A long-standing problem in developmental biology, closely associated with pattern formation, is how continuous morphogen gradients are translated into sharp response borders that define clearcut territories in which cells express different sets of genes. To understand how gradients can generate thresholds, several mechanisms have been investigated over the years. Initially, most of these studies were of a theoretical nature, unrelated to any specific example based on solid molecular background. In an elegant study currently published in Molecular Systems Biology (Melen et al, 2005), blending experiment with theory, Shilo, Barkai and co-workers address this question within the context of a well-selected developmental system. They resort to computational systems biology to uncover the role of a phosphorylation–dephosphorylation cycle in generating a sharp boundary separating distinct developmental domains in the Drosophila embryo.
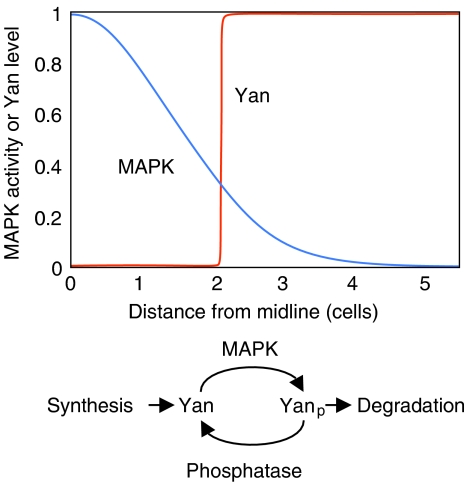
The phenomenon considered underlies pattern formation in the Drosophila embryonic ventral ectoderm. There, a graded MAPK activation results from a graded activation of the EGF receptor, via the binding of its ligand Spitz secreted by a single row of cells positioned along the midline of the ventral ectoderm. Degradation of the transcriptional repressor Yan is triggered upon phosphorylation by MAPK. Melen et al show that the boundaries of Yan degradation on the two sides of the midline are extremely sharp. The Yan protein is absent from 1–2 cell rows on each side of the midline, whereas the adjacent rows display high levels of Yan comparable to those seen in most lateral cells (Figure 1). How the graded MAPK activation is converted into an all-or-none degradation switch of Yan is the topic of this study.
Figure 1.
(Top) Schematic representation of MAPK activity and total Yan level as a function of distance from midline of ventral ectoderm in the Drosophila embryo (adapted from Melen et al, 2005). The smooth gradient in MAPK translates into an all-or-none transition in the level of Yan protein, which creates a sharp boundary between adjacent territories in the ventral ectoderm. The Yan protein is fully degraded when MAPK activity exceeds a critical value. Experiments described by Melen et al in this issue of Molecular Systems Biology indicate that the threshold-generating mechanism relies on zero-order ultrasensitivity in Yan phosphorylation. (Bottom) The Yan phosphorylation–dephosphorylation network involves the kinase MAPK, a yet unidentified phosphatase, as well as synthesis of Yan and degradation of the phosphorylated form of the protein, Yanp. Zero-order ultrasensitivity responsible for the threshold dependence of Yan on MAPK (see top panel) arises in the network when the kinase and phosphatase are saturated by their respective substrate and function in the zero-order kinetic domain.
The authors begin by establishing the experimental bases of the threshold phenomenon. Building on previous results, they first show, by using a variety of mutants, that graded MAPK activation is converted to a sharp border of Yan degradation and that the threshold-generating mechanism is post-transcriptional. The authors establish that MAPK activation is both essential and sufficient for Yan degradation, and that the level of activated MAPK determines the position of the degradation border. They proceed to show that Yan phosphorylation by MAPK is the critical step in Yan degradation.
To gain insights into the molecular mechanism underlying the Yan degradation switch, Melen et al turn to the mathematical modeling of the Yan degradation network. A strong aspect of this study is the thorough comparison of a variety of mechanisms for Yan phosphorylation by MAPK. The goal set forth by the authors is to test the distinctive predictions of these mechanisms so as to establish which one(s) can best account for the generation of the threshold observed in the experiments. Four models are compared: (1) non-cooperative, first-order (linear) phosphorylation of Yan by MAPK; (2) cooperative multiple phosphorylation, linear with respect to Yan; (3) non-cooperative, zero-order phosphorylation; and (4) positive feedback in Yan phosphorylation. Threshold generation in mechanism (3) rests on the phenomenon of ‘zero-order ultrasensitivity' previously described by Goldbeter and Koshland (1981) in the general context of protein covalent modification.
Of the four mechanisms considered initially, Melen et al show that a single one is capable of accounting for all the tests suggested by the models. Solving for the steady state of phosphorylated Yan (marked for degradation) as a function of the distance from midline cells from which the graded distribution of activated MAPK originates, the authors first show that mechanisms (2), (3) and (4) can all generate a threshold. Discriminating between these three models of Yan degradation is made in two ways, which both rely on perturbing the steady-state level of Yan. Specifically, Yan overexpression is shown to lead to distinctive changes in the boundaries of Yan degradation and to different times of evolution to the steady state, depending on the mechanism considered.
Next, the authors test experimentally the theoretical predictions, to distinguish between the various mechanisms. Upon overexpressing Yan in transgenic flies, they find that the position of the sharp boundary does not change with respect to midline cells. Of the three models that can generate a threshold, only the linear cooperative model (2) and the zero-order ultrasensitivity mechanism (3) can account for this observation, which eliminates mechanism (4) based on positive feedback. To further differentiate between the cooperative and zero-order models, the authors examine the time course of Yan degradation, which significantly increases following overexpression. They show that the various experiments can only be accounted for by the zero-order ultrasensitivity mechanism.
The original finding (Goldbeter and Koshland, 1981) that a cycle of phosphorylation–dephosphorylation can serve as a switch is itself an illustration of the use of computational systems biology. Indeed, it was the numerical study of a biochemical model that led to the prediction that a quasi-discontinuous switch in the steady-state level of a phosphorylated protein can result from a progressive change in the maximum rate of the kinase versus that of the phosphatase acting on this protein. Such a switch only occurs in the presence of both kinase and phosphatase, when the enzymes operate in the zero-order kinetic domain (i.e. the protein substrate concentration is much larger than the Km of the modifying enzymes)—hence the name of zero-order ultrasensitivity given to this threshold-generating phenomenon. The possibility that zero-order switches play a role in defining developmental thresholds in pattern formation has been proposed (Goldbeter and Wolpert, 1990). The great interest of the study published in this issue by Melen et al is twofold: it provides for the first time a concrete example of a developmental threshold associated with zero-order ultrasensitivity, and it offers a methodology to characterize the threshold-generating mechanism by discriminating between possible models on the basis of their specific predictions, and by testing the latter experimentally. The interest of these results extends beyond the case of Yan degradation in Drosophila, given the widespread occurrence of the control of transcriptional regulators through reversible phosphorylation.
To demonstrate zero-order ultrasensitivity in vitro in a phosphorylation–dephosphorylation system, it is necessary to vary the ratio of kinase to phosphatase activity at varying levels of protein substrate. This approach was applied to glycogen phosphorylase (Meinke and Edstrom, 1991). A key advantage of studying the threshold in Yan phosphorylation is that it occurs in vivo, in physiological conditions. Both the mechanism and function of the phenomenon can be addressed at the same time. Moreover, the gradient in MAPK established in the Drosophila embryonic ventral ectoderm naturally provides a continuous variation of the kinase to phosphatase activity ratio. Thus, the existence of a sharp boundary separating distinct developmental domains allows a spatial readout of the zero-order ultrasensitivity threshold in Yan phosphorylation.
An alternative threshold-generating mechanism considered for long in relation to developmental thresholds (Lewis et al, 1977; Meinhardt, 1982) relies on all-or-none transitions between two simultaneously stable steady states. This phenomenon, known as bistability, occurs in a variety of chemical and biological systems as a result of positive feedback or mutual inhibition (see Ferrell, 2002, for a recent discussion of bistable behavior in signal transduction). In the context of pattern formation, model studies showed that bistable transitions can occur in a critical range within a gradient of controlling factor. Initially, the studies of developmental thresholds based on bistability did not pertain to an identifiable mechanism in a specific developmental system. A recent study based on a computational model (A Goldbeter and O Pourquié, submitted) suggests that sharp developmental thresholds defined through bistability may underlie the formation of boundaries between somites in the presomitic mesoderm in chicken or mouse embryos. In that system, bistability would arise from mutual inhibition between antagonistic gradients of retinoic acid and fibroblast growth factor (Diez del Corral et al, 2004). Verifying these predictions in the context of somitogenesis would illustrate that formation of sharp boundaries in development may exploit a variety of fundamental mechanisms, including zero-order ultrasensitivity and bistability.
References
- Diez del Corral R, Olivera-Martinez I, Goriely A, Gale E, Maden M, Storey K (2004) Opposing FGF and retinoid pathways: a signaling switch that controls differentiation and patterning onset in the extending vertebrate body axis. BioEssays 26: 857–869 [DOI] [PubMed] [Google Scholar]
- Ferrell JE Jr (2002) Self-perpetuating states in signal transduction: positive feedback, double negative feedback and bistability. Curr Opin Cell Biol 14: 140–148 [DOI] [PubMed] [Google Scholar]
- Goldbeter A, Koshland DE Jr (1981) An amplified sensitivity arising from covalent modification in biological systems. Proc Natl Acad Sci USA 78: 6840–6844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbeter A, Wolpert L (1990) Covalent modification of proteins as a threshold mechanism in development. J Theor Biol 142: 243–250 [DOI] [PubMed] [Google Scholar]
- Lewis J, Slack J, Wolpert L (1977) Thresholds in development. J Theor Biol 65: 579–590 [DOI] [PubMed] [Google Scholar]
- Meinhardt H (1982) Models of Biological Pattern Formation. London: Academic Press [Google Scholar]
- Meinke MH, Edstrom RD (1991) Muscle glycogenolysis. Regulation of the cyclic interconversion of phosphorylase a and phosphorylase b. J Biol Chem 266: 2259–2266 [PubMed] [Google Scholar]
- Melen GJ, Levy S, Barkai N, Shilo BZ (2005) Threshold responses to morphogen gradients by zero-order ultrasensitivity. Mol Syst Biol 13 December 2005; 2005.0028. doi:10.1038/msb4100036 [DOI] [PMC free article] [PubMed] [Google Scholar]