Abstract
Von Willebrand factor (VWF) is a multimeric protein that mediates platelet adhesion at sites of vascular injury, and ADAMTS13 (a disintegrin and metalloprotease with thrombospondin)is a multidomain metalloprotease that limits platelet adhesion by a feedback mechanism in which fluid shear stress induces proteolysis of VWF and prevents disseminated microvascular thrombosis. Cleavage of the Tyr1605–Met1606 scissile bond in the VWF A2 domain depends on a Glu1660–Arg1668 segment in the same domain and on the noncatalytic spacer domain of ADAMTS13, suggesting that extensive enzyme–substrate interactions facilitate substrate recognition. Based on mutagenesis and kinetic analysis, we find that the ADAMTS13 spacer domain binds to an exosite near the C terminus of the VWF A2 domain. Deleting the spacer domain from ADAMTS13 or deleting the exosite from the VWF substrate reduced the rate of cleavage ≈20-fold. A cleavage product containing the exosite was a hyperbolic mixed-type inhibitor of ADAMTS13 proteolysis of either VWF multimers or model peptide substrates but only if the ADAMTS13 enzyme contained the spacer domain. The specificity of this unique mechanism depends on tension-induced unfolding of the VWF A2 domain, which exposes the scissile bond and exosite for interaction with complementary sites on ADAMTS13.
Keywords: enzyme kinetics, thrombotic thrombocytopenic purpura, fluid shear stress
After vascular injury, platelets adhere to von Willebrand factor (VWF), a multimeric blood protein that can exceed 20,000 kDa in mass. VWF mediates platelet adhesion to exposed connective tissue, endothelial cells, and other adherent platelets (1, 2). To prevent thrombosis, platelet adhesion is regulated by a unique feedback mechanism involving tension-induced proteolysis. Flowing blood exerts a force on the growing platelet-rich thrombus that stretches VWF and exposes a cleavage site for plasma ADAMTS13, a member of a disintegrin and metalloprotease with thrombospondin type I repeat family (3–5). The metalloprotease domain (M domain) of ADAMTS13 cleaves the Tyr1605–Met1606 bond in the A2 domain of VWF (Pro1480–Gly1672, numbered from the initiation codon) (6–8), severing the VWF multimer and releasing adherent platelets. ADAMTS13 deficiency causes thrombotic thrombocytopenic purpura, a life-threatening disease characterized by disseminated microvascular thrombosis (4, 9, 10). Conversely, mutations in the VWF A2 domain that lead to excessive proteolysis cause von Willebrand disease type 2A, an inherited bleeding disorder (1).
Achieving balanced regulation of platelet adhesion is a formidable challenge under the conditions prevailing in blood. VWF and ADAMTS13 occur at low concentrations of ≈10 μg/ml and ≈1 μg/ml, respectively, compared with the total plasma protein concentration of ≈80,000 μg/ml. Nevertheless, VWF is the only known substrate of ADAMTS13 in plasma, even though ADAMTS13 is constitutively active (11). Furthermore, VWF is resistant to ADAMTS13 until it is subjected to high fluid shear stress (7), adsorbed onto a surface (12), or treated with chaotropic agents such as urea (8) or guanidine hydrochloride (7).
Several mechanisms contribute to the remarkable specificity of ADAMTS13. First, the activation of cleavage by tensile stress indicates that the scissile bond is inaccessible in native VWF. The modeled structure of the VWF A2 domain supports this conclusion, suggesting that the cleavage site is buried in a central β-sheet (13) and exposed on unfolding. Furthermore, von Willebrand disease mutations in the A2 domain that cause increased proteolysis are predicted to destabilize the folded structure (13). Second, binding of platelet glycoprotein Ibα (GPIbα) or heparin to the VWF A1 domain allosterically promotes cleavage of the adjacent A2 domain by ADAMTS13 (14, 15). Finally, VWF cleavage is markedly impaired by deletion of the ADAMTS13 spacer domain (16, 17) or by deletion of residues E1660APDLVLQR1668 from the VWF A2 domain (18), suggesting that contacts between “exosites” on the enzyme and substrate, located some distance from the active site and scissile bond, facilitate substrate recognition. To date, these exosite interactions have not been characterized.
We now demonstrate by mutagenesis and kinetic analysis that the spacer domain of ADAMTS13 binds to an exosite at the C terminus of the VWF A2 domain that is separated by ≈50 aa residues from the cleaved Tyr1605–Met1606 bond. This exosite appears to be cryptic in native VWF, and its interaction with the ADAMTS13 spacer domain is essential for feedback inhibition of VWF-dependent platelet adhesion. This elegant mechanism appears to be unique in its use of tension-induced conformational changes to expose cryptic exosites and cleavage sites, activating the proteolysis of VWF within platelet-rich thrombi in flowing blood and preventing microvascular thrombosis.
Results
Differential Cleavage of GST-VWF73 and GST-VWF64 by Plasma ADAMTS13.
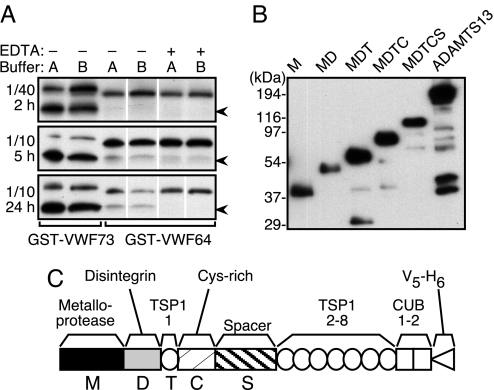
Variations in VWF unfolding complicate the analysis of ADAMTS13 enzyme kinetics, but cleavage can be studied independently of substrate unfolding with fragments of VWF domain A2 (18) that do not have significant secondary structure (19). As reported in ref. 18, a low concentration of plasma ADAMTS13 (≈0.07 nM) cleaved GST-VWF73, which contains amino acid residues Asp1596–Arg1668 from VWF domain A2 but not GST-VWF64, which lacks 9 aa residues (E1660APDLVLQR1668) that are present at the C terminus of GST-VWF73. However, detectable cleavage of GST-VWF64 did occur on increasing the enzyme concentration 4-fold and increasing the reaction time from 2 h to 5 or 24 h (Fig. 1A). Similar results were obtained in a buffer commonly used for ADAMTS13 assays (5 mM Tris·HCl, pH 8.0/10 mM BaCl2) (18) and in a more physiological buffer with optimized divalent metal ion concentrations (50 mM Hepes, pH 7.4/150 mM NaCl/5 mM CaCl2/0.1 μM ZnCl2) (20). GST-VWF64 preparations with and without a C-terminal His6 tag were cleaved at similar rates (data not shown), indicating that slow cleavage is attributable to the absence of the amino acid residues Glu1660–Arg1668 and not to relocation of the His6 tag.
Fig. 1.
Plasma and recombinant ADAMTS13. (A) GST-VWF73 and GST-VWF64 were incubated for the indicated time with plasma diluted 1/40 or 1/10 without (−) or with (+) 10 mM EDTA in buffer A (5 mM Tris·HCl, pH 8.0/10 mM BaCl2) or buffer B (50 mM Hepes, pH 7.4/150 mM NaCl/5 mM CaCl2/0.1 μM ZnCl2). Plasma contains ≈3 nM ADAMTS13. Substrates and 28-kDa cleaved products (arrowhead) were detected by gel electrophoresis and Western blotting with anti-GST antibody. Results are representative of at least three experiments. (B) ADAMTS13 variants expressed in 293 cell lines and in conditioned media were quantitated by SDS/PAGE, Western blotting with anti-V5 antibody and densitometry. M, 83 nM; MD, 58 nM; MDT, 218 nM; MDTC, 143 nM; MDTCS, 71 nM; ADAMTS13, 209 nM. Results are representative of at least three experiments. (C) ADAMTS13 consists of a metalloprotease domain (M), a disintegrin domain (D), a thrombospondin type 1 repeat (TSP1), a Cys-rich domain (C), a spacer domain (S), seven additional thrombospondin type 1 repeats (TSP1 2–8), and two complement Clr/Cls, sea urchin epidermal growth factor, bone morphogenetic protein or CUB domains (CUB). Constructs contain a V5 epitope and His6 tag (V5-H6) at the C terminus. Constructs truncated at domain boundaries encode ADAMTS13 through amino acid residue Gln289 (M), Gly385 (MD), Glu439 (MDT), Cys555 (MDTC), and Ala685 (MDTCS).
Cleavage of GST-VWF73 and GST-VWF64 by Recombinant ADAMTS13 Variants.
Molecular modeling suggests that the critical amino acids missing from VWF64 comprise a C-terminal α-helix in the VWF A2 domain (13), which could provide an exosite for substrate recognition by ADAMTS13. Deletion of the ADAMTS13 spacer domain also markedly impairs the cleavage of VWF (16, 17), suggesting that the spacer domain might bind such an exosite. To assess this potential interaction, recombinant ADAMTS13 and selected variants (Fig. 1B) were compared for activity with the substrates GST-VWF73 and GST-VWF64.
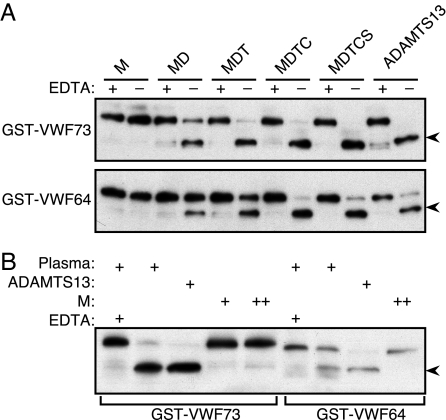
When used at the concentration of plasma ADAMTS13 (3 nM), all recombinant forms of ADAMTS13 except for the isolated metalloprotease domain (M) cleaved both GST-VWF73 and GST-VWF64 during a 5-h reaction (Fig. 2A). The M construct also did not cleave either substrate when reactions were extended to 24 h and the enzyme concentration was increased to 30 nM (Fig. 2B). No aberrant cleavage products were observed for any construct, and EDTA blocked cleavage completely. The amino acid sequence of the C-terminal GST-VWF64 cleavage product, determined by Edman degradation, began with MVTGNPASD. Thus, ADAMTS13 cleaves the Tyr1605–Met1606 bond in GST-VWF64, GST-VWF73 (21), and plasma VWF (6–8).
Fig. 2.
Substrate cleavage by ADAMTS13 and variants. (A) Samples containing 3 nM ADAMTS13 or variants were incubated for 5 h with 65 nM GST-VWF73 or GST-VWF64 in reaction buffer B with (+) or without (−) 10 mM EDTA. Results are representative of three independent experiments. (B) Comparison of plasma and recombinant ADAMTS13. Substrates (65 nM) were incubated with plasma (≈0.3 nM ADAMTS13), full-length recombinant ADAMTS13 (3 nM), or construct M (+, 3 nM; ++, 30 nM) for 24 h. Substrates and 28-kDa cleaved products (arrowheads) were detected by gel electrophoresis and Western blotting with anti-GST antibody.
The ADAMTS13 Spacer Domain Accelerates GST-VWF73 Cleavage.
In preliminary studies by an ELISA method (22), the reaction rate depended on specific domains of ADAMTS13, which differed for the two substrates. GST-VWF73 was cleaved ≈60% in 30 min by full-length ADAMTS13 and even more rapidly by construct MDTCS. Deletion of the spacer domain markedly decreased the cleavage rate, and construct M was inactive (Fig. 3A). As expected, GST-VWF64 was cleaved slowly by full-length ADAMTS13. The largest differences in rate were attributable to the spacer domain. In particular, MDTCS cleaved GST-VWF73 >8-fold faster than GST-VWF64, whereas MDTC cleaved both substrates at the same rate.
Fig. 3.
Kinetic studies of ADAMTS13 and variants. (A) Cleavage of immobilized GST-VWF73 and GST-VWF64 by 3 nM ADAMTS13 (▵), MDTCS (□), MDTC (●), MDT (○), MD (▴), and M (■) was performed by using an ELISA method to detect the remaining uncleaved substrate. Mean values for three independent experiments are shown. (B) The N-terminal ADAMTS13 cleavage product for substrate VWF73 or VWF64 (GPLGSDREQAPNLVY; 1,615 Da) was quantitated by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) by using an internal standard synthetic peptide having Leu at position 3 isotopically substituted with 13C and 15N, which increases the peptide mass to 1,622 Da. The product concentration was calculated from the ratio of peak areas for product peptide to standard peptide, times the internal standard concentration ([P] = (Areap/Areais) × [standard]). (C) Initial rates were determined for the cleavage of VWF73 and VWF64 by full-length ADAMTS13 (▵), MDTCS (□), and MDTC (●). Each point represents the average of at least two independent experiments. The lines represent the nonlinear least-squares fit of the Michaelis–Menten equation.
Kinetics of VWF73 and VWF64 Cleavage.
A quantitative mass spectrometry assay was developed to evaluate the role of the ADAMTS13 spacer domain and the C-terminal Glu1660–Arg1668 segment of VWF domain A2 in substrate recognition. Substrates VWF73 and VWF64 were prepared by digesting GST-VWF73 and GST-VWF64, respectively, with rhinovirus 3C protease to remove the GST moiety. Subsequent cleavage by ADAMTS13 released a small N-terminal 1,615-Da product peptide that was detected by MALDI-TOF MS. The amount of product was determined with reference to an internal standard peptide identical in sequence but with 7 Da greater mass resulting from isotopic substitution (Fig. 3B). The assay was linear over the 2.5- 250-fmol product, and the interassay coefficient of variation was 8.5% (n = 12).
With this assay, kinetic constants were determined for full-length ADAMTS13, MDTCS, and MDTC (Fig. 3C and Table 1). ADAMTS13 cleaved VWF73 with Km of 1.7 μM, kcat of 1.3 s−1, and specificity constant (kcat/Km) of 7.5 × 105 M−1 s−1. Similar values of kcat were observed for ADAMTS13, MDTCS, and MDTC with both substrates VWF73 and VWF64, suggesting that all of the recombinant proteases are properly folded and have comparable activity. As judged by specificity constants (kcat/Km), all enzymes cleaved VWF64 with similar efficiency. MDTC cleaved VWF73 and VWF64 with a similar kcat/Km of 1.1 × 105 M −1 s−1 and 0.89 × 105 M−1 s−1, respectively. In contrast, ADAMTS13 and MDTCS cleaved substrate VWF73 15-fold to 20-fold more readily than substrate VWF64. Therefore, rapid substrate cleavage depends on the presence of both the C-terminal Glu1660–Arg1668 segment of VWF73 and the spacer domain of ADAMTS13.
Table 1.
Kinetic parameters for cleavage of VWF73 and VWF64
| Enzyme | VWF73 |
VWF64 |
VWF73/VWF64 |
||||
|---|---|---|---|---|---|---|---|
| Km, μM | kcat, s−1 | kcat/Km, μM−1 s−1 | Km, μM | kcat, s−1 | kcat/Km, μM−1 s−1 | kcat/Km:kcat/Km | |
| ADAMTS13 | 1.7 ± 0.4 | 1.3 ± 0.1 | 0.75 ± 0.16 | 37.7 ± 12.8 | 1.8 ± 0.4 | 0.05 ± 0.02 | 15.3 |
| MDTCS | 0.8 ± 0.2 | 1.7 ± 0.1 | 2.0 ± 0.6 | 5.5 ± 1.4 | 0.56 ± 0.04 | 0.10 ± 0.03 | 20.5 |
| MDTC | 16.0 ± 4.5 | 1.8 ± 0.3 | 0.11 ± 0.04 | 17.9 ± 6.3 | 1.6 ± 0.3 | 0.09 ± 0.04 | 1.2 |
Values are the mean ± SD of n = 3 experiments, except for VWF73 cleavage by ADAMTS13 and MDTC, where n = 2.
Kinetic Evidence for Exosite-Mediated Substrate Binding to the Spacer Domain.
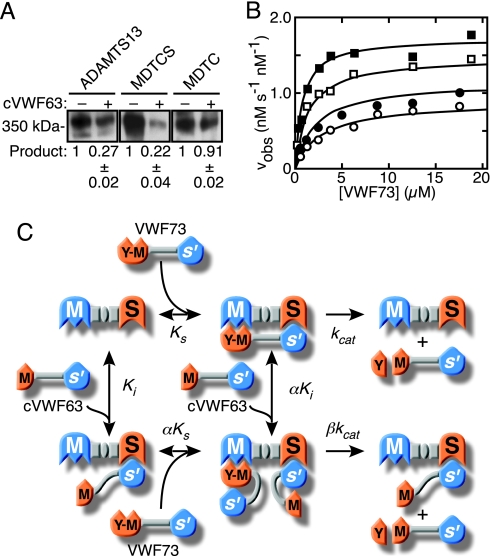
Differences in the values for Km account for most of the observed differences in reaction rates, suggesting that the Glu1660–Arg1668 segment of VWF73 and the ADAMTS13 spacer domain contribute significantly to substrate binding affinity and might interact directly. If so, the C-terminal cleavage product of VWF73 (cVWF63) would be expected to inhibit MDTCS but not MDTC, which lacks a spacer domain. When tested at a fixed concentration (7.5 μM), cVWF63 did inhibit MDTCS activity toward either substrate VWF73 or VWF64 (1.5 μM) by >82%, but it had no effect on the activity of MDTC (Table 2). Product cVWF63 also inhibited ADAMTS13 and MDTCS cleavage of multimeric plasma VWF by 73–78%, but it inhibited MDTC <10% (Fig. 4A), demonstrating a role for the ADAMTS13 spacer in recognizing full-length VWF as well as model peptide substrates.
Table 2.
Product inhibition of MDTCS and MDTC by cVWF63
| Enzyme | VWF73 |
VWF64 |
||
|---|---|---|---|---|
| −cVWF63 | +cVWF63 | −cVWF63 | +cVWF63 | |
| nM product s−1 | nM ADAMTS13 s−1 | |||
| MDTCS | 1.2 ± 0.1 | 0.13 ± 0.01 | 0.11 ± 0.02 | 0.02 ± 0.001 |
| MDTC | 0.14 ± 0.015 | 0.13 ± 0.01 | 0.09 ± 0.01 | 0.08 ± 0.005 |
Initial cleavage rates without (−) and with (+) 7.5 μM cVWF63. The concentration of substrate was 1.5 μM. Values are the mean ± SD for at least three independent experiments.
Fig. 4.
Product inhibition and exosite binding. (A) Plasma VWF (20 nM) was pretreated with 1.2 M guanidine HCl to induce a protease-sensitive conformation (20), diluted 10-fold into reactions containing ADAMTS13 (1 nM), MDTCS (1 nM), or MDTC (5 nM), without (−) or with (+) 2 μM cVWF63, and incubated for 30 min (ADAMTS13, MDTCS) or 60 min (MDTC). The expected dimeric 350-kDa product band was detected by Western blotting and densitometry. A representative blot is shown. Exposure was 15 s for ADAMTS13 and MDTCS and 5 min for MDTC. Product generation with cVWF63 was normalized to the value obtained without cVWF63. Results are expressed as the mean ± SD for three independent experiments. (B) Initial reaction rates were measured by MALDI-TOF MS with 0.11 nM MDTCS in the absence (■; data of Fig. 2C) or presence of 0.5 μM (□), 1.5 μM (●), or 3.0 μM (○) cVWF63, the C-terminal product of VWF73 cleavage. Each point represents the average of at least two independent experiments. The lines represent the global least-squares fit with a mixed-type modifier model (C) in which the Tyr1605–Met1606 region (Y-M) of substrate VWF73 engages the metalloprotease domain (M) of MDTCS, and an exosite involving C-terminal residues Glu1660–Arg1668 engages the spacer domain (S). Cleavage of VWF73 produces a small N-terminal peptide (Y) and larger C-terminal cVWF63. Product cVWF63 binds to the spacer domain, which inhibits the binding and cleavage of VWF73.
Kinetic analysis (Fig. 4B) showed that product cVWF63 inhibited the cleavage of VWF73 in a manner consistent with mixed-type inhibition of enzyme MDTCS (Fig. 4C). In this model, the inhibitor cVWF63 binds through an exosite interaction to the enzyme MDTCS with a Ki of 0.44 ± 0.11 μM and to the MDTCS-VWF73 complex with αKi of 2.4 ± 1.1 μM, where the errors represent the SD for the global fit of the data to Eq. 1 (see Methods). Similarly, substrate VWF73 binds to MDTCS with a Ks of 0.77 ± 0.11 μM and to the complex of MDTCS and cVWF63 with αKs of 4.3 ± 1.8 μM. The MDTCS–VWF73 enzyme–substrate complex yields product at a rate kcat of 1.7 ± 0.05 s−1. The ternary cVWF63–MDTCS–VWF73 inhibitor–enzyme–substrate complex yields product at a much slower rate that approaches zero at sufficiently high concentrations of cVWF63 (βkcat of 0.2 ± 0.2 s−1). This exosite-dependent mechanism accounts for the observed kinetics of product inhibition and for the dependence of reaction rates on both the Glu1660–Arg1668 segment of VWF73 and the ADAMTS13 spacer domain.
Discussion
The balanced regulation of VWF cleavage in vivo depends on several cooperative interactions with ADAMTS13. The metalloprotease domain of ADAMTS13 must recognize and cleave the Tyr1605–Met1606 bond of VWF domain A2, but the isolated metalloprotease domain does not cleave VWF multimers or fragments of the VWF A2 domain at a significant rate (Figs. 2 and 3) (16, 17), and it may cut the wrong bond (23). Construct MD slowly cleaves the correct Tyr1605–Met1606 bond of GST-VWF73 (Figs. 2 and 3) (23), suggesting that the disintegrin domain may help to position the substrate in the active site. In addition, removal of the spacer (e.g., construct MDTC) markedly impairs substrate cleavage, whereas constructs containing the spacer domain (e.g., MDTCS) cleave multimeric VWF, GST-VWF73 (Fig. 3A), or VWF73 (Fig. 3C and Table 1) at essentially a normal rate (16, 17, 23). Therefore, the spacer domain is a major determinant of catalytic efficiency.
ADAMTS13 binds with high affinity and cleaves either multimeric VWF or GST-VWF73, provided the spacer domain is present (12, 23). Therefore, the structural requirements for ADAMTS13 to cleave small substrates like VWF73 are similar to those for cleaving multimeric VWF, which implies that interactions limited to the A2 domain are sufficient to determine the substrate specificity of ADAMTS13. Furthermore, our kinetic data, particularly the effects of product inhibition (Fig. 4 and Table 2), indicate that the ADAMTS13 spacer domain binds to an exosite within VWF domain A2 that is distant from the scissile bond and requires residues Glu1660–Arg1668. Disruption of this exosite in the VWF substrate or deletion of the spacer domain from ADAMTS13 causes a similar marked reduction in the rate of substrate cleavage. In addition, ADAMTS13 does not bind native multimeric VWF unless it is treated with chaotropic agents or adsorbed to a surface (12), suggesting that the exosite, like the scissile bond, is buried within the folded A2 domain.
At maximum extension, the distance between the scissile bond (Tyr1605–Met1606) and the exosite (up to Arg1668) could be as much as 220 Å (3.5 Å per residue), and ADAMTS13 appears able to accommodate a substrate of this length. For example, a combination of a metalloprotease domain [ADAM33, protein data base (PDB) ID code 1R54; ≈26 × ≈38 × ≈52 Å] (24), a disintegrin (trimestatin, PDB ID code 1J2L; ≈15 × ≈22 × ≈48 Å) (25), and a TSP1 repeat (PDB ID code 1LSL; ≈15 × ≈20 × ≈55 Å) (26) might span ≈150 Å. The Cys-rich and spacer domains, comprising 246 aa residues and 27.5 kDa, would complete the substrate binding surface. ADAMTS13 domains between the metalloprotease and spacer presumably would interact with VWF domain A2 sequences between the scissile bond and exosite, and the first TSP1 repeat is a likely candidate based on the effects of mutagenesis. Deletion of the ADAMTS13 spacer increases the Kd for binding immobilized VWF from ≈14 nM to ≈200 nM, and further deletion of the first TSP1 domain abolishes specific binding (12).
Structures other than the proximal ADAMTS13 domains and the VWF A2 domain also contribute to the regulation of VWF proteolysis. Deletion of the C-terminal TSP1 repeats and CUB domains has small but significant effects on binding affinity for multimeric VWF (12) and on the cleavage of both VWF73 and VWF64 (Table 1), suggesting that distal ADAMTS13 domains can influence the recognition of substrates by more proximal domains. The VWF A3 domain, just C-terminal to the homologous A2 domain, also may bind to the CUB domains of ADAMTS13 and help to localize it in flowing blood (27, 28), although the VWF A3 domain has no effect on substrate cleavage under static conditions (14, 15, 29).
The VWF A1 domain regulates ADAMTS13 through yet another mechanism, by providing a docking site for activating cofactors. We have shown that VWF domain A1 inhibits the cleavage of the adjacent domain A2, and this inhibition can be alleviated by the interaction of domain A1 with platelet GPIbα or glycosaminoglycans (14). A recent study also shows that VWF domain A1 binds chloride ions, which inhibits the cleavage of the Tyr1605–Met1606 bond in domain A2 by ADAMTS13 (15). In addition, the conformation of domain A1 changes when it binds to GPIbα, and a VWD type 2B mutation within the A1 domain that stabilizes this bound structure reduces chloride binding and accelerates the cleavage of the adjacent A2 domain (15). Thus, cofactor binding to VWF domain A1 appears to modify the A2 domain allosterically, promoting the exposure of its cryptic scissile bond and exosite to ADAMTS13.
Our findings, together with results from several other approaches, support a model for regulating platelet adhesion primarily by tension-activated proteolysis. Membrane GPIbα on adherent platelets binds to the A1 domain of VWF, and this interaction allosterically destabilizes the adjacent domain A2 (14, 15). In addition, fluid shear stress in flowing blood acts on the adherent platelets and applies tensile stress to the VWF multimers, which unfolds the A2 domain and exposes the Tyr1605–Met1606 bond to the ADAMTS13 active site (7, 13) as well as a C-terminal exosite for binding to the ADAMTS13 spacer domain (12, 16, 17, 23). ADAMTS13 severs the VWF multimer, releasing the adherent platelets and limiting thrombus growth. These cooperative interactions between the active site and exosite limit the action of ADAMTS13 in time and space, confining it to platelet-rich thrombi that experience enough fluid shear stress. The physiologic importance of the VWF exosite–ADAMTS13 spacer interaction is emphasized by the finding that autoantibodies to the spacer domain inhibit ADAMTS13 and are common causes of thrombotic thrombocytopenic purpura (17, 30, 31).
The VWF-ADAMTS13 system appears to be unique in its use of tension-induced protein unfolding as the signal for biologically essential feedback inhibition, in this case mediated by proteolysis. However, similar mechanisms might evolve wherever tension on a protein sensor could expose cleavage sites or exosites to a suitable protease. Such a mechanism is particularly appropriate to the circulatory system, where pathologically increased fluid shear stress is characteristic of injured blood vessels that are in danger of thrombotic occlusion.
Methods
Preparation of ADAMTS13 Substrates.
Plasmids were constructed as described in ref. 18 to express proteins composed of Schistosoma japonicum GST, a cleavage site for human rhinovirus 3C protease, a segment of VWF domain A2, and a C-terminal His6 tag. The desired VWF coding sequence was amplified by PCR using the template pSVHvWF1 (32), Pfu Turbo DNA polymerase (Stratagene, La Jolla, CA), and appropriate oligonucleotide primers (18). PCR products were inserted between the BamHI and EcoRI sites of plasmid pGEX-6P-1 (Amersham Biosciences, Piscataway, NJ). Plasmid pGST-VWF73 encodes VWF residues Asp1596–Arg1668, and plasmid pGST-VWF64 encodes VWF residues Asp1596–Arg1659.
GST-VWF73 and GST-VWF64 were expressed in Escherichia coli BL21 (18). Bacteria were grown to log phase in LB medium at 37°C and induced for 4 h with 0.25 mM isopropyl β-d-thiogalactoside. Cells were harvested, and soluble fractions were prepared by using CelLytic B (Sigma, St. Louis, MO). Proteins from a 0.8-liter culture were adsorbed onto 3 ml of Ni2+-nitrilotriacetic acid-agarose beads (Qiagen, Valencia, CA) and eluted with 50 mM sodium phosphate, pH 8.0/250 mM imidazole. Proteins were dialyzed against 20 mM Tris·HCl, pH 8.0/150 mM NaCl, adsorbed onto a 2-ml column of glutathione-agarose (Amersham Biosciences), and eluted with buffer containing 10 mM glutathione. After dialysis against 20 mM Tris·HCl, pH 8.0/150 mM NaCl, the protein concentration was determined with a BCA protein assay kit (Pierce, Rockford, IL) and a BSA standard.
To obtain VWF73 and VWF64 peptides, GST-VWF73 and GST-VWF64 were cleaved with PreScission protease (Amersham Biosciences) at 2 units of enzyme per 100 μg of substrate for 10 h at 4°C. PreScission protease and released GST were removed by adsorption onto 2 ml of glutathione-agarose. Products in the supernatant were purified by HPLC on a C18 column (10 × 150 mm 218TP; Vydac, Hesperia, CA) with an acetonitrile gradient (0–80%) in 0.1% trifluoroacetic acid. Peptides were concentrated by using vacuum and dialyzed against 50 mM Hepes, pH 7.4/150 mM NaCl. Peptide concentrations were determined by amino acid analysis and absorbance at 280 nm. Substrates were stored at −80°C.
ADAMTS13 Variants.
Human ADAMTS13 (FL) and variants truncated after the Cys-rich domain (MDTC), first TSP1 repeat (MDT), disintegrin domain (MD), and metalloprotease domain (M) were expressed in stably transfected T-REx 293 cell lines as described in refs. 12 and 20. Recombinant ADAMTS13 truncated after the spacer domain (MDTCS) was expressed by transient transfection. T-REx 293 cells were cultured in DMEM containing 10% Tet-System Approved FBS (Invitrogen, Carlsbad, CA), 5 μg/ml blasticidin S, and 2 mM l-glutamine. Cells were transfected at 90% confluence by using 4 μg of plasmid DNA and 60 μl of Lipofectamine 2000 in 250 μl of Opti-MEM (Invitrogen). After 5 h, the medium was replaced with Freestyle serum-free medium (Invitrogen) containing 1 μg/ml tetracycline. Conditioned medium was collected after 24 h, and 1 μl/ml protease inhibitor mixture (Sigma) and 1 mM phenylmethylsulfonyl fluoride were added. Medium was centrifuged at 800 × g for 10 min, and the supernatant was concentrated 20-fold by ultrafiltration with a Centriprep YM-30 (Millipore Corp., Bedford, MA). ADAMTS13 protein concentrations were determined by Western blotting on PVDF membranes with anti-V5 antibody and standardized with V5-tagged Positope reference protein (Invitrogen) as described in ref. 16. Signals were detected by chemifluorescence (ECL Plus; Amersham Biosciences) with a STORM Imager and analyzed with the program ImageQuantTL (Amersham Biosciences).
Substrate Cleavage by ADAMTS13.
Reactions with plasma ADAMTS13 contained 40 μl of buffer A [5 mM Tris·HCl, pH 8.0/10 mM BaCl2 (ref. 18)] and 0.9 μl of BSA (5 mg/ml) or buffer B [50 mM Hepes, pH 7.4/150 mM NaCl/5 mM CaCl2/0.1 μM ZnCl2 (ref. 20)], 0.1 μl of 4-(2-aminoethyl)benzenesulfonyl fluoride (40 mM), and 0.1 μg of substrate. Cleavage of GST-VWF73 or GST-VWF64 was initiated by adding 1 μl or 4 μl, respectively, of citrated normal human plasma and buffer sufficient to make a total volume of 45 μl. Reactions were incubated at 37°C for 2 h, 5 h, or 24 h and stopped by adding an equal volume of 2× SDS sample buffer containing 30 mM EDTA. Samples (10 μl) were analyzed by SDS/PAGE on 10–20% gradient gels (Invitrogen), electrotransfer onto PVDF membranes, and incubation with a 1:10,000 dilution of horseradish peroxidase-conjugated rabbit anti-GST (Amersham Biosciences). Bands were detected by chemifluorescence (ECL Plus). Reactions with recombinant ADAMTS13 or variants were performed similarly by using reaction buffer B, substrate concentration of 3 or 30 nM, and an incubation time of 5 h or 24 h.
Alternatively, an ELISA method was used (22) with minor modification. Microtiter plates precoated with anti-GST antibodies (Pierce) were incubated 1 h at room temperature with GST-VWF73 or GST-VWF64 (0.25 μg/ml). After washing, ADAMTS13 or a variant (3 nM) in reaction buffer B (50 μl) was added and incubated at 37°C for different times. After three washes, the remaining uncleaved substrates were detected with horseradish peroxidase-conjugated anti-His antibodies (Invitrogen) or Indian His-Pro (Pierce) for 1 h at room temperature. Plates were developed by an illumination kit (Pierce) and read at 450 nm.
Cleavage Site Characterization.
GST-VWF64 (5 μg) was incubated in reaction buffer B with recombinant ADAMTS13 (30 nM) at 37°C for 5 h. Components were separated by SDS/PAGE on a 10–20% gradient gel, transferred onto a PVDF membrane (Bio-Rad, Hercules, CA), and stained with SimplyBlue SafeStain (Invitrogen). The 7.0-kDa product band was excised and sequenced by automated Edman degradation (Protein and Nucleic Acid Laboratory, Washington University).
Kinetics Studies by MALDI-TOF MS.
Reactions were performed at room temperature (25°C) in reaction buffer B containing varying concentrations of VWF73 or VWF64, and ADAMTS13 or a variant (0.056 nM enzyme for VWF73 cleavage or 0.28 nM enzyme for VWF64 cleavage). Reactions were desalted by adsorption on C18 micropipette tips (Glygen Corp., Columbia, MD) and elution with 60% acetonitrile/0.1% formic acid. Samples (1 μl) were mixed with 250 fmol (1 μl) of synthetic peptide standard (Sigma) having leucine at position 3 isotopically labeled with 13C and 15N (GP([13C15N]L)GSDREQAPNLVY; mass, 1,622). Aliquots (0.5 μl) were mixed with an equal volume of α-cyano-4-hydroxycinnamic acid matrix (6.2 mg/ml in methanol/acetonitrile/water = 36/56/8; Agilent Technologies, Santa Clara, CA) and analyzed by MALDI-TOF MS (33). Linearity of the assay was confirmed by constructing standard curves with a similar product peptide (CDREQAPNLVY; mass, 1,307) and the standard peptide. Synthetic peptide concentrations were determined by amino acid analysis. Initial rates of product generation were determined by sampling reactions at five to seven time points between 0 and 30 min (<8% substrate cleavage) and linear regression analysis. Rates were fitted to the Michaelis–Menten equation to obtain the apparent Michaelis constant (Km) and catalytic rate constant (kcat).
Inhibition of Substrate Cleavage by Product cVWF63.
GST-VWF73 (8 mg) was digested to completion by recombinant ADAMTS13 (0.3 nM) in buffer B (10 ml) for 5 h at 37°C. The C-terminal 63-aa residue product peptide (cVWF63) was purified by HPLC as described for VWF73. To assess product inhibition, reactions were performed in buffer B with varying concentrations of cVWF63, VWF73, or VWF64, and ADAMTS13 or variants. Initial rates were determined by MALDI-TOF MS analysis. Product generation as a function of enzyme (E), cVWF63 (I), and substrate (S) were analyzed according to a general model in which cVWF63 is a hyperbolic mixed-type inhibitor of ADAMTS13 (34) as shown in Fig. 4C. Eq. 1 describes the dependence of the initial velocity (vobs) on the initial concentration of substrate [S], enzyme [E], and inhibitor [I]. The equation reduces to the Michaelis–Menten equation when [I] = 0.
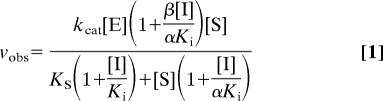 |
Cleavage of plasma VWF by ADAMTS13, MDTCS, or MDTC was assayed in reaction buffer B by Western blotting and densitometry of the 350-kDa dimer of C-terminal VWF subunit fragments (7, 20) in the presence or absence of cVWF63.
Acknowledgments
We thank the Alvin J. Siteman Cancer Center at Barnes–Jewish Hospital and Washington University School of Medicine and the Washington University Department of Medicine for the use of the Proteomics Core, particularly Alan Davis for extensive technical assistance in MALDI-TOF MS analysis. This work was supported by National Institutes of Health Grant HL72917 (to J.E.S.), American Heart Association National Scientist Development Award 053011N (to P.J.A.), and American Heart Association Fellow to Faculty Transition Award 047503N (to E.M.M.). The Siteman Cancer Center is supported in part by National Cancer Institute Cancer Center Support Grant P30 CA91842.
Abbreviations
- ADAMTS
a disintegrin and metalloprotease with thrombospondin
- cVWF63
cleavage product of VWF73
- GPIbα
glycoprotein Ibα
- TSP1
thrombospondin type 1 repeat
- VWF
von Willebrand factor.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Sadler JE. Annu Rev Biochem. 1998;67:395–424. doi: 10.1146/annurev.biochem.67.1.395. [DOI] [PubMed] [Google Scholar]
- 2.Ruggeri ZM. J Thromb Haemostasis. 2003;1:1335–1342. doi: 10.1046/j.1538-7836.2003.00260.x. [DOI] [PubMed] [Google Scholar]
- 3.Zheng X, Chung D, Takayama TK, Majerus EM, Sadler JE, Fujikawa K. J Biol Chem. 2001;276:41059–41063. doi: 10.1074/jbc.C100515200. [DOI] [PubMed] [Google Scholar]
- 4.Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, McGee BM, Yang AY, Siemieniak DR, Stark KR, Gruppo R, et al. Nature. 2001;413:488–494. doi: 10.1038/35097008. [DOI] [PubMed] [Google Scholar]
- 5.Soejima K, Mimura N, Hirashima M, Maeda H, Hamamoto T, Nakagaki T, Nozaki C. J Biochem. 2001;130:475–480. doi: 10.1093/oxfordjournals.jbchem.a003009. [DOI] [PubMed] [Google Scholar]
- 6.Dent JA, Berkowitz SD, Ware J, Kasper CK, Ruggeri ZM. Proc Natl Acad Sci USA. 1990;87:6306–6310. doi: 10.1073/pnas.87.16.6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsai H-M. Blood. 1996;87:4235–4244. [PubMed] [Google Scholar]
- 8.Furlan M, Robles R, Lämmle B. Blood. 1996;87:4223–4234. [PubMed] [Google Scholar]
- 9.Furlan M, Robles R, Galbusera M, Remuzzi G, Kyrle PA, Brenner B, Krause M, Scharrer I, Aumann V, Mittler U, et al. N Engl J Med. 1998;339:1578–1584. doi: 10.1056/NEJM199811263392202. [DOI] [PubMed] [Google Scholar]
- 10.Tsai HM, Lian EC. N Engl J Med. 1998;339:1585–1594. doi: 10.1056/NEJM199811263392203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majerus EM, Zheng X, Tuley EA, Sadler JE. J Biol Chem. 2003;278:46643–46648. doi: 10.1074/jbc.M309872200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Majerus EM, Anderson PJ, Sadler JE. J Biol Chem. 2005;280:21773–21778. doi: 10.1074/jbc.M502529200. [DOI] [PubMed] [Google Scholar]
- 13.Sutherland JJ, O'Brien LA, Lillicrap D, Weaver DF. J Mol Model. 2004;10:259–270. doi: 10.1007/s00894-004-0194-9. [DOI] [PubMed] [Google Scholar]
- 14.Nishio K, Anderson PJ, Zheng XL, Sadler JE. Proc Natl Acad Sci USA. 2004;101:10578–10583. doi: 10.1073/pnas.0402041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Cristofaro R, Peyvandi F, Baronciani L, Palla R, Lavoretano S, Lombardi R, Di Stasio E, Federici AB, Mannucci PM. J Biol Chem. 2006;281:30400–30411. doi: 10.1074/jbc.M603321200. [DOI] [PubMed] [Google Scholar]
- 16.Zheng X, Nishio K, Majerus EM, Sadler JE. J Biol Chem. 2003;278:30136–30141. doi: 10.1074/jbc.M305331200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soejima K, Matsumoto M, Kokame K, Yagi H, Ishizashi H, Maeda H, Nozaki C, Miyata T, Fujimura Y, Nakagaki T. Blood. 2003;102:3232–3237. doi: 10.1182/blood-2003-03-0908. [DOI] [PubMed] [Google Scholar]
- 18.Kokame K, Matsumoto M, Fujimura Y, Miyata T. Blood. 2004;103:607–612. doi: 10.1182/blood-2003-08-2861. [DOI] [PubMed] [Google Scholar]
- 19.Sadler JE, Moake JL, Miyata T, George JN. Hematology. 2004:407–423. doi: 10.1182/asheducation-2004.1.407. [DOI] [PubMed] [Google Scholar]
- 20.Anderson PJ, Kokame K, Sadler JE. J Biol Chem. 2006;281:850–857. doi: 10.1074/jbc.M504540200. [DOI] [PubMed] [Google Scholar]
- 21.Wu JJ, Fujikawa K, Lian EC, McMullen BA, Kulman JD, Chung DW. J Thromb Haemostasis. 2006;4:129–136. doi: 10.1111/j.1538-7836.2005.01677.x. [DOI] [PubMed] [Google Scholar]
- 22.Zhou W, Tsai HM. Thromb Haemostasis. 2004;91:806–811. doi: 10.1160/TH03-11-0675. [DOI] [PubMed] [Google Scholar]
- 23.Ai J, Smith P, Wang S, Zhang P, Zheng XL. J Biol Chem. 2005;280:29428–29434. doi: 10.1074/jbc.M505513200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orth P, Reichert P, Wang W, Prosise WW, Yarosh-Tomaine T, Hammond G, Ingram RN, Xiao L, Mirza UA, Zou J, et al. J Mol Biol. 2004;335:129–137. doi: 10.1016/j.jmb.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 25.Fujii Y, Okuda D, Fujimoto Z, Horii K, Morita T, Mizuno H. J Mol Biol. 2003;332:1115–1122. doi: 10.1016/s0022-2836(03)00991-4. [DOI] [PubMed] [Google Scholar]
- 26.Tan K, Duquette M, Liu JH, Dong Y, Zhang R, Joachimiak A, Lawler J, Wang JH. J Cell Biol. 2002;159:373–382. doi: 10.1083/jcb.200206062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong JF, Moake JL, Bernardo A, Fujikawa K, Ball C, Nolasco L, Lopez JA, Cruz MA. J Biol Chem. 2003;278:29633–29639. doi: 10.1074/jbc.M301385200. [DOI] [PubMed] [Google Scholar]
- 28.Tao Z, Peng Y, Nolasco L, Cal S, Lopez-Otin C, Li R, Moake JL, Lopez JA, Dong JF. Blood. 2005;106:4139–4145. doi: 10.1182/blood-2005-05-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zanardelli S, Crawley JT, Chion CK, Lam JK, Preston RJ, Lane DA. J Biol Chem. 2005;281:1555–1563. doi: 10.1074/jbc.M508316200. [DOI] [PubMed] [Google Scholar]
- 30.Klaus C, Plaimauer B, Studt JD, Dorner F, Lammle B, Mannucci PM, Scheiflinger F. Blood. 2004;103:4514–4519. doi: 10.1182/blood-2003-12-4165. [DOI] [PubMed] [Google Scholar]
- 31.Luken BM, Turenhout EA, Hulstein JJ, Van Mourik JA, Fijnheer R, Voorberg J. Thromb Haemostasis. 2005;93:267–274. doi: 10.1160/TH04-05-0301. [DOI] [PubMed] [Google Scholar]
- 32.Matsushita T, Sadler JE. J Biol Chem. 1995;270:13406–13414. doi: 10.1074/jbc.270.22.13406. [DOI] [PubMed] [Google Scholar]
- 33.Houston CT, Taylor WP, Widlanski TS, Reilly JP. Anal Chem. 2000;72:3311–3319. doi: 10.1021/ac991499m. [DOI] [PubMed] [Google Scholar]
- 34.Segel IH. Enzyme Kinetics: Behavior and Analysis of Raid Equilibrium and Steady-State Enzyme Systems. New York: Wiley; 1993. pp. 178–192. [Google Scholar]