Abstract
We have developed a wet lab DNA microarray simulation as part of a complete DNA microarray module for high school students. The wet lab simulation has been field tested with high school students in Illinois and Maryland as well as in workshops with high school teachers from across the nation. Instead of using DNA, our simulation is based on pH indicators, which offer many ideal teaching characteristics. The simulation requires no specialized equipment, is very inexpensive, is very reliable, and takes very little preparation time. Student and teacher assessment data indicate the simulation is popular with both groups, and students show significant learning gains. We include many resources with this publication, including all prelab introductory materials (e.g., a paper microarray activity), the student handouts, teachers notes, and pre- and postassessment tools. We did not test the simulation on other student populations, but based on teacher feedback, the simulation also may fit well in community college and in introductory and nonmajors' college biology curricula.
INTRODUCTION
Every day, newspapers publish stories about discoveries made using genomic methods. One popular method is the DNA microarray, which allows investigators to measure the level of gene activity for an entire genome. Recent research has revealed that DNA microarrays can be used to better diagnose diseases such as cancer. Soon, microarrays will be a part of clinical diagnosis (Kakiuchi et al., 2004). It is increasingly important for more people to understand genomic methods. Fortunately, undergraduates are gaining access to DNA microarrays through a number of initiatives (Brewster et al., 2004; Campbell et al., 2006; Campbell and Heyer, 2006a, 2006b; Genome Consortium for Active Teaching [GCAT], 2006a). However, high school students often miss out on learning the newest methods such as microarrays because of constraints on time and funding (National Research Council [NRC], 2002).
We have developed a wet lab simulation that is part of a 2- to 3-d DNA microarray module that teaches high school students about DNA microarrays. Our goals included 1) providing an interactive way to experience how microarrays are used to study gene expression; 2) teaching students that DNA microarrays can measure the activity of many genes simultaneously; and 3) enabling students to discover that genes are differentially regulated (expressed differently under different conditions). This module uses a wet lab simulation (in combination with a paper lab exercise; Zanta, 2004, 2006) to teach students how DNA microarray experiments are performed. Furthermore, through the simulation, students learn that genes are differentially regulated. In preliminary testing, the microarray simulation facilitated active, hands-on learning; the students enjoyed the lab; and they significantly improved their scores on surveys conducted before and after the simulation. The simulation is very reliable and fits within a 45-min class period. The reagents are inexpensive and can be prepared once for multiple classes. In today's educational testing climate, for a new biology module to be incorporated into the curriculum, it must address some of the National Science Education Standards (NRC, 1996) that appear on standardized tests. The microarray simulation uses a case study in cancer biology to help students address several education standards covered in end-of-year tests (see Supplemental Material 1).
A recent study by the NRC (2005) found that U.S. high school classrooms frequently lack challenging and meaningful laboratory experiences for students. The NRC report outlined what constitutes a good lab. Students should have hands-on and minds-on opportunities to learn. Furthermore, college faculty often complain that entering students are poorly prepared for modern biology. Therefore, there is a need for curricular materials that will help high school teachers provide high-quality, interesting lab experiences for their students and help prepare them for college biology courses. This article describes a simulation that can be used for large numbers of students. The simulation has been used with >100 high school teachers in national workshops, with two teachers and 338 students in Hinsdale, IL, and with four high school teachers and ∼150 students in the Montgomery County School District in Maryland. The appendices in the Supplemental Material online provide the wet lab handouts and all assessment tools; the paper lab exercise is also available online (Zanta, 2004). The simulation has been commercialized by Genisphere (Hatfield, PA) with the agreement that we would be able to publish a “how to create your own” version.
DNA Microarray Methodology
DNA microarrays are a high-throughput method used to survey the relative amount of transcription (gene expression) for every gene in a genome. DNA microarrays (sometimes referred to as gene chips) do not allow absolute levels of quantification of gene expression (e.g., 250 mRNA molecules per cell). However, DNA chips do allow investigators to determine how much mRNA was produced in a sample relative to the amount of mRNA produced by a control population. An example study might compare lung cancer tissue to healthy lung tissue in order to determine whether there is an increase (induction) or decrease (repression) of gene expression and, if so, by how much. The relative amounts of mRNA produced by a particular gene in two samples can be used to produce a ratio that indicates the differences in transcription. For example, if Gene A produced 250 mRNA molecules in healthy lung cells and 1000 mRNAs in lung cancer cells, then Gene A is induced fourfold in lung cancer (i.e., 1000 ÷ 250 = 4). Conversely, perhaps healthy cells produce 4000 mRNA molecules from Gene B but lung cancer cells only produce 500, then Gene B is repressed eightfold in cancer cells (i.e., 4000 ÷ 500 = 8). However, some genes will not show differences in the level of transcription between the lung cancer cells and healthy lung cells, and thus their ratio of gene regulation will be approximately one. If we wanted to understand the causes of lung cancer, we might want to focus on Gene A and Gene B rather than the hundreds that were equivalent in the two tissues.
Scientists and clinicians are collaborating to improve the diagnosis of diseases such as lung cancer. Currently, diagnoses are made in broad categories based on clinical observations, and all patients are treated the same within a category. Using DNA chips, many investigators believe medicine can become personalized such that each patient will be prescribed medical treatment that will best match his or her illness. Within their lifetimes, today's high school students will probably benefit clinically from DNA chip–based diagnosis. Approximately 1 of 3 women and 1 of 2 men in the United States will develop cancer (American Cancer Society, 2006), and many of them may be diagnosed with DNA microarrays.
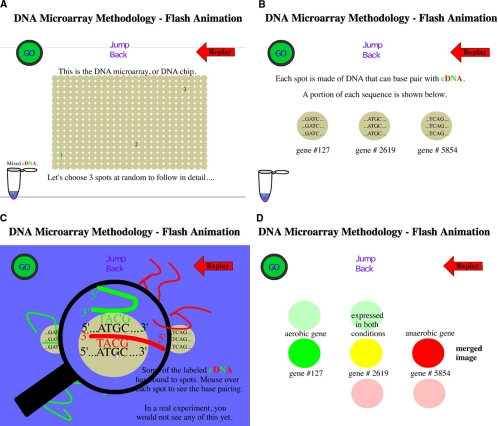
With this great potential for social impact, it is important that high school students understand gene chips, regardless of their post–high school career plans. The wet lab simulation and accompanying case study provide a realistic scenario that is easy for students to follow and makes the cancer scenario relevant to their lives. The student-friendly microarray module that we have developed can easily be integrated into high school, community college, and introductory college biology curricula. As an introduction to the gene chip methodology, students read the handouts we have produced (see Supplemental Material 3) and view a free animation (Campbell, 2000; Figure 1). DNA microarrays that measure gene activity require many steps, all of which are too small to observe by eye. Therefore, a paper microarray lab (Zanta, 2004) combined with the animation provide a good foundation that prepares students for the wet lab simulation.
Figure 1.
Screen shots of DNA microarray animation. Students are directed to view this animation along with reading some brief introductory materials (see Supplemental Material 3) before the first class session. (A–D) Different stages of the animation after cDNA synthesis.
MATERIALS AND METHODS
Simulated Spotted DNA
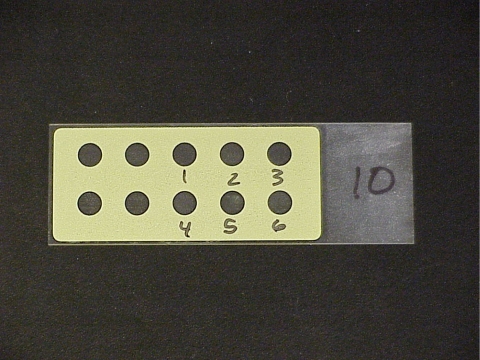
We used two different pH indicators that are colorless at neutral pH and colored at high pH (pH >10). In particular, we used thymolphthalein (Acros Organics, Pittsburgh, PA; 151460100) and phenolphthalein (Fisher Scientific, Pittsburgh, PA; P79–100). Fifty milligrams of phenolphthalein was dissolved in 50 ml ethanol and then mixed with 50 ml of water. Fifty milligrams of thymolphthalein was dissolved in 50 ml ethanol and then mixed with 50 ml of water. These pH indicators were mixed with molten agarose (Promega, Madison, WI; V312A) and allowed to cool. They could be placed in a hot water bath (65°C) and kept molten if they were to be used that day. If used days later, the cooled agarose and dye indicator mixtures were microwaved until all the agarose was melted. Pipettes were used to apply small circles of the simulated DNA onto glass slides (Figure 2).
Figure 2.
Picture of empty slide before spotting. Circles are numbered to indicate gene position for printing. The yellow mask is hydrophobic, and holes are hydrophilic to improve morphology of spots (i.e., prevent the spots from spreading).
Desired colors were produced by mixing water and the pH indicator solutions until the 2% wt/vol agarose stock solution reached a final concentration of 1.5% wt/vol. The volume of each pH indicator and water mixed with 1000 μL of agarose to produce each spot color is shown in Table 1.
Table 1.
pH indicator and water solutions for producing desired colors
| Spot color | Thymolphthalein | Phenolphthalein | Water |
|---|---|---|---|
| Intense blue | 500 μL | 0 μL | 0 μL |
| Intense red | 0 μL | 500 μL | 0 μL |
| Intense purple | 250 μL | 250 μL | 0 μL |
| Light blue | 100 μL | 0 μL | 400 μL |
| Light red | 0 μL | 100 μL | 400 μL |
| Clear | 0 μL | 0 μL | 500 μL |
It is worth noting that the red dye phenolphthalein is light sensitive and will fade to colorless upon exposure to light. Therefore, the dye solution should be kept in the dark (wrapped in foil) and photographed shortly after exposure to the base. This shortcoming has been alleviated in the commercialized version.
Glass Slides
Any glass slide might work, but we have good results with slides that are covered with a hydrophobic mask with holes in the mask; these are reusable. We chose the 10 × 5-mm hole mask because it provided a compromise of spot size and number of spots per slide (Figure 2). There are multiple suppliers, such as Tekdon (Myakka City, FL), Scientific Devices (Perth Amboy, NJ), and Proscitech (Kirwan, Queensland, Australia; respectively, at http://www.tekdon.com/Microscope_slides2.html, http://www.scientificdevice.com/intl_product_pages/iprinted_microscope_slides.htm, and http://www.proscitech.com.au/catalogue/g1.asp). Alternatively, permanent marker or waxed pencil could be used to draw masks for each spot on a regular glass microscope slide.
Simulated cDNA Probes
The hybridization solution is simply 0.1 M NaOH. This is the only potential hazard in the simulation because a strong base can be caustic. For this reason, students and teachers should wear gloves during the lab and wash their hands at the end of lab.
Student Assessment
Formal assessment was conducted after the paper and wet lab exercises with 158 high school Honors Biology students (primarily in ninth grade) in Hinsdale, IL. Formal assessment consisted of pre- and posttests to measure student knowledge of microarrays and to get objective feedback on the microarray module (see Supplemental Material 3). A total of 138 students completed the pretest and 75 completed the posttest. Each question had a total value of 2 points and was scored for 0, 1, or 2 points based on the quality of the answer. Total pretest scores ranged from 0 to 6 (0–75% correct). Total posttest scores ranged from 1 to 8 points (12.5–100% correct). Fifty-five students took both tests and recorded their names on their papers, which allowed us to measure their results for individual student gains using a paired t test. For the entire dataset, significant differences were calculated using a Z-test for each question, and the totals are shown in Table 2. Since that time, an additional 180 students have used the microarray module, but they did not participate in the formal assessment. Informal assessment was conducted in the Maryland high schools, and anecdotal evidence supported the findings from the formal assessment in Illinois.
Table 2.
Comparison of pre- and posttest student results
| Questions | Pretest average(n = 138) | Posttest average(n = 75) | Significant difference |
|---|---|---|---|
| 1. What is a microarray? | 0.14 | 0.84 | p = 1 × 10−9 |
| 2. Describe one use of a microarray. | 0.24 | 1.15 | p = 3 × 10−14 |
| 3. Analysis of microarray experimental results | 0.24 | 1.31 | p= 4 × 10−24 |
| 4. Place steps of microarray procedure in order (multiple correct answers). | 0.36 | 1.48 | p = 3 × 10−20 |
| Totals (8 possible points) | 0.98a | 4.78a | p = 2 × 10−26 |
a 8.2% correct.
b 59.8% correct.
Teacher Assessment
We surveyed 12 high school teachers in a Summer 2005 Howard Hughes Medical Institute Biotechnology Education and Outreach Program Genomics workshop at the University of Illinois at Urbana-Champaign (Howard Hughes Medical Institute, Chevy Chase, MD), and two teachers who tested the lab activities in their high school classrooms in February 2005. During the workshop, the teachers were asked to complete the pretest and the posttest objective section. Analysis of the pretest revealed that 50% of these outstanding teachers did not have an adequate understanding of DNA microarray technology before undertaking the microarray unit.
RESULTS AND DISCUSSION
Microarray Simulation
The entire DNA microarray module was designed to fit two class periods of 45 min each (Figure 3). To measure what they knew already, students took a pretest the day before the microarray module began (see Supplemental Material 2). For homework the night before the paper exercise, they were assigned reading that introduced microarrays (see Supplemental Material 2) and were to watch a microarray animation. The first day in class included an introductory presentation of the homework. This introduction was followed by the paper microarray exercise and a follow-up discussion on how the DNA microarray method works. The paper exercise is available online (Zanta, 2004). The second day in class was devoted to the wet lab DNA microarray simulation.
Figure 3.
Students in the lab working with the annotated timeline of the Microarray Module.
- Students take microarray pretest.
- Homework assignment requires students to read handout and view the DNA Microarray Methodology Animation created by A.M.C. at Davidson College (http://www.bio.davidson.edu/courses/genomics/chip/chip.html).
- Teacher reviews the microarray uses and procedures using the animation or handout.
- Students work on the paper microarray lab created by C.A.Z. (Zanta, 2004).
- The teacher facilitates discussion of how the microarray measures gene expression, why gene expression is important, and how this relates to the cancer scenario of the activity.
- Homework assignment: Outline the wet lab microarray simulation procedure.
- Students work on the microarray simulation and analyze and record their results in groups.
- Teacher facilitates discussion of how the microarray measures gene expression, why gene expression is important, and how this relates to the cancer scenario of the activity.
- The posttest using same exam as the pretest, plus additional open-ended feedback on the entire unit.
To perform the microarray simulation, students worked in groups of 2–4 students. Each group was provided with a special glass slide, and all groups shared six tubes of “genes” for printing their microarrays. All groups also had access to the “mixed-labeled cDNA hybridization solution” for applying to the spotted genes once the microarrays were printed and cooled.
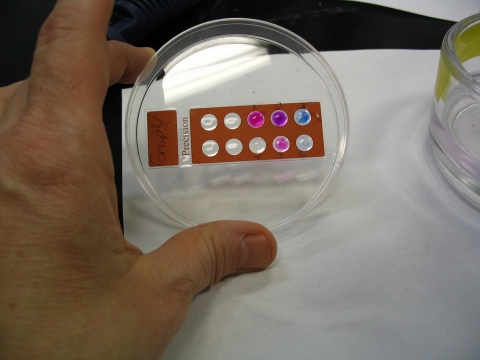
The students followed a detailed protocol that explained the technical aspects of the simulation (see Supplemental Material 4). In brief, single drops of melted agarose with pH indicators were placed on a microscope slide in circles prenumbered 1–6. The spots cooled and gelled in <1 min. Students placed their slides in containers of “mixed labeled cDNA hybridization solution” and watched the colors develop. After 1 min, the colors were recorded. Digital images were taken to accurately recall the colors (Figure 4). When the agarose and dyes were reheated because the master solutions were made in advance, sometimes the dyes were not evenly mixed, and the gene spots looked speckled.
Figure 4.
Results of the microarray simulation carried out by students at Hinsdale Central High School in Hinsdale, IL. The colors in this microarray simulation are red, blue, and purple (red and blue combined) instead of red, green, and yellow seen in a typical DNA microarray. Note differences in color intensities between spots 1 and 5 (both red) and 3 and 6 (both blue). Spot 2 is purple and 4 is colorless. The speckled coloration is due to incomplete mixing after reheating the master solution of spotting solutions.
A variety of different scenarios can be used in this simulation. The scenario described in this study was a popular one in which the students were told that the experimental sample contains labeled probes taken from lung cancer biopsies and the control from noncancerous lung cells. Most students know someone who has cancer, and because real microarrays are being developed for cancer diagnosis, it is easy for students to see the relevance of the simulation to their daily lives. Students are allowed to compare results to see if their data are reproducible. Many of the Maryland students used a scenario studying yeast in space instead of the lung cancer scenario (Adler, 2006).
Students can be asked to answer a series of questions that focus their attention on the meaning of the colors: Which genes were induced? Which genes were repressed? Were any genes transcribed similarly in the two samples? What were the molecular causes for their color differences? Were all the induced genes activated to the same degree? What about the repressed genes? Once students have answered these questions, they can be queried to explain how two lung samples could display differential gene regulation—a major educational objective of this module. They can be reminded that all cells contain the same genes, but different tissues transcribe genes differently. From this discussion, they can be guided to realize that loss of gene regulation is a key component of cancer formation—another important educational objective. Advanced students could be encouraged to learn about oncogenes and tumor suppressors, which are described in textbooks used in advanced placement (AP) biology courses. Once they have learned about tumor suppressors and oncogenes, students can be asked to speculate if any of the six genes in their simulation might be oncogenes or tumor suppressors. From this point, students may ask how some cancers can run in families, which can lead to a discussion of how many mutations are required for cancers to form (at least two recessive mutations of tumor suppressors and at least one dominant oncogene mutation).
Students can be encouraged to convert their colors to quantitative ratios (Campbell and Heyer, 2006b). Note that colors will not always fall exactly into one of the colors in the online chart so students should be encouraged to estimate the best ratio. This may help them realize that the ratios are not all multiples of 2 and are actually part of a continuum composed of fractions as well (e.g., 3.5-fold repressed). An additional mathematical module allows students to deepen their understanding of DNA microarrays. The mathematical module lets students analyze their ratios for variance, which they visualize graphically, use their ratios for clustering genes and tissue samples, and use their wet lab simulation data to diagnose a cancer sample with the aid of mathematical methods (Heyer and Campbell, 2006). This mathematical module provides a real-world context for basic math skills and emphasizes the need to integrate biology with mathematics.
Assessment Data
Student Assessment.
Assessment was carried out at Hinsdale Central High School in Illinois. Students completed a pretest before carrying out the complete microarray module (introduction/animation, paper activity, and wet lab simulation) and a posttest after completing the module (see Figure 3). Only 2 of the 55 Illinois students who were tracked on both pre- and posttests had worse scores on the posttest than on the pretest, and these scores varied by only 1 and 2 points. The average score for the pretest was 1 point out of a total of 8 possible points (n = 138), whereas the posttest average was 4.8 points (n = 75). Each question in the test showed a significant improvement from the pretest average to the posttest average (Table 1). When the learning gains were measured for the 55 students who signed both tests, we found p values ranging from 1 × 10−11 to essentially zero. Students had little prior knowledge of microarrays and gained an understanding of microarrays after carrying out the paper and wet lab activities.
Open-ended feedback from the posttest survey was overwhelmingly positive for these laboratory activities (see Box 1). One student commented to her teacher that she really liked the microarray simulation—it was her favorite lab because she saw how it connected with what they were studying in class. When asked what they did not like about the activity, one student replied “There is nothing I liked least. Every part was great and I enjoy doing lab experiments.” Regarding the prelab paper microarray activity, one student commented, “I felt that I understood everything better because of the prelab. I enjoyed actually understanding this lab.” Several students noted that they enjoyed doing the lab on cancer because it affects their lives.
Box 1. Anonymous student comments when asked “What did you enjoy most about this microarray unit?”
“I enjoyed doing this because, although we did a much simplified version, we were doing an experiment that real scientists use to learn more about a disease.”
“It made it easier to understand how microarrays work and it was interesting. Also, we didn't have to wait very long for results.”
“It was very interesting using the cDNA and being able to understand everything that was happening at a molecular level.”
“I liked the outcome where we could compare the amount of gene expression in different cells!”
“I enjoyed actually understanding this lab.”
“Feeling like a scientist and seeing what a microarray is. The color part was also very cool. Easy to understand.”
“Seeing that different genes are working when you have cancer and when you don't.”
“I liked how clear the results were.”
We were surprised to see so few negative comments about the laboratory when the students were asked for their least favorite part of the lab and suggestions for improvement. When asked what they liked least about the activities and suggestions for improvement, the majority of students noted that there was nothing to improve. Many said the entire module was great and wrote nothing in this space. On the 75 posttests, the major suggestions for improvement were as follows:
provide more introduction to microarrays—to better understand results (8)
provide more details on uses of microarrays in medicine and other fields (2)
When asked what they liked least about the microarray unit, the only feedback was as follows:
answering the questions after the lab (5)
having the reverse transcription step prepared for them before lab (1)
the lab wasn't very independent (1)
the paper microarray activity (1)
the preparation we had to do before the activity (1)
disliked wearing safety goggles, gloves, and aprons (12)
The quantitative results show that after completing these activities, the Illinois students (and presumably the Maryland students as well) gained a greater understanding of the use of DNA microarrays to study gene expression. The students seemed to be deeply engaged in the activity that used lung cancer as an example scenario. Because this is a complex topic, we suggest that teachers use prelab exercises to facilitate deeper understanding of the concepts before the wet lab microarray simulation. After the wet lab microarray simulation, a teacher could facilitate a thoughtful class discussion to ensure that students have a thorough understanding of microarrays, differential gene regulation, and gene expression in cancer. Experience has shown us that repetition of concepts can lead to a more meaningful understanding of complex topics.
Teacher Assessment.
Teachers who have used this wet lab simulation reported that it was easy to do, would easily fit into their class periods, and was realistic enough that students could understand the key steps in a DNA microarray experiment and data analysis. Combining the teacher responses with student assessment data above, the simulation provides a needed boost to modernize a typical high school curriculum.
Of the 14 teachers who tested the full microarray module at the University of Illinois Biotechnology Education and Outreach Program Genomics workshop, all were enthusiastic about the learning activities. One teacher noted that the “simple prelabs make you really consider what is happening during the activity.” Some additional positive comments on the microarray module included the following:
“Better understanding of how microarrays work. Hands-on is always better.”
“Helps explain the use in a simple manner and extends a more complex means to study proteins.”
“Explanation of something I didn't know about before, and a practical example to use in the classroom.”
“Clear color analysis allows you to see intensity, mix, or no color.”
“A practical example to use in the classroom”
“Useful—would like to do this one in class.”
“Visual activity [that] shows gene expression of normal and cancerous cells. Colors made it easy to understand.”
“Hands on—understood [microarrays] better after the lab activity”
“Simulates the real thing.”
“Very easy—I will use [this in my classroom].”
“The color changes were a good ‘oooh!’ factor. I thought it was all useful.”
“Quick and easy!”
“Easy to do and understand.”
The suggestions for improvement and negative comments were:
“More background.”
“Better paper instructions.”
“Use more genes than 6—real microarrays are more complex.”
“To[o] simple.”
Only one teacher noted that he/she would like to use the microarray activities but felt it might be too difficult to fit these extra exercises into the existing curriculum. The remaining teachers were positive about incorporating the microarray module into their curricula. One teacher commented that he/she was “not sure about [using the microarray simulation in the classroom]—I don't like to mislead students.” This comment is interesting in that it could apply to many molecular biology laboratories that are used in high school classrooms and could provide an opportunity to discuss the benefits and limitations of laboratory simulations. Many of these activities are simulations that do not utilize the same reagents and equipment used in current scientific laboratories because of time and financial constraints. However, in order to offer an engaging laboratory experience within the time frame of a typical 45- to 55-min high school class, a simulation is sometimes the best option. Schools that have block schedules (classes of 80–90 min) may be able to do the entire module in a single day, although spreading it over 2 days may prevent student overload and enhance student comprehension.
CONCLUSIONS
Science education is in need of modernization in high schools and introductory biology courses (for majors and nonmajors) in the United States and in other parts of the world (Chattopadhyay, 2005). Students benefit from hands-on and minds-on activities, even if they are simulations of more complex methods. We have developed a wet lab simulation of DNA microarrays that is very reliable, inexpensive, and meets several criteria outlined in the National Science Education Standards. In some cases, it may be cost effective for one employee of the district to prepare kits for all schools rather than purchasing the commercial kit. For example, the Montgomery County Public School District in Maryland was able to use Howard Hughes Medical Institute funding to provide such support. However, the pH indicators are expensive, cannot be purchased in small quantities, and most school districts do not have the personnel in place to produce kits for every school. The Genisphere commercial kit was designed to keep the costs under $1.00 per simulation, the results are better, more reproducible, and the kit has a longer shelf life because of proprietary improvements. Nevertheless, the authors felt it was important to publish this article so teachers could create their own kits if they felt a do-it-yourself solution fit their needs better. We recently learned of a commercial microarray kit distributed by Fotodyne (New Berlin, WI), but it is much more expensive and requires scanning by a specialized piece of equipment, and the entire process is less student-friendly because of the complexity of the protocol.
ACKNOWLEDGMENTS
We thank Mr. Jarod Honeycutt for integrating the pilot study into his honors biology classes and all the Hinsdale Central High School students for their enthusiastic participation in the study, as well as students of Catherine Ulicny (one section biotechnology at Paint Branch High School), Sanford Herzon (two sections of forensics at Wootton High School), Angelique Bosse (one section of AP biology at Montgomery Blair High School), and John Fitz (two sections of molecular biology at Wootton High School) in the Montgomery County School District. This work is partially supported by an Undergraduate Biological Science Education Program grant from the Howard Hughes Medical Institute to C.A.Z. Authors from Davidson College were supported by the Davidson College Biology Department and by grants from the National Science Foundation (DBI-0099720) and the Howard Hughes Medical Institute.
Footnotes
Competing interests statement: Genisphere has commercialized the microarray simulation into a kit based on the method described in this article. The Genisphere kit differs from the protocol described here and contains technical modifications that prevent the dyes from fading as quickly. A.M.C., C.A.Z., and K.M.G. give free workshops around the country to high school teachers and are compensated by Genisphere for these workshops. A.M.C. and Genisphere agreed that the original method developed in his laboratory would be published in an open access journal as a condition for commercializing the microarray simulation module.
REFERENCES
- Adler L. Cells in Space: Cell Biology and DNA Microarrays. [(accessed 22 June 2006)];2006 www.bio.davidson.edu/projects/GCAT/HSChips/Yeast_combined.doc. [Google Scholar]
- American Cancer Society. Cancer Facts and Figures 2006. [(accessed 19 June 2006)];2006 http://www.cancer.org/downloads/STT/CAFF2006PWSecured.pdf.
- Brewster J. L., Beason K. B., Eckdahl T. T., Evans I. M. The microarray revolution: perspectives from educators. Biochem. Mol. Biol. Educ. 2004;32(4):217–227. doi: 10.1002/bmb.2004.494032040362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A. M. [(accessed 12 June 2006)];DNA Microarray Animation. 2000 www.bio.davidson.edu/Courses/genomics/chip/chip.html. [Google Scholar]
- Campbell A. M., Eckdahl T. T., Fowlks E., Heyer L. J., Hoopes L.L.M., Ledbetter M. L., Rosenwald A. G. Genome Consortium for Active Teaching (GCAT) Science. 2006;311:1103–1104. doi: 10.1126/science.1121955. [DOI] [PubMed] [Google Scholar]
- Campbell A. M., Heyer L. J. 2nd ed. San Francisco: Benjamin Cummings; 2006a. Discovering Genomics, Proteomics, and Bioinformatics; p. 447 pp.. [Google Scholar]
- Campbell A. M., Heyer L. J. [(accessed 13 June 2006)];Quantifying Gene Chip Colors. 2006b www.bio.davidson.edu/people/macampbell/LRSD/colors.html. [Google Scholar]
- Chattopadhyay A. Understanding of genetic information in higher secondary students in northeast India and the implications for genetics education. Cell Biol. Educ. 2005;4:97–104. doi: 10.1187/cbe.04-06-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genome Consortium for Active Teaching (GCAT). GCAT Home Page. [(accessed 12 June 2006)];2006a doi: 10.1126/science.1121955. www.bio.davidson.edu/GCAT. [DOI] [PubMed]
- Genome Consortium for Active Teaching. GCAT DNA Chip Simulations: Dry Lab and Wet Lab Curricula. [(accessed 13 June 2006)];2006b www.bio.davidson.edu/projects/GCAT/HSChips/HSchips.html.
- Heyer L. J., Campbell M. A. Value Added: Blending Math into a High School Genomics Lab. [(accessed 10 August 2006)];2006 http://gcat.davidson.edu/Online_Genomics/hs_kit_math_module.pdf.
- Kakiuchi S. Prediction of sensitivity of advanced non-small cell lung cancers to gefitinib (Iressa, ZD1839) Hum. Mol. Genet. 2004;13((24)):3029–3043. doi: 10.1093/hmg/ddh331. [DOI] [PubMed] [Google Scholar]
- National Research Council. Washington, DC: National Academy Press; 1996. National Science Education Standards. [Google Scholar]
- National Research Council. Learning and Understanding: Improving Advanced Study of Mathematics and Science in U.S. High Schools: Report of the Content Panel for Biology, by the Committee on Programs for Advanced Study of Mathematics and Science in American High Schools. In: Wood W. B., editor. Washington, DC: National Academy Press; 2002. [Google Scholar]
- National Research Council. America's Lab Report: Investigations in High School Science. In: Singer S. R., Hilton M. L., Schweingruber H. A., editors. Washington, DC: National Research Council; 2005. [Google Scholar]
- Zanta C. A. [(accessed July 5 2006)];Gene Chips for High Schools—Paper Exercise. 2004 www.bio.davidson.edu/people/macampbell/LRSD/LRSD_ChipsPaper.html.
- Zanta C. A. Using Gene Chips to Study the Genetics of Lung Cancer: A DNA Macroarray Lab. [(accessed 5 July 2006)];2006 http://www.bio.davidson.edu/people/macampbell/LRSD/Full_Handout.doc.