Abstract and Introduction
Abstract
Background
Ovarian transposition is a surgical maneuver used to protect ovarian function before delivery of gonadocidal doses of radiation therapy. Ovarian transposition has been performed in patients whose treatment includes pelvic radiotherapy as a part of management for Hodgkin's disease and other gynecologic malignancies.
Case
Laparoscopic ovarian transposition was performed on a 28-year-old female with rectal cancer. At 13-month follow-up from completion of chemoradiation treatments, normal menstrual cycles at regular monthly intervals are reported. FSH, LH, and progesterone levels obtained during the second half of menstrual cycles were within normal ranges.
Conclusion
Ovarian transposition is an effective surgical procedure for preserving ovarian function in patients at risk of radiotherapy-induced ovarian failure. Laparoscopic techniques can be used to move the ovaries outside of the radiation portal. Consideration should be given for ovarian transposition for other abdominal and pelvic malignancies before onset of radiation treatments in patients who desire preservation of ovarian function.
Introduction
Preservation of ovarian function is an important concern in premenopausal cancer patients whose treatments include radiation therapy to pelvic structures that incorporate the ovaries. Ovarian transposition was developed to displace the ovaries from their normal anatomic position and away from the direct radiation portal, with the goal of maintaining long-term ovarian function.
Historically, ovarian transposition was typically performed for premenopausal patients undergoing pelvic irradiation for Hodgkin's disease at the time of staging laparotomy. With the popularization of laparoscopic surgical techniques, ovarian transposition can now be performed in other clinical settings. Thus, ovarian transposition should be considered in all premenopausal women undergoing pelvic irradiation, including gastrointestinal malignancies for which preoperative radiation therapy is employed.
In this report, we describe the use of laparoscopic ovarian transposition as a means to preserve ovarian function in a 28-year-old woman with rectal carcinoma who underwent preoperative combined modality therapy with chemotherapy and radiation therapy.
Patient and Methods
An otherwise healthy 28-year-old female, 19 months postpartum, noted intermittent rectal bleeding after delivery. At presentation, she complained of tenesmus and described a 10-pound weight loss. A colonoscopy revealed an ulcerated mass in the distal rectum, at approximately 3 cm from the anal verge, extending approximately 7 cm proximally. Multiple biopsies of the mass revealed an invasive, moderately differentiated adenocarcinoma.
Physical examination (a digital rectal exam) revealed a 75% circumferential mass with partial fixation abutting the anorectal ring. The mass could be felt through the vagina, although it did not penetrate into it. The remainder of the gynecologic examination was normal. An anoscopy was subsequently performed, which revealed the most distal portion of the lesion to be on the left anterior wall, situated approximately 1.0 to 1.5 cm from the dentate line. The extent of the disease work-up revealed no evidence of metastatic disease.
Because of the partial fixation of the mass and the proximity to the anal verge, preoperative neoadjuvant chemotherapy was recommended in an attempt to convert the need for an abdominoperineal resection to a low anterior resection.
Before the onset of radiation treatments, the patient was referred for laparoscopic ovarian transposition. The extent of the radiation field was discussed with the gynecologic oncologist before the procedure to ensure that the transposed ovaries would be outside of the treatment portals. The ovaries were transposed laparoscopically to the paracolic gutters without complications.
Laparoscopy was performed in the usual fashion. The patient received general endotracheal anesthesia and was placed in steep Trendelenburg's position, in stirrups. A Foley catheter was placed into the bladder, and a uterine manipulator was placed in the endometrial cavity. Four 1-cm incisions were made and the trocar was introduced into the peritoneal cavity at the umbilicus, suprapubic region, and both lower quadrants. The peritoneal cavity was inspected and found to be normal. The peritoneum of both pelvic sidewalls was incised, and the retroperitoneal spaces were developed. The common, external, and internal iliac vessels were identified. The ovarian vessels and ureters were traced on both sides. Under the direct vision of the ureters, the utero-ovarian ligaments were separated with an Endo-GIA. The peritoneum under and lateral to the ovarian vessels were incised to an area outside of the true pelvis, under direct vision of the ureters. At this point, the ovarian vessels could be turned laterally with a sufficient angle to maintain appropriate blood supply. The ovaries and tubes were fixed high in the paracolic gutters, below the spleen and the liver, with 2–0 silk sutures, in 3 points, to prevent torsion. The upper and lower poles of the ovaries were marked with hemoclips. At the end of the procedure, good blood supply to both ovaries was confirmed by a small incision of the fallopian tubes, which was cauterized. The metal clips around the ovaries helped to verify that they would be out of the radiation portals on radiation verification films.
Radiation Therapy
The patient was simulated in a prone position in an immobilization device to ensure daily set-up reproducibility. A radiopaque marker was placed at the anus, and barium was inserted to delineate the rectum on simulation films. A standard 3-field rectal radiation technique by means of posterior-anterior (PA) and right and left lateral portals was used. Custom blocks were created to shape the radiation fields and shield areas not at risk. The dose delivered to each ovary from all 3 radiation fields (as denoted by the radiographic clips) was calculated.
The patient received a total dose of 4500 cGy to the pelvis (including the rectum and draining lymph nodes) in 25 daily fractions of 180 cGy each over 5 weeks. Treatment was delivered using 10 MV photons, 5 days a week.
Chemotherapy
During Weeks 1 and 5 of the radiation, the patient received fluorouracil (5-FU), 350 mg/m2 by intravenous push (IVP) for 5 days. After her surgery, she received once weekly 5-FU, 400 mg/m2 IVP, and leucovorin, 200 mg/m2 IV soluset over 1 hour. The adjuvant chemotherapy regimen was delivered over 6 months.
Surgery
One month after completion of her radiation treatments, the patient underwent a low anterior resection. A pull-through technique using a hand-sewn colo-anal anastomosis with colonic J pouch and mobilization of splenic flexure was used. The procedure was tolerated without complications.
Pathology
Pathologic review of the segment of colon revealed microscopic foci of adenocarcinoma in the bowel wall. Distal and proximal margins were free of disease. The 9 lymph nodes sampled were all without evidence of metastatic disease.
Results and Follow-up
After ovarian transposition, the ovaries were determined to be outside of the radiation fields, as visualized by the radiographic clips placed at the time of laparoscopy (Figures 1–4). The doses calculated to the clips from the PA, right lateral, and left lateral portals were 74.9 cGy for the right ovary and 59.9 cGy for the left ovary.
Figure 1.
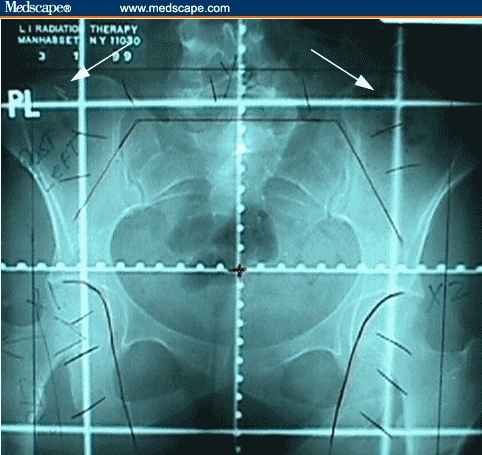
PA simulation field with clips marking transposed ovaries (arrows).
Figure 4.
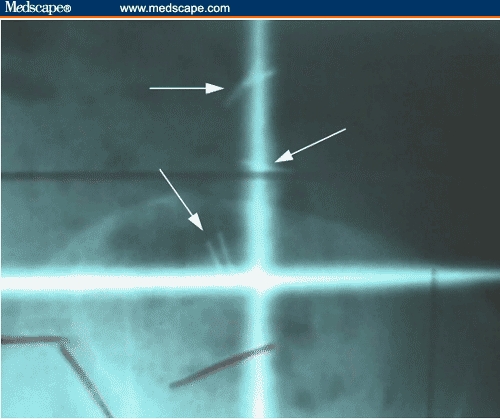
Close up of clips (arrows) denoting transposed right ovary of the radiation portal (PA film).
Thirteen months after completion of her radiation and chemotherapy, the patient is without evidence of disease. She continues to have regular menstrual cycles with normal flow and without dysmenorrhea. She denies any further episodes of rectal bleeding. Physical examination has revealed no evidence of recurrent disease. A repeat colonoscopy advanced to the ileum demonstrated the surgical anastomosis to be intact and without evidence of recurrent disease.
Laboratory values of FSH, LH, and progesterone during the second half of the patient's menstrual cycle were obtained 13 months after completion of the radiotherapy. The values were 8.0 mIU/mL, 4.9 mIU/mL, and 1.54 ng/mL, respectively – all within the normal limits.
Discussion
Preservation of ovarian function by laparoscopic transposition of the ovaries before pelvic irradiation has been demonstrated to be a safe and effective procedure for patients with Hodgkin's disease, as well as in the treatment of a variety of gynecologic malignancies.[1–6] Historically, surgical exploration of the abdomen or pelvis as part of staging or resection procedures has allowed for access to the ovaries for direct-open transposition of the ovaries for planned subsequent radiation therapy. As laparoscopic techniques have improved, consideration for ovarian transposition in other clinical settings is more attractive. In our case, the patient was a 28-year-old woman who presented with a fixed rectal lesion. If she had been treated with an initial surgical approach, she would have required an abdominoperineal resection. On the other hand, neoadjuvant combined modality therapy would have resulted in ovarian ablation.
Neoadjuvant chemoradiation has been used preoperatively in low-lying or fixed rectal lesions to improve resectability and attempt sphincter preservation.[7–9] Bilateral laparoscopic ovarian transposition before radiotherapy in this patient allowed for preservation of ovarian function. The laparoscopic approach enabled the patient to return to normal function and start radiation within days of the procedure.
Techniques for ovarian transposition using a laparoscopic approach vary according to the radiation field shape, size, and location. Tinga and colleagues[3] have described transposition of the ovaries in a fixed position behind the uterus (to lie beneath an external midline block) as well as a superior transposition to the level of the iliac crest. They contend that the disadvantage of the midline oophoropexy is a higher level of internal radiation scatter, as the area is generally surrounded by in-field radiation. Morice and colleagues[6] reported a series of 24 patients who underwent ovarian transposition to the paracolic gutters, before radiation for gynecologic malignancies. The authors concluded that this procedure was a safe and effective method of preserving ovarian function. Complications in both of these reports were rare.
In today's era of reproductive technology, the ovaries can be stimulated to produce numerous eggs, which can later be retrieved under ultrasound guidance from the relocation site in the paracolic gutter. These eggs can be fertilized with sperm and the embryo re-implanted into a uterus. This may allow a patient, such as ours, to have her own biological child or a biological child through a surrogate mother, after being treated for rectal cancer with chemoradiation and radical colorectal surgery.
Doses of radiation delivered to the ovary following successful laparoscopic oophoropexy have been determined and reported by several authors. Covens and colleagues[11] studied 3 patients in whom ovarian transposition was performed and determined the radiation dose expected to be received by the ovaries after delivery by either an external beam radiation dose of 4500 cGy or via brachytherapy. The mean dose of radiation received by the transposed ovary was 175 cGy (range, 40–370 cGy) after a mean follow-up of 2 years. The iatrogenic menopause rate was 10% overall, but iatrogenic menopause did not occur in any of the patients younger than 40 years of age. In the present case, the ovarian dose received after transposition was 74.9 cGy for the right ovary and 59.9 cGy for the left ovary, both below the dose expected to be gonadocidal.
Numerous studies have attempted to specify radiation therapy tolerance doses for the various tissues and structures of the body. Common dose definitions that are used to describe various tissue tolerances are the minimal tolerance dose, TD 5/5, and the maximal tolerance dose, TD 50/5, which refer to a severe complication rate of 5% and 50%, respectively, with 5 years of radiation completion. For the ovary, these values are approximately 300 cGy and 1200 cGy, with sterility being the endpoint of severe complication. Tolerance doses for other tissues are significantly higher. In clinical practice, however, the tolerance doses for the ovary and other tissues may actually be lower, given the now common treatment regimens that typically include chemotherapy or altered radiation fractionation schemes. In general, and as was observed in our case, if the ovaries can be appropriately shielded from the direct radiation beam via an oophoropexy maneuver, a dose below the TD 50/5 (300 cGy) can be achieved with a reasonable expectation of ovarian function preservation post treatment.
The typical outcome measure in ovarian transposition following radiation therapy is ovarian function. This is often measured by quantitative analysis of ovary-stimulating or ovary-producing hormones, as well as fertility outcomes. Spontaneous pregnancies are possible if tubal function is preserved as part of the oophoropexy. Morice and colleagues[10] reported on 37 consecutive cases of ovarian transposition. In these cases, 16 pregnancies occurred spontaneously, 12 of which did not have the ovaries repositioned from the adnexae.
Quantitative analysis of ovarian function has been reported by Covens and colleagues[11] in 3 patients undergoing ovarian transposition. Serum FSH was found to be normal in 2 of 3 patients menstruating regularly 24 to 32 months after radiation. Treissman and colleagues[12] reported on a patient in whom laparoscopic ovarian transposition was performed before definitive treatment for an anal carcinoma. Tulandi and Al-Took[13] reported that normal menstruation returned after irradiation in a 34-year-old woman who underwent laparoscopic ovarian transposition before radiation for treatment of rectal carcinoma. Although postmenopausal symptoms and elevation of serum gonadotropins initially indicated ovarian failure, normal menstruation resumed and correlated with normal FSH levels 8 months after treatment. A subsequent report by Tulandi[14] documented a successful spontaneous pregnancy in this woman. In the current case report, ovarian and uterine functions are shown to be preserved 13 months after treatment, as determined by normal menstruation and normal levels of FSH, LH, and progesterone. As of publication of this report, the patient has by choice not attempted to become pregnant.
The primary benefit of ovarian transposition is prevention or delay of premature menopause, not preservation of fertility. In fact, with “curative” doses in the range of 8500 cGy with external beam plus intracavitary brachytherapy, the resultant endometrial damage essentially precludes successful pregnancy, either spontaneously or with in vitro technique.
Conclusion
Ovarian transposition is a relatively easy and highly effective surgical procedure used to preserve ovarian function in patients who are at risk of iatrogenic ovarian failure as a result of radiation therapy-induced function ablation. Using laparoscopic techniques, the ovaries can be moved to a position outside of the direct radiation portal with virtually no postoperative complications. Ovarian function is preserved in most patients with successful transposition given direct radiation field exclusion, especially in patients younger than 40 years. Subsequent intrauterine pregnancy after laparoscopic ovarian transposition has been reported.[15] Consideration should be given for ovarian transposition laparoscopically, when feasible, for other abdominal and pelvic malignancies, before the onset of radiation treatments in patients in whom preservation of ovarian function is desired. Furthermore, the placement of surgical clips is a useful addition to help ensure that the radiation dose to the ovaries is kept at a minimum level and to facilitate treatment planning.
Figure 2.
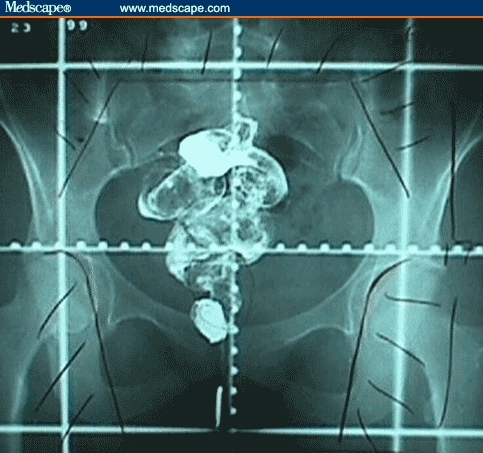
PA simulation field with anal marker and rectal barium contrast.
Figure 3.
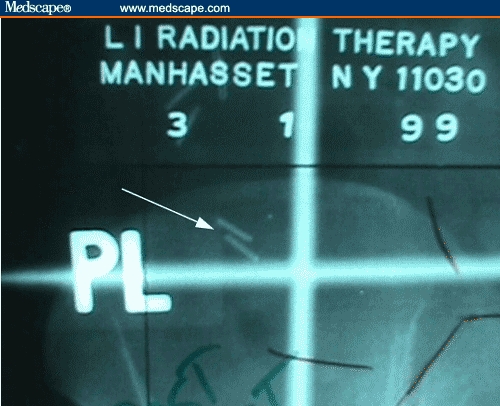
Close up of clips (arrow) denoting transposed left ovary outside of the radiation portal (PA film).
Contributor Information
Leonard A Farber, Radiation Oncology, Long Island Radiation Therapy/Nassau Radiologic Group, New York, NY.
John W Ames, Radiation Oncology, Long Island Radiation Therapy/Nassau Radiologic Group, New York, NY.
Stephen Rush, Radiation Oncology, Long Island Radiation Therapy/Nassau Radiologic Group, New York, NY.
David Gal, Gynecologic Oncology, Long Island College Hospital, New York, NY.
References
- 1.Classe JM, Mahe M, Moreau P, et al. Ovarian transposition by laparoscopy before radiotherapy in the treatment of Hodgkin's disease. Cancer. 1998;83:1420–1424. [PubMed] [Google Scholar]
- 2.Howard FM. Laparoscopic lateral ovarian transposition before radiation treatment of Hodgkin disease. J Am Assoc Gynecol Laparosc. 1997;4:601–604. doi: 10.1016/s1074-3804(05)80096-9. [DOI] [PubMed] [Google Scholar]
- 3.Tinga DJ, Dolsma WV, Tamminga RY, et al. Preservation of ovarian function in 2 young women with Hodgkin disease by laparoscopic transposition of the ovaries prior to abdominal irradiation. Ned Tijdschr Geneeskd. 1999;143:308–312. [PubMed] [Google Scholar]
- 4.Schulz-Lobmeyr I, Schratter-Sehn A, Huber J, Wenzl R. Laparoscopic lateral ovarian transposition before pelvic irradiation for a Non Hodgkin lymphoma. Acta Obstet Gynecol Scand. 1999;78:350–352. [PubMed] [Google Scholar]
- 5.Clough KB, Goffinet F, Labib A, et al. Laparoscopic unilateral ovarian transposition prior to irradiation: prospective study of 20 cases. Cancer. 1996;77:2638–2645. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2638::AID-CNCR30>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 6.Morice P, Castaigne D, Haie-Meder C, et al. Laparoscopic ovarian transposition for pelvic malignancies: indications and functional outcomes. Fertil Steril. 1998;70:956–960. doi: 10.1016/s0015-0282(98)00284-2. [DOI] [PubMed] [Google Scholar]
- 7.Minsky BD, Cohen AM, Enker WE, et al. Preoperative 5-FU, low dose leucovorin, and concurrent radiation therapy for rectal cancer. Cancer. 1993;73:273. doi: 10.1002/1097-0142(19940115)73:2<273::aid-cncr2820730207>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 8.Minsky BD, Cohen AM, Kemeny N, et al. Efficacy or pre-operative 5-FU, high dose leucovorin, and sequential radiation therapy for unresectable rectal cancer. Cancer. 1993;71:3486. doi: 10.1002/1097-0142(19930601)71:11<3486::aid-cncr2820711105>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 9.Bosset JF, Pavy JJ, Hamers HP, et al. Determination of the optimal dose of 5-fluorouracil when combined with low dose D,L-leucovorin and irradiation in rectal cancer. Results of three consecutive phase II studies. Eur J Cancer. 1993;29:476. doi: 10.1016/0959-8049(93)90012-5. [DOI] [PubMed] [Google Scholar]
- 10.Morice P, Thiam-Ba R, Castaine D, et al. Fertility results after ovarian transposition for pelvic malignancies treated by external irradiation or brachytherapy. Hum Reprod. 1998;13:660–663. doi: 10.1093/humrep/13.3.660. [DOI] [PubMed] [Google Scholar]
- 11.Covens AL, van der Putten HW, Fyles AW, et al. Laparoscopic ovarian transposition. Eur J Gynaecol Oncol. 1996;17:177–182. [PubMed] [Google Scholar]
- 12.Treissman MJ, Miller D, McComb PF. Laparoscopic lateral ovarian transposition. Fertil Steril. 1996;65:1229–1231. doi: 10.1016/s0015-0282(16)58344-7. [DOI] [PubMed] [Google Scholar]
- 13.Tulandi T, Al-Took S. Laparoscopic ovarian suspension before irradiation. Fertil Steril. 1998;70:381–383. doi: 10.1016/s0015-0282(98)00155-1. [DOI] [PubMed] [Google Scholar]
- 14.Tulandi T. Profile: Dr. Togas Tulandi. McGill University Health Centre Health Perspectives. August/September 2002, p 5. Available at: http://www.muhcfoundation.com/publications/perspectives/Issue_2.eng.pdf.
- 15.Dabirashrafi H, Moghadami-Tabrizi N, Zandinejad K. Laparoscopic ovarian transposition with subsequent intrauterine pregnancy. J Am Assoc Gynecol Laparosc. 1996;3:515–517. doi: 10.1016/s1074-3804(05)80160-4. [DOI] [PubMed] [Google Scholar]


