Abstract and Introduction
Abstract
Objectives
To describe allergic asthma and allergic rhinitis pathophysiology and review the pharmacologic, pharmacokinetic, pharmacodynamic, efficacy, and safety data for omalizumab.
Methods
MEDLINE, In-Process & Other Non-Indexed Citations, and EMBASE Drugs & Pharmacology were searched using olizumab, omalizumab, E25, rhuMAb-E25, and anti-IgE. Combinations of rhinitis, asthma, and IgE captured information on disease pathophysiology.
Results
Omalizumab is a monoclonal antibody targeting the high-affinity receptor binding site on human immunoglobulin (Ig)E. Bound IgE is not available for basophil binding, degranulation is attenuated, and allergic symptoms are reduced. In asthma trials, omalizumab reduced inhaled corticosteroid and rescue medication requirements and improved asthma control and asthma quality of life in moderate to severe allergic asthmatics with disease poorly controlled by inhaled corticosteroids. In trials of patients with poorly controlled moderate to severe seasonal allergic rhinitis (SAR), omalizumab reduced the severity of exacerbations and rescue medication use, and improved rhinitis-related quality of life. Benefits were also observed in trials utilizing combinations of immunotherapy and omalizumab for SAR and in trials of perennial allergic rhinitis (PAR). Omalizumab has been well tolerated. Although malignant neoplasms have been observed in treated patients, they were likely not related to omalizumab therapy.
Conclusions
Omalizumab has demonstrated efficacy in children, adults, and adolescents with uncontrolled moderate to severe allergic asthma and allergic rhinitis. Long-term safety beyond 52 weeks needs continued evaluation.
Introduction
Diseases such as asthma and allergic rhinitis have a significant societal impact. These conditions affect a substantial population of patients and impose a burden in terms of treatment costs, productivity loss, and reduced quality of life.[1–5] Although medications are available to treat these conditions, some focus only on symptom relief, others are nonspecific in their mechanism of action (and, therefore, produce substantial side effects), and none provide symptom relief in all patients.[6–9] Because of IgE's role in the manifestation of these conditions (see discussion below), there has been interest in developing therapies that specifically target this immunoglobulin to treat allergic asthma and allergic rhinitis. The recent introduction of the monoclonal anti-IgE antibody omalizumab (Xolair, Genentech, South San Francisco, California) provides clinicians with an additional unique option for treating these conditions. This review will present information on the pathophysiology of allergic asthma and allergic rhinitis and describe the pharmacology, pharmacokinetics, pharmacodynamics, clinical efficacy data, and safety profile for omalizumab. The focus of this review is a function of the fact that most published information with omalizumab addresses its use for these conditions. However, future applications for anti-IgE therapy are likely forthcoming for other IgE-mediated conditions such as food allergies and atopic dermatitis.[10,11] In particular, recent information suggests that anti-IgE therapy can prevent the manifestations of inadvertent peanut exposure in patients with peanut allergy.[11]
Pathophysiology of Allergic Diseases
Although not all cases of asthma or rhinitis are clearly attributable to atopy, it is accepted that atopy does play an etiologic role in the pathophysiology of these conditions. The reported proportion of asthma and rhinitis cases attributed to atopy varies among studies and populations. The attributable risk is also highly dependent on whether researchers utilize a more or less conservative definition of atopy. Researchers who have reviewed this literature to calculate the weighted mean population attributable risk suggest that approximately 40% of asthma cases and 50% of noninfectious rhinitis cases can be attributed to atopy.[12,13] Additionally, atopy is one of the strongest currently identified predisposing factors for the development of asthma.[6,14] In light of the common pathophysiologic basis for allergic asthma and allergic rhinitis, it is not surprising that these conditions often co-exist. This has led researchers to postulate that these conditions may actually be manifestations of one syndrome.[15]
Discussion of the pathophysiology of allergic conditions begins with exposure of an allergen to antigen-presenting cells (macrophages, dendritic cells). These cells engulf the allergen, process it, and display the peptide epitope of the allergen on its cell surface for presentation to T and B lymphocytes. This is followed by direct interactions between T and B lymphocytes, which initiate B-lymphocyte activation and subsequent allergen-specific IgE production.[16] During the progression from an inactive B lymphocyte to an IgE-secreting plasma cell, B lymphocytes express membrane-bound IgE (mIgE), which assists in antigen processing and the transduction of signals that drive this progression.[17] Plasma cells may then secrete IgE that is available for binding to its receptors on other cells.
IgE binds to high-affinity (Fc-epsilon-RI) and low-affinity (Fc-epsilon-RII; also known as CD23) receptors on several immune system cells. The site whereby IgE binds to Fc-epsilon-RI is located on the Fc fragment in the area where the C-epsilon-3 region (or domain) adjoins the C-epsilon-2 domain (Figure 1).[18,19] Although distinct from the Fc-epsilon-RI binding site, the IgE Fc-epsilon-RII binding site is also on the C-epsilon-3 domain.[20] During subsequent antigen exposure, there is cross-linking of antigen by multiple Fc-epsilon-RI-bound IgE molecules on basophils and mast cells (basophil-like cells located in tissues).
Figure 1.
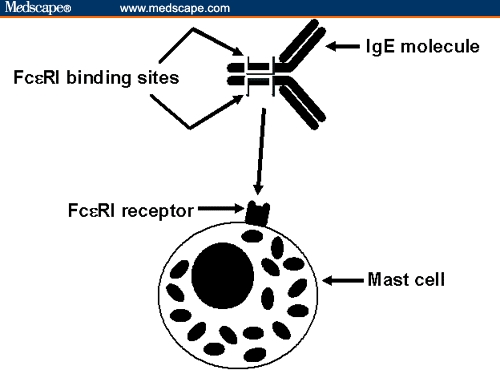
Graphic representation of IgE binding to Fc-epsilon-RI on a mast cell. Binding occurs with IgE “lying on its side,” producing a conformational change in the unbound Fc-epsilon-RI binding site and precluding binding by omalizumab.
This triggers degranulation of these cells, resulting in the release of preformed inflammatory mediators (histamine, tryptase) and the synthesis and release of newly generated mediators (prostaglandins, leukotrienes) and cytokines (tumor necrosis factor [TNF], interleukin [IL]-4, IL-5, IL-6). Released mediators initiate an early-phase response within minutes after allergen exposure. In the bronchial mucosa, this manifests as an asthmatic exacerbation (mucosal edema, mucous production, bronchial smooth muscle spasm); in the nasal mucosa, sneezing, itching, rhinorrhea, and nasal congestion are observed.[6,7,21–23] Some mediators released during the acute-phase response act as chemoattractants and promote the infiltration of mucosal surfaces with immune cells, particularly eosinophils.[7,22,24] With subsequent release of eosinophil and newly generated mast cell products, a second wave of allergic symptoms can be observed over the 6–12 hours following the early-phase response. Persisting late-phase inflammatory responses may then be responsible for the clinical and histologic findings reported in chronic allergic diseases.
In addition to activating basophils through Fc-epsilon-RI binding, IgE may interact with other immune cells in less defined ways.[25] IgE binds to Fc-epsilon-RI on antigen-presenting cells and eosinophils. Such interactions appear to facilitate antigen presentation by antigen-presenting cells. Although the effect on eosinophils is less clear, it is postulated that such interactions may regulate local tissue IgE concentrations.[23] The other IgE receptor, Fc-epsilon-RII, is found on B lymphocytes; binding to this receptor may augment antigen presentation by these cells.[21,26]
Omalizumab Description
Omalizumab is a monoclonal antibody identified through conventional somatic cell hybridization techniques.[27] Through this process, researchers identified a murine monoclonal anti-human IgE antibody, MAE11, whose paratope was directed toward the site that binds Fc-epsilon-RI on basophils and mast cells.[18,19] MAE11 was humanized in a process involving transplantation of the complementarity-determining regions (CDRs; specific areas within the paratope that interact with an antigen – in this instance, human IgE) onto a human IgG1 antibody framework.[28] Additional MAE11 amino acid sequences were also incorporated into the humanized antibody to maintain the proper CDR spatial arrangement. This process resulted in a humanized monoclonal antihuman IgE antibody, rhuMAb-E25 (later named omalizumab), which contained approximately 5% nonhuman amino-acid residues (Figure 2).
Figure 2.
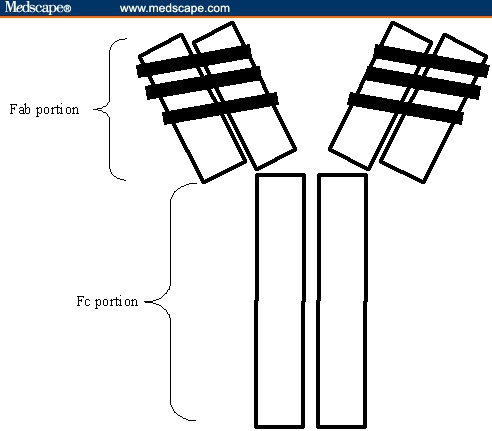
Graphic representation of omalizumab. The black areas of the Fab portion represent the complementarity-determining regions transplanted from the murine monoclonal antibody MAE11.
Omalizumab Pharmacology
The clinical effect of omalizumab on allergic diseases is the result of several pharmacologic interactions. Omalizumab binds free human IgE with a binding affinity higher than that observed between IgE and Fc-epsilon-RI[29–31]; it does not bind to Fc-epsilon-RI on basophils (these are IgE receptors; omalizumab has a human IgG1 antibody framework) or IgE that is already bound either to Fc-epsilon-RI on basophils or Fc-epsilon-RII on other cells (lymphocytes, monocytes, eosinophils, platelets).[16,28,31–34] These binding characteristics allow omalizumab to neutralize IgE-mediated responses without causing basophil degranulation that could occur with binding to basophils or cross-linking with basophil-bound IgE.[18] In vitro, omalizumab prevents lung mast cells from becoming sensitized with IgE by binding this immunoglobulin before it can attach to Fc-epsilon-RI on these cells,[32,33] yet it does not cause histamine release from basophils and lung mast cells that have been sensitized to ragweed antigen (ie, have ragweed IgE on their surface).[32,33] Additionally, the inability of omalizumab to bind Fc-epsilon-RII-bound IgE may decrease the likelihood of cytotoxic hypersensitivity reactions that might be observed if such binding occurred on cells with these receptors (ie, platelets).[31]
Although IgE actually contains 2 copies of its Fc-epsilon-RI and Fc-epsilon-RII binding sites,[19,34] omalizumab does not bind to the second site of an IgE antibody that is already bound to Fc-epsilon-RI or Fc-epsilon-RII on a cell surface. During binding to Fc-epsilon-RI on a basophil, IgE undergoes a conformational change.[19,34] This alters the conformation of the second site, thus precluding binding by omalizumab.[19,31,34] The mutual exclusion of omalizumab and Fc-epsilon-RII binding to IgE is the result of that fact that 2 lectin heads from Fc-epsilon-RII occupy both of the Fc-epsilon-RII binding sites on the IgE antibody, thus precluding binding by omalizumab.[34,35]
Neutralization of IgE antibodies does not completely explain the pharmacologic effect of omalizumab. Omalizumab also promotes Fc-epsilon-RI downregulation on basophils because of the close direct correlation between free serum IgE and the number of Fc-epsilon-RI expressed on basophils.[36–38] Subsequently, the amount of basophil-bound IgE is reduced. Ultimately, these effects may represent dissociation of IgE from existing basophils with subsequent receptor downregulation in response to low free IgE serum concentrations or the replacement of the original pool of basophils with new basophils that have not experienced upregulation by high serum free IgE concentrations.[38]
In vitro, omalizumab and human IgE form several immune complexes that vary in size as the 2 components' molar ratios are changed.[29] The largest complex, a stable cyclical hexameric structure consisting of 3 IgE and 3 omalizumab molecules, is formed at a 1:1 molar ratio. With excesses of either IgE or omalizumab, the distribution of complexes is dominated by a trimer consisting of 1 IgE and 2 omalizumab molecules or vice versa (Figure 3).
Figure 3.
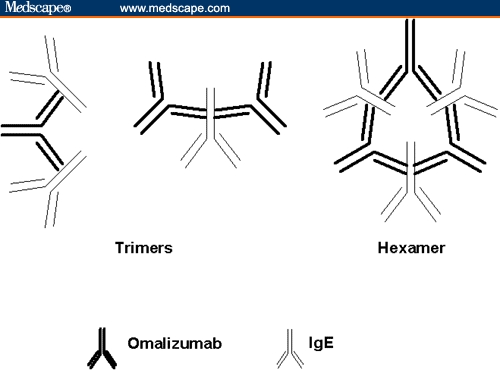
Graphic representation of immune complexes formed between omalizumab and IgE. The hexamer is predominant when components are present in a 1:1 molar ratio; the trimers predominate when one of the components is in excess of the other.
These findings are consistent with those reported in cynomolgus monkeys, a species whose IgE is bound by omalizumab with an affinity similar to that observed with human IgE. The complexes produced in these monkeys are of limited size (no greater than a hexamer); with doses that produce unequal IgE:omalizumab ratios, the dominant complex is a trimer.[39] It is postulated that the IgE:omalizumab immune complexes themselves may add to the clinical effect of omalizumab.[31] Omalizumab binding of IgE may not preclude IgE binding to allergens. Hence, IgE:omalizumab complexes may compete with basophil-bound IgE for allergens, thereby reducing the chance of basophil degranulation with allergen re-exposure.
Additional, less well defined pharmacologic interactions may contribute to the action of omalizumab. In vitro and animal studies suggest that omalizumab binding to mIgE may inhibit or lyse mIgE-expressing B cells and reduce IgE synthesis.[31,32] Although omalizumab does not bind basophil-bound IgE, omalizumab may bind mIgE on mIgE-expressing B lymphocytes because mIgE expression on the surface of these cells occurs in such a way that the IgE Fc-epsilon-RI binding sites remain exposed.[35,40] Since antigen-presenting cells and eosinophils also express Fc-epsilon-RI, omalizumab also likely inhibits IgE binding to these cells, affecting their immunologic response.[41] Omalizumab therapy has been shown to downregulate Fc-epsilon-RI expression on the dendritic cells of SAR patients.[42] Additionally, there is evidence that Fc-epsilon-RI and Fc-epsilon-RII expression on other immune cells varies with IgE concentrations.[43–45] Hence, reductions in IgE concentrations with omalizumab therapy may downregulate the expression of these receptors, thereby reducing the immunologic response of these cells. The full implications of these effects have not been evaluated in human clinical studies of omalizumab.
Clinical Trials
Allergic Asthma
Early Clinical Trials
Two small early omalizumab studies focused on omalizumab's ability to attenuate the asthmatic response to an inhaled aeroallergen challenge.[46,47] Patients had stable mild asthma, a positive skin prick test to at least 1 common aeroallergen, and an asthmatic response to aeroallergen challenge. Patients were randomized to intravenous omalizumab or placebo for 8–10 weeks. Comparisons to placebo recipients in each study revealed that omalizumab treatment reduced the early-phase asthmatic response during the 2 hours following aeroallergen challenge. Although no changes were observed in response to methacholine challenges, forced expiratory volume in 1 second (FEV1) in the absence of aeroallergen challenge, symptoms, or bronchodilator use, this was expected given that these patients were already well controlled at baseline. FEV1 measurements carried out to 7 hours after challenge also demonstrated less of a fall in FEV1 during the 2- to 7-hour time period, an indication that omalizumab attenuates the late-phase asthmatic response.[47] Although the underlying pathophysiology suggests that this finding should be associated with reduced lung eosinophils, this was not observed in comparisons of induced sputum samples attained at baseline and at the end of treatment. The percentage of eosinophils was significantly lower at the end of therapy for both groups, but the magnitude of the reduction was not different between placebo and omalizumab recipients.[47]
Adult-adolescent Trials
A phase 2 study by Milgrom and colleagues[48] provided the earliest large-scale, randomized, placebo-controlled, double-blinded clinical study of omalizumab in adolescent and adult allergic asthmatic patients. A total of 317 patients with moderate or severe persistent asthma were randomized to placebo, high-dose intravenous omalizumab, or low-dose intravenous omalizumab. Enrolled patients had to have a positive skin prick test to 2 or more perennial allergens and an FEV1 that was 50% to 90% of predicted. Patients also had to be symptomatic over the week prior to randomization despite use of their inhaled corticosteroids (ICS) with or without rescue beta-agonist therapy. Thirty-five patients were also receiving oral corticosteroids. Patients received 12 weeks of placebo or omalizumab every 2 weeks in addition to their ICS therapy (steroid-stable phase). Study drug was continued for another 8 weeks while ICS therapy was tapered (steroid-reduction phase). At week 12 of treatment, there was a significant difference in the mean reduction in asthma symptom scores in the 2 omalizumab treatment groups relative to placebo; this was also observed at week 20, but only among the high-dose recipients. These findings were observed despite trends for more successful tapering of inhaled and oral corticosteroids among omalizumab recipients.
Omalizumab was subsequently evaluated in randomized, placebo-controlled, double-blinded, phase 3 clinical trials involving adolescents and adults with moderate to severe allergic asthma.[49–52] Two of these studies were similar in design to the earlier trial, with some notable differences.[49,50,53] Doses were administered subcutaneously and standardized such that patients received an approximate dose of at least 0.016 mg/kg per IU of IgE/mL per 4 weeks. Smaller patients received 150 mg or 300 mg every 4 weeks; larger patients were dosed every 2 weeks with 225 mg, 300 mg, or 375 mg. Additional changes included: conversion of patients to inhaled beclomethasone dipropionate (BDP) titrated to previous asthma control; extension of the steroid-stable phase to 16 weeks and the steroid-reduction phase to 12 weeks; and use of exacerbation frequency as the primary end point. Eligibility characteristics included: a positive skin prick test to at least 1 common allergen, total serum IgE 30–700 IU/mL, baseline FEV1 40% to 80% of predicted, and residual asthma symptoms during the 2 weeks prior to randomization despite treatment with high-dose ICS (no other asthma medications other than an inhaled rescue beta-agonist).
These studies enrolled 1068 patients; average baseline FEV1 and asthma symptom scores were approximately 70% of predicted and 4 (0–9 scale; symptom severity proportional to score), respectively.[49,50] Outcomes were comparable, despite disease severity differences (21.7% and 99.4% of patients were classified as having severe persistent asthma in the studies by Soler and colleagues and Busse and colleagues, respectively). There was a significant reduction in exacerbation frequency among omalizumab recipients during the steroid-stable and steroid-reduction phases of both trials when compared with placebo recipients (Table 1). Among the secondary end points, omalizumab recipients had better asthma symptom scores, required less beta-agonist rescue therapy (most weekly intervals), recorded higher FEV1 measurements (most weekly intervals), and fewer patients experienced an exacerbation. Differences were observed despite more successful ICS tapering among omalizumab recipients. In the study by Soler and colleagues, the median ICS daily dose was lower (100 mcg vs 300 mcg, P < .001).[49] Additionally, a greater proportion of omalizumab recipients were able to reduce their BDP dose (P < .001); 79% reduced it by >/= 50% (vs 55% of placebo recipients; P value not reported), and 43% were able to discontinue BDP (vs 19% of placebo recipients; P value not reported). Among omalizumab recipients in the study by Busse and colleagues, the median ICS dose reduction was greater (75% vs 50%, P < .001), more patients achieved a >/= 50% reduction (72.4% vs 54.9%, P < .01), and more patients were able to discontinue ICS therapy (39.6% vs 19.1%, P < .001).[50] In a substudy of 35 patients from the study by Soler and colleagues, omalizumab recipients had significantly lower peripheral eosinophil counts and IL-13 concentrations, airways resistance was significantly less, and the acetylcholine concentration required to provoke an FEV1 20% reduction was significantly higher at the end of the steroid-stable phase.[54]
Table 1.
Frequency and Incidence of Asthma Exacerbations in the Adult/Adolescent Clinical Trials of Omalizumab by Soler and Colleagues and Busse and Colleagues
| Steroid-Stable Phase | Steroid-Reduction Phase | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Soler et al[49] | Busse et al[50] | Soler et al[49] | Busse et al[50] | ||||||||
| Treatment group | Oa | Pb | P value | Oa | Pb | P value | Oa | Pb | P value | Oa | Pb | P value |
| Exacerbations per patient | 0.28 | 0.66 | < .001 | 0.28 | 0.54 | .006 | 0.36 | 0.75 | < .001 | 0.39 | 0.66 | .003 |
| Patients with >/= 1 exacerbation (%) | 12.8 | 30.5 | < .001 | 14.6 | 23.3 | .0009 | 15.7 | 29.8 | < .001 | 21.3 | 32.3 | .0004 |
Oa = omalizumab; Pb = placebo
Following the steroid-reduction phase of these studies, patients were eligible to enter a 24-week double-blind extension phase in which they continued their study treatment and the lowest effective BDP dose.[55,56] Investigators could make BDP dose changes and prescribe additional asthma medications. The primary findings of the core studies persisted. With omalizumab treatment, the exacerbation frequency was lower, and fewer patients experienced an exacerbation despite ICS requirements that were significantly lower than those of placebo recipients.
A third omalizumab adult-adolescent allergic asthma clinical trial involved 246 patients with severe asthma (determined by a requirement for daily treatment with high doses of inhaled corticosteroids).[51] The study structure and dosing were identical to the above studies with a few exceptions: patients were converted to inhaled fluticasone at doses that provided disease control, a subset of patients were also receiving oral corticosteroids, use of long-acting beta-agonists was allowed, the steroid-reduction phase was 16 weeks, and the primary end point was the percent reduction in the fluticasone dose needed to maintain disease control. At the end of the steroid-reduction phase, omalizumab recipients only on inhaled fluticasone (not requiring oral corticosteroids at enrollment) had a greater reduction in fluticasone requirements (mean reduction of 57.2% vs 43.3%, P = .003). Furthermore, a greater percentage of omalizumab recipients were able to reduce their fluticasone dose by at least 50% (74% vs 51%, P = .001). Although the number of patients experiencing an exacerbation was similar in the omalizumab and placebo groups, rescue medication use for the omalizumab recipients (compared with baseline) was significantly lower at all measured time points (changes in the placebo group were not significant). Asthma symptom scores among omalizumab recipients were either lower or no different from those of placebo recipients.
To better evaluate omalizumab utility in clinical practice, Ayres and colleagues,[57] in a multicenter, randomized, open-label study, randomized 312 poorly controlled (defined as at least 1 emergency room visit or hospitalization or at least 1 course of oral corticosteroids for asthma over the prior year) adult and adolescent patients with persistent moderate-to-severe allergic asthma, to best standard care (BSC) plus omalizumab or BSC only. The model for BSC was the guideline published by the National Heart, Lung, and Blood Institute.[58] Upon enrollment, patients were being treated according to steps 3 or 4 of this guideline; 21% of patients were receiving daily systemic corticosteroid therapy. Over the 12-month study period, omalizumab recipients experienced 49.6% fewer asthma deterioration-related events per patient-day (ADRIs, asthma exacerbations requiring 2 or more days of systemic corticosteroids or a supplemental burst course of systemic steroids, 2 or more days of antibiotic therapy, 2 or more lost work/school days, unscheduled physician visits, an emergency room visit, or hospitalization). Additionally, more omalizumab recipients remained ADRI-free (36.1% vs 20.2%), and fewer experienced multiple ADRIs (40.8% vs 66.3%, P = .001). Omalizumab recipients also required less rescue medication, had better morning FEV1 measurements and asthma symptoms scores, and had a greater reduction in the mean daily ICS dose.
Pediatric Trials
Omalizumab therapy was evaluated in 6- to 12-year-old children with moderate to severe allergic asthma in a trial whose structure and dosing were identical to that described by Soler and colleagues and Busse and colleagues. The primary end point was the measurement of omalizumab's steroid-sparing effects.[59] Eligibility criteria included: stable asthma that was well controlled with low-dose ICS, a positive skin prick test to at least 1 common allergen, total serum IgE 30–1300 IU/mL, and a baseline FEV1 >/= 60% of predicted. A total of 334 patients were enrolled; the mean FEV1 was approximately 85% of predicted. After 28 weeks of therapy (12 in the steroid-stable and 16 in the steroid-reduction phases), the median BDP dose reduction in omalizumab recipients was 100% (vs 66.7% among placebo recipients, P = .001). A significantly greater proportion of omalizumab recipients reduced their ICS dose, and more were able to withdraw ICS therapy (55% vs 39%, P = .004). Regarding secondary end points, during the steroid-reduction phase, a smaller proportion of omalizumab recipients experienced an exacerbation (18.2% vs 38.5%, P < .001), and the exacerbation frequency was significantly lower. With omalizumab treatment, there were fewer urgent, unscheduled outpatient physician visits (12.9% vs 30.3%, P = .001), less variability in the morning peak expiratory flow rates, fewer 2- or 3-consecutive-night awakenings requiring rescue medications (11.6% vs 21.1%, P = .002), fewer requirements for rescue beta-agonist therapy, and fewer school days missed (0.65 days vs 1.21 days, P = .04). BDP dose requirements and asthma control measurements were similar during the subsequent 24-week extension phase.[60]
Asthma-related Quality of Life and Perceptions of Treatment Efficacy
Because conventional clinical measures of asthma are not complete descriptions of the functional impairments or improvements experienced in clinical trials,[61] investigators also included measures of quality of life and perceptions of treatment efficacy. Quality of life was evaluated via an Asthma Quality of Life Questionnaire.[62,63] Impressions of therapy effectiveness were evaluated by asking patients and investigators to rate treatment efficacy as excellent, good, moderate, poor, or worse.
In the trials reported by Soler and colleagues and Busse and colleagues, overall asthma quality-of-life (AQoL) scores among omalizumab recipients showed significantly greater improvement (relative to baseline) during all 3 treatment phases. Additionally, in each phase, a significantly greater proportion of patients experienced a clinically relevant change in their overall AQoL score; a significantly greater proportion also experienced a large improvement (greater than a clinically relevant change).[64,65] Similar improvements were reported in the third adult asthma trial.[51] In the childhood asthma trial, changes in AQoL scores were similar between groups at the end of the steroid-stable phase.[66] However, omalizumab recipients experienced significantly greater improvements in overall AQoL scores at the end of the steroid-reduction phase. Additionally, a significantly greater proportion of patients experienced a clinically relevant change in their overall AQoL score at the end of this phase.
Patients' and investigators' impressions of therapy effectiveness were consistent with AQoL evaluations. With assessments performed at the end of the steroid-reduction phases, ratings by patients and investigators were more likely to be good or excellent for the omalizumab recipients. The percentage of patients indicating that response was good or excellent among omalizumab and placebo recipients, respectively, was: 70% vs 40% (P < .001) in the study by Soler and colleagues[64]; 60.6% vs 38.1% (P < .001) in the study by Busse and colleagues[50,65]; and 76.2% vs 49% (P < .001) in the study by Milgrom and colleagues.[59,66] Investigator responses were similar to those of patients.
Secondary Analyses of Clinical Trial Data
Corren and colleagues[67] pooled data from Soler and colleagues,[49] Busse and colleagues,[50] and the childhood allergic asthma study[59] to assess the effect of omalizumab treatment on the incidence of serious asthma exacerbations and the need for asthma-related unscheduled outpatient visits, emergency room treatments, and overnight hospitalizations. With omalizumab treatment, there was a lower incidence of serious asthma exacerbations requiring unscheduled outpatient visits (21.3 vs 35.5 visits per 100 patient-years; relative risk [RR] = 0.60; P < .01) or emergency room visits (1.8 vs 3.8 visits per 100 patient-years; RR = 0.47; P = .05); overnight hospitalizations were less common (2 during 779.5 patient-years of treatment with omalizumab, 18 for placebo recipients during 526.7 patient-years of treatment); and the total number of asthma-related hospitalization days was less for omalizumab recipients who required such care (4 vs 97 days, P < .002). Although omalizumab may prove cost-effective for patients routinely requiring such high-intensity healthcare resources, a cost-effectiveness analysis of data from the trials by Solar and colleagues[49] and Busse and colleagues[50] indicates that the cost of omalizumab therapy may be prohibitive for all but the most severe poorly controlled asthmatics.[68] Because of omalizumab's cost, its use may be cost-effective only in patients who are regularly hospitalized for prolonged periods. Bousquet and colleagues[69] also pooled data from these trials to identify the baseline patient characteristics that are predictive of response to omalizumab. Logistic regression analysis of the data from the steroid-stable phase of these trials revealed that the following characteristics were predictive of response: a history of emergency asthma treatment in the prior year, a baseline requirement for high doses (>/= 800 mcg/day) of inhaled BDP, and a baseline FEV1 of </= 65% of predicted. Baseline IgE concentrations were not predictive of response. Among the omalizumab recipients who responded to omalizumab by the end of this 16-week phase, 61% showed a response at 4 weeks, and 87% had responded by 12 weeks.
With the data from the steroid-stable phases of 3 adult-adolescent trials,[49–51] Holgate and colleagues[70] performed a meta-analysis to evaluate the impact of omalizumab on an annualized rate of all asthma exacerbation episodes (AEEs) and significant AEEs (sAEEs; an exacerbation requiring doubling of ICS dose or use of systemic steroids) among patients who were at high risk of serious asthma-related morbidity and mortality. The rates of AEEs and sAEEs were significantly lower (reductions of 51% and 55%, respectively; P < .1) with omalizumab treatment. The absolute difference (in favor of omalizumab) in sAEE rates increased dramatically with baseline FEV1 severity. Differences in the risk of sAEEs translated into the need to treat 5.7 patients with omalizumab to maintain 1 patient free of sAEEs.
Allergic Rhinitis
The nasal response to a nasal allergen challenge is attenuated by omalizumab treatment. Hanf and colleagues[71] randomized 23 patients with allergic rhinitis (seasonal and/or perennial) to 16 weeks of subcutaneous omalizumab (at least 0.016 mg/kg per IU of IgE/mL per 4 weeks) or placebo. At 16 weeks, nasal symptom scores after a nasal allergen challenge were significantly lower in omalizumab recipients (compared with placebo recipients and with scores from the pretreatment nasal challenge). Analysis of nasal lavage fluid postchallenge showed a greater percent reduction in human serum albumin and TNF-alpha compared with fluid from the baseline challenge, findings suggestive of reduced nasal inflammation.
Seasonal Allergic Rhinitis
The earliest reports on the use of omalizumab for SAR are randomized, placebo-controlled, double-blinded, dose-ranging studies in adolescent and adult patients with ragweed-induced SAR.[72,73] The earlier trial did not show differences in daily SAR symptom scores (the primary efficacy variable), possibly because of smaller than predicted changes in symptom exacerbation during the pollen season, variability in symptom reporting, and too few patients demonstrating adequate IgE suppression.[72]
In the second ragweed SAR study, 536 patients with a 2-year history of moderate to severe ragweed-induced rhinitis, a positive skin prick test to ragweed allergen, and a total IgE concentration of 30–700 IU/mL were randomized to placebo or one of several omalizumab regimens.[73] Over a 12-week period, subcutaneous omalizumab doses of 50 mg, 150 mg, or 300 mg were administered every 3–4 weeks (depending on the patient's baseline IgE concentration). All patients received their first treatment approximately 2 weeks before the ragweed pollen season. Compared with placebo recipients, significantly better results were consistently observed with the 300-mg dose for most outcome parameters over the duration of the study; this occurred inconsistently with the 50-mg and 150-mg doses. Among the 300-mg omalizumab recipients, significant advantages were observed with regard to: average nasal (primary outcome parameter) and ocular symptom severity scores, percentage of days with minimal nasal symptoms, proportion of days requiring rescue medications (12% vs 21%, P = .005), good or excellent treatment effectiveness ratings by patients (70.7% vs 40.8%, P < .001) and investigators (65.3% vs 35.4%, P < .001), mean overall Rhinitis Quality of Life (RQoL) scores, and mean number of days missed from work or school (0.1 vs 0.4, P = .005). Although patients receiving the 300-mg omalizumab dose experienced little change in their nasal symptom severity scores throughout the trial, placebo recipients experienced a 50% increase during the season peak.
Omalizumab efficacy has also been assessed in patients with moderate to severe birch pollen SAR.[74] A total of 251 patients were randomized to subcutaneous omalizumab or placebo in a 2:1 ratio; 300 mg was administered every 3 weeks for 3 doses or every 4 weeks for 2 doses, depending on the baseline serum IgE concentration. Efficacy was similar to that reported for ragweed SAR. In a substudy of 30 patients from this trial, omalizumab recipients had significantly smaller increases in total peripheral blood and nasal mucosal eosinophils. There was a significant positive correlation between free serum IgE concentrations and peripheral blood eosinophil counts, biopsy eosinophil numbers, and biopsy Fc-epsilon-RI-expressing cell numbers. A significant positive correlation was also found between symptom severity scores (nasal, ocular, and total scores) and the eosinophil marker specific for activated eosinophils.[75]
Omalizumab has also been evaluated in combination with specific immunotherapy (SIT) in a randomized, placebo-controlled, double-blinded study involving children and adolescents (6–17 years of age) with documentation of IgE specific for birch and grass pollen and a clinical history of SAR induced by both of these allergens.[76] A total of 255 patients were randomized into 1 of 4 arms: birch SIT/placebo, birch SIT/omalizumab, grass SIT/placebo, or grass SIT/omalizumab. Weekly SIT was initiated at least 14 weeks prior to the start of the birch pollen season (which preceded the grass pollen season) with a 24-week regimen of subcutaneous placebo or omalizumab (at least 0.016 mg/kg per IU of IgE/mL every 4 weeks) being initiated at week 12. The primary efficacy variable, the daily symptom load, was calculated as the sum of the mean daily symptom and the mean daily rescue medicine scores. The median symptom load scores for the entire study period (data from both pollen seasons) were 48% lower (P < .001 for each analysis) in both of the omalizumab treatment arms relative to their respective placebo treatment arms (ie, birch SIT/omalizumab vs birch SIT/placebo). Analysis of individual pollen season data demonstrated that combining the season-appropriate SIT (ie, birch SIT during birch pollen season) with omalizumab resulted in lower median symptom load scores compared with those of patients receiving season-appropriate SIT and placebo. This effect of omalizumab was observed regardless of the efficacy of the season-appropriate SIT alone. In a substudy of 92 children from this trial, in vitro allergen stimulation of leukocytes from study end samples revealed that leukotriene release was lower among the leukocytes attained from omalizumab recipients. This observation was independent of the allergen stimulus and the SIT received; leukotriene release was less in the grass SIT/omalizumab group vs the grass SIT/placebo group when leukocytes were stimulated with either birch or grass pollen allergen (the same holds true for patients in the birch SIT groups). Multiple regression analysis revealed that omalizumab treatment (but not SIT therapy) had a significant effect on stimulated leukotriene release.[77]
Perennial Allergic Rhinitis
The first published trial of omalizumab in PAR was a randomized, double-blinded, placebo-controlled study in which 289 patients with symptomatic moderate to severe PAR of 2 years duration, a positive skin prick test to a common perennial allergen (dust mites, dog, or cat allergen), and a total serum IgE concentration of 30–700 IU/mL were randomized to receive 16 weeks of subcutaneous placebo or omalizumab.[78] Doses were administered once or twice monthly to provide at least 0.016 mg/kg of omalizumab per IU of IgE/mL per 4 weeks. Assessment of the primary efficacy variable, mean daily nasal severity score, revealed that omalizumab recipients had significantly lower values than placebo recipients at each 4-week time point. Post hoc analyses of subgroups that had been unresponsive to immunotherapy or intranasal corticosteroids showed that these patients also had significant improvements. Omalizumab recipients also needed fewer rescue medication tablets per day and experienced a greater reduction in the number of days/month requiring rescue medication. Additionally, more patients experienced a clinically important improvement in their RQoL scores for all components of the RQoL questionnaire, and symptoms were more frequently rated as under complete control or markedly improved (53% vs 34%, P = .001).
Concomitant Asthma and PAR
In light of the common pathophysiology of allergic asthma and allergic rhinitis, it is expected that patients suffering from these conditions concomitantly would receive relief of both conditions with omalizumab therapy. Vignola and colleagues[79] evaluated the efficacy of omalizumab in a randomized, double-blinded, placebo-controlled trial involving 405 adults and adolescents with concomitant allergic asthma and PAR. Enrollment criteria included an IgE concentration of 30–1300 IU/mL, a positive skin-prick test to an indoor allergen, a history of moderate-to-severe PAR for at least 2 years, asthma requiring ICS therapy, and a history of 2–3 unscheduled medical visits for asthma over the prior 1–2 years. Patients were also required to have RQoL and AQoL scores indicative of greater than mild symptoms. After being converted to budesonide Turbuhaler ICS therapy, patients were randomized to subcutaneous omalizumab (at least 0.016 mg/kg per IU of IgE/mL per 4 weeks) or placebo for 28 weeks. End-of-trial comparisons to placebo revealed that fewer omalizumab recipients had experienced an asthma exacerbation (30.1% vs 20.6%, P = .02), and the mean rate of exacerbations was lower. Additionally, more omalizumab recipients reported clinically relevant changes and large improvements in quality of life as measured by the AQoL and RQoL questionnaires. Analysis of secondary end points revealed that omalizumab recipients had more significant reductions in their asthma and rhinitis symptom scores, and more patients and investigators rated asthma and rhinitis treatment effectiveness as excellent or good.
Pharmacokinetics
After subcutaneous administration, omalizumab is slowly absorbed over several days with an average absolute bioavailability of 62% (range, 53% to 71%).[52,80,81] Measurements of omalizumab serum concentrations reflect the combination of free omalizumab and that which is bound to IgE. Although area under the serum concentration profile differs between intravenous and subcutaneous dosing, equal doses produce similar omalizumab trough concentrations.[72] Peak concentrations are reached after an average of 7–8 days following a single subcutaneous dose[52]; with multiple dosing, accumulation occurs with steady-state serum concentrations reportedly being reached after 14–28 days.[52,72] With intravenous administration, the pharmacokinetic profile is described by a 2-compartment central elimination model; after subcutaneous administration, it is described by a 1-compartment first-order absorption model.[82]
The apparent volume of distribution for omalizumab approximates that of the plasma volume.[39,52,80,83] Distribution studies are limited to reports from intravenous administration of radiolabeled omalizumab in cynomolgus monkeys.[39] Blood sampling and harvesting of organs and tissues revealed that most of the drug remains in the central intravascular compartment. Little to no accumulation was observed in the harvested tissues and organs. This animal model is thought to be predictive of what is expected in humans with elevated IgE concentrations because these animals have high endogenous IgE concentrations (presumable because of parasitic infections) and omalizumab binds to cynomolgus IgE with similar affinity to that of human IgE.
The pathways for metabolism and clearance of omalizumab are incompletely understood. Clearance appears to be a function of IgG and IgG:antigen clearance processes.[39,52] IgG elimination involves degradation via the liver reticuloendothelial system (RES) and endothelial cells. Intact IgG is also excreted via the bile. Omalizumab:IgE complexes are cleared faster than uncomplexed omalizumab, but slower than free IgE.[81] Clearance rates decrease with higher doses and increase in patients with higher baseline IgE concentrations, possibly because of the differences observed in the clearance of complexed and uncomplexed omalizumab.[82] The terminal half-life of omalizumab is variable, with investigators reporting values ranging from 1 to 4 weeks.[52,71,79–84] This is similar to the 21-day half-life reported for human IgG1.[85] Differences in age, sex, race, and indication for use do not result in clinically significant differences in the pharmacokinetic profile of omalizumab.[80,81]
Pharmacodynamics
The dynamics of Fc-epsilon-RI expression on basophils during treatment and upon discontinuation of intravenous omalizumab is described in 2 phase 1 trials involving patients with PAR. In the first study, 12 patients received biweekly intravenous omalizumab.[38] Three months into treatment, the basophil Fc-epsilon-RI density decreased by 97%, the average number of IgE molecules/basophil decreased by 99%, and free IgE serum concentrations averaged 1% of pretreatment concentrations. The reversibility of the receptor changes was evaluated in a subsequent study by these investigators.[86] Eleven patients received intravenous omalizumab for 46 weeks. Low or undetectable amounts of IgE were detected on basophils obtained within 4 weeks of the last omalizumab dose. By the time patients were terminated from the study (20–53 weeks after the last omalizumab infusion, depending on how fast IgE serum concentrations recovered), basophil surface IgE was 47% of baseline while free serum IgE concentrations were 62% of baseline. Basophil Fc-epsilon-RI expression also increased over this time period. In a subsequent SAR trial, inhibition of basophil Fc-epsilon-RI expression was observed within 7 days of a standard omalizumab dose (0.016 mg/kg per IU of IgE/mL); maximum inhibition was reported to occur within 14 days.[87]
Changes in free IgE and total IgE with omalizumab treatment have been well described. After intravenous administration, free IgE concentrations drop substantially within 1–2 hours.[38,47,48] Following a small rebound, subsequent free IgE concentrations remain low for the duration of therapy.[46–48,72] The onset of the effect on IgE can be observed within 3 days of therapy initiation, as demonstrated after a standard subcutaneous omalizumab dose (0.016 mg/kg per IU of IgE/mL) in a trial in patients with SAR; IgE concentrations were 96% lower than baseline.[87] With subcutaneous administration, 300 mg of omalizumab reduced free IgE concentrations to </= 10.4 IU/mL (significance discussed below) in 63% to 69% of SAR patients within 3–4 weeks of their initial dose.[73,74] Over the course of subcutaneous omalizumab therapy, the free IgE changes are sustained. In the adult/adolescent trials of allergic asthma, free IgE concentrations were 89% to 99% of baseline[48–50]; the reduction in the pediatrics trial was 95% to 99%.[59] In the trials of allergic asthma by Soler and colleagues and Busse and colleagues and the pediatric trial by Milgrom and colleagues, median free IgE concentrations were below 10.4 IU/mL across all omalizumab dose ranges.[88]
In contrast to free IgE concentrations, total IgE concentrations (free IgE plus omalizumab-bound IgE) increase with omalizumab therapy because omalizumab:IgE complexes are cleared slower than free IgE.[46–48,50,59,72,80] The magnitude of increase has been 2–5 times baseline measurements,[46,59,72] with the actual increase in individual patients dependent upon the omalizumab dose and the baseline IgE concentrations.[72] With omalizumab discontinuation, free and total IgE concentrations approach baseline slowly. In the initial dose-ranging study involving patients with ragweed SAR, average free IgE concentrations returned to baseline within 8 weeks of the last omalizumab infusion.[72] Total IgE concentrations generally take longer to approach baseline after therapy termination.[72,73]
The best associations among omalizumab dose, suppression of free IgE concentration, and outcome are described in clinical trials involving patients with SAR. In the initial dose-ranging study described previously, the reduction in free IgE concentrations was more pronounced with higher doses and during the first few weeks of the trial, before the initial 7-day dosing interval was extended to 14 days.[72] Although significant between-group differences were not observed in average daily SAR symptom scores, the authors noted that these scores “appeared to be lower” in patients with free IgE concentrations < 16.7 IU/mL (statistical analysis not described). Dose-response curves revealed that consistent suppression of free IgE concentrations to the lowest levels of detection was achieved when the steady-state trough omalizumab concentration:total IgE concentration was 10–15:1. This correlated with administration of an omalizumab dose of approximately 0.005 mg/kg/week per IU of IgE/mL.
These findings were validated in the second dose-ranging study involving SAR patients.[73] IgE suppression appeared to be dependent on the dose of omalizumab, with greater suppression occurring with higher doses and more frequent dosing. Additionally, at the peak of the pollen season, patients with free IgE trough concentrations (defined as the IgE concentration measured prior to the next omalizumab dose) </= 10.4 IU/mL had significantly better average daily nasal symptom severity scores and used significantly fewer daily rescue medication tablets than those with free IgE trough concentrations of > 62.5 IU/mL. As described previously, significant improvement in outcome parameters was consistently observed only among patients receiving the highest omalizumab dose (300 mg every 3–4 weeks). These findings are consistent with those in patients receiving omalizumab for birch pollen-induced SAR.[74] In this study, the average daily nasal symptom severity score, average number of daily rescue medication tablets, and the proportion of days requiring rescue medication were lower in patients with free IgE concentrations < 10.4 IU/mL (compared with patients with values > 62.5 IU/mL).
Safety and Tolerability
In the Xolair package insert, the descriptions of all reported adverse events and those considered drug-related in allergic asthma trials indicate that such events have occurred with similar frequency among omalizumab and placebo recipients. Most reactions were mild to moderate in severity.[52] The most commonly reported adverse events with omalizumab therapy were injection site reactions (45%), viral infections (23%), upper respiratory tract infections (20%), sinusitis (16%), headache (15%), and pharyngitis (11%). Injection site reactions of any severity occurred in 45% of omalizumab recipients and 43% of placebo recipients. Reactions included: bruising, redness, warmth, burning, stinging, itching, hive formation, pain, induration, mass, and inflammation. Most of these reactions occurred within 1 hour of injection, resolved within 8 days, and generally decreased in frequency with subsequent dosing. Severe injection-site reactions (details not provided) were reported in 12% of omalizumab and 9% of placebo recipients.[52]
In the published reports of the adult-adolescent allergic asthma trials, there also were not differences between omalizumab and placebo recipients with regard to adverse event reporting. The types of events reported are consistent with the package insert.[49–51,55,56] Local injection site reactions were associated with 8.6% to 20.4% of omalizumab injections and 6.5% to 10.3% of placebo injections.[49–51,55] In the trial by Soler and colleagues, bruising was the most common reaction reported by both omalizumab and placebo recipients; redness, warmth, and itching were more common among omalizumab recipients.[49]
In the first 28 weeks of the childhood allergic asthma trial, the incidence of adverse events among omalizumab recipients was similar to that reported by placebo recipients.[59] Most reactions were mild to moderate in severity, with the most common being headache, pharyngitis, upper respiratory tract infections, and viral infections. Adverse events judged as drug-related did occur more frequently in the omalizumab recipients (6.2% vs 0.9% of placebo recipients, P = .029). These events included urticaria, maculopapular rash, flushing, pruritis, and arm pain. Local injection site reactions occurred in 37.5% of omalizumab and 36.6% of placebo recipients; these tended to decrease in frequency and severity over the course of the study. Four omalizumab (1.7%) and 2 placebo (1.8%) recipients withdrew because of pain and/or fear of injections. These findings are similar to those reported during the 24-week extension phase of the study.[60]
The adverse event profiles described in trials of allergic rhinitis are similar to those reported in the allergic asthma trials.[73,74,76,78] Of note, in the SAR trial where patients were randomized to different subcutaneous omalizumab doses (50 mg, 150 mg, or 300 mg), there was not evidence of a dose-response relationship with any of the reported adverse events.[73] The safety of omalizumab readministration in a subsequent ragweed pollen season was also assessed in a follow-up study involving 287 omalizumab recipients from this trial.[88] In a 12-week, open-labeled, safety trial, patients received 300 mg of subcutaneous omalizumab every 3–4 weeks (frequency dependent on serum IgE concentrations). The adverse event profile was similar to that described in the original study, with headache and upper respiratory tract infections being most frequently reported.
Laboratory Values
In studies that include descriptions of laboratory monitoring with omalizumab treatment, clinically significant changes in such values have not been observed.[50,56,59,60,73,74,78,89] However, in 2000, the US Food and Drug Administration requested that new omalizumab trials be suspended because of concerns about thrombocytopenia reported in monkey studies after exposure to concentrations in excess of the maximum human exposure in clinical trials.[90] Based on submitted supplementary data, the short hold on clinical trials was lifted. Platelet monitoring has been specifically described in 3 separate clinical trials. In the SAR trial comparing combinations of SIT and omalizumab, isolated occurrences of platelet counts below 150,000/mm3 (range, 47–148,000/mm3) occurred in 10 patients (9 omalizumab recipients).[76] Platelet counts were normal with subsequent sampling, and there were no reports of bleeding. In the childhood allergic asthma trial, 3 omalizumab recipients had clinically significant reductions in platelets (vs 1 placebo recipient). These were isolated occurrences, and episodes of bleeding were not observed.[60] The manufacturer reports that among the > 4000 patients treated with omalizumab in their clinical trials (16 trials over a 6-year period), investigators have not observed any cases of sustained thrombocytopenia in patients with normal platelet counts at baseline (data on file, Genentech, Inc.). Additionally, in a recent report of an open-labeled safety trial in 864 patients with moderate-severe asthma, platelet counts of < 100000/mm3 occurred in 4.8% of omalizumab recipients and 5.7% of control patients (standard therapy group); a 50% drop was observed in 0.86% and 0.71%, respectively.[91] None of the patients had platelet counts < 75000/mm3, all reductions were isolated and transient, and there were no reports of bleeding.
Anaphylaxis and Immunogenicity
According to the Xolair package insert, anaphylaxis was reported in 3 (incidence of < 0.1%) patients. These reactions occurred within 2 hours of a first or subsequent omalizumab dose. Symptoms included urticaria and throat and/or tongue edema. Respiratory failure was not observed, and all patients survived.[52] Although urticaria is described in patients involved in the discussed clinical trials, it was not described as a common adverse event.[46,48–50,59,60,73,74,76,78,89] These reactions were typically mild or moderate in severity and usually resolved quickly with therapy discontinuation or despite continued therapy. Except for the trial involving children with allergic asthma,[59] the incidence of urticaria among omalizumab and placebo recipients has been similar. In this study, urticaria was reported in 9 omalizumab (4%) and 1 placebo (0.9%) recipients. The urticaria was considered to be drug-related in 3 (1.3% incidence) omalizumab recipients.
In clinical trials of omalizumab, several investigators included analysis for the development of anti-omalizumab antibodies. Such antibodies were not detected in any of these trials.[47–50,55,56,72–74,78,89] Additionally, omalizumab skin testing was performed at baseline and at study completion in one of the early SAR trials.[72] The incidence of positive results was similar between omalizumab and placebo recipients (data not provided). In the Xolair package insert, it is reported that low titers of anti-omalizumab antibodies have been detected in 1 of 1723 treated patients.[52]
Although omalizumab administration results in immune complex formation, investigators have not observed evidence of patients experiencing reactions that would be considered manifestations of complex precipitation or complement activation.[55,56,59,73,74,78,89] In the cynomolgus monkey model, omalizumab:IgE complexes did not interact with blood cells and were not observed to accumulate in tissues or organs.[39]
Cancer Risk
Among the warnings in the Xolair package insert is mention of malignant neoplasms.[52] Malignancies were observed in 20/4127 (0.5%) omalizumab and 5/2236 (0.2%) placebo recipients involved in clinical studies. The observed malignancies in the omalizumab recipients included several instances of skin (11 occurrences in 6 patients, 2 being melanomas), breast (5), and prostate (initial, 1; recurrence, 1) cancer; non-Hodgkin's lymphoma, thyroid (recurrence), metastatic adenocystic parotid, bladder, parotid, pancreas, and colorectal cancer each occurred in 1 patient (data on file, Genentech, Inc.). In 18 of these patients, the events occurred within 12 months of therapy initiation; approximately 60% were within 6 months. Several patients had a history of previous cancers, premalignant conditions, or other risk factors for development of a malignancy. Although it is hypothesized that the immune systems of atopic persons may be better able to identify and reject clones of malignant cells, a link between IgE and cancer has not been established.[92,93] Nevertheless, since the majority of patients in clinical trials have had no more than a year's exposure to omalizumab, the risk for malignancy with more prolonged treatment needs to be studied, particularly in individuals who may be at higher risk for malignancies.
Parasitic Infections
Concern for increased susceptibility to parasitic infections while on omalizumab occurs because IgE is thought to induce an antibody-dependent, cell-mediated cytotoxic response against helminthic parasites.[93] In clinical trials, only a few cases of such infections have been observed in omalizumab recipients.[94] The risk for susceptibility to parasitic infections will need continued assessment.
Conclusions
Omalizumab is a subcutaneously administered monoclonal anti-IgE antibody that reduces free IgE concentrations and promotes downregulation of IgE receptors on basophils. In patients with allergic asthma poorly controlled with inhaled steroids, omalizumab improves asthma symptom control and allows patients to be managed with lower inhaled steroid doses. Omalizumab reduces the severity of SAR exacerbations during ragweed and birch pollen seasons in patients susceptible to these allergens. Additionally, omalizumab improves symptom control in patients with PAR. Omalizumab has been well tolerated in clinical trials that have extended as long as 52 weeks in duration. Safety beyond that time remains to be established, particularly with regard to the occurrence of neoplastic diseases while on therapy.
References
- 1.Weiss KB, Sullivan SD, Lyttle CS. Trends in the cost of illness for asthma in the United States, 1985–1994. J Allergy Clin Immunol. 2000;106:493–499. doi: 10.1067/mai.2000.109426. [DOI] [PubMed] [Google Scholar]
- 2.Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma — United States, 1980–1999. MMWR Surveill Summ. 2002;51(SS01):1–13. [PubMed] [Google Scholar]
- 3.Ford ES, Mannino DM, Homa DM, et al. Self-reported asthma and health related quality of life. Findings from the Behavioral Risk Factor Surveillance System. Chest. 2003;123:119–127. doi: 10.1378/chest.123.1.119. [DOI] [PubMed] [Google Scholar]
- 4.Fineman SM. The burden of allergic rhinitis: beyond dollars and cents. Ann Allergy Asthma Immunol. 2002;88(Suppl):2–7. doi: 10.1016/s1081-1206(10)62022-4. [DOI] [PubMed] [Google Scholar]
- 5.Baroody FM. Allergic rhinitis: broader disease effects and implications for management. Otolaryngol Head Neck Surg. 2003;128:616–631. doi: 10.1016/S0194-59980300257-2. [DOI] [PubMed] [Google Scholar]
- 6.National Asthma Education and Prevention Program. Expert panel report: Guidelines for the diagnosis and management of asthma. Updated selected topics-2002. J Allergy Clin Immunol. 2002;110(Pt 2):S141–219. [PubMed] [Google Scholar]
- 7.Bousquet J, Van Cauwenberge P, Khaltaev N, Aria Workshop Group Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108(Suppl):S147–334. doi: 10.1067/mai.2001.118891. [DOI] [PubMed] [Google Scholar]
- 8.Garcia G, Adler M, Humbert M. Difficult asthma. Allergy. 2003;58:114–121. doi: 10.1034/j.1398-9995.2003.02171.x. [DOI] [PubMed] [Google Scholar]
- 9.White P, Smith H, Baker N, Davis W, Frew A. Symptom control in patients with hay fever in UK general practice: how well are we doing and is there a need for allergen immunotherapy? Clin Exp Allergy. 1998;28:266–270. doi: 10.1046/j.1365-2222.1998.00237.x. [DOI] [PubMed] [Google Scholar]
- 10.Sicherer SH, Leung DYM. Advances in allergic skin disease, anaphylaxis, and hypersensitivity reactions to foods, drugs, and insect stings. J Allergy Clin Immunol. 2004;114:118–124. doi: 10.1016/j.jaci.2004.03.056. [DOI] [PubMed] [Google Scholar]
- 11.Leung DYM, Sampson HA, Yunginger JW, et al. Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med. 2003;348:986–993. doi: 10.1056/NEJMoa022613. [DOI] [PubMed] [Google Scholar]
- 12.Pearce N, Pekkanen J, Beasley R. How much asthma is really attributable to atopy? Thorax. 1999;54:268–272. doi: 10.1136/thx.54.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zacharasiewicz A, Douwes J, Pearce N. What proportion of rhinitis symptoms is attributable to atopy? J Clin Epidemiol. 2003;56:385–390. doi: 10.1016/s0895-4356(03)00043-x. [DOI] [PubMed] [Google Scholar]
- 14.Burrows B, Martinez FD, Halonen M, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320:271–277. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 15.Togias A. Rhinitis and asthma: evidence for respiratory system integration. J Allergy Clin Immunol. 2003;111:1171–1183. doi: 10.1067/mai.2003.1592. [DOI] [PubMed] [Google Scholar]
- 16.Goldsby RA, Kindt TJ, Osborne BA, Kuby J, editors. Immunology. 5th edition. New York: WH Freeman and Company; 2003. [Google Scholar]
- 17.Tarlinton D. Enhanced: antigen presentation by memory B cells – the sting is in the tail. Science. 1997;276:374–375. doi: 10.1126/science.276.5311.374. [DOI] [PubMed] [Google Scholar]
- 18.Hook WA, Zinsser FU, Berenstein EH, Siraganian RP. Monoclonal antibodies defining epitopes on human IgE. Mol Immunol. 1991;28:631–639. doi: 10.1016/0161-5890(91)90132-4. [DOI] [PubMed] [Google Scholar]
- 19.Presta L, Shields R, O'Connell L, et al. The binding site on human immunoglobulin E for its high affinity receptor. J Biol Chem. 1994;269:26368–26373. [PubMed] [Google Scholar]
- 20.Nissim A, Schwarzbaum S, Siraganian R, Eshhar Z. Fine specificity of the IgE interaction with the low and high affinity Fc receptor. J Immunol. 1993;150:1365–1374. [PubMed] [Google Scholar]
- 21.Oettgen HC, Geha RS. IgE in asthma and atopy: cellular and molecular connections. J Clin Invest. 1999;104:829–835. doi: 10.1172/JCI8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Broide DH. Molecular and cellular mechanisms of allergic disease. J Allergy Clin Immunol. 2001;108:S65–71. doi: 10.1067/mai.2001.116436. [DOI] [PubMed] [Google Scholar]
- 23.Kay AB. Allergy and allergic diseases. N Engl J Med. 2001;344:30–37. doi: 10.1056/NEJM200101043440106. [DOI] [PubMed] [Google Scholar]
- 24.Pearlman DS. Pathophysiology of the inflammatory response. J Allergy Clin Immunol. 1999;104:S132–137. doi: 10.1016/s0091-6749(99)70308-8. [DOI] [PubMed] [Google Scholar]
- 25.Novak N, Kraft S, Bieber T. IgE receptors. Curr Opin Immunol. 2001;13:721–726. doi: 10.1016/s0952-7915(01)00285-0. [DOI] [PubMed] [Google Scholar]
- 26.Gustavsson S, Hjulstrom S, Tianmin L, Heyman B. CD23/IgE-mediated regulation of the specific antibody response in vivo. J Immunol. 1994;152:4793–4800. [PubMed] [Google Scholar]
- 27.Breedveld FC. Therapeutic monoclonal antibodies. Lancet. 2000;355:735–740. doi: 10.1016/s0140-6736(00)01034-5. [DOI] [PubMed] [Google Scholar]
- 28.Presta LG, Lahr SJ, Shields RL, et al. Humanization of an antibody directed against IgE. J Immunol. 1993;151:2623–2632. [PubMed] [Google Scholar]
- 29.Liu J, Lester P, Builder S, Shire SJ. Characterization of complex formation by humanized anti-IgE monoclonal antibody and monoclonal human IgE. Biochemistry. 1995;34:10474–10482. doi: 10.1021/bi00033a020. [DOI] [PubMed] [Google Scholar]
- 30.Meng YG, Singh N, Wong WL. Binding of cynomolgus monkey IgE to a humanized anti-human IgE antibody and human high affinity IgE receptor. Mol Immunol. 1996;33:635–642. doi: 10.1016/0161-5890(96)00024-7. [DOI] [PubMed] [Google Scholar]
- 31.Chang TW. The pharmacological basis of anti-IgE therapy. Nat Biotechnol. 2000;18:157–162. doi: 10.1038/72601. [DOI] [PubMed] [Google Scholar]
- 32.Shields RL, Whether WR, Zioncheck K, et al. Inhibition of allergic reactions with antibodies to IgE. Int Arch Allergy Immunol. 1995;107:308–312. doi: 10.1159/000237010. [DOI] [PubMed] [Google Scholar]
- 33.Saban R, Haak-Frendscho M, Zine M, et al. Human FcERI-IgG and humanized anti-IgE monoclonal antibody MaE11 block passive sensitization of human and rhesus monkey lung. J Allergy Clin Immunol. 1994;94:836–843. doi: 10.1016/0091-6749(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 34.Sechi S, Roller PP, Willette-Brown J, Kinet JP. A conformational rearrangement upon binding of IgE to its high affinity receptor. J Biol Chem. 1996;271:19256–19263. doi: 10.1074/jbc.271.32.19256. [DOI] [PubMed] [Google Scholar]
- 35.Sutton BJ, Gould HJ. The human IgE network. Nature. 1993;366:421–428. doi: 10.1038/366421a0. [DOI] [PubMed] [Google Scholar]
- 36.Malveaux FJ, Conroy MC, Adkinson NF, Lichtenstein LM. IgE receptors on human basophils. Relationship to serum IgE concentration. J Clin Invest. 1978;62:176–181. doi: 10.1172/JCI109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacGlashan D, McKenzie-White J, Chichester K, et al. Blood. 1998;91:1633–1643. [PubMed] [Google Scholar]
- 38.MacGlashan DW, Bochner BS, Adelman DC, et al. Down-regulation of FcepsilonRI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J Immunol. 1997;158:1438–1445. [PubMed] [Google Scholar]
- 39.Fox JA, Hotaling TE, Struble C, Ruppel J, Bates DJ, Schoenhoff MB. Tissue distribution and complex formation with IgE of an anti-IgE antibody after intravenous administration in cynomolgus monkeys. J Pharmacol Exp Ther. 1996;279:1000–1008. [PubMed] [Google Scholar]
- 40.Chen HY, Liu F-T, Hou CMH, Huang JSW, Sharma BB, Chang TW. Monoclonal antibodies against the CepsilonmX domain of human membrane-bound IgE and their potential use for targeting IgE-expressing B cells. Int Arch Allergy Immunol. 2002;128:315–324. doi: 10.1159/000063860. [DOI] [PubMed] [Google Scholar]
- 41.Milgrom H. Is there a role for treatment of asthma with omalizumab? Arch Dis Child. 2003;88:71–74. doi: 10.1136/adc.88.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prussin C, Griffith DT, Boesel KM, Lin H, Foster B, Casale TB. Omalizumab treatment downregulates dendritic cell FcepsilonRI expression. J Allergy Clin Immunol. 2003;112:1147–1154. doi: 10.1016/j.jaci.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Kisselgof AB, Oettgen HC. The expression of murine B cell CD23, in vivo, is regulated by its ligand, IgE. Int Immunol. 1998;10:1377–1384. doi: 10.1093/intimm/10.9.1377. [DOI] [PubMed] [Google Scholar]
- 44.Novak N, Tepel C, Koch S, Brix K, Bieber T, Kraft S. Evidence for a differential expression of the FcepsilonRIgamma chain in dendritic cells of atopic and nonatopic donors. J Clin Invest. 2003;111:1047–1056. doi: 10.1172/JCI15932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shira BS, Kon OM, Grant JA, Kay AB. Expression of high-affinity IgE receptors (FcepsilonRI) on peripheral blood basophils, monocytes, and eosinophils in atopic and nonatopic subjects: relationship to total serum IgE concentrations. J Allergy Clin Immunol. 1997;99:699–706. doi: 10.1016/s0091-6749(97)70033-2. [DOI] [PubMed] [Google Scholar]
- 46.Boulet L-P, Chapman KR, Cote J, et al. Inhibitory effects of an anti-IgE antibody E25 on allergen-induced early asthmatic response. Am J Respir Crit Care Med. 1997;155:1835–1840. doi: 10.1164/ajrccm.155.6.9196083. [DOI] [PubMed] [Google Scholar]
- 47.Fahy JV, Fleming HE, Wong HH, et al. The effect of an anti-IgE monoclonal antibody on the early- and late-phase responses to allergen inhalation in asthmatic subjects. Am J Respir Crit Care Med. 1997;155:1828–1834. doi: 10.1164/ajrccm.155.6.9196082. [DOI] [PubMed] [Google Scholar]
- 48.Milgrom H, Fick RB, Su JQ, et al. Treatment of allergic asthma with monoclonal anti-IgE antibody. N Engl J Med. 1999;341:1966–1973. doi: 10.1056/NEJM199912233412603. [DOI] [PubMed] [Google Scholar]
- 49.Soler M, Matz J, Townley R, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001;18:254–261. doi: 10.1183/09031936.01.00092101. [DOI] [PubMed] [Google Scholar]
- 50.Busse W, Corren J, Lanier BQ, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe asthma. J Allergy Clin Immunol. 2001;108:184–190. doi: 10.1067/mai.2001.117880. [DOI] [PubMed] [Google Scholar]
- 51.Holgate ST, Chuchalin A, Herbert J, et al. Efficacy and safety a recombinant anti-immunoglobulin E antibody (omalizumab) in severe allergic asthma. Clin Exp Allergy. 2004;34:632–638. doi: 10.1111/j.1365-2222.2004.1916.x. [DOI] [PubMed] [Google Scholar]
- 52.Genentech, Inc. Xolair(R) (omalizumab for subcutaneous use) package insert. South San Francisco, Calif.: 2003. Jun, [Google Scholar]
- 53.Busse WW. Anti-immunoglobulin E (omalizumab) therapy in allergic asthma. Am J Respir Crit Care. 2001;164:S12–17. doi: 10.1164/ajrccm.164.supplement_1.2103026. [DOI] [PubMed] [Google Scholar]
- 54.Noga O, Hanf G, Kunkel G. Immunological and clinical changes in allergic asthmatics following treatment with omalizumab. Int Arch Allergy Immunol. 2003;131:46–52. doi: 10.1159/000070434. [DOI] [PubMed] [Google Scholar]
- 55.Buhl R, Soler M, Matz J, et al. Omalizumab provides long-term control in patients with moderate-to-severe asthma. Eur Respir J. 2002;20:73–78. doi: 10.1183/09031936.02.00278102. [DOI] [PubMed] [Google Scholar]
- 56.Lanier BQ, Corren J, Lumry W, Liu J, Fowler-Taylor A, Gupta N. Omalizumab is effective in the long-term control of severe allergic asthma. Ann Allergy Asthma Immunol. 2003;91:154–159. doi: 10.1016/S1081-1206(10)62170-9. [DOI] [PubMed] [Google Scholar]
- 57.Ayres JG, Higgins B, Chilvers ER, Ayre G, Blogg M, Fox H. Efficacy and tolerability of anti-immunoglobulin E therapy with omalizumab in patients with poorly controlled (moderate-to-severe allergic asthma) Allergy. 2004;59:701–708. doi: 10.1111/j.1398-9995.2004.00533.x. [DOI] [PubMed] [Google Scholar]
- 58.National Institutes of Health National Heart, Lung, and Blood Institute. Expert panel report 2. Guidelines for the diagnosis and management of asthma. 1997. Jul, NIH publication no. 97–4051. [PubMed]
- 59.Milgrom H, Berger W, Nayak A, et al. Treatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab) [serial online] Pediatrics. 2001;108 doi: 10.1542/peds.108.2.e36. [cited 2003 September 5]. Available from URL: http://www.pediatrics.org/cgi/content/full/108/2/e36. [DOI] [PubMed] [Google Scholar]
- 60.Berger W, Gupta N, McAlary M, Fowler-Taylor A. Evaluation of long-term safety of the anti-IgE antibody, omalizumab, in children with allergic asthma. Ann Allergy Asthma Immunol. 2003;91:182–188. doi: 10.1016/S1081-1206(10)62175-8. [DOI] [PubMed] [Google Scholar]
- 61.Buhl R. Omalizumab (Xolair[R]) improves quality of life in adult patients with allergic asthma: a review. Respir Med. 2003;97:123–129. doi: 10.1053/rmed.2003.1442. [DOI] [PubMed] [Google Scholar]
- 62.Juniper EF, Guyatt GH, Epstein RS, Ferrie PJ, Jaeschke R, Hiller TK. Evaluation of impairment of health-related quality of life in asthma: development of a questionnaire for use in clinical trials. Thorax. 1992;47:76–83. doi: 10.1136/thx.47.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific quality of life questionnaire. J Clin Epidemiol. 1994;47:81–87. doi: 10.1016/0895-4356(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 64.Buhl R, Hanf G, Soler M, et al. The anti-IgE antibody omalizumab improves asthma-related quality of life in patients with allergic asthma. Eur Respir J. 2002;20:1088–1094. doi: 10.1183/09031936.02.00016502. [DOI] [PubMed] [Google Scholar]
- 65.Finn A, Gross G, van Bavel J, et al. Omalizumab improves asthma-related quality of life in patients with severe allergic asthma. J Allergy Clin Immunol. 2003;111:278–284. doi: 10.1067/mai.2003.54. [DOI] [PubMed] [Google Scholar]
- 66.Lemanske RF, Nayak A, McAlary M, Everhard F, Fowler-Taylor A, Gupta N. Omalizumab improves asthma-related quality of life in children with allergic asthma [serial online] Pediatrics. 2002;110 doi: 10.1542/peds.110.5.e55. [cited 2003 September 5]. Available from URL: http://www.pediatrics.org/cgi/content/full/110/5/e55. [DOI] [PubMed] [Google Scholar]
- 67.Corren J, Casale T, Deniz Y, Ashby M. Omalizumab, a recombinant humanized anti-IgE antibody, reduces asthma-related emergency room visits and hospitalizations in patients with allergic asthma. J Allergy Clin Immunol. 2003;111:87–90. doi: 10.1067/mai.2003.49. [DOI] [PubMed] [Google Scholar]
- 68.Oba Y, Salzman GA. Cost-effectiveness analysis of omalizumab in adults and adolescents with moderate-to-severe allergic asthma. J Allergy Clin Immunol. 2004;114:265–269. doi: 10.1016/j.jaci.2004.05.049. [DOI] [PubMed] [Google Scholar]
- 69.Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest. 2004;125:1378–1386. doi: 10.1378/chest.125.4.1378. [DOI] [PubMed] [Google Scholar]
- 70.Holgate S, Bousquet J, Wenzel S, Fox H, Liu J, Castellsague J. Efficacy of omalizumab, an anti-immunoglobulin E antibody in patients with allergic asthma at high risk of serious asthma-related morbidity and mortality. Curr Med Res Opin. 2001;17:233–240. [PubMed] [Google Scholar]
- 71.Hanf G, Noga O, OConnor A, Kunkel G. Omalizumab inhibits allergen challenge-induced nasal response. Eur Respir J. 2004;23:414–418. doi: 10.1183/09031936.04.00024504. [DOI] [PubMed] [Google Scholar]
- 72.Casale TB, Berstein IL, Busse WW, et al. Use of an anti-IgE humanized monoclonal antibody in ragweed-induced allergic rhinitis. J Allergy Clin Immunol. 1997;100:110–121. doi: 10.1016/s0091-6749(97)70202-1. [DOI] [PubMed] [Google Scholar]
- 73.Casale TB, Condemi J, LaForce C, et al. Effect of omalizumab on symptoms of seasonal allergic rhinitis. A randomized controlled trial. JAMA. 2001;286:2956–2967. doi: 10.1001/jama.286.23.2956. [DOI] [PubMed] [Google Scholar]
- 74.Adelroth E, Rak S, Haahtela T, et al. Recombinant humanized mAb-E25, an anti-IgE mAb, in birch pollen-induced seasonal allergic rhinitis. J Allergy Clin Immunol. 2000;106:253–259. doi: 10.1067/mai.2000.108310. [DOI] [PubMed] [Google Scholar]
- 75.Plewako H, Arvidsson M, Petruson K, et al. The effect of omalizumab on nasal allergic inflammation. J Allergy Clin Immunol. 2002;110:68–71. doi: 10.1067/mai.2002.125488. [DOI] [PubMed] [Google Scholar]
- 76.Kuehr J, Brauburger J, Zielen S, et al. Efficacy of combination treatment with anti-IgE plus specific immunotherapy in polysensitized children and adolescents with seasonal allergic rhinitis. J Allergy Clin Immunol. 2002;109:274–280. doi: 10.1067/mai.2002.121949. [DOI] [PubMed] [Google Scholar]
- 77.Kopp MV, Brauburger J, Riedinger F, et al. The effect of anti-IgE treatment on in vitro leukotriene release in children with seasonal allergic rhinitis. J Allergy Clin Immunol. 2002;110:728–735. doi: 10.1067/mai.2002.128804. [DOI] [PubMed] [Google Scholar]
- 78.Chervinsky P, Casale T, Townley R, et al. Omalizumab, an anti-IgE antibody, in the treatment of adults and adolescents with perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2003;91:160–167. doi: 10.1016/S1081-1206(10)62171-0. [DOI] [PubMed] [Google Scholar]
- 79.Vignola AM, Humbert M, Bousquet J, et al. Efficacy and tolerability of anti-immunoglobulin E therapy with omalizumab in patients with concomitant allergic asthma and persistent allergic rhinitis: SOLAR. Allergy. 2004;59:709–717. doi: 10.1111/j.1398-9995.2004.00550.x. [DOI] [PubMed] [Google Scholar]
- 80.Marian M, Sun Y-N, Sinclair BS, et al. Clinical pharmacokinetics (PK) and IgE pharmacodynamics (PD) of omalizumab, a recombinant humanized monoclonal antibody to IgE. Clin Pharmacol Ther. 2001;69:P7. [Google Scholar]
- 81.Bisberg D, Froehlich J, Schoenhoff M, Mendelson J. Multiple administrations of the anti-IgE recombinant humanized monoclonal antibody E25 (rhuMAb-E25) reduces free IgE levels in a dose dependent manner in adolescents and children with moderate to severe allergic asthma. J Clin Pharmacol. 1996;36:859. [Google Scholar]
- 82.Froehlich J, Schoenhoff M, Tremblay T, Ruppel J, Jardieu P. Initial human study with a humanized recombinant anti-IgE monoclonal antibody: safety, tolerance, and pharmacokinetic (PK)/dynamic profile. Clin Pharmacol Ther. 1995;157:162. [Google Scholar]
- 83.Schoenhoff M, Lin Y, Froehlich J, Fick R, Bates D. A pharmacodynamic model describing free IgE concentrations following administration of a recombinant humanized monoclonal anti IgE antibody in humans. Pharm Res. 1995;12(Suppl):S411. [Google Scholar]
- 84.Schoenhoff M, Bates D, Ruppel J, et al. Pharmacokinetics/dynamics following administration of a recombinant humanized monoclonal anti IgE antibody in the cynomolgus monkey. J Allergy Clin Immunol. 1995;95:356. [Google Scholar]
- 85.Morell A, Terry W, Waldmann T. Metabolic properties of IgG subclasses in man. J Clin Invest. 1970;49:673–680. doi: 10.1172/JCI106279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saini SS, MacGlashan DW, Sterbinsky SA, et al. Down-regulation of human basophil IgE and FCepsilonRIalpha surface densities and mediator release by anti-IgE-infusions is reversible in vitro and in vivo. J Immunol. 1999;162:5624–5630. [PubMed] [Google Scholar]
- 87.Lin H, Boesel KM, Griffith DT, et al. Omalizumab rapidly decreases nasal allergic response and FcepsilonRI on basophils. J Allergy Clin Immunol. 2004;113:297–302. doi: 10.1016/j.jaci.2003.11.044. [DOI] [PubMed] [Google Scholar]
- 88.Hochhaus G, Brookman L, Fox H, et al. Pharmacodynamics of omalizumab: implications for optimized dosing strategies and clinical efficacy in the treatment of allergic asthma. Curr Med Res Opin. 2003;19:491–498. doi: 10.1185/030079903125002171. [DOI] [PubMed] [Google Scholar]
- 89.Nayak A, Casale T, Miller SD, et al. Tolerability of retreatment with omalizumab, a recombinant humanized monoclonal anti-IgE antibody, during a second ragweed pollen season in patients with seasonal allergic rhinitis. Allergy Asthma Proc. 2003;24:323–329. [PubMed] [Google Scholar]
- 90.Omalizumab. Anti-IgE monoclonal antibody E25, E25, humanised anti-IgE MAb, IgE 025, monoclonal antibody E25, olizumab, Xolair, rhuMAb-E25. BioDrugs. 2002;16:380–386. doi: 10.2165/00063030-200216050-00009. [DOI] [PubMed] [Google Scholar]
- 91.Israel E, Cohn J, Meltzer E, McCarty J, Zheng B, Carroll A. Omalizumab does not induce thrombocytopenia in the treatment of asthma. J Allergy Clin Immunol. 2003;111:S146–147. [Google Scholar]
- 92.Rosenbaum JT, Dwyer JM. The role of IgE in the immune response to neoplasia: a review. Cancer. 1977;39:11–20. doi: 10.1002/1097-0142(197701)39:1<11::aid-cncr2820390104>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 93.Winter WE, Hardt NS, Fuhrman S. Immunoglobulin E. Importance in parasitic infections and hypersensitivity responses. Arch Pathol Lab Med. 2000;124:1382–385. doi: 10.5858/2000-124-1382-IE. [DOI] [PubMed] [Google Scholar]
- 94.Johansson SGO, Haahtela T, O'Byrne PM. Omalizumab and the immune system: an overview of preclinical and clinical data. Ann Allergy Asthma Immunol. 2002;89:132–138. doi: 10.1016/S1081-1206(10)61928-X. [DOI] [PubMed] [Google Scholar]


