphetamine-induced changes in the Vary component, which would be confounded with the typical finding that variable behavior is more persistent (see e.g., Doughty & Lattal, 2001). Thus, the lag was increased to decrease the percentage of reinforced sequences in the Vary component.
Each component was presented four times in a session and ended after five reinforcers were earned or 10 min, whichever occurred first. At the beginning of each daily session, the first component was selected at random, after which the components alternated. Sessions terminated after 40 reinforcers or 200 sequences (see Measures), whichever occurred first. Sessions usually ended after 40 reinforcers in about 40 min, including the blackout at the beginning.
Drug Administration
d-Amphetamine administration (Phase 1)
Assessment of the effects of d-amphetamine began after response rates and sequence variability (as measured by the U-value statistic, see Measures below) in both components were judged stable by visual inspection over the last 10 sessions. Behavior of all pigeons was judged stable after 44 baseline sessions. d-Amphetamine sulfate (Sigma-Aldrich, St. Louis, MO) was dissolved in a 0.9% NaCl (saline) solution and administered in a volume of 1.0 ml/kg of the 80% free-feeding weight. Drug and vehicle were administered via i.m. injections into the breast of the pigeon immediately before it was placed into the experimental chamber. Prior to drug administration, several preliminary saline injections were given to accustom the subjects to the injection procedure. Results from these preliminary injections were excluded from the analyses.
Following the preliminary injections, d-amphetamine and vehicle were given in the following order: 3.0 mg/kg, 1.0 mg/kg, saline, 0.3 mg/kg, 0.1 mg/kg, and saline. The effects of saline and each drug dose were determined in this order three times, followed by additional replications of the 3.0 and 1.0 mg/kg doses for P98 and P99. Tests were separated by at least three consecutive baseline sessions. The session immediately preceding a d-amphetamine or saline test session was designated a control session. Data for P154 in all analyses reflect only 2 determinations of 3.0 mg/kg d-amphetamine as this pigeon did not respond during the third determination of this dose. Each pigeon also received one dose of 5.6 mg/kg d-amphetamine. Data from this session are not included as none of the pigeons pecked the keys following this dose.
Ethanol administration
Following Phase 1 of d-amphetamine administration, ethanol administration began. At least six sessions (6–10 sessions, across pigeons) followed the last administration of d-amphetamine before ethanol was administered. A 95% v/v ethanol solution was diluted with water to make a 20% ethanol solution and administered intragastrically (i.e., by gavage) 20 min before the session. A 10 ml syringe was connected to 12 cm of flexible tubing which was passed down the esophagus to deliver the ethanol. Prior to ethanol administration, one water (vehicle) gavage was administered to accustom the subjects to the administration procedure. Results from this administration were excluded from the analyses.
Following the water gavage, ethanol and water were given in the following order: 2.0 g/kg, 1.0 g/kg, 1.5 g/kg, and water. The volume of the 20% ethanol solution was adjusted for each pigeon to produce each dose (range 2.3–6.0 ml, across pigeons). The effects of ethanol and water were determined in this order four times. Tests were separated by at least three consecutive baseline sessions prior to which no ethanol was administered. The session immediately preceding an ethanol or water test session was designated a control session.
d-Amphetamine administration (Phase 2)
Following completion of ethanol administration, the doses of d-amphetamine used in the first condition were administered twice in the manner described above for Phase 1. At least 10 sessions (13–19 sessions, across pigeons) followed the last administration of ethanol before d-amphetamine was again administered. Data from P154 are absent from the ethanol condition and Phase 2 of d-amphetamine administration because this pigeon's performance deteriorated for unknown reasons following Phase 1 of d-amphetamine administration, and baseline was never reestablished. Consequently, P154 was removed from the study before the ethanol condition. Data from P98 and P99 for the 3.0 mg/kg dose in all analyses of Phase 2 of d-amphetamine administration reflect only one value because the pigeons pecked the keys following only one determination of this dose.
Measures
To obtain a measure of overall response rates, we calculated sequences per minute. A sequence was defined as four responses and the scheduled consequence (food presentation for correct sequences or flashing houselight for incorrect sequences). Sequences/min was calculated by dividing the number of sequences completed in a component by the total number of min spent in the component separately for the Vary and Repeat components.
We calculated U value as a measure of variability. The U value (Miller & Frick, 1949; Page & Neuringer, 1985) is an index of overall sequence variability and is calculated as
where RFi represents the relative frequency of each of the 16 possible key-peck sequences. If each of the 16 possible sequences occurred an equal number of times throughout the session, the resulting U value would be 1.0, indicating maximum variability. If only one of the sequences occurred during the session, the U value would be 0.0. Data for sequences/min and U value are expressed relative to control performance. Percent control sequences/min and U value were calculated by dividing values by their corresponding mean control values.
To examine the effects of d-amphetamine and ethanol on the relative frequency of specific sequences, we calculated the frequency of each of the 16 possible key-peck sequences. Relative frequency was calculated by dividing the number of occurrences of each sequence by the total number of completed sequences in the session separately for the Vary and Repeat components. Mean relative frequencies for control sessions and from each dose of d-amphetamine and ethanol were obtained for each pigeon.
We also calculated the percentage of reinforced sequences by dividing the number of sequences that produced food by the total number of sequences during the session separately for the Vary and Repeat components.
Results
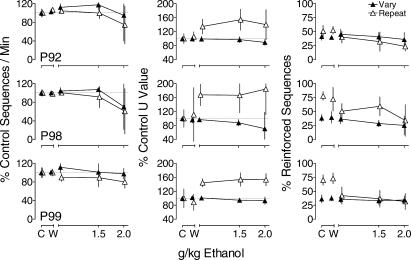
During control sessions (averaged over all sessions immediately preceding each dose of d-amphetamine and the vehicle), sequences/min was similar during the Vary and Repeat components for all pigeons (Table 1). Figure 1 shows that the highest dose of d-amphetamine decreased sequences/min (left column) in both components with the exception of P92 in the Vary component, whereas saline administration had no systematic effects. U values during control sessions throughout the study were relatively high during the Vary component and were lower during the Repeat component for all pigeons (Table 1), indicating that overall sequence variability was higher in the Vary component than the Repeat component. The center column of Figure 1 shows that saline had no systematic effect on U value in either component. Administration of d-amphetamine had little effect on U value during the Vary component, except for a decrease at the highest dose for P98. Sequence variability during the Repeat component was more affected; the highest dose of d-amphetamine increased U value for all pigeons, with increases to at least 120% of control for 3 of the 4 pigeons. During control sessions throughout the study, the percentages of reinforced sequences (e.g., Figure 1, right column) were higher during the Repeat component than during the Vary component. The right column shows that saline had no systematic effects on the percentage of reinforced sequences. During the Repeat component, the highest dose of d-amphetamine decreased the percentage of reinforced sequences for 2 of 4 pigeons (P92 and P98). In the Vary component, the percentage of reinforced sequences was relatively unaffected by d-amphetamine.
Table 1. Mean sequences per min and U value (SD in parentheses) in the Vary and Repeat components during control sessions for all pigeons in all conditions of the experiment.
| Subject | Component |
d-Amphetamine (1st) |
Ethanol |
d-Amphetamine (2nd) |
|||
| Seqs/Min | U value | Seqs/Min | U value | Seqs/Min | U value | ||
| P92 | Vary | 7.39 (0.41) | 0.83 (0.04) | 7.00 (0.60) | 0.86 (0.03) | 7.53 (0.36) | 0.84 (0.02) |
| Repeat | 8.03 (0.44) | 0.37 (0.06) | 7.68 (0.55) | 0.36 (0.05) | 7.98 (0.49) | 0.39 (0.05) | |
| P98 | Vary | 8.09 (0.47) | 0.83 (0.04) | 8.17 (0.53) | 0.84 (0.05) | 7.68 (0.67) | 0.86 (0.05) |
| Repeat | 7.04 (0.47) | 0.35 (0.10) | 7.80 (0.51) | 0.24 (0.06) | 7.82 (0.47) | 0.31 (0.09) | |
| P99 | Vary | 8.65 (0.56) | 0.83 (0.03) | 7.63 (0.81) | 0.82 (0.04) | 8.36 (0.21) | 0.78 (0.05) |
| Repeat | 7.79 (0.52) | 0.38 (0.06) | 7.16 (0.74) | 0.34 (0.09) | 6.76 (0.71) | 0.27 (0.05) | |
| P154 | Vary | 6.89 (0.43) | 0.70 (0.03) | — | — | — | — |
| Repeat | 5.90 (0.40) | 0.46 (0.05) | — | — | — | — | |
Fig 1. Mean sequences/min (left column), U value (center column; see text for calculation), and percent reinforced sequences (right column) as a function of d-amphetamine for each pigeon during the Vary (filled triangles) and Repeat (unfilled triangles) components during the first three determinations of the effects of d-amphetamine (Phase 1).
Data for sequences/min and U value are expressed as percent control. Unconnected points show means for all control (C) and saline (S) sessions. Lines connect points showing means across doses of d-amphetamine. Dotted lines indicate 100% of control performance. Vertical bars indicate one standard deviation above and below the mean. In some cases, the variability around a point is obscured by the point. Data points for the Vary and Repeat components are offset slightly on the x-axis for clarity.
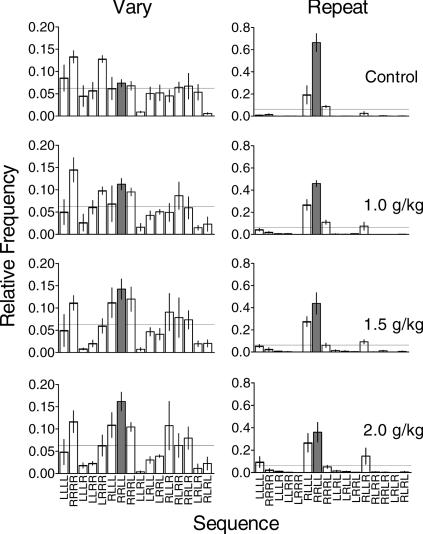
Figure 2 shows that during control sessions in the Vary component (top left panel), relative sequence frequency (averaged across pigeons) was more evenly distributed among the 16 possible sequences as compared to the sequence distribution in the Repeat component (top right panel). Individual data are presented in the Appendix.1 Sequences that required zero or one changeover from one response key to the other occurred more than those that required two or more changeovers. d-Amphetamine (lower left panels) tended to increase the relative frequency of the sequences RLLR, RLRR, and RRLR, and LLLL at the highest dose. Aside from these increases, the relative frequency distributions under d-amphetamine in the Vary component remained similar to the control distributions. During control sessions in the Repeat component (top right panel) the most frequently emitted sequences were the target sequence (RRLL) and RLLL. Aside from these sequences, only RRRL and RLLR occurred relatively frequently. The highest dose of d-amphetamine decreased the relative frequency of the target sequence and increased the relative frequency of other sequences (RRRR, LLLL) somewhat.
Fig 2. Mean relative frequency of each possible four-response sequence averaged across pigeons in the Vary (left column) and Repeat (right column) components during control sessions and across doses of d-amphetamine.
Dashed lines indicate the relative frequency of each possible sequence predicted by chance (.0625). All sequences are plotted on the x-axis from left to right in order of the number of key changeovers required with the leftmost sequence requiring no changeovers and the rightmost sequence requiring the greatest number of changeovers (cf. Doughty & Lattal, 2001). Vertical bars indicate one standard error above and below the mean. For clarity, y-axes are scaled differently for the Vary and Repeat components. In all panels, the target sequence from the Repeat component (RRLL) is shaded for ease of inspection.
During control sessions (averaged over all sessions immediately preceding each dose of ethanol and the vehicle) in the ethanol-administration condition, pigeons completed a similar number of sequences/min during both the Vary and Repeat components (Table 1). The left column of Figure 3 shows that water and ethanol administration had relatively little effect on the sequences/min. Although there were some decreases evident at the highest dose of ethanol for all pigeons, this dose also produced a substantial increase in variability across determinations for 2 of the 3 pigeons. The center column of Figure 3 shows that water administration had no systematic effect on U values. Ethanol produced large increases in U value during the Repeat component at all doses (to nearly 160% of control for all pigeons), but had little effect on U value in the Vary component. The right column of Figure 3 shows that water administration had little effect on the percentage of reinforced sequences in either component for all pigeons. Ethanol decreased the percentage of reinforced sequences during the Repeat component, but had little effect on this measure in the Vary component.
Fig 3. Mean sequences/min (left column), U value (center column), and percent reinforced sequences (right column) as a function of ethanol.
Unconnected points show means for all control (C) and water (W) sessions. Other details as in Figure 1.
Figure 4 shows that during control sessions in the Vary component (top left panel), mean relative sequence frequency was fairly evenly distributed among the 16 possible sequences. Individual data are presented in the Appendix. Ethanol increased the relative frequency of the sequences RRLL, RRRL, RLLL, and decreased the relative frequency of single-switch sequences beginning with L (i.e., LLLR, LLRR, LRRR). In addition, the frequency of RLLR was somewhat increased (left panels). During control sessions in the Repeat component (top right panel), the target sequence occurred most frequently, with RLLL and RRRL also occurring at relative frequencies above chance. Aside from these sequences, few other sequences occurred. Ethanol decreased the relative frequency of the target sequence and increased the relative frequency of other sequences, in particular LLLL and RLLR (right panels).
Fig 4. Mean relative frequency of each possible four-response sequence in the Vary (left column) and Repeat (right column) components during control sessions and across all doses of ethanol.
Other details as in Figure 2.
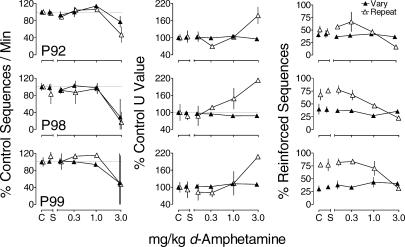
During Phase 2 of d-amphetamine administration, as in Phase 1, control sequences/min (calculated as in Phase 1) was similar during both components (Table 1). The left column of Figure 5 shows that the highest dose of d-amphetamine decreased sequences/min and increased variability across determinations for all pigeons, whereas saline administration had no systematic effects on sequences/min. The center column of Figure 5 shows that saline had negligible effects on U values in both components. The highest dose of d-amphetamine increased U values in the Repeat component to greater than 165% of control for all pigeons, while U value in the Vary component was unaffected. The highest dose of d-amphetamine decreased the percentage of reinforced sequences during the Repeat component (right column), while the percentage of reinforced sequences in the Vary component remained similar to that observed during control sessions across all doses of d-amphetamine. Saline had relatively little effect on the percentage of reinforced sequences.
Fig 5. Mean sequences/min (left column), U value (center column), and percent reinforced sequences (right column) as a function of d-amphetamine for each pigeon during the fourth and fifth determinations of the effects of d-amphetamine (Phase 2).
Other details as in Figure 1.
Figure 6 shows that sequence distribution during control sessions in both components in Phase 2 was similar to that in Phase 1 of d-amphetamine administration. In the Vary component (left column) d-amphetamine increased the relative frequency of sequences requiring one or two changeovers. In addition, the relative frequency of the sequence RRRR was increased by the highest dose. In the Repeat component (right column) the highest dose of d-amphetamine decreased the relative frequency of the target sequence and increased the relative frequency of other sequences somewhat, particularly RRRR and RLLR.
Fig 6. Mean relative frequency of each possible four-response sequence in the Vary (left column) and Repeat (right column) components during control sessions and across doses of d-amphetamine during the fourth and fifth determinations (Phase 2).
Other details as in Figure 2.
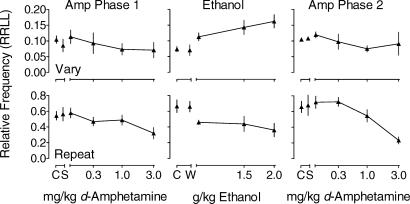
Figure 7 focuses on changes in the relative frequency of the sequence RRLL and shows that in the Repeat component, the relative frequency of RRLL (the target sequence in this component) was substantially higher than in the Vary component during control sessions in both Phases 1 and 2 of d-amphetamine administration. In addition, during Phase 2 in the Repeat component, the control relative frequency of RRLL was higher than during Phase 1. Saline administration had no effect on the relative frequency of the target sequence. d-Amphetamine decreased the relative frequency of this sequence in both Phase 1 and Phase 2. In the Vary component the relative frequency of RRLL was near 0.10 during control sessions in both Phase 1 and Phase 2 of d-amphetamine administration, and neither saline nor d-amphetamine had substantial effects on the relative frequency of RRLL in either phase.
Fig 7. Mean relative frequency of the sequence RRLL averaged across pigeons in the Vary (top row) and Repeat (bottom row) components as a function of d-amphetamine during Phase 1 (left column) and Phase 2 (right column) of d-amphetamine administration and as a function of ethanol (center column).
The data are taken from the Appendix (with the exception of saline). In all panels, unconnected data points show means for all control and vehicle sessions. Lines connect data points showing means across doses of d-amphetamine and ethanol. Vertical lines indicate one standard error above and below the mean.
During ethanol administration (Figure 7, center column), the relative frequency of RRLL during control sessions in the Vary component was about 0.07. Ethanol dose-dependently increased the relative frequency of RRLL. In the Repeat component under control conditions, the relative frequency of RRLL was higher, around 0.70. Ethanol dose-dependently decreased the relative frequency of this sequence, with vehicle having no effect in either component.
Discussion
During control sessions in all conditions of the experiment, sequences/min during both the Vary and Repeat components were similar. The contingencies on variability were effective, as evidenced by higher U values in the Vary component. These results replicate those from previous studies (e.g., Cohen et al., 1990; Page & Neuringer, 1985). d-Amphetamine and ethanol had little effect on sequences/min, except for some decreases at the highest doses tested. The major findings were in the effects of these drugs on sequence variability. First, both d-amphetamine and ethanol increased the overall measure of variability, U value, in the Repeat component but had little effect on this measure in the Vary component. These effects of ethanol replicate those that Cohen et al. (1990) obtained with rats. Second, the increase in variability as indexed by U value during Phase 2 of d-amphetamine administration was considerably larger than the increase during Phase 1. Third, administration of both drugs changed the relative frequency of particular response sequences, with more systematic changes occurring under ethanol. We consider each of these results below.
Both ethanol and d-amphetamine increased U value in the Repeat component, indicating an increase in overall variability, but had little effect on U value in the Vary component. We obtained the same results when we examined the proportion of the 16 possible key-peck sequences made per session (analysis not shown; raw data in the Appendix). Inspection of the relative frequency distributions under both d-amphetamine and ethanol (Figures 2, 4, and 6), however, shows that there also were shifts in the relative frequencies of particular response sequences. Particularly apparent under d-amphetamine is the large decrease in the relative frequency of the target sequence in the Repeat component (RRLL). In the Vary component, d-amphetamine increased the relative frequency of LLLL (Phase 1) and RRRR (Phase 2). In the Repeat component, ethanol decreased the relative frequency of RRLL and increased the relative frequency of RLLR. In addition, ethanol also increased the relative frequency of specific sequences in the Vary component (RRLL, RLLL, RRRL; see discussion below). Thus, although both d-amphetamine and ethanol increased overall variability in the Repeat component and had less of an effect in the Vary component as indexed by U value, inspection of the relative frequency distributions suggests that both drugs increased the relative frequency of a few sequences in both components, with more systematic increases occurring under ethanol. U value does not capture these changes due to the molar nature of the U-value measure, which renders it relatively insensitive to systematic changes in the molecular structure of behavior (see Machado, 1997).
Both phases of d-amphetamine administration increased U value in the Repeat component and had little effect on this measure in the Vary component. The magnitude of the effects, however, differed between these phases. The maximum increase averaged across pigeons (excluding P154 as this pigeon did not participate in Phase 2) in Phase 1 was 124.4 percent of control, compared to an increase of 199.5 percent of control in Phase 2. One potential explanation for this result is that the intervening ethanol-administration condition somehow influenced the subsequent effects of d-amphetamine in Phase 2. Manley and Little (1997) provide some evidence that a history of dietary ethanol administration can sensitize locomotor behavior to the subsequent effects of d-amphetamine. In one condition, they exposed mice to chronic ethanol administration (about 28 g/kg/day) by liquid diet for 3 weeks and then subsequently measured locomotor activity under d-amphetamine. Significant differences emerged between groups on day 10 of d-amphetamine treatment. In another condition, mice were given 7 days of ethanol exposure in which they drank about the same amount of ethanol per day as in the previous condition. No differences in locomotor activity were found between the control and ethanol groups when they then were tested with d-amphetamine. These results show that under some conditions, ethanol can sensitize behavior to the subsequent effects of d-amphetamine, but under other conditions it does not. The many differences between Manley and Little and the present study, as well as the lack of a no-ethanol control group, indicate the need for further research before conclusions regarding any role of ethanol in the change in effects of d-amphetamine across phases in the present study can be made.
Both d-amphetamine and ethanol decreased the relative frequency of the target sequence (RRLL) in the Repeat component, resulting in fewer reinforced sequences under this contingency. Similar results have been reported when variable and repetitive behavior is disrupted by other manipulations. For example, Neuringer (1991) assessed the effects of increasing the minimum time between responses in a sequence on reinforced variation and repetition. The percentage of reinforced sequences in the Repeat group fell as the required time between responses increased, but no such performance decrements were observed in the Vary group. Based on these results, Neuringer concluded that effective Repeat performance depends on remembering previous responses in a sequence. Conversely, he suggested that behavior that effectively meets Vary contingencies is relatively independent of, and may actually be hindered by, remembering previous responses in a sequence. Other results are amenable to this interpretation. For example, Page and Neuringer (1985) showed that pigeons' response sequences became more variable when the lag requirement was increased from 5 to 50 (Exp. 3), as well as when the number of required responses in a sequence was increased from four to eight (Exp. 4). Thus, one interpretation is that accurate Repeat performance requires remembering previous responses in a sequence, whereas accurate Vary performance does not (see Neuringer, 2004; Page & Neuringer, 1985, for discussion).
The present results are consistent with this interpretation. d-Amphetamine and ethanol increased variability and decreased the percentage of reinforced sequences during the Repeat component, but both drugs had little effect on overall sequence variability or percentage of reinforced sequences during the Vary component. d-Amphetamine and ethanol have both been reported to decrease measures of remembering in other experimental preparations (e.g., Baron, Wright, & Wenger, 1998; Givens, 1995; Givens & McMahon, 1997; Hoffman & Matthews, 2001; Schoblock, Maisonneuve, & Glick, 2003). Comparison of the present results to the results from these studies, however, must be made with caution in light of procedural differences. Future experiments could employ procedures and methods that can explicitly assess the role of remembering in performance under contingencies that produce variable and repetitive behavior.
An alternative interpretation is that repetitive behavior simply may be more easily disrupted than variable behavior. Selective disruption of repetition has also been reported when reinforced variable and repetitive behavior is disrupted by nonpharmacological means, such as prefeeding and within-session response-independent food presentations (Doughty & Lattal, 2001), extinction (Neuringer, Kornell, & Olufs, 2001), increasing minimum interresponse times between responses in a sequence (Neuringer, 1991), and inserting a delay between correct sequences and the consequent reinforcer (Odum, Ward, Barnes, & Burke, in press). Together, these results suggest that the selective disruption of repetition by drugs in the present study may not be related to pharmacological effects on remembering per se, but may reflect a more general susceptibility of repetition to disruption. Future research may elucidate the mechanism responsible for this common outcome.
The effects of d-amphetamine on relative sequence frequency largely were specific to the Repeat component. d-Amphetamine decreased the relative frequency of the target sequence RRLL in this component, suggesting decreased discriminative control of sequence responses by previous responses (which could be conceptualized as remembering). In addition, the relative frequency of RRRR may have increased during Phase 2. In the Vary component, although there were some increases in the relative frequency of particular sequences (LLLL and RRRR), the relative frequency distributions under d-amphetamine otherwise remained similar to those under control conditions.
Ethanol also decreased the relative frequency of the target sequence in the Repeat component and in addition increased the relative frequency of other sequences in both components. Whereas the effects of d-amphetamine on relative frequencies suggest decreased discriminative control of sequence responses by previous responses in the Repeat component, the sequences that increased in both the Vary and Repeat components under ethanol suggest an interaction of the effects of ethanol with two types of stimulus control. In the Repeat component, the large decrease in the relative frequency of the target sequence (Figure 7) and the increase in the relative frequency of RLLR, the sequence which, when repeated, makes up the target sequence (i.e., RLLRRLLRRLLR) suggest a decrease in discriminative control of sequence responses by previous responses. During the Vary component, the large increase in the relative frequency of RRLL (the target sequence in the Repeat component), together with the increase in the relative frequency of RLLL and RRRL (sequences that are similar to RRLL, but with an early or late right to left switch), suggests a decrease of discriminability between components as well as decreased discriminative control of subsequent sequence responses by previous responses within components. Thus, whereas d-amphetamine may have produced decrements in within-component stimulus control (i.e., discriminative control of sequence responses by previous responses) in the Repeat component, ethanol may have produced decrements in both within-component and between-component (Vary vs. Repeat) stimulus control. Support for this interpretation may be found in reports that ethanol decreases measures of stimulus control in conditional discrimination experimental preparations (e.g., Melia & Elhers, 1989; Roache, Spiga, & Burt, 1993).
The results of the present experiment are relevant to several other areas of investigation. The ethanol-induced response-sequence repetition in both components (Figure 4) is consistent with other reports of alcohol increasing behavioral repetition in both operant (e.g., Crow, 1982, 1988) and radial-arm maze (e.g., Devenport, 1984; Devenport & Merriman, 1983) experimental preparations. When variability is indexed as changes in U value (Figure 3), however, our results also are consistent with reports of alcohol increasing behavioral variability (Cohen et al., 1990; McElroy & Neuringer, 1990). Thus, characterization of the present results depends on whether conclusions are based on the overall measure of variability or on inspection of more molecular changes in behavior. As noted by McElroy and Neuringer, there are several ways that variability can be defined and measured. The present results suggest that assessing overall variability using molar measures without examining systematic changes in the molecular structure of behavior may lead to incomplete characterization of the effects of some drugs on reinforced variability and repetition.
The effects of d-amphetamine in this procedure merit additional discussion. Many studies have shown that d-amphetamine administration produces profound locomotor stereotypies. In the present experiment, however, d-amphetamine increased overall sequence variability in the Repeat component, and did not produce systematic stereotypy in the key-peck sequences across phases in the Vary component (Figures 2 and 6). There are several possible explanations for this result. First, in the present procedure, unlike traditional experimental preparations used to assess d-amphetamine-induced stereotypy, reinforcement depended on behaving with an appropriate degree of variability or repetition. The different results produced by d-amphetamine under the two types of procedures suggest that d-amphetamine may have differential effects depending on the procedural contingencies on variability. Wolgin and Wade (1995) showed that d-amphetamine-induced stereotypy of rats was suppressed if it interfered with ingestive behavior. This result, and the lack of systematic or substantial d-amphetamine-induced stereotypy in either component in the present study, suggest that operant contingencies can modulate the expression of d-amphetamine-induced stereotypies (see also Pinkston & Branch, 2003, for related discussion). Alternatively, different types of behavior such as locomotor activity and key-peck sequences may be differentially affected by d-amphetamine. Future research should attempt to examine more clearly how the type of behavior being measured may contribute to or limit the expression of drug-induced behavioral stereotypies under different experimental contingencies.
Acknowledgments
The authors thank Katie Burke for assistance in the conduct of the experiment. We thank Tim Shahan, Corina Jimenez-Gomez, and Chris Podlesnik for comments on an earlier version of the manuscript. Portions of these data were presented at the 2004 annual meeting of the Association for Behavior Analysis in Boston, Massachusetts and the 2005 annual meeting of the Association for Behavior Analysis in Chicago, Illinois.
Appendix
Mean relative frequency of the 16 possible sequences during the Vary and Repeat components for all pigeons during control sessions and across increasing drug dose in all conditions of the experiment. Also shown is the number of sessions contributing to each mean in each condition.
| # of Sessions: |
d-amphetamine Phase 1 |
|||||||
| Control | ||||||||
| P92 |
P98 |
P99 |
P154 |
|||||
| 19 |
21 |
21 |
19 |
|||||
| Sequence | Vary | Repeat | Vary | Repeat | Vary | Repeat | Vary | Repeat |
| LLLL | 0.140 | 0.005 | 0.022 | 0.007 | 0.067 | 0.000 | 0.196 | 0.018 |
| RRRR | 0.142 | 0.005 | 0.161 | 0.007 | 0.150 | 0.003 | 0.179 | 0.063 |
| LLLR | 0.089 | 0.001 | 0.045 | 0.001 | 0.006 | 0.002 | 0.116 | 0.001 |
| LLRR | 0.108 | 0.000 | 0.063 | 0.001 | 0.013 | 0.000 | 0.147 | 0.000 |
| LRRR | 0.101 | 0.000 | 0.110 | 0.000 | 0.117 | 0.000 | 0.122 | 0.003 |
| RLLL | 0.067 | 0.379 | 0.024 | 0.129 | 0.109 | 0.224 | 0.118 | 0.324 |
| RRLL | 0.097 | 0.509 | 0.113 | 0.668 | 0.131 | 0.586 | 0.076 | 0.409 |
| RRRL | 0.074 | 0.072 | 0.066 | 0.075 | 0.071 | 0.072 | 0.031 | 0.164 |
| LLRL | 0.005 | 0.001 | 0.006 | 0.001 | 0.001 | 0.000 | 0.000 | 0.001 |
| LRLL | 0.028 | 0.004 | 0.037 | 0.007 | 0.075 | 0.002 | 0.002 | 0.003 |
| LRRL | 0.026 | 0.000 | 0.066 | 0.002 | 0.061 | 0.000 | 0.001 | 0.004 |
| RLLR | 0.061 | 0.019 | 0.073 | 0.094 | 0.037 | 0.108 | 0.007 | 0.006 |
| RLRR | 0.035 | 0.001 | 0.071 | 0.003 | 0.062 | 0.004 | 0.003 | 0.002 |
| RRLR | 0.016 | 0.001 | 0.109 | 0.005 | 0.046 | 0.000 | 0.001 | 0.000 |
| LRLR | 0.009 | 0.000 | 0.032 | 0.000 | 0.039 | 0.000 | 0.001 | 0.000 |
| RLRL | 0.003 | 0.001 | 0.002 | 0.001 | 0.015 | 0.001 | 0.000 | 0.000 |
| # of Sessions: | 0.1 mg/kg | |||||||
| P92 | P98 | P99 | P154 | |||||
| 3 | 3 | 3 | 3 | |||||
| Sequence | Vary | Repeat | Vary | Repeat | Vary | Repeat | Vary | Repeat |
| LLLL | 0.115 | 0.000 | 0.010 | 0.014 | 0.050 | 0.000 | 0.197 | 0.030 |
| RRRR | 0.150 | 0.013 | 0.185 | 0.024 | 0.167 | 0.008 | 0.172 | 0.028 |
| LLLR | 0.058 | 0.000 | 0.013 | 0.000 | 0.000 | 0.000 | 0.120 | 0.000 |
| LLRR | 0.082 | 0.000 | 0.071 | 0.000 | 0.023 | 0.000 | 0.129 | 0.000 |
| LRRR | 0.117 | 0.000 | 0.106 | 0.000 | 0.103 | 0.000 | 0.093 | 0.000 |
| RLLL | 0.112 | 0.348 | 0.083 | 0.077 | 0.157 | 0.291 | 0.114 | 0.314 |
| RRLL | 0.071 | 0.521 | 0.156 | 0.767 | 0.143 | 0.520 | 0.082 | 0.506 |
| RRRL | 0.092 | 0.096 | 0.065 | 0.063 | 0.047 | 0.058 | 0.057 | 0.117 |
| LLRL | 0.000 | 0.000 | 0.005 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| LRLL | 0.044 | 0.000 | 0.051 | 0.000 | 0.067 | 0.000 | 0.010 | 0.000 |
| LRRL | 0.043 | 0.000 | 0.076 | 0.000 | 0.057 | 0.000 | 0.000 | 0.005 |
| RLLR | 0.059 | 0.022 | 0.037 | 0.056 | 0.020 | 0.114 | 0.020 | 0.000 |
| RLRR | 0.030 | 0.000 | 0.058 | 0.000 | 0.053 | 0.000 | 0.006 | 0.000 |
| RRLR | 0.014 | 0.000 | 0.070 | 0.000 | 0.047 | 0.010 | 0.000 | 0.000 |
| LRLR | 0.013 | 0.000 | 0.013 | 0.000 | 0.050 | 0.000 | 0.000 | 0.000 |
| RLRL | 0.000 | 0.000 | 0.000 | 0.000 | 0.020 | 0.000 | 0.000 | 0.000 |
| # of Sessions: |
0.3 mg/kg |
|||||||
| P92 |
P98 |
P99 |
P154 |
|||||
| 3 |
3 |
3 |
3 |
|||||
| Sequence | Vary | Repeat | Vary | Repeat | Vary | Repeat | Vary | Repeat |
| LLLL | 0.098 | 0.071 | 0.037 | 0.000 | 0.043 | 0.000 | 0.152 | 0.020 |
| RRRR | 0.077 | 0.000 | 0.153 | 0.000 | 0.160 | 0.000 | 0.204 | 0.045 |
| LLLR | 0.051 | 0.011 | 0.071 | 0.008 | 0.007 | 0.000 | 0.098 | 0.000 |
| LLRR | 0.089 | 0.000 | 0.112 | 0.000 | 0.027 | 0.000 | 0.238 | 0.007 |
| LRRR | 0.122 | 0.002 | 0.136 | 0.000 | 0.103 | 0.000 | 0.095 | 0.007 |
| RLLL | 0.054 | 0.428 | 0.028 | 0.197 | 0.087 | 0.195 | 0.113 | 0.310 |
| RRLL | 0.074 | 0.329 | 0.053 | 0.548 | 0.190 | 0.564 | 0.054 | 0.436 |
| RRRL | 0.020 | 0.065 | 0.084 | 0.150 | 0.027 | 0.077 | 0.037 | 0.153 |
| LLRL | 0.014 | 0.013 | 0.000 | 0.000 | 0.007 | 0.000 | 0.000 | 0.000 |
| LRLL | 0.069 | 0.013 | 0.012 | 0.008 | 0.023 | 0.000 | 0.009 | 0.000 |
| LRRL | 0.069 | 0.002 | 0.029 | 0.000 | 0.050 | 0.000 | 0.000 | 0.000 |
| RLLR | 0.079 | 0.040 | 0.035 | 0.075 | 0.050 | 0.155 | 0.000 | 0.008 |
| RLRR | 0.110 | 0.000 | 0.073 | 0.016 | 0.070 | 0.000 | 0.000 | 0.007 |
| RRLR | 0.048 | 0.000 | 0.127 | 0.000 | 0.070 | 0.000 | 0.000 | 0.000 |
| LRLR | 0.017 | 0.000 | 0.045 | 0.000 | 0.080 | 0.000 | 0.000 | 0.000 |
| RLRL | 0.007 | 0.026 | 0.007 | 0.000 | 0.007 | 0.009 | 0.000 | 0.007 |
| # of Sessions: | 1.0 mg/kg | |||||||
| P92 | P98 | P99 | P154 | |||||
| 3 | 3 | 3 | 3 | |||||
| Sequence | Vary | Repeat | Vary | Repeat | Vary | Repeat | Vary | Repeat |
| LLLL | 0.026 | 0.043 | 0.045 | 0.000 | 0.040 | 0.008 | 0.224 | 0.050 |
| RRRR | 0.052 | 0.000 | 0.165 | 0.000 | 0.153 | 0.000 | 0.197 | 0.118 |
| LLLR | 0.033 | 0.007 | 0.079 | 0.000 | 0.017 | 0.000 | 0.126 | 0.013 |
| LLRR | 0.076 | 0.006 | 0.086 | 0.008 | 0.010 | 0.000 | 0.225 | 0.012 |
| LRRR | 0.094 | 0.000 | 0.122 | 0.000 | 0.057 | 0.000 | 0.128 | 0.000 |
| RLLL | 0.096 | 0.387 | 0.006 | 0.151 | 0.087 | 0.205 | 0.066 | 0.300 |
| RRLL | 0.097 | 0.417 | 0.071 | 0.634 | 0.103 | 0.545 | 0.023 | 0.357 |
| RRRL | 0.021 | 0.075 | 0.054 | 0.055 | 0.067 | 0.026 | 0.004 | 0.124 |
| LLRL | 0.007 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.004 | 0.000 |
| LRLL | 0.049 | 0.007 | 0.012 | 0.000 | 0.040 | 0.000 | 0.000 | 0.007 |
| LRRL | 0.037 | 0.000 | 0.030 | 0.000 | 0.040 | 0.000 | 0.000 | 0.007 |
| RLLR | 0.150 | 0.053 | 0.075 | 0.132 | 0.077 | 0.200 | 0.004 | 0.007 |
| RLRR | 0.165 | 0.000 | 0.103 | 0.000 | 0.147 | 0.000 | 0.000 | 0.000 |
| RRLR | 0.030 | 0.000 | 0.153 | 0.021 | 0.107 | 0.008 | 0.000 | 0.006 |
| LRLR | 0.039 | 0.000 | 0.000 | 0.000 | 0.030 | 0.000 | 0.000 | 0.000 |
| RLRL | 0.028 | 0.007 | 0.000 | 0.000 | 0.023 | 0.008 | 0.000 | 0.000 |
| # of Sessions: |
3.0 mg/kg |
|||||||
| P92 |
P98 |
P99 |
P154 |
|||||
| 3 |
3 |
3 |
2 |
|||||
| Sequence | Vary | Repeat | Vary | Repeat | Vary | Repeat | Vary | Repeat |
| LLLL | 0.081 | 0.050 | 0.265 | 0.066 | 0.053 | 0.021 | 0.295 | 0.129 |
| RRRR | 0.054 | 0.000 | 0.320 | 0.605 | 0.163 | 0.016 | 0.128 | 0.000 |
| LLLR | 0.027 | 0.015 | 0.073 | 0.008 | 0.007 | 0.020 | 0.128 | 0.032 |
| LLRR | 0.075 | 0.000 | 0.088 | 0.019 | 0.003 | 0.000 | 0.167 | 0.032 |
| LRRR | 0.094 | 0.002 | 0.116 | 0.023 | 0.007 | 0.000 | 0.077 | 0.032 |
| RLLL | 0.066 | 0.411 | 0.000 | 0.040 | 0.143 | 0.187 | 0.077 | 0.323 |
| RRLL | 0.065 | 0.285 | 0.011 | 0.165 | 0.130 | 0.510 | 0.077 | 0.323 |
| RRRL | 0.026 | 0.019 | 0.049 | 0.052 | 0.083 | 0.048 | 0.038 | 0.065 |
| LLRL | 0.014 | 0.021 | 0.006 | 0.000 | 0.007 | 0.000 | 0.000 | 0.000 |
| LRLL | 0.054 | 0.017 | 0.010 | 0.008 | 0.027 | 0.008 | 0.000 | 0.000 |
| LRRL | 0.074 | 0.002 | 0.023 | 0.000 | 0.003 | 0.000 | 0.000 | 0.032 |
| RLLR | 0.125 | 0.141 | 0.011 | 0.014 | 0.083 | 0.156 | 0.013 | 0.000 |
| RLRR | 0.165 | 0.000 | 0.006 | 0.000 | 0.097 | 0.000 | 0.000 | 0.032 |
| RRLR | 0.040 | 0.000 | 0.022 | 0.000 | 0.127 | 0.000 | 0.000 | 0.000 |
| LRLR | 0.027 | 0.000 | 0.000 | 0.000 | 0.017 | 0.000 | 0.000 | 0.000 |
| RLRL | 0.013 | 0.038 | 0.000 | 0.000 | 0.043 | 0.035 | 0.000 | 0.000 |
| # of Sessions: | Ethanol Control | |||||
| P92 | P98 | P99 | ||||
| 16 | 16 | 16 | ||||
| Sequence | Vary | Repeat | Vary | Repeat | Vary | Repeat |
| LLLL | 0.108 | 0.014 | 0.027 | 0.010 | 0.120 | 0.004 |
| RRRR | 0.127 | 0.010 | 0.159 | 0.007 | 0.113 | 0.029 |
| LLLR | 0.089 | 0.000 | 0.037 | 0.000 | 0.009 | 0.001 |
| LLRR | 0.089 | 0.000 | 0.059 | 0.000 | 0.024 | 0.002 |
| LRRR | 0.124 | 0.000 | 0.117 | 0.000 | 0.143 | 0.000 |
| RLLL | 0.058 | 0.356 | 0.020 | 0.132 | 0.107 | 0.089 |
| RRLL | 0.066 | 0.509 | 0.065 | 0.780 | 0.090 | 0.703 |
| RRRL | 0.059 | 0.097 | 0.088 | 0.064 | 0.057 | 0.098 |
| LLRL | 0.006 | 0.000 | 0.011 | 0.000 | 0.011 | 0.000 |
| LRLL | 0.047 | 0.003 | 0.029 | 0.000 | 0.077 | 0.001 |
| LRRL | 0.019 | 0.000 | 0.060 | 0.000 | 0.077 | 0.000 |
| RLLR | 0.066 | 0.012 | 0.049 | 0.007 | 0.020 | 0.057 |
| RLRR | 0.085 | 0.000 | 0.065 | 0.000 | 0.044 | 0.000 |
| RRLR | 0.033 | 0.000 | 0.124 | 0.000 | 0.045 | 0.009 |
| LRLR | 0.023 | 0.000 | 0.083 | 0.000 | 0.054 | 0.000 |
| RLRL | 0.002 | 0.000 | 0.005 | 0.000 | 0.009 | 0.006 |
| # of Sessions: |
1.0 g/kg |
|||||
| P92 |
P98 |
P99 |
||||
| 4 |
4 |
4 |
||||
| Sequence | Vary | Repeat | Vary | Repeat | Vary | Repeat |
| LLLL | 0.042 | 0.039 | 0.006 | 0.025 | 0.102 | 0.065 |
| RRRR | 0.094 | 0.009 | 0.187 | 0.013 | 0.153 | 0.036 |
| LLLR | 0.064 | 0.003 | 0.010 | 0.013 | 0.005 | 0.003 |
| LLRR | 0.089 | 0.000 | 0.036 | 0.013 | 0.058 | 0.007 |
| LRRR | 0.090 | 0.000 | 0.115 | 0.000 | 0.089 | 0.000 |
| RLLL | 0.042 | 0.298 | 0.016 | 0.172 | 0.147 | 0.330 |
| RRLL | 0.088 | 0.443 | 0.122 | 0.513 | 0.128 | 0.423 |
| RRRL | 0.084 | 0.147 | 0.112 | 0.097 | 0.089 | 0.088 |
| LLRL | 0.033 | 0.009 | 0.011 | 0.000 | 0.005 | 0.000 |
| LRLL | 0.045 | 0.006 | 0.025 | 0.000 | 0.058 | 0.000 |
| LRRL | 0.044 | 0.003 | 0.047 | 0.009 | 0.061 | 0.007 |
| RLLR | 0.081 | 0.040 | 0.054 | 0.147 | 0.013 | 0.037 |
| RLRR | 0.097 | 0.000 | 0.134 | 0.000 | 0.029 | 0.000 |
| RRLR | 0.028 | 0.000 | 0.108 | 0.000 | 0.042 | 0.000 |
| LRLR | 0.025 | 0.000 | 0.012 | 0.000 | 0.008 | 0.000 |
| RLRL | 0.054 | 0.003 | 0.004 | 0.000 | 0.010 | 0.003 |
| # of Sessions: | 1.5 g/kg | |||||
| P92 | P98 | P99 | ||||
| 4 | 4 | 4 | ||||
| Sequence | Vary | Repeat | Vary | Repeat | Vary | Repeat |
| LLLL | 0.029 | 0.077 | 0.000 | 0.027 | 0.119 | 0.058 |
| RRRR | 0.079 | 0.003 | 0.133 | 0.013 | 0.121 | 0.057 |
| LLLR | 0.012 | 0.006 | 0.007 | 0.013 | 0.005 | 0.005 |
| LLRR | 0.029 | 0.009 | 0.027 | 0.000 | 0.005 | 0.000 |
| LRRR | 0.028 | 0.000 | 0.070 | 0.000 | 0.082 | 0.000 |
| RLLL | 0.058 | 0.314 | 0.105 | 0.185 | 0.173 | 0.325 |
| RRLL | 0.110 | 0.321 | 0.186 | 0.630 | 0.132 | 0.366 |
| RRRL | 0.096 | 0.072 | 0.089 | 0.023 | 0.174 | 0.089 |
| LLRL | 0.012 | 0.019 | 0.000 | 0.000 | 0.010 | 0.022 |
| LRLL | 0.043 | 0.018 | 0.032 | 0.000 | 0.064 | 0.003 |
| LRRL | 0.017 | 0.006 | 0.061 | 0.000 | 0.045 | 0.006 |
| RLLR | 0.154 | 0.130 | 0.106 | 0.078 | 0.012 | 0.070 |
| RLRR | 0.158 | 0.000 | 0.072 | 0.006 | 0.005 | 0.000 |
| RRLR | 0.107 | 0.016 | 0.077 | 0.018 | 0.037 | 0.000 |
| LRLR | 0.031 | 0.000 | 0.019 | 0.000 | 0.009 | 0.000 |
| RLRL | 0.036 | 0.010 | 0.015 | 0.008 | 0.010 | 0.000 |
| # of Sessions: |
2.0 g/kg |
|||||
| P92 |
P98 |
P99 |
||||
| 4 |
4 |
4 |
||||
| Sequence | Vary | Repeat | Vary | Repeat | Vary | Repeat |
| LLLL | 0.034 | 0.187 | 0.008 | 0.027 | 0.103 | 0.069 |
| RRRR | 0.069 | 0.017 | 0.129 | 0.004 | 0.152 | 0.048 |
| LLLR | 0.011 | 0.010 | 0.013 | 0.008 | 0.030 | 0.018 |
| LLRR | 0.030 | 0.000 | 0.022 | 0.006 | 0.016 | 0.003 |
| LRRR | 0.019 | 0.000 | 0.100 | 0.000 | 0.069 | 0.000 |
| RLLL | 0.087 | 0.178 | 0.075 | 0.189 | 0.164 | 0.430 |
| RRLL | 0.125 | 0.236 | 0.163 | 0.527 | 0.198 | 0.319 |
| RRRL | 0.087 | 0.026 | 0.106 | 0.061 | 0.121 | 0.068 |
| LLRL | 0.004 | 0.024 | 0.000 | 0.004 | 0.007 | 0.016 |
| LRLL | 0.031 | 0.009 | 0.044 | 0.018 | 0.017 | 0.003 |
| LRRL | 0.033 | 0.003 | 0.042 | 0.004 | 0.042 | 0.003 |
| RLLR | 0.215 | 0.273 | 0.069 | 0.144 | 0.038 | 0.024 |
| RLRR | 0.098 | 0.009 | 0.080 | 0.008 | 0.011 | 0.000 |
| RRLR | 0.100 | 0.011 | 0.109 | 0.000 | 0.030 | 0.000 |
| LRLR | 0.004 | 0.000 | 0.031 | 0.000 | 0.000 | 0.000 |
| RLRL | 0.051 | 0.017 | 0.008 | 0.000 | 0.008 | 0.000 |
| # of Sessions: |
d-amphetamine Phase 2 |
|||||
| Control | ||||||
| P92 |
P98 |
P99 |
||||
| 12 |
12 |
12 |
||||
| Sequence | Vary | Repeat | Vary | Repeat | Vary | Repeat |
| LLLL | 0.125 | 0.002 | 0.057 | 0.005 | 0.090 | 0.000 |
| RRRR | 0.153 | 0.004 | 0.161 | 0.018 | 0.147 | 0.027 |
| LLLR | 0.079 | 0.000 | 0.024 | 0.000 | 0.010 | 0.000 |
| LLRR | 0.090 | 0.000 | 0.076 | 0.000 | 0.018 | 0.000 |
| LRRR | 0.095 | 0.000 | 0.129 | 0.003 | 0.129 | 0.000 |
| RLLL | 0.063 | 0.216 | 0.036 | 0.191 | 0.146 | 0.069 |
| RRLL | 0.102 | 0.519 | 0.110 | 0.700 | 0.103 | 0.757 |
| RRRL | 0.057 | 0.085 | 0.097 | 0.053 | 0.016 | 0.120 |
| LLRL | 0.005 | 0.000 | 0.005 | 0.000 | 0.010 | 0.000 |
| LRLL | 0.025 | 0.000 | 0.049 | 0.002 | 0.125 | 0.000 |
| LRRL | 0.031 | 0.000 | 0.048 | 0.000 | 0.079 | 0.000 |
| RLLR | 0.052 | 0.171 | 0.021 | 0.026 | 0.040 | 0.026 |
| RLRR | 0.066 | 0.000 | 0.058 | 0.000 | 0.021 | 0.000 |
| RRLR | 0.040 | 0.002 | 0.084 | 0.003 | 0.018 | 0.000 |
| LRLR | 0.010 | 0.000 | 0.027 | 0.000 | 0.031 | 0.000 |
| RLRL | 0.007 | 0.000 | 0.018 | 0.000 | 0.018 | 0.000 |
| # of Sessions: | 0.1 mg/kg | |||||
| P92 | P98 | P99 | ||||
| 2 | 2 | 2 | ||||
| Sequence | Vary | Repeat | Vary | Repeat | Vary | Repeat |
| LLLL | 0.108 | 0.000 | 0.038 | 0.018 | 0.056 | 0.000 |
| RRRR | 0.174 | 0.015 | 0.195 | 0.000 | 0.113 | 0.000 |
| LLLR | 0.038 | 0.000 | 0.008 | 0.000 | 0.011 | 0.000 |
| LLRR | 0.074 | 0.000 | 0.072 | 0.000 | 0.008 | 0.000 |
| LRRR | 0.110 | 0.000 | 0.156 | 0.000 | 0.130 | 0.000 |
| RLLL | 0.072 | 0.169 | 0.050 | 0.116 | 0.114 | 0.043 |
| RRLL | 0.111 | 0.564 | 0.109 | 0.774 | 0.138 | 0.819 |
| RRRL | 0.046 | 0.044 | 0.108 | 0.054 | 0.033 | 0.058 |
| LLRL | 0.000 | 0.000 | 0.000 | 0.000 | 0.008 | 0.000 |
| LRLL | 0.045 | 0.000 | 0.061 | 0.000 | 0.165 | 0.000 |
| LRRL | 0.029 | 0.000 | 0.054 | 0.000 | 0.086 | 0.000 |
| RLLR | 0.079 | 0.193 | 0.000 | 0.039 | 0.057 | 0.038 |
| RLRR | 0.069 | 0.000 | 0.065 | 0.000 | 0.011 | 0.000 |
| RRLR | 0.044 | 0.000 | 0.064 | 0.000 | 0.022 | 0.019 |
| LRLR | 0.000 | 0.000 | 0.008 | 0.000 | 0.016 | 0.000 |
| RLRL | 0.000 | 0.015 | 0.010 | 0.000 | 0.033 | 0.022 |
| # of Sessions: |
0.3 mg/kg |
|||||
| P92 |
P98 |
P99 |
||||
| 2 |
2 |
2 |
||||
| Sequence | Vary | Repeat | Vary | Repeat | Vary | Repeat |
| LLLL | 0.157 | 0.000 | 0.037 | 0.000 | 0.141 | 0.000 |
| RRRR | 0.088 | 0.000 | 0.157 | 0.019 | 0.158 | 0.042 |
| LLLR | 0.078 | 0.000 | 0.009 | 0.000 | 0.017 | 0.000 |
| LLRR | 0.128 | 0.000 | 0.018 | 0.000 | 0.000 | 0.000 |
| LRRR | 0.157 | 0.000 | 0.119 | 0.019 | 0.144 | 0.000 |
| RLLL | 0.029 | 0.244 | 0.056 | 0.091 | 0.056 | 0.020 |
| RRLL | 0.050 | 0.663 | 0.128 | 0.673 | 0.114 | 0.835 |
| RRRL | 0.070 | 0.053 | 0.210 | 0.128 | 0.030 | 0.082 |
| LLRL | 0.020 | 0.000 | 0.000 | 0.000 | 0.026 | 0.000 |
| LRLL | 0.010 | 0.000 | 0.009 | 0.000 | 0.094 | 0.000 |
| LRRL | 0.060 | 0.000 | 0.055 | 0.000 | 0.164 | 0.000 |
| RLLR | 0.058 | 0.040 | 0.019 | 0.034 | 0.000 | 0.022 |
| RLRR | 0.058 | 0.000 | 0.046 | 0.000 | 0.009 | 0.000 |
| RRLR | 0.029 | 0.000 | 0.082 | 0.037 | 0.008 | 0.000 |
| LRLR | 0.010 | 0.000 | 0.045 | 0.000 | 0.017 | 0.000 |
| RLRL | 0.000 | 0.000 | 0.009 | 0.000 | 0.024 | 0.000 |
| # of Sessions: |
1.0 mg/kg |
|||||
| P92 |
P98 |
P99 |
||||
| 2 |
2 |
2 |
||||
| Sequence | Vary | Repeat | Vary | Repeat | Vary | Repeat |
| LLLL | 0.074 | 0.000 | 0.014 | 0.023 | 0.080 | 0.000 |
| RRRR | 0.084 | 0.011 | 0.173 | 0.034 | 0.155 | 0.045 |
| LLLR | 0.032 | 0.000 | 0.007 | 0.000 | 0.013 | 0.000 |
| LLRR | 0.074 | 0.000 | 0.154 | 0.000 | 0.013 | 0.000 |
| LRRR | 0.126 | 0.000 | 0.110 | 0.000 | 0.108 | 0.000 |
| RLLL | 0.105 | 0.240 | 0.014 | 0.225 | 0.058 | 0.015 |
| RRLL | 0.094 | 0.463 | 0.070 | 0.471 | 0.061 | 0.703 |
| RRRL | 0.063 | 0.089 | 0.049 | 0.091 | 0.040 | 0.161 |
| LLRL | 0.000 | 0.000 | 0.007 | 0.000 | 0.022 | 0.000 |
| LRLL | 0.031 | 0.000 | 0.000 | 0.000 | 0.144 | 0.000 |
| LRRL | 0.053 | 0.000 | 0.034 | 0.000 | 0.110 | 0.000 |
| RLLR | 0.074 | 0.198 | 0.021 | 0.156 | 0.059 | 0.075 |
| RLRR | 0.127 | 0.000 | 0.104 | 0.000 | 0.022 | 0.000 |
| RRLR | 0.021 | 0.000 | 0.223 | 0.000 | 0.034 | 0.000 |
| LRLR | 0.021 | 0.000 | 0.007 | 0.000 | 0.074 | 0.000 |
| RLRL | 0.021 | 0.000 | 0.014 | 0.000 | 0.009 | 0.000 |
| # of Sessions: |
3.0 mg/kg |
|||||
| P92 |
P98 |
P99 |
||||
| 2 |
1 |
1 |
||||
| Sequence | Vary | Repeat | Vary | Repeat | Vary | Repeat |
| LLLL | 0.037 | 0.006 | 0.000 | 0.075 | 0.061 | 0.047 |
| RRRR | 0.216 | 0.073 | 0.341 | 0.321 | 0.204 | 0.000 |
| LLLR | 0.000 | 0.000 | 0.000 | 0.038 | 0.041 | 0.016 |
| LLRR | 0.019 | 0.000 | 0.068 | 0.000 | 0.041 | 0.000 |
| LRRR | 0.066 | 0.000 | 0.091 | 0.000 | 0.061 | 0.000 |
| RLLL | 0.102 | 0.276 | 0.091 | 0.189 | 0.082 | 0.344 |
| RRLL | 0.065 | 0.160 | 0.045 | 0.226 | 0.163 | 0.313 |
| RRRL | 0.009 | 0.084 | 0.068 | 0.038 | 0.041 | 0.094 |
| LLRL | 0.019 | 0.018 | 0.023 | 0.000 | 0.000 | 0.031 |
| LRLL | 0.037 | 0.018 | 0.000 | 0.000 | 0.082 | 0.000 |
| LRRL | 0.028 | 0.000 | 0.045 | 0.000 | 0.082 | 0.000 |
| RLLR | 0.131 | 0.149 | 0.045 | 0.057 | 0.000 | 0.156 |
| RLRR | 0.094 | 0.036 | 0.114 | 0.038 | 0.000 | 0.000 |
| RRLR | 0.056 | 0.103 | 0.068 | 0.000 | 0.061 | 0.000 |
| LRLR | 0.046 | 0.012 | 0.000 | 0.000 | 0.061 | 0.000 |
| RLRL | 0.074 | 0.066 | 0.000 | 0.019 | 0.020 | 0.000 |
Footnotes
There are different numbers of sessions contributing to the means shown in different columns in the Appendix. These differences arise in part because each drug or saline test session was preceded by a control session. Therefore, there are many more control sessions than the number of determinations of the effects of a particular dose. The calculations of the mean relative sequence frequencies from control and drug-test sessions presented in the Appendix, therefore, are based on different numbers of sessions. This detail should be noted when interpreting changes in the relative frequencies of particular response sequences under drug. Of particular importance in this regard is interpreting changes in very small relative-frequency values (i.e., <0.05) from control to drug conditions. Such changes reflect the averaging of a different number of sessions in the calculation of control- and drug-session means and, therefore, must be interpreted with caution.
References
- Baron S.P, Wright D, Wenger G.R. Effects of drugs of abuse and scopolamine on memory in rats: Delayed spatial alternation and matching to position. Psychopharmacology. 1998;137:7–14. doi: 10.1007/s002130050587. [DOI] [PubMed] [Google Scholar]
- Blough D.S. The reinforcement of least frequent interresponse times. Journal of the Experimental Analysis of Behavior. 1966;9:581–591. doi: 10.1901/jeab.1966.9-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant D, Church R.M. The determinants of random choice. Animal Learning & Behavior. 1974;2:245–248. [Google Scholar]
- Cohen L, Neuringer A, Rhodes D. Effects of ethanol on reinforced variations and repetitions by rats under a multiple schedule. Journal of the Experimental Analysis of Behavior. 1990;54:1–12. doi: 10.1901/jeab.1990.54-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow L.T. Ethanol-induced response stereotypy: Simple alternation, fixed-interval rates of response, and response location. Bulletin of the Psychonomic Society. 1982;19:169–172. [Google Scholar]
- Crow L.T. Alcohol effects on variability-contingent operant responding in the rat. Bulletin of the Psychonomic Society. 1988;26:126–128. [Google Scholar]
- Denney J, Neuringer A. Behavioral variability is controlled by discriminative stimuli. Animal Learning & Behavior. 1998;26:154–162. [Google Scholar]
- Devenport L.D. Extinction-induced spatial dispersion in the radial arm maze: Arrest by ethanol. Behavioral Neuroscience. 1984;98:979–985. doi: 10.1037//0735-7044.98.6.979. [DOI] [PubMed] [Google Scholar]
- Devenport L.D, Merriman V.J. Ethanol and behavioral variability in the radial-arm maze. Psychopharmacology. 1983;79:21–24. doi: 10.1007/BF00433010. [DOI] [PubMed] [Google Scholar]
- Doughty A.H, Lattal K.A. Resistance to change of operant variation and repetition. Journal of the Experimental Analysis of Behavior. 2001;76:195–215. doi: 10.1901/jeab.2001.76-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givens B. Low doses of ethanol impair spatial working memory and reduce hippocampal theta activity. Alcohol Clinical & Experimental Research. 1995;19:763–767. doi: 10.1111/j.1530-0277.1995.tb01580.x. [DOI] [PubMed] [Google Scholar]
- Givens B, McMahon K. Effects of ethanol on non-spatial working memory and attention in rats. Behavioral Neuroscience. 1997;111:275–282. doi: 10.1037//0735-7044.111.2.275. [DOI] [PubMed] [Google Scholar]
- Hoffman S.E, Matthews D.B. Ethanol-induced impairments in spatial working memory are not due to deficits in learning. Alcohol Clinical & Experimental Research. 2001;25:856–861. [PubMed] [Google Scholar]
- Lyon M, Randrup A. The dose-response effect of d-amphetamine upon avoidance behavior in the rat seen as a function of increasing stereotypy. Psychopharmacologia. 1972;23:334–347. doi: 10.1007/BF00406736. [DOI] [PubMed] [Google Scholar]
- Machado A. Operant conditioning of behavioral variability using a percentile schedule of reinforcement. Journal of the Experimental Analysis of Behavior. 1989;52:155–166. doi: 10.1901/jeab.1989.52-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado A. Increasing the variability of response sequences in pigeons by adjusting the frequency of switching between two keys. Journal of the Experimental Analysis of Behavior. 1997;68:1–25. doi: 10.1901/jeab.1997.68-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley S.J, Little H.J. Enhancement of d-amphetamine- and cocaine-induced locomotor activity after chronic ethanol administration. Journal of Pharmacology and Experimental Therapeutics. 1997;281:1330–1339. [PubMed] [Google Scholar]
- McElroy E, Neuringer A. Effects of alcohol on reinforced repetitions and reinforced variations in rats. Psychopharmacology. 1990;102:49–55. doi: 10.1007/BF02245743. [DOI] [PubMed] [Google Scholar]
- Melia K.F, Ehlers C.L. Signal detection analysis of ethanol effects on a complex conditional discrimination. Pharmacology, Biochemistry & Behavior. 1989;33:581–584. doi: 10.1016/0091-3057(89)90391-2. [DOI] [PubMed] [Google Scholar]
- Miller G.A, Frick F.C. Statistical behavioristics and sequences of responses. Psychological Review. 1949;56:311–324. doi: 10.1037/h0060413. [DOI] [PubMed] [Google Scholar]
- Mook D.M, Neuringer A. Different effects of d-amphetamine on reinforced variations versus repetitions in Spontaneously Hypertensive rats (SHR). Physiology & Behavior. 1994;56:939–944. doi: 10.1016/0031-9384(94)90327-1. [DOI] [PubMed] [Google Scholar]
- Neuringer A. Can people behave “randomly”?: The role of feedback. Journal of Experimental Psychology: General. 1986;115:62–75. [Google Scholar]
- Neuringer A. Operant variability and repetition as functions of interresponse time. Journal of Experimental Psychology: Animal Behavior Processes. 1991;17:3–12. [Google Scholar]
- Neuringer A. Operant variability: Evidence, functions, and theory. Psychonomic Bulletin & Review. 2002;9:672–705. doi: 10.3758/bf03196324. [DOI] [PubMed] [Google Scholar]
- Neuringer A. Reinforced variability in animals and people: Implications for adaptive action. American Psychologist. 2004;59:891–906. doi: 10.1037/0003-066X.59.9.891. [DOI] [PubMed] [Google Scholar]
- Neuringer A, Kornell N, Olufs M. Stability and variability in extinction. Journal of Experimental Psychology: Animal Behavior Processes. 2001;27:79–94. [PubMed] [Google Scholar]
- Odum A.L, Ward R.D, Barnes C.A, Burke K.A. The effects of delayed reinforcement on variability and repetition of response sequences. Journal of the Experimental Analysis of Behavior. in press doi: 10.1901/jeab.2006.58-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page S, Neuringer A. Variability is an operant. Journal of Experimental Psychology: Animal Behavior Processes. 1985;11:429–452. doi: 10.1037//0097-7403.26.1.98. [DOI] [PubMed] [Google Scholar]
- Pinkston J.W, Branch M.N. Sensitization to cocaine in pigeons: Interaction with an operant contingency. Experimental & Clinical Psychopharmacology. 2003;11:102–109. doi: 10.1037//1064-1297.11.1.102. [DOI] [PubMed] [Google Scholar]
- Pryor K.W, Haag R, O'Reilly J. The creative porpoise: Training for novel behavior. Journal of the Experimental Analysis of Behavior. 1969;12:653–661. doi: 10.1901/jeab.1969.12-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randrup A, Munkvad I. Stereotyped activities produced by d-amphetamine in several animal species and man. Psychopharmacologia. 1967;11:300–310. doi: 10.1007/BF00404607. [DOI] [PubMed] [Google Scholar]
- Roache J.D, Spiga R, Burt D.B. Triazolam and ethanol effects on human matching-to-sample performance vary as a function of pattern size and discriminability. Drug & Alcohol Dependence. 1993;32:219–229. doi: 10.1016/0376-8716(93)90086-6. [DOI] [PubMed] [Google Scholar]
- Schoblock J.R, Maisonneuve I.M, Glick S.D. Differences between d-methamphetamine and d-amphetamine in rats: Working memory, tolerance, and extinction. Psychopharmacology. 2003;170:150–156. doi: 10.1007/s00213-003-1522-y. [DOI] [PubMed] [Google Scholar]
- Wolgin D.L, Wade J.V. Learned suppression of stereotypy in d-amphetamine-treated rats: Implications for understanding tolerance to d-amphetamine “anorexia.”. Behavioural Pharmacology. 1995;6:254–262. [PubMed] [Google Scholar]