Abstract
Synaptic complexes in prokaryotic transposons occur when transposase monomers bind to each of two specific end-binding sequences and then associate to bring the proteins and the two ends of the transposon together. It is within this complex of proteins and DNA that identical catalytic reactions are carried out by transposase on each of the ends of the transposon. In this study, we perform in vitro transposition reactions by combining the methylated inside end (IEME) biased hyperactive Tn5 transposase, Tnp sC7 version 2.0, and the outside end (OE) biased hyperactive Tn5 transposase, Tnp EK/LP, with plasmid DNA containing a transposon defined by one IEME and one OE. These two proteins cooperate to facilitate double end cleavage of the transposon from the plasmid and conversion into transposition products via strand transfer. When one of the hyperactive Tnps is replaced with a catalytically inactive version containing the mutation EA326 (DDE mutant), the predominant reaction product is a linearized plasmid resulting from single end cleavage. Restriction analysis of these linear products reveals that cleavage is occurring on the end distal to that which is bound by the transposase with an intact active site or in trans. Similar in vitro experiments performed with precut transposons and a supercoiled target plasmid demonstrated that the strand transfer reaction is also facilitated by a trans active DDE motif.
Catalytic reactions facilitated by DNA rearrangement enzymes such as transposases, retroviral integrases, and site-specific recombinases take place within the context of nucleoprotein synaptic complexes. The function of these complexes presumably is to coordinate reactions that are occurring on two or more DNA substrates that would not otherwise be in close proximity to one another. This prerequisite for complex formation also prevents partial reactions that could otherwise take place by single proteins bound to only one DNA site. A fundamental question about these complexes that can be addressed is: What is it about the synaptic complex that allows catalysis to occur and prevents catalysis in its absence?
In prokaryotic transposition, monomers of transposase bind to the ends of the transposon and then bring them together into a protein–DNA complex termed a synaptic complex. Transposase then catalyzes identical chemical reactions of both ends of the transposon within the context of this complex. These transposase proteins contain a series of three carboxylic acid residues, the DDE motif, that are specifically required for catalysis. Studies of the replicative transposon bacteriophage Mu have demonstrated that the DDE motif is supplied in trans for both catalytic reactions in the replicative mechanism, namely nicking of the 3′ ends and their transfer into a target DNA molecule (1–6). In other words, the transposase active site does not catalyze cleavage of the end of the transposon that it binds to but rather catalyzes cleavage of the opposite end within the context of the synaptic complex. Whether the DDE motif is donated in trans for other classes of transposases has not been reported previously.
The prokaryotic transposon Tn5 is a member of the IS4 family of bacterial cut-and-paste transposons (7). The Tn5 transposase acts differently than the Mu transposase in that it must cleave both the 3′ and 5′ strands of the transposon before strand transfer. This mechanism has recently been shown to involve three catalytic steps and to use the formation of a hairpin intermediate during the cleavage stage both in Tn5 transposition (8) and previously in the IS4 relative Tn10 (9). We know further that the synaptic complex in Tn5 transposition contains two monomers of transposase that are responsible for carrying out both the cleavage and strand transfer reactions (10). The next step in our understanding of the Tn5 synaptic complex and how catalysis occurs within it is to answer the question: Does each protein function catalytically at its specific DNA-binding site (in cis) or is the active site being donated to the other DNA end in the complex (in trans)?
Mutation of amino acid 326 of Tn5 transposase to alanine (EA326) results in a loss of in vivo function of the protein. This residue has been directly shown by crystallography to exist in the catalytic pocket of the protein (11). In vitro analysis has also confirmed that the Tnp EA326 protein is specifically disabled for catalysis but is unaffected in its ability to form synaptic complexes, which is the expected phenotype of a transposase DDE mutant (unpublished results).
The Tn5 transposase mutation EK54 was previously isolated as an N-terminal mutation that increases transposition efficiency (12). Analysis of this mutation revealed that it increases the affinity of transposase binding to the outside end (OE) sequences while decreasing the protein's ability to bind to the inside end (IE) (13). This mutation, in combination with the mutation LP372 (14), results in an in vitro active form of Tn5 transposase that facilitates movement of OE/OE transposons (15).
Recently, we have isolated a hyperactive mutant of Tn5 transposase, Tnp sC7 version 2.0 (Tnp sC7), which has a different binding-site recognition specificity than Tnp EK/LP (unpublished results). Tnp sC7 preferentially uses methylated inside ends (IEME) and not OE as the transposon-defining site. The ability of these two hyperactive proteins to preferentially recognize different specific end-binding sequences has allowed us to perform in vitro transposition experiments in which a transposon with two different end sequences can be converted into transposition products by addition of both proteins. By adding the DDE mutation EA326 to one of the two proteins, we have been able to form heterodimeric synaptic complexes in which only one active site is functional. In addition, we know which end of the transposon the catalytically active transposase is bound to. Thus we can ask whether the catalytic active site functions in cis or in trans. The results reported here clearly indicate that the DDE motif in Tn5 transposition is donated in trans for both cleavage and strand transfer reactions.
Materials and Methods
Media and Reagents.
Bacterial cultures for cloning, mating-out, and protein purification were grown in Luria broth (16). Antibiotics were purchased from Sigma and added at the following concentrations where applicable: ampicillin, 100 μg/ml; chloramphenicol, 20 μg/ml; nalidixic acid, 20 μg/ml; and gentamycin, 5 μg/ml. Restriction enzymes and T4 ligase were purchased from New England Biolabs and Promega. Taq polymerase used during construction of pRZ9900 (LP372/EA326) was purchased from Promega. The site-directed mutagenesis procedure used to generate pRZ9905(sC7/EA326) required native Pfu polymerase, which was purchased from Strategene. Oligonucleotides for site-directed mutagenesis were purchased from either Research Genetics (Huntsville, AL) or Integrated DNA Technologies (Coralville, IA). Sequencing was performed by using sequenase Ver. 2.0 (United States Biochemical) and radionucleotides purchased from Amersham or performed by the DNA sequencing facility at University of Wisconsin Madison Biotechnology Center.
Construction of Plasmid Vectors.
Plasmid vector pRZ9900 was constructed by inserting the EcoRI-BglII fragment of pRZ701 into pUC19 (New England Biolabs) that was digested with EcoRI and BamHI. The NheI-BglII fragment of this plasmid was then replaced with the LP372 containing fragment from pRZ4856 to form pRZ9900(LP372). The mutation EA326 was introduced into this plasmid by site-directed mutagenesis following the procedure of Martin et al. (17). The NotI-BglII fragment of this plasmid (pRZ9900(LP372/EA326) was then used to replace the same fragment in pGRTYB35 to become pGRTYB35(EA326). This expression construct was used to purify transposase with the mutations EK54/MA56/EA326/LP372 (Tnp EK/LP/EA326).
Plasmid pRZ9905 was constructed for use in the directed evolution study (unpublished results). The small EcoRI-NotI insert of pRZ5412 was used to replace the same fragment of pRZ9900 to form pRZ9901. This places an AflII restriction site between the promoter and coding sequence of transposase and also introduces the mutation MA56, which stops the expression of inhibitor and is necessary for later protein purification. The SphI-SapI fragment of this plasmid was then removed to form pRZ9905. This plasmid was used in a directed evolution study that resulted in the isolation of pRZ9905(sC7). Analysis of this protein led to the construction of Tnp sC7 version 2.0, a transposase mutant with the following four mutations: RC8, EV58, EK344, and LQ372.The mutation EA326 was added to this plasmid by site-directed mutagenesis by using the single-end overlap procedure (18). The resulting plasmid was designated pRZ9905(sC72.0/EA326). The transposase coding sequence of this plasmid and that of pRZ9905(sC72.0) were then cloned into pTYB4 (New England Biolabs). These constructs were isolated and designated as pGRTYB35(sC72.0) and pGRTYB35(sC72.0/EA326). These expression constructs were used for overexpression and purification of Tnp sC7 and Tnp sC7/EA326, respectively. Plasmids pGRTYB35(Δ369) and pGRTYB35(sC7v2.0Δ369), for purification of Tnp EK/LP Δ369 and Tnp sC7 Δ369, were constructed by deleting the C-terminal sequence from pGRTYB35 and pGRTYB35(sC7v2.0) via PCR cloning.
Plasmid pGRT7(IE/OE) was constructed by replacing the mosaic end sequences of pGRT7105 with one OE sequence and one IE sequence by standard cloning methods.
Bacterial Strains.
All cloning involved in constructing pGRTYB35(EA326) was performed in DH5α. All cloning and mutagenesis involved in the creation of pGRTYB35(sC7) and pGRTYB35(sC7/EA326) were performed in JM109. Escherichia coli strains RZ212[Δ(lac-proA,B), ara, str, recA56, srl, thi/pOX38-Gen], and 14R525[F- nalr] were used in the mating-out assays.
In Vivo Transposition Assays.
Comparison of the in vivo activity of Tnp EK/LP and Tnp sC7 in conjunction with different end sequences was performed by trans mating-out assays (19). Transposase was expressed from either pGRTYB35 (Tnp EK/LP) or pGRTYB35(sC72.0) (Tnp sC7). The data given are the average of data sets of n = 3.
Purification of Transposase and Substrate Plasmid.
The mutant transposase proteins Tnp EK/LP, Tnp EK/LP/EA326, Tnp sC7, Tnp sC7/EA326, Tnp EK/LP Δ369, and Tnp sC7 Δ369 were purified by using the IMPACT T7 system from New England Biolabs. The conditions used for expression of the proteins and the details of the purification were described previously (I. Goryshin, personal communication; ref. 6). The concentrations of the proteins were determined by running aliquots on SDS/PAGE gels along with known standards and by staining with SYPRO orange stain (Bio-Rad) followed by quantitation with a Flourimager SI and with image quant software (Molecular Dynamics).
Substrate plasmid pRZ5412, which was used for in vitro cleavage assays, was purified by using a quiafilter plasmid maxi kit (Qiagen, Chatsworth, CA). The plasmid concentration was determined by gel electrophoresis along with known DNA concentration standards followed by ethidium bromide staining and quantitation with a Flourimager SI and with image quant software (Molecular Dynamics).
Plasmid pUC19 was used as the supercoiled target plasmid for in vitro strand transfer assays. It was isolated from cells by using a Quiafilter plasmid mega kit (Qiagen). The DNA was then electrophoresed on a 1% agarose gel and purified by using Quiaquick spin columns. The linear precut transposon was made by digesting pGRT7(IE/OE) plasmid with PvuII followed by gel purification as above.
In Vitro Transposition Assays.
Gel-shift assays were performed as described by York and Reznikoff (20). The DNA substrates were 60 bp long and differed only in the end sequence (IE or OE). They were prepared by annealing complimentary oligonucleotides. Methylation of IE was achieved by incubation with dam methylase according to the manufacturer's protocol (NEB).
In vitro transposition reactions were performed in 200-μl volumes under conditions described previously (15). The concentration of plasmid pRZ5412 used for all cleavage reactions was 0.25 nM. The concentration of active transposase was 1.5 nM. Inactive transposase protein was added at 12 nM.
All reactions were incubated at 37°C for 3 h. The reactions were then heated at 68°C for 20 min to disable transposase. This was followed by digestion to completion with ScaI. The digested reactions were then concentrated by ethanol precipitation. These products were visualized by electrophoresis on a 1% agarose gel followed by ethidium bromide staining.
For the strand transfer reactions, all tubes contained plasmid target DNA at a concentration of 30 nM and linear precut transposon at 10 nM. The EK/LP-alone reaction contained Tnp EK/LP at 30 nM. The two protein experiments also contained 120 nM Tnp sC7/EA326. Reactions were incubated at 37°C for 3 h and then heated for 20 min at 68°C to disable transposase. Where indicated, aliquots of these reactions were digested with either ClaI or SnaBI restriction endonuclease. Samples were treated with SDS and heat to remove transposase and then analyzed on a 1.1% agarose gel by ethidium staining.
Results
The IE sequence of transposon Tn5 is a specific binding site for transposase that defines the internal border of the IS50 insertion sequences (Fig. 1). In E. coli, transposase is able to facilitate transposition of DNA sequences flanked by two IEs but only in dam hosts (21). In strains that have functional dam methylase, the ability of transposase to function with the inside end sequence (IEME) is strongly inhibited, presumably because of the methylation of two dam target sequences within the 19 bp of IE. Through the use of a directed evolution approach, we have been able to isolate a mutant transposase protein, Tnp sC7 version 2.0, that exhibits hyperactivity in the presence of IEME sequences. Furthermore, this protein is not hyperactive in the presence of wild-type OE sequences. An analysis of this mutant and the description of its isolation are being described elsewhere (unpublished results).
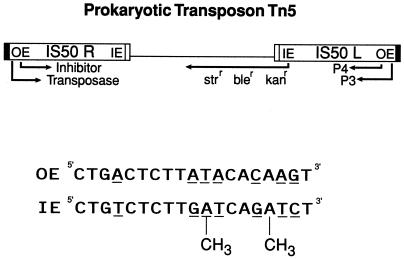
Figure 1.
The prokaryotic composite transposon Tn5 consists of two insertion sequences that flank three antibiotic resistance genes. IS50R encodes both the 476-aa transposase protein and a truncated form called the inhibitor. Movement of the transposon by transposase depends on binding of the transposase to either OE or IE. The ability of transposase to recognize IE in E. coli is greatly inhibited by the presence of two dam methylation sights (GATC), which result in the presence of four methyl groups per end.
The Hyperactive Tnp Mutants EK/LP and sC7 Have Strong OE vs. IEME Activity Differences in Vivo.
The mating-out assay is a standard in vivo assay that is used to compare quantitatively the relative transposition level between different mutant proteins when assayed with different transposon end sequences (22). We performed trans mating-out assays (the Tnp is expressed on one plasmid, and the transposon is present on another plasmid) for both Tnp EK/LP and Tnp sC7 with the transposon containing plasmids with either two OE or two IEME sequences. The plasmid used to express the transposases differed only in the sequence of the transposase protein, and the transposon plasmids differed only in the end sequences present.
Results confirm that the two hyperactive proteins have opposite substrate specificities when activity is compared in the presence of two OEs vs. in the presence of two IEMEs. Tnp EK/LP has an activity difference of nearly 104:1 in favor of OE (Tnp EK/LP + OE frequency = 9.9 × 10−5; Tnp EK/LP + IEME frequency = 1.2 × 10−8). The difference exhibited by Tnp sC7 is 100:1 in the opposite direction (Tnp sC7 + OE frequency = 2.4 × 10−7; Tnp sC7 + IEME frequency = 2.6 × 10−5). This difference itself is substantial, given that the protein is 100 times more active with a substrate that is not conducive to transposition when it is tested with wild-type transposase.
Primary Binding of Mutant Tnps to IEME and OE.
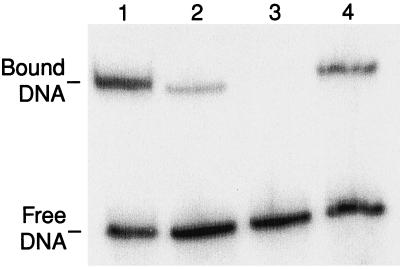
We next tested the ability of these mutant Tnp proteins for their binding to IEME and OE end sequences by gel-shift assay. We tested the ability of a C-terminally truncated form of each Tnp that is 369 aa long. This was necessary because full length Tnp does not form stable monomer complexes, whereas the truncated form does (20). The results shown in Fig. 2 reveal that Tnp sC7 (Δ369) has a higher affinity for binding to IEME than OE (42% to 9% shifted). Tnp EK/LP (Δ369) is able to shift oligonucleotides with OE sequences (lane 4, 23% shifted) but is impaired for recognition of IEME containing DNAs (lane 3).
Figure 2.
Sequence-specific binding of truncated forms of Tnp sC7 and Tnp EK/LP to IEME and OE end sequences. Tnp sC7 Δ369 binds preferentially to IEME (lane 1) while having a lower affinity for binding OE sequences (lane 2). Binding of Tnp EK/LP Δ369 to IEME is undetectable (lane 3), whereas the protein binds readily to OE DNAs (lane 4).
Heterodimeric Synaptic Complexes with Only One Catalytically Active Transposase Linearize Plasmids via Catalysis in Trans.
Previously in our laboratory, a DDE motif mutant of Tnp EK/LP (Tnp EK/LP/EA326) was constructed by site-directed mutagenesis and analyzed by both in vivo and in vitro activity assays performed with purified protein (unpublished results). This protein fails to promote transposition in vivo and in vitro but behaves like active Tnp EK/LP when tested for the ability to form paired-end complexes, indicating that it is unchanged in its ability to perform synapsis. These results are not surprising given that DDE motif mutants in other transposase proteins also exhibit this phenotype (23–26). It is widely believed that this triad of residues is specifically necessary for the binding of divalent metal ions in the catalytic pocket and that without these cofactors, the protein is unable to promote catalysis (27, 28).
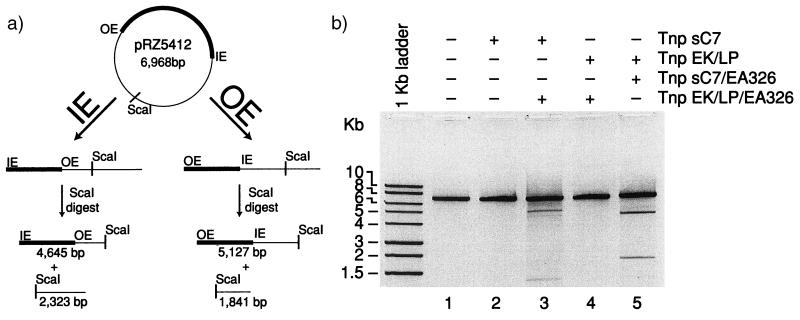
Both the hyperactive altered specificity mutant Tnp sC7 and the catalysis inactivating mutant Tnp EK/LP/EA326 were developed to allow us to perform in vitro protein mixing experiments with substrate plasmid containing a transposon defined by OE and IEME. This experiment was designed to form heterodimeric synaptic complexes in which only a single targeted protein within the synapse contains a functional active site. After the overexpression and purification of Tnp sC7 and Tnp EK/LP/EA326 (as described in Materials and Methods), in vitro transposition reactions were performed with either Tnp sC7 alone or Tnp sC7 and an 8-fold excess of the catalytically inactive Tnp EK/LP/EA326. After incubation for 3 h at 37°C, the reactions were heated to 68°C to inactivate transposase. The reactions were then returned to 37°C, and DNA was digested to completion with ScaI restriction endonuclease. The products were then analyzed by separation on a 1% agarose gel followed by ethidium bromide staining. The expected products that would be formed by heterodimeric complexes are shown in Fig. 3a. One monomer in the synapse contains a functional active site that is able to catalyze cleavage at an end of the transposon, resulting in a linear DNA molecule. Digestion with ScaI, which cleaves only once in the plasmid, converts these linear molecules into two fragments. Plasmids cleaved by Tnp at IEME and digested with ScaI result in fragments of 4,645 bp and 2,323 bp. Plasmids cleaved by Tnp at OE and subsequently by ScaI result in fragments of 5,127 bp and 1,841 bp. The results of Tnp sC7 reactions are shown in Fig. 3b, lanes 2 and 3. Incubation of pRZ5412 with Tnp sC7 does not result in the formation of cleavage products (lane 2); it is unable to efficiently promote reactions on the OE/IEME defined transposon. The addition of the OE-specific catalytically inactive Tnp EK/LP/EA326 restores the ability of Tnp sC7 to cleave the plasmid at OE (lane 3). This indicates that Tnp sC7 (bound at IEME) forms a synapse with the help of the inactive Tnp EK/LP/EA326 (bound at OE) and results in cleavage at OE. Therefore the transposase active site is functioning in trans.
Figure 3.
Cleavage within the Tn5 synaptic complex is facilitated by a trans acting active site. (a) The OE/IEME transposon containing plasmid pRZ5412 was incubated with transposase for 3 h at 37°C. After heat treatment to kill transposase, the samples were digested with ScaI restriction endonuclease. This enzyme cleaves the substrate plasmid at a unique location on the donor backbone. (b) Reaction aliquots were run on a 1% agarose gel and then visualized by ethidium bromide staining next to a DNA marker (1 kb ladder; Promega). When the IEME biased transposase Tnp sC7 is used as the active transposase, the catalytically inactive transposes Tnp EK/LP/EA326 helps it to facilitate cleavage at OE (lane 3). When the OE biased transposase Tnp EK/LP is used, it can facilitate cleavage of IEME with the help of the catalytically inactive protein Tnp sC7/EA326 (lane 5).
If this interpretation is true, the same reaction should be able to cleave pRZ5412 at IEME by removing the EA326 mutation from Tnp EK/LP and adding it into Tnp sC7. This essentially “moves” the functional active site present within the heterodimer to the other protein. Site-directed mutagenesis was used to change the codon for amino acid 326 of Tnp sC7 from one encoding glutamate to one encoding alanine (Tnp sC7/EA326). The catalytically inactive form of Tnp sC7, Tnp sC7/EA326, was then purified.
New in vitro reactions with pRZ5412 were performed as before, but this time active Tnp EK/LP was tested for the ability to cleave pRZ5412 in both the absence (Fig. 3b, lane 4) and presence (lane 5) of catalytically inactive Tnp sC7/EA326. The results show that Tnp EK/LP cannot cleave pRZ5412 alone, but with the help of IEME biased Tnp sC7/EA326 it is able to form synapsis and cleave at IEME in trans.
Precleaved OE/IEME Transposons Undergo Single-Ended Insertion in Trans.
We next designed a similar experiment to determine the cis/trans orientation of the transposase active site during strand transfer. The reaction included a mixture of a supercoiled target plasmid, a linear precut transposon with one OE and one IEME, active Tnp EK/LP, and the catalytically inactive transposase protein Tnp sC7/EA326.
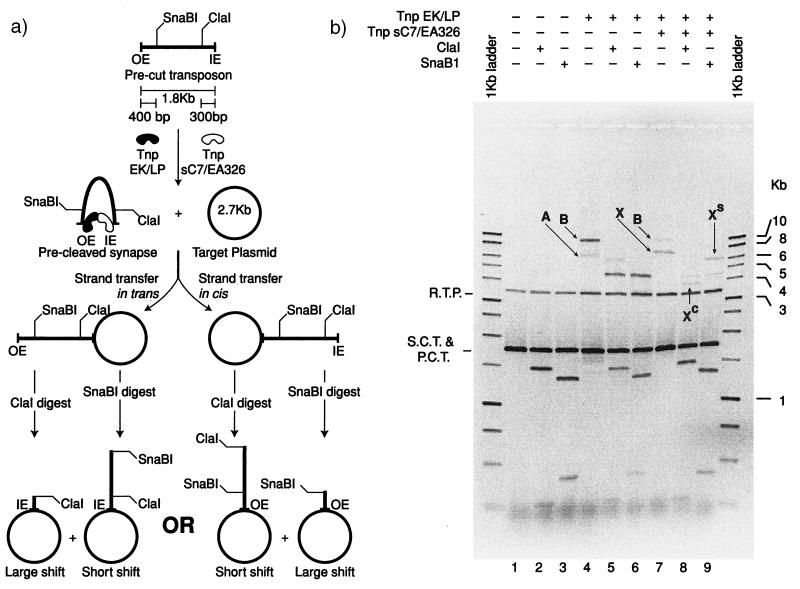
The results of the experiment are shown in Fig. 4b. In lane 1, the negative control, two DNA bands are present. The slow migrating band represents nicked-target plasmid. The faster migrating complex is composed of a mixture of supercoiled target DNA and the linear precut transposon. When this control reaction DNA is digested by either ClaI (lane2) or SnaBI (lane 3), the precut transposon is cleaved into two fragments, and the target DNA remains uncut. When the DNA is incubated with Tnp EK/LP, two products can be seen (lane 4). The less efficiently formed complex (A) migrates at the position predicted if two precut molecules form a synapse and are inserted into a target plasmid at both ends. This results in a linear DNA molecule consisting of a linearized target with a transposon inserted at each end. The ClaI digest (lane 5) results in a shift in the size of this linear molecule that corresponds to the insertion of two transposons at OE. After SnaBI digestion, the band shifts to its predicted 3.5-kb position, and the signal is lost in that of the nicked plasmid DNA (lane 6). The major product (B) corresponds to the double-ended insertion of the precut transposon into the target plasmid. This corresponds to a “normal” integration of the transposon into target DNA. The resulting product is a relaxed plasmid consisting of the target plasmid and an inserted transposon. Digestion of this product with either ClaI or SnaBI results in linearization of the relaxed plasmid to form the predicted 4.5-kb molecule (lanes 5 and 6).
Figure 4.
Strand transfer of a precut OE/IEME transposon is catalyzed in trans. The rationale for the experiment is shown in a. A linear precut transposon with one OE and one IEME, when incubated with Tnp EK/LP and Tnp sC7/EA326 and a target plasmid, facilitates single-ended insertion of the transposon into target DNA. To determine whether the reaction is inserting the transposon at IEME (in trans) or OE (in cis), the reaction products are digested with either ClaI or SnaBI. Strand transfer reactions shown in Fig. 3b were incubated for 3 h followed by heat treatment of transposase (68°C for 20′). Where indicated, aliquots of the reaction were digested with either ClaI or SnaBI restriction endonuclease. Undigested and digested samples were then treated with SDS and heat to remove transposase followed by electrophoresis on a 1.1% agarose gel. The results of the reaction reveal that the single-ended insertion [lane 7 (X)] is shifted to a position near the relaxed plasmid band after ClaI digestion [lane 8 (XC)], whereas the SnaBI digestion leads to a small shift [lane 9 (XS)]. These results indicate in trans catalysis during strand transfer.
The production of product B, the double-ended insertion, is somewhat surprising because it means that in this assay, Tnp EK/LP is functioning at both the OE and IEME sequences. However, other experiments have shown that the use of a precleaved substrate reduces the differential affinity of Tnp EK/LP (I. Goryshin, personal communication).
Addition of the inactive Tnp sC7/EA326 to the incubation mixture has two major effects: (i) The formation of products A and B is inhibited and (ii) a new product band (X) is visible as the major product. We assume this molecule represents nicked-target plasmids with linear transposon tails that are the result of single-ended strand transfer. Digestion of this reaction product with either ClaI or SnaBI should result in a complex that migrates faster than the original product but slower than the relaxed target plasmid alone. Furthermore, the asymmetric location of these two restriction sites along the transposon DNA should result in one “large shift” (product closer to relaxed target DNA) and one “small shift” (product closer to original undigested product), depending on which end is being inserted. Digestion with ClaI results in a shift of product X so that the band now runs relatively close to the relaxed target plasmid band (XC), indicating that the precut transposon has undergone strand transfer at IEME or in trans (lane 8). This result is confirmed by digestion of the same strand transfer reaction with SnaBI in which the band shifts only a small amount (XS in lane 9). Thus, even though the differential affinity is lowered for precleaved substrate DNA, it is still sufficient, so that in the presence of Tnp sC7/EA326, Tnp EK/LP is predominantly bound to OE.
We wish to mention in further proof of this interpretation that products from these reactions were run on numerous agarose gels of differing percentages and at different voltages. The relative mobility of product X and its ClaI and SnaBI digest products (as well as the undigested product B) in relation to the linear marker was highly variable depending on gel conditions, indicating that they all contain a circular DNA component.
Discussion
In this manuscript, we report that the active-site DDE motif functions catalytically in trans. Initial in vitro experiments involving plasmid pRZ5412 and the IEME specific hyperactive transposase sC7 mixed with Tnp EK/LP/EA326 demonstrated that the two proteins function together to cleave DNA at the outside end of the transposon. Secondly, by performing in vitro experiments with plasmid pRZ5412 in the presence of the OE hyperactive transposase EK/LP and Tnp sC7/EA326, the proteins worked in cooperation to cleave the DNA at the inside end of the transposon. Therefore the plasmid cleavage occurs at the end of the transposon that is recognized by the catalytically inactive form of transposase.
We further demonstrate that the active site functions in trans during strand transfer. In vitro reactions performed with precleaved OE/IEME transposons and supercoiled target plasmids with a mixture of Tnp EK/LP and Tnp sC7/EA326 resulted in the insertion of the IEME into the target, thus indicating that the intact active site is functioning on the opposite end of the transposon. This result, coupled with the cleavage results, directly proves that both the cleavage and strand transfer reactions on an end of the transposon are catalyzed by the same monomer within the Tn5 synaptic complex. We cannot claim, however, to have demonstrated the orientation of active sites during the third chemical step of the reaction (hairpin resolution), because our experiments do not distinguish between single-end break complexes that have undergone hairpin resolution and those that still contain unresolved hairpins. However, the results presented (cleavage and strand transfer catalyzed by the same protein monomer) lend support to the existence of the two-metal ion mechanism that has been proposed for transposase and integrase proteins, as this mechanism predicts that all reactions occurring on a single end are catalyzed by the same monomer active site (9, 28).
Implications of Trans Catalysis on the Mechanism of Tn5 Transposition.
Our results indicate the cleavage steps of Tn5 transposition are facilitated by an active-site DDE motif that is donated in trans. This feature alone is enough to prevent chemical reactions from being facilitated by transposase in the absence of the formation of a competent synaptic complex. The absence of such a requirement for catalysis could lead to cleavage of single ends of the transposon in the absence of any reaction at the opposite end, which would be detrimental to the survival of the transposon. The mechanistic elegance of the synaptic complex allows the catalytic events that lead to excision from each end and subsequent strand transfer into product DNA to be coordinated both spatially and temporally in a reaction that is highly down-regulated.
Subsequent to the completion of this work, a protein–DNA cocrystal of Tnp EK/LP complexed with precut OE DNA in a synaptic complex was solved (30). In this structure, the DNA molecule that contacts the transposase primary binding region (including amino acids 54 and 58) is bound in the active site of the opposite transposase molecule. Thus the crystallographic results are in agreement with the biochemical work presented here.
Similarities Between Mu and Tn5.
Previous experiments with the replicative phage Mu transposon have indicated that despite the presence of four monomers in the synaptic complex only the active-site DDE of the monomers bound nearest to the ends of the transposon function catalytically (2, 5, 6). Each of these monomers is responsible for catalyzing both reactions on a single end. It was also shown that the DDE motif in these reactions is donated in trans (2–6).
The results we have obtained with the conservative cut-and-paste transposon Tn5 yielded the same results, despite the differences in architecture of the two complexes. Assembly of the synaptic complex in Tn5 requires only two specific DNA-binding sequences and transposase (15). Assembly of the precleavage synaptic complex in Mu is more elaborate, requiring six binding sites, divalent metal ions, and two host proteins. Despite this difference in assembly, the realization that only two monomers donate DDE motifs during catalysis for Mu and Tn5 transposition and that the active site of both functions in trans suggests that the two are closely related catalytically. In fact, comparison of the crystal structures of the Mu transposase catalytic core (31) and Tn5 transposase catalytic core (11, 30) shows striking structural similarities.
Synapse Data from Site-Specific Recombinases.
Although the idea of an in trans component playing a role in regulating reactions that occur in synaptic complexes is conceptually appealing, it is not a universal component of all synapses. The most striking counterexample is provided by the crystal structure of the Cre recombinase synapse in which the protein active site is completely derived from a single monomer that catalyzes cleavage in cis (32). In this example, it is believed that the catalytically competent conformation of the protein is achieved only on formation of the synapse. Previous biochemical evidence has also shown that λ integrase contains all necessary catalytic activities within a single active site that functions in cis (33). However, it is interesting to note that studies of the related Flp recombinase of yeast indicate the critical tyrosine that functions as the initial nucleophile in the reaction is donated in trans (34, 35). Therefore it is not possible to assume that all proteins of a certain class will use the same regulatory mechanism within the synapse.
Acknowledgments
We thank Dr. Igor Goryshin for invaluable help in designing the strand transfer assay. We also thank Dr. Goryshin and A. Bhasin for critical review of the manuscript. This work was funded by National Institutes of Health (NIH) Grant GM50692 awarded to W.S.R. Partial support for T.A.N. was provided by NIH Training Grant GM08349.
Abbreviations
- OE
outside end
- IE
inside end
- IEME
methylated inside end
- Tnp EK/LP
OE hyperactive Tn5 transposase
- Tnp sC7
IEME hyperactive Tn5 transposase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.160107997.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.160107997
References
- 1.Aldaz H, Schuster E, Baker T A. Cell. 1996;85:257–269. doi: 10.1016/s0092-8674(00)81102-2. [DOI] [PubMed] [Google Scholar]
- 2.Yang J Y, Kim K, Yayaram M, Harshey R M. EMBO J. 1995;14:2374–2384. doi: 10.1002/j.1460-2075.1995.tb07232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savilahti H, Mizuuchi K. Cell. 1996;85:271–280. doi: 10.1016/s0092-8674(00)81103-4. [DOI] [PubMed] [Google Scholar]
- 4.Yang J, Makkuni J, Harshey R M. Cell. 1996;85:447–455. doi: 10.1016/s0092-8674(00)81122-8. [DOI] [PubMed] [Google Scholar]
- 5.Namgoong S Y, Harshey R M. EMBO J. 1998;17:3775–3785. doi: 10.1093/emboj/17.13.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams T L, Jackson E L, Carritte A, Baker T A. Genes Dev. 1999;13:2725–2737. doi: 10.1101/gad.13.20.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rezsohazy R, Hallet B, Delcour J, Mahillon J. Mol Microbiol. 1993;9:1283–1295. doi: 10.1111/j.1365-2958.1993.tb01258.x. [DOI] [PubMed] [Google Scholar]
- 8.Bhasin A, Goryshin I Y, Reznikoff W S. J Biol Chem. 1999;274:37025–37029. doi: 10.1074/jbc.274.52.37021. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy A K, Guhathakurta A, Kleckner N, Haniford D B. Cell. 1998;95:125–134. doi: 10.1016/s0092-8674(00)81788-2. [DOI] [PubMed] [Google Scholar]
- 10.Bhasin, A., Goryshin, I. Y., Steiniger-White, M., York, D. & Reznikoff, W. S. (2000) J. Mol. Biol., in press. [DOI] [PubMed]
- 11.Davies D R, Braam L M, Reznikoff W S, Rayment I. J Biol Chem. 1999;274:11904–11913. doi: 10.1074/jbc.274.17.11904. [DOI] [PubMed] [Google Scholar]
- 12.Zhou M, Reznikoff W S. J Mol Biol. 1997;271:362–373. doi: 10.1006/jmbi.1997.1188. [DOI] [PubMed] [Google Scholar]
- 13.Zhou M, Bhasin A, Reznikoff W S. J Mol Biol. 1997;276:913–925. doi: 10.1006/jmbi.1997.1579. [DOI] [PubMed] [Google Scholar]
- 14.Weinreich M D, Gash A, Reznikoff W S. Genes Dev. 1994;8:2362–2374. doi: 10.1101/gad.8.19.2363. [DOI] [PubMed] [Google Scholar]
- 15.Goryshin I Y, Reznikoff W S. J Biol Chem. 1998;273:7367–7374. doi: 10.1074/jbc.273.13.7367. [DOI] [PubMed] [Google Scholar]
- 16.Sambrook J, Fritsch E, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 17.Martin A, Toselli E, Rosier M, Auffray C, Devignes M. Nucleic Acids Res. 1995;23:1642–1643. doi: 10.1093/nar/23.9.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horton R M, Hunt H D, Ho S N, Pullen J K, Pease L R. Gene. 1989;77:61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- 19.Goryshin I Y, Kil Y V, Reznikoff W S. Proc Natl Acad Sci USA. 1994;91:10834–10838. doi: 10.1073/pnas.91.23.10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.York D, Reznikoff W S. Nucleic Acids Res. 1996;24:3790–3796. doi: 10.1093/nar/24.19.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin J C P, Krebs M P, Reznikoff W S. J Mol Biol. 1988;199:35–45. doi: 10.1016/0022-2836(88)90377-4. [DOI] [PubMed] [Google Scholar]
- 22.Johnson R C, Yin J C, Reznikoff W S. Cell. 1982;30:873–882. doi: 10.1016/0092-8674(82)90292-6. [DOI] [PubMed] [Google Scholar]
- 23.Baker T A, Luo L. Proc Natl Acad Sci USA. 1994;91:6654–6658. doi: 10.1073/pnas.91.14.6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bolland S, Kleckner N. Cell. 1996;84:223–233. doi: 10.1016/s0092-8674(00)80977-0. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy A K, Haniford D B. J Mol Biol. 1996;256:533–547. doi: 10.1006/jmbi.1996.0106. [DOI] [PubMed] [Google Scholar]
- 26.Sarnovski R J, May E W, Craig N L. EMBO J. 1996;15:6348–6361. [PMC free article] [PubMed] [Google Scholar]
- 27.Allingham J S, Pribil P A, Haniford D B. J Mol Biol. 1999;289:1195–1206. doi: 10.1006/jmbi.1999.2837. [DOI] [PubMed] [Google Scholar]
- 28.Reznikoff W S, Bhasin A, Davies D R, Goryshin I Y, Mahnke L A, Naumann T, Rayment I, Steiniger-White M, Twining S S. Biochem Biophys Res Commun. 2000;266:729–734. doi: 10.1006/bbrc.1999.1891. [DOI] [PubMed] [Google Scholar]
- 29.Beese L S, Steitz T A. EMBO J. 1991;10:25–33. doi: 10.1002/j.1460-2075.1991.tb07917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davies, D. R., Goryshin, I. Y., Reznikoff, W. S. & Rayment, I. (2000) Science, in press. [DOI] [PubMed]
- 31.Rice P, Mizuuchi K. Cell. 1995;82:209–220. doi: 10.1016/0092-8674(95)90308-9. [DOI] [PubMed] [Google Scholar]
- 32.Guo F, Gopaul D N, Van Duyne G D. Nature (London) 1997;389:40–46. doi: 10.1038/37925. [DOI] [PubMed] [Google Scholar]
- 33.Nunes-Duby S E, Tirumalai R S, Dorgai L, Yagil E, Weisberg R A, Landy A. EMBO J. 1994;13:4421–4430. doi: 10.1002/j.1460-2075.1994.tb06762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee J, Jayaram M. Genes Dev. 1997;11:2438–2447. doi: 10.1101/gad.11.18.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee J, Jayaram M, Grainge I. EMBO J. 1999;18:784–791. doi: 10.1093/emboj/18.3.784. [DOI] [PMC free article] [PubMed] [Google Scholar]