Abstract
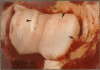
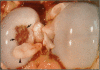
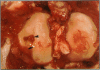
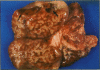
Fifty-two joints from pigs with nonsuppurative joint disease from a local abattoir were examined grossly, histologically, and microbiologically in order to establish macroscopic differences between degenerative arthropathy and arthritis due to an infectious organism. The joints were grouped grossly according to the type and severity of lesions of the synovial membrane and cartilage, and microscopically according to the severity of synovial membrane lesions. Osteochondrosis and Erysipelothrix rhusiopathiae were the most common causes of nonsuppurative joint disease in the joints examined. The major macroscopic differences between these two arthropathies were in the nature and severity of the synovial and cartilaginous lesions and involvement of the lymph node draining the diseased joint. Typically, in osteochondrosis, the changes are feathery hypertrophy of villi, focal full-thickness cartilage buckles, ulcers or flaps, and no change in the draining lymph node, whereas in Erysipelothrix- caused arthritis, the villous hypertrophy is severe and polypoid in nature, there is diffuse erosion of articular cartilage, and the draining lymph node is consistently hypertrophic and often cystic.
Keywords: Porcine, polyarthritis, osteochondrosis, Erysipelothrix rhusiopathiae
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bond M. P. Polyarthritis of pigs in western Australia: the role of Erysipelothrix rhusiopathiae. Aust Vet J. 1976 Oct;52(10):462–467. doi: 10.1111/j.1751-0813.1976.tb05395.x. [DOI] [PubMed] [Google Scholar]
- Cross G. M., Edwards M. J. The detection of arthritis in pigs in an abattoir and its public health significance. Aust Vet J. 1981 Apr;57(4):153–158. doi: 10.1111/j.1751-0813.1981.tb00500.x. [DOI] [PubMed] [Google Scholar]
- Cross G. M., Penny R. H., Claxton P. D. The abattoir incidence of polyarthritis in pigs in Australia. Aust Vet J. 1971 Mar;47(3):126–126. doi: 10.1111/j.1751-0813.1971.tb14763.x. [DOI] [PubMed] [Google Scholar]
- FREEMAN M. J., BERMAN D. T. HYPERSENSITIVITY IN ERYSIPELAS ARTHRITIS OF SWINE. II. HYPERSENSITIZATION OF SWINE WITH STERILE ERYSIPELOTHRIX ANTIGENS. Am J Vet Res. 1964 Jan;25:145–150. [PubMed] [Google Scholar]
- FREEMAN M. J. EFFECTS OF VACCINATION ON THE DEVELOPMENT OF ARTHRITIS IN SWINE WITH ERYSIPELAS: CLINICAL, HEMATOLOGIC, AND GROSS PATHOLOGIC OBSERVATIONS. Am J Vet Res. 1964 May;25:589–598. [PubMed] [Google Scholar]
- FREEMAN M. J., SEGRE D., BERMAN D. T. HYPERSENSITIVITY IN ERYSIPELAS ARTHRITIS OF SWINE. I. HYPERSENSITIZATION OF SWINE WITH VIABLE AND NONVIABLE ERYSIPELOTHRIX. Am J Vet Res. 1964 Jan;25:135–144. [PubMed] [Google Scholar]
- Farnum C. E., Wilsman N. J., Hilley H. D. An ultrastructural analysis of osteochondritic growth plate cartilage in growing swine. Vet Pathol. 1984 Mar;21(2):141–151. doi: 10.1177/030098588402100202. [DOI] [PubMed] [Google Scholar]
- Grøndalen T. Osteochondrosis and arthrosis in Norwegian slaughter-pigs in 1980 compared to 1970. Nord Vet Med. 1981 Sep-Nov;33(9-11):417–422. [PubMed] [Google Scholar]
- Harris E. D., Jr, DiBona D. R., Krane S. M. Collagenases in human synovial fluid. J Clin Invest. 1969 Nov;48(11):2104–2113. doi: 10.1172/JCI106177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M. A., Ruth G. R., Hilley H. D., Torrison J. L., Bagent J. K., Leman A. D. Dyschondroplasias of growth cartilages (osteochondrosis) in crossbred commercial pigs at one and 15 days of age: radiological, angiomicrographical and histological findings. Vet Rec. 1985 Jan 12;116(2):40–47. doi: 10.1136/vr.116.2.40. [DOI] [PubMed] [Google Scholar]
- Hogg A. A review of lameness in swine. Mod Vet Pract. 1981 Sep;62(9):689–693. [PubMed] [Google Scholar]
- Johansson H. E., Rejnö S. Light and electron microscopic investigation of equine synovial membrane. A comparison between healthy joints and joints with intraarticular fractures and osteochondrosis dissecans. Acta Vet Scand. 1976;17(2):153–168. doi: 10.1186/BF03547924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid S. A., Lidvall E. R. Communicating cartilage canals of the physis of the distal part of the ulna of growing swine and their potential role in healing of metaphyseal dysplasia of osteochondrosis. Am J Vet Res. 1982 Jun;43(6):938–944. [PubMed] [Google Scholar]
- Kincaid S. A., Lidvall E. R. Observations on the postnatal morphogenesis of the porcine humeral condyle and the pathogenesis of osteochondrosis. Am J Vet Res. 1983 Nov;44(11):2095–2103. [PubMed] [Google Scholar]
- Lipowitz A. J., Wong P. L., Stevens J. B. Synovial membrane changes after experimental transection of the cranial cruciate ligament in dogs. Am J Vet Res. 1985 May;46(5):1166–1170. [PubMed] [Google Scholar]
- NEHER G. M., SWENSON C. B., DOYLE L. P., SIKES D. The incidence of arthritis in swine following vaccination for swine erysipelas. Am J Vet Res. 1958 Jan;19(70):5–14. [PubMed] [Google Scholar]
- Olsson S. E., Reiland S. The nature of osteochondrosis in animals. Summary and conclusions with comparative aspects on osteochondritis dissecans in man. Acta Radiol Suppl. 1978;358:299–306. [PubMed] [Google Scholar]
- Oronsky A., Ignarro L., Perper R. Release of cartilage mucopolysaccharide-degrading neutral protease from human leukocytes. J Exp Med. 1973 Aug 1;138(2):461–472. doi: 10.1084/jem.138.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIKES D., NEHER G. M., DOYLE L. P. Studies on arthritis in swine. I. Experimental erysipelas and chronic arthritis in swine. Am J Vet Res. 1955 Jul;16(60):349–366. [PubMed] [Google Scholar]
- SIKES D., NEHER G. M., DOYLE L. P. Swine erysipelas. I. A discussion of experimentally induced disease. J Am Vet Med Assoc. 1956 Mar 15;128(6):277–281. [PubMed] [Google Scholar]
- Sikes D., Crimmins L. T., Fletcher O. J., Jr Rheumatoid arthritis of swine: a comparative pathologic study of clinical spontaneous remissions and exacerbations. Am J Vet Res. 1969 May;30(5):753–769. [PubMed] [Google Scholar]
- Sikes D. Experimental production of rheumatoid arthritis of swine: physiopathologic changes of tissues. Am J Vet Res. 1968 Sep;29(9):1719–1731. [PubMed] [Google Scholar]
- Sikes D., Fletcher O., Jr, Papp E. Experimental production of pannus in a rheumatoid-like arthritis of swine. Am J Vet Res. 1966 Jul;27(119):1017–1025. [PubMed] [Google Scholar]
- Sikes D. New Concepts of Arthritis in Swine. Can J Comp Med Vet Sci. 1960 Dec;24(12):347–351. [PMC free article] [PubMed] [Google Scholar]
- Tittiger F., Alexander D. C. Studies on the bacterial floral of condemmed portions from arthritic hogs. Can J Comp Med. 1971 Jul;35(3):244–248. [PMC free article] [PubMed] [Google Scholar]
- Turner G. V. A microbiological study of polyarthritis in slaughter pigs. J S Afr Vet Assoc. 1982 Jun;53(2):99–101. [PubMed] [Google Scholar]
- Turner G. V. Porcine arthritis and meat hygiene in South Africa. J S Afr Vet Assoc. 1978 Mar;49(1):40–44. [PubMed] [Google Scholar]
- Turner G. V. The pathology of infectious polyarthritis in slaughter pigs. J S Afr Vet Assoc. 1982 Jun;53(2):95–98. [PubMed] [Google Scholar]
- ZIFF M., GRIBETZ H. J., LOSPALLUTO J. Effect of leukocyte and synovial membrane extracts on cartilage mucoprotein. J Clin Invest. 1960 Feb;39:405–412. doi: 10.1172/JCI104051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziff M. Factors involved in cartilage injury. J Rheumatol Suppl. 1983 Dec;11:13–25. [PubMed] [Google Scholar]
- Zvaifler N. J. The immunopathology of joint inflammation in rheumatoid arthritis. Adv Immunol. 1973;16(0):265–336. doi: 10.1016/s0065-2776(08)60299-0. [DOI] [PubMed] [Google Scholar]