Abstract
Recognition of pathogen-associated molecular signatures is critically important in proper activation of the immune system. The toll-like receptor (TLR) signaling network is responsible for innate immune response. In mammalians, there are 11 TLRs that recognize a variety of ligands from pathogens to trigger immunological responses. In this paper, we present a comprehensive map of TLRs and interleukin 1 receptor signaling networks based on papers published so far. The map illustrates the possible existence of a main network subsystem that has a bow-tie structure in which myeloid differentiation primary response gene 88 (MyD88) is a nonredundant core element, two collateral subsystems with small GTPase and phosphatidylinositol signaling, and MyD88-independent pathway. There is extensive crosstalk between the main bow-tie network and subsystems, as well as feedback and feedforward controls. One obvious feature of this network is the fragility against removal of the nonredundant core element, which is MyD88, and involvement of collateral subsystems for generating different reactions and gene expressions for different stimuli.
Keywords: bow-tie structure, robustness, toll-like receptor
Introduction
The toll-like receptor (TLR) signaling pathway is the front-line subsystem against invasive microorganisms for both innate and adaptive immunity (Iwasaki and Medzhitov, 2004). To sense innumerable and various pathogenic threats, TLRs have evolved to recognize pathogen-associated molecular patterns (PAMPs), which represent molecular features on the surface of pathogens. The TLR gene family and their pathways have been evolutionarily well conserved in both invertebrates and vertebrates (Hoffmann and Reichhart, 2002; Roach et al, 2005). One of the fundamental questions is how pathogenic stimuli in the form of PAMPs induce various responses that ultimately protect the host. Each TLR binds to a variety of PAMPs that work as molecular markers of potential pathogens that the host shall be defended against. For example, TLR4 was found to be a receptor for lipolysaccharide (LPS) and essential to generate responses to Gram-negative bacteria in which LPS is a part of the outer membrane (Poltorak et al, 1998), TLR9 responds to DNA-containing unmethylated CpG motifs (Hemmi et al, 2000), TLR3 is activated by double-stranded RNA (Alexopoulou et al, 2001), and bacteria flagellin activates TLR5 (Hayashi et al, 2001). There are extensive reviews on ligand receptor relationships for further reference (Akira and Takeda, 2004; Beutler, 2004; Iwasaki and Medzhitov, 2004). TLRs and interleukin 1 receptors (IL-1Rs) have a conserved region of amino acids, which is known as the toll/IL-1R (TIR) domain (Slack et al, 2000). Signaling of the TLR/IL-1R superfamily is mediated through myeloid differentiation primary response gene 88 (MyD88), IL-1R-associated kinases (IRAKs), transforming growth factor beta-activated kinase 1 (TAK1), TAK1-binding protein 1 (TAB1), TAB2, tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF6), etc. (Akira and Takeda, 2004). It should be mentioned that TLR1, TLR2, TLR6, TLR4, and TLR5 are located on the plasma membrane, whereas TLR3, TLR7, and TLR9 are not located on the cell surface (Akira and Takeda, 2004). While ligands for each TLR and interactions downstream of receptors are now being identified at a dramatic pace, doubt is now being cast on the global logic behind all TLR pathways. It was argued that the TLR pathway forms an hour-glass structure (Beutler, 2004), but the precise shape of the global TLR signaling network and its functional implications has not been elucidated. Since TLRs activate innate immunity and influence the nature of adaptive immunity (Hoebe et al, 2004), understanding the logic behind TLR signaling is the most important topic in immunology.
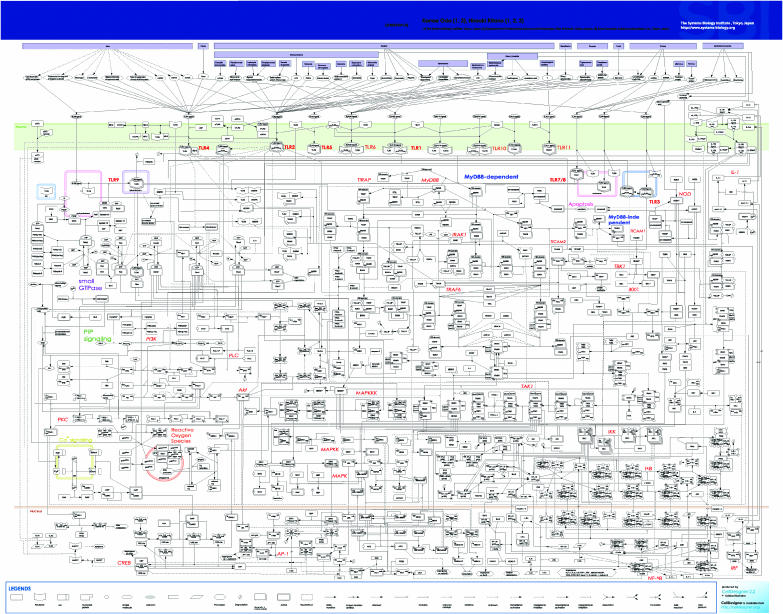
Therefore, we present a map of TLR and IL-1R signaling networks (Figure 1). We manually assembled molecular interactions based on published papers and constructed a TLR map that incorporates the possible pathways in mammalians using a modeling support software, CellDesigner ver.2.2 (http://celldesigner.org/) (Funahashi and Kitano, 2003). The map comprises 652 species and 444 reactions. The species shown on the TLR map can be categorized as follows: 340 proteins, 170 oligomers, 79 simple molecules, 18 genes, eight RNA, three ions, 18 degraded products, and 16 phenotypes. The breakdown of reactions is as follows: 242 state transitions, 106 associations, 25 dissociations, 33 transports, 24 unknown transitions, and 14 omitted transitions. Out of 444 reactions, there are 397 interactions: 270 catalyses, 75 unknown catalyses, 20 inhibitions, nine unknown inhibitions, and 23 transcriptional activations. All the 411 references used for constructing the map are listed in the ‘References for TLR Pathway Map' and the CellDesigner software allows the user to access references that are used as grounds of individual reaction using PubMed ID. It should be noted that the map is a best effort based on existing papers and was created manually. The criteria for inclusion into the map are similar to those for the previous epidermal growth factor receptor (EGFR) signaling map (Oda et al, 2005), and we did our best to reconstruct a reliable map. However, errors and missing interactions are inevitable, and we must assume that there are interactions that have yet to be identified. Obviously, the map will be continuously updated and possible errors will be corrected. This correction and updating process has to be a continuous process involving the community of TLR signaling experts.
Figure 1.
A comprehensive molecular interaction map of TLR signaling network. The SBML and PDF files of the map are available from the Supplementary information. The map can be best viewed in the PDF format. All of the species, proteins, and reactions included in the map are listed in the SBML file when opened by CellDesigner (http://celldesigner.org/). Abbreviations: A20, tumor necrosis factor-inducible protein A20; Akt, v-akt murine thymoma viral oncogene homolog; ASK, apoptosis signal-regulating kinase; ATF, activating transcription factor; Bcl, B-cell CLL/lymphoma; beta-TrCP, beta-transducin repeat-containing protein; BTK, Bruton agammaglobulinemia tyrosine kinase; CaM, calmodulin; CaMKI, calcium/calmodulin-dependent protein kinase; CBP, CREB-binding protein; c-Cbl, Casitas B-lineage lymphoma proto-oncogene; CD, cluster of differentiation; Cdc42, cell division cycle 42 (GTP-binding protein, 25 kDa); CK, casein kinase; c-Myc, v-myc myelocytomatosis viral oncogene homolog; CRE, cAMP response element; CREB, cAMP response element-binding protein; CsgA, major curlin subunit precursor, Salmonella enterica; c-Src, v-src sarcoma (Schmidt–Ruppin A-2) viral oncogene homolog (avian); C-TAK1, MAP/microtubule affinity-regulating kinase 3; CYLD, cylindromatosis (turban tumor syndrome); DAG, diacylglycerol; dsRNA, double-strand RNA; ECSIT, evolutionarily conserved signaling intermediate in toll pathway; EEA, early endosome antigen; eIF, eukaryotic translation initiation factor; Elk-1, ETS domain protein Elk-1; ERK, extracellular signal-regulated kinase; FADD, Fas-associated via death domain; Fos, v-fos FBJ murine osteosarcoma viral oncogene homolog; gp91phox, glycoprotein of 91 kDa from phagocyte oxidase; GSK, glycogen synthase kinase; HDAC, histone deacetylase, HMG, high-mobility group nucleosome-binding domain; hnRNP, heterogeneous nuclear ribonucleoprotein; HSP, heat-shock protein; Ibtk, inhibitor of Bruton agammaglobulinemia tyrosine kinase; ICE, interleukin 1-B-converting enzyme; IκB, nuclear factor of κ light polypeptide gene enhancer in B-cells inhibitor; IKK, I-κ-B kinase; IL, interleukin; IL-1ra, interleukin 1 receptor antagonist; IL-1RAcP, interleukin 1 receptor accessory protein; IP3, inositol 1,4,5-triphosphate; IP3R, inositol 1,4,5-triphosphate receptor; IRAK, interleukin 1 receptor-associated kinase; IRF, interferon-regulatory factor; ISRE, interferon-a-stimulated response element; JNK, c-Jun N-terminal kinase; Jun, v-jun sarcoma virus 17 oncogene homolog (avian); KSR, kinase suppressor of ras; LBP, lipopolysaccharide-binding protein; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinase; MAPKAPK, mitogen-activated protein kinase-activated protein kinase; MBP, myelin basic protein; MD-2, lymphocyte antigen 96; MEKK, MAPK/ERK kinase kinase; MKK, mitogen-activated protein kinase kinase; MKP, MAP kinase phosphatase; MMT virus, mouse mammary tumor virus; Mnk, MAP kinase interacting serine/threonine kinase; MSK, mitogen- and stress-activated protein kinase; MyD88, myeloid differentiation primary response gene 88; NF-κB, nuclear factor κB; NIK, nuclear factor κB-inducing kinase; NOD, nucleotide-binding oligomerization domain; NSF, N-ethylmaleimide-sensitive factor; NUR77, nuclear receptor subfamily 4, group A, member 1; p62, phosphotyrosine-independent ligand for the Lck SH2 domain p62; PAK, p21-activated kinase; PDK, 3-phosphoinositide-dependent protein kinase; pellino, pellino (Drosophila) homolog; PI(4)P5K, phosphatidylinositol-5-kinase; PI, phosphatidylinositol; Pi, phosphoric ion; PI3,4,5-P3, phosphatidylinositol-3,4,5-triphosphate; PI3,4-P2, phosphatidylinositol-3,4-bisphosphate; PI3K, phosphatidylinositol-3-kinase; PI3-P, phosphatidylinositol-3-phosphate; PI4,5-P2, phosphatidylinositol-4,5-bisphosphate; PI4-P, phosphatidylinositol-4-phosphate; PKA, protein kinase A; PKC, protein kinase C; PKR, eukaryotic translation initiation factor 2-α kinase; PLC, phospholipase C; PLD, phospholipase D; PP, protein phosphatase; Rab, RAS-associated protein; Rabaptin, RAB GTPase-binding effector protein; Rabex, RAB guanine nucleotide exchange factor; Rac, ras-related C3 botulinum toxin substrate; Raf, v-raf-1 murine leukemia viral oncogene homolog; Ras, rat sarcoma viral oncogene homolog; Rho, ras homolog gene family; RhoGDI, GDP dissociation inhibitor; Rin, Ras interaction; RIP, receptor-interacting serine–threonine kinase; RKIP, Raf kinase inhibitor protein; RS virus, respiratory syncytial virus; Sab, SH3-domain-binding protein 5 (BTK-associated); SERCA, sarcoplasmic/endoplasmic reticulum calcium ATPase; SIGIRR, single immunoglobulin and toll-interleukin 1 receptor (TIR) domain; SOCS, suppressor of cytokine signaling; ssRNA, single-strand RNA; ST2L, interleukin 1 receptor-like 1; STF, soluble tuberculosis factor; TAB, transforming growth factor beta-activated kinase-binding protein; TAK, transforming growth factor beta-activated kinase; TBK, TRAF family member-associated nuclear factor κB activator-binding kinase; TICAM, toll-like receptor adaptor molecule; TIFA, TRAF-interacting protein with a forkhead-associated domain; TIR, toll-interleukin 1 receptor; TIRAP, toll-interleukin 1 receptor domain-containing adaptor protein; TLR, toll-like receptor; TOLLIP, toll-interacting protein; TPL, tumor progression locus; TRAF, tumor necrosis factor receptor-associated factor; TRAILR, tumor necrosis factor-related apoptosis-inducing ligand receptor; TRIAD3A, ubiquitin-conjugating enzyme 7-interacting protein 1, isoform A; TRIP, thyroid hormone receptor interactor; Trx, thioredoxin; Ubc, ubiquitin-conjugating enzyme; Uev, ubiquitin-conjugating enzyme E2 variant; Vav1, vav 1 oncogene.
One of the issues in constructing maps of molecular interactions is the reliability of the map. But what does map accuracy mean, what are the justifications for including specific interactions but excluding others, and how should conflicting and uncertain reports be dealt with? There are at least two major sources of inaccuracies: inaccuracy within each paper of reference, and inaccuracy of interpretation of papers. The former problem is inherent in many pathway databases based on manual curation, and only way to mitigate the problem is to set a certain standard on which papers to be used for map construction. As in the case of the EGFR signaling map, we have included interactions that have been experimentally verified in multiple reports. We may include interactions that are reported in a single paper if there are no conflicting reports. But almost all experiments in them were performed under the distinct conditions at each laboratory. Hence, it is inevitable that drawing the pathway is like the mosaic woodwork that is gathering the ‘possible' interactions. The selection of the information on the pathway map must be entrusted to the users according to their purposes, and which interpretation to be widely agreed may rest on the community-wide discusions. For some readers, some interactions may be viewed as premature hypotheses, whereas the same interactions may be considered more plausible by others. The certaintity rating may be used to illustrate how much each interaction is hypothetical or the level of confidence, but such a rating itself may be subjective without a sophisticated evaluation method. Thus, at present the map could be skeptically viewed as merely representing ‘The View of the World' of the authors, rather like the ‘New Yorkers' View of the World' map sold to tourists. Nevertheless, we consider our map to be useful because it does represent one comprehensive view of the network, the map is based on published articles, and publication of such a map can initiate a community-wide interactive process for creating a more accurate and information-rich map. We are currently working on a scheme to accept community-wide feedback on the map, so that the map can be iteratively improved in both coverage and quality.
In order to make the map a practical and accessible resource, it has to be provided in a standard format. Thus, the map complies with Systems Biology Markup Language (SBML) for machine readable representation (Hucka et al, 2003), and adopts a specific graphical notation system called the process diagram, which intends to provide a standard for representing molecular interactions in an unambiguous way (Kitano et al, 2005). The main symbols used to represent molecules and interactions in this map are the same as those of the EGFR map (Oda et al, 2005), which is based on the process diagram of Systems Biology Graphical Notation (SBGN: http://www.sbgn.org/) (Kitano et al, 2005). The compounds, except proteins, genes, RNAs, and ions, such as lipids and carbohydrates, although they are complicated, are all shown as ‘simple molecule' for the sake of convenience. Because the TLR system has numerous combinations of protein complex, we adopted another local rule in the TLR Pathway Map to enhance the readability of the map. A protein with ‘*' at the end of its name means that it binds to other molecules and often makes a conformational change. The circle-headed ‘catalysis' arrow towards a state transition of a protein with ‘*' means binding with it. Readers may notice that there are substantial numbers of molecular components appearing in both the EGFR map and TLR map. In future, CellDesigner will provide a powerful means to merge several large-scale maps so that an integrated map, possibly genome-wide in scale, can be created and used by researchers to navigate through the network.
Architectural features of the TLR map
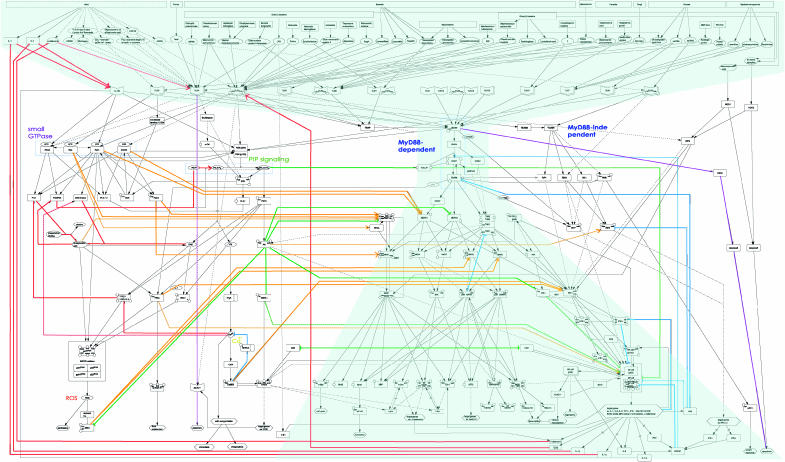
It is important to construct a comprehensive map of molecular interactions in order to understand the possible logic behind the network. Even without kinetic parameters to run a dynamic simulation, the map provides information that can be used to analyze architectural features of the network. In order to analyze the global network architecture, a simpler diagram that focuses on the flow of information and causal relationships is needed. Figure 2 is a reduced version of Figure 1 in which only flows of activations and inhibitions are shown for the sake of readability. In Figure 2, filled arrows indicate activation and bar-headed arrows indicate inhibition.
Figure 2.
The architecture of the TLR signaling network. A reduced map of TLR signaling network that extracts the flow of activation and inhibition. This diagram is created based on the comprehensive TLR signaling network map as shown in Figure 1. Filled arrows in this figure indicate ‘activation', and other arrows such as bar-headed, circle-headed, dashed, and dot-dot-dashed lines are the same as Figure 1. The double line denotes ‘binding' in this figure. A molecule shown to connect with both ‘activation' and ‘inhibition' arrows can act oppositely according to the condition. A line of mutual inhibition that connects two CREB-binding protein (CBP) molecules means the competition for limiting amounts of CBP, which is a transcriptional coactivator that interacts with both NF-κ B p65 and CREB. TLR signaling network consists of the main bow-tie network and three collateral subnetworks that involves small GTPase, PIPs, and MyD88-independent pathways. There are extensive crosstalk regulations between the main bow-tie network and other subsystems, as well as multiple feedback and feedforward controls. Notable interactions are color-coded: red, positive feedback loop; blue, negative feedback loop; purple, inhibitory feedforward path; orange, positive crosstalk from subsystems to the bow-tie network; and green, negative crosstalk from subsystems to the bow-tie network. High resolution file for this figure is available from the Supplementary information.
It shows that TLR signaling pathways are roughly divided into four possible subsystems. The first is the main system with MyD88–IRAK4–IRAK1–TRAF6 as a bow-tie core process to activate nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) cascade, leading to the induction of many target genes such as cytokines that are essential for the innate immune response and the maturation and proliferation of the cell. Almost all TLRs utilize this core process and so various distinct signals from pathogens are assembled to only a handful of proteins. The second and third systems seem to be subsystems with a small GTPase module and phosphatidylinositol phosphate (PIP) signaling module, respectively. We consider the small GTPase module and PIP signaling module to be distinct modules, rather than merging them into a central MyD88 module. This is because both the small GTPase module and PIP signaling module receive extensive inputs directly from receptors and transmit them to various molecules downstream of MyD88 as well as outside of downstream of MyD88. For example, the small GTPase module receives inputs from IL-1R, TLR9, TLR4, and TLR2, and the PIP signaling module receives inputs from IL-1R, small GTPase module, TLR2, TLR3, and MyD88, whereas components within the MyD88 module such as IRAK4, IRAK1, IRAK2, and TRAF6 are only activated through MyD88 activation. At the same time, small GTPase and PI3 kinase (PI3K) activates NF-κB and MAPK (Arbibe et al, 2000; Xu et al, 2003; Sarkar et al, 2004). Thus, the small GTPase and PIP signaling modules shall be considered as collateral modules, instead of merging into the central MyD88 module. These subsystems are essential for the battle against invaders. Their pathways are merged at several points and cooperate with each other to exclude pathogens by actin reorganization leading to chemotaxis and phagocytosis and the production of reactive oxygen species (ROS) to kill them. The last subsystem is limited to TLR3 and TLR4, which can stimulate another pathway called MyD88-independent pathway through the TLR adaptor molecule (TICAM)1/2 (Yamamoto et al, 2003). It remains to be investigated how it signals to MAPK cascade (Chu et al, 1999; Goh et al, 2000), but it can activate NF-κB on the late phase as well as the interferon-regulatory factor family that induces potential cytokines, type I interferon, and the induction of IL-1 activates autocrinely MyD88-dependent pathways and two subsystems leading to the full activation of the whole system. Thus, this pathway would appear to be a detour.
One of the notable features of the TLR signaling network is the possible existence of a bow-tie structure as the central subsystem of the TLR network in which MyD88 is a nonredundant core. The bow-tie structure has also been observed in the EGFR signaling network (Oda et al, 2005), and has been considered to be a characteristic architectural feature of robust systems (Csete and Doyle, 2004; Kitano, 2004). At the same time, the TLR signaling network is different from the EGFR signaling network as it has extensive collateral pathways that may modulate downstream behaviors of the main bow-tie network.
Multiple system controls
As shown in Figure 2, there are multiple system controls in the TLR system. In total, seven positive feedback and seven negative feedback loops are identified (shown in red and blue, respectively). Among positive feedback loops, the four loops (Nos. 1–4 in Table I) are the regulation from the output to the input, and one (No. 5) is in the bow-tie lower wing. Six negative feedback loops are classified as follows: two (Nos. 8 and 9) are in the bow-tie lower wing, two (Nos. 10 and 11) are from the output to the lower wing, one (No. 12) is from the output to the bow-tie core process, and the last one (No. 13) is from the output to the input. The remaining two positive (Nos. 6 and 7) and one negative (No. 14) feedback loops exist in the subsystems involved in the regulation of concentration of the cytosol calcium. There are conflicting feedback loops. For example, feedback from IL-1α and IL-1β to IL-1RI (Nos. 1 and 2, respectively) provides postitive feedbacks, whereas feedback from interleukin 1 receptor antagonist (IL-1ra) to IL-1RI (No. 13) provides a negative feedback. The map predicts balance of activation between IL-1 and IL-1ra affects proinflammatory response of the system. A recent paper reports this is actually a case(Matsuki et al, 2006).
Table 1.
Feedback and feedforward controls in the TLRs system
| No. | Origin | Destination | Note | |||
|---|---|---|---|---|---|---|
| Feedback | ||||||
| Positive | 1 | IL-1α | Transcriptional target of NF-κB | IL-1RI | Activates NF-κB via the MyD88-dependent pathway | |
| 2 | IL-1β | Transcriptional target of NF-κB | IL-1RI | Activates NF-κB via the MyD88-dependent pathway | ||
| 3 | TLR2 | Transcriptional target of NF-κB | MyD88, PI3K, small GTPase | Activates NF-κB | ||
| 4 | β-Defensin2 | Transcriptional target of NF-κB | TLR4 | Activates NF-κB via the MyD88-dependent and -independent pathway | Controversial | |
| 5 | IKKβ | Activated by NIK | TPL2(p58) | Activates NIK | Through the process yet identified | |
| 6 | PLD | Activated by PKC alpha, beta II, and cytosol Ca2+ | PI4,5-P2 | Material of IP3 which increases cytosol Ca2+ via IP3R | ||
| 7 | DAG kinase | Activated by cytosol Ca2+ | PI4,5-P2 | Material of IP3 which increases cytosol Ca2+ via IP3R | Through the process yet identified | |
| Negative | 8 | NF-κB1(p105) | Activated by IKKβ | TPL2(p58) | Activates IKKβ via NIK | |
| 9 | p38αMAPK | Activated by MKK3 | TAK1 | Activates MKK3 | ||
| 10 | IκBα | Transcriptional target of NF-κB | NF-κB | Transcriptional factor of A20 | ||
| 11 | A20 | Transcriptional target of NF-κB | NF-κB | Transcriptional factor of A20 | ||
| 12 | A20 | Transcriptional target of NF-κB | TRAF6 | Activates NF-κB via the MyD88-dependent pathway | ||
| 13 | IL-1ra | Transcriptional target of NF-κB | IL-1RI | Activates NF-κB via the MyD88-dependent pathway | ||
| 14 | CaMKII | Activated by cytosol Ca2+ | SERCA | Decreases cytosol Ca2+ | ||
| Feedforward | ||||||
| Negative | 15 | TLR2 | NUR77 | Causes apoptosis | ||
| 16 | TLR4 | NUR77 | Causes apoptosis | |||
| 17 | MyD88 | FADD | Causes apoptosis via the activation of caspase-8 | |||
| 18 | Src kinases | Activates BTK | BTK | Inhibited by Src kinases via c-Cbl | Through the process yet identified | |
In addition to these feedback controls, there is a possible negative feedforward control (shown in purple). MyD88 also mediates apoptosis via a Fas-associated death domain–caspase-8-dependent pathway, and TLR4 and TLR2 can induce apoptosis through an orphan nuclear receptor Nur77 by a caspase-independent pathway, although its precise mechanism is unclear (Kim et al, 2003). Thus, the TLR system induces the activation of the immunity to survive, while it prepares cell death at the same time. At a cell-level view, this mechanism could be considered as a negative feedforward control (Table I).
Regulations between main and subsystems
There are many crosstalk regulations between the main bow-tie pathway and two subsystems. Especially, we identified a lot of crosstalk regulations from a subsystem to the main bow-tie pathway; positive and negative regulations are shown in orange and green, respectively. There are 13 positive and seven negative crosstalk regulations, and interestingly all the crosstalk regulations go towards the bow-tie lower wing. For example, small GTPases and ROS can stimulate MAPK cascade by nine ways, and v-akt murine thymoma viral oncogene homolog (Akt) can inhibit both MAPK cascade and NF-κB activation by means of five distinct mechanisms. Thus, the fact that regulations from other systems concentrate in the bow-tie lower wing is highly suggestive.
Possible undiscovered negative regulations
The bow-tie structure has extensive system controls to govern the system's dynamics. In this paper, we demonstrate that TLR pathway forms a bow-tie structure and the two related subsystems with multiple positive/negative system controls and crosstalk regulations. However, we could identify no negative regulations from the lower wing and/or the outputs to the upper wing and/or inputs in the TLR system while constructing this map. While both ‘inhibition' and ‘activation' usually exist to regulate the balance, there may be undiscovered negative regulations in this pathway. For example, NF-κB induces both IL-1 and IL-1ra, an inhibitor of IL-1R, so there must be negative regulations from the lower wing and/or outputs against each TLR. Recently, many negative regulators of TLRs such as soluble TLR4 (Iwami et al, 2000) have been reported (reviewed by Liew et al, 2005) and, although their regulations remains to be investigated, they must be strong candidates. It is important to understand the TLR system in depth to research the negative regulations that seem to be lacking in a system-level view.
Naturally, there is a huge cytokine/chemokine network in the downstream region of the TLR system and it is regulated from the network both positively and negatively. For example, suppressor of cytokine signaling 1, which is the downstream element of cytokine signaling such as interferon and IL-6, has been found to inhibit both NF-κB (p65) and IRAK1 activation. (Kinjyo et al, 2002; Nakagawa et al, 2002; Ryo et al, 2003) We are planning to construct and analyze the complicated cytokine/chemokine networks and their interactions in the future.
Mechanisms for differential responses for different stimuli
Since MyD88 is the single core element in the bow-tie structure, any inputs that converge into this network are only able to change the activation level of MyD88. This subsystem alone cannot make different responses regardless of different stimuli.
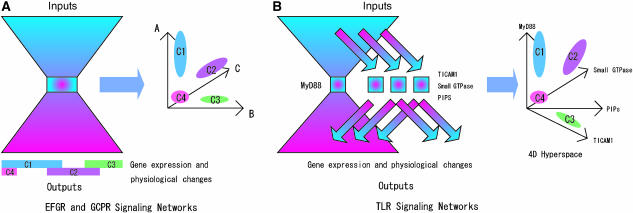
One of the major questions in signal-transduction research is how a specific signal-transduction network generates different responses for each set of combinatorial stimuli. Recently, an extensive study has been made to demonstrate some signaling pathway function as classifier of stimuli (Janes et al, 2005). What is the logic behind such processes? Previously, we have created a comprehensive map of the EGFR signaling network in which the core of the bow-tie structure consists of PIPs, small GTPase, nonreceptor tyrosine kinase (non-RTK), and possibly signal transducer and activator of transcription 1/2. There are three or four possible elements in the core of the bow-tie architecture. A similar structure may be found in the G-protein-coupled receptor (GPCR) signaling network where calcium, cyclic AMP, and inositol phosphate are likely candidates for core elements of the bow-tie structure. In these networks, we can assume the existence of hyperspace, a mathematical term referring to N-dimensional space, created by activation levels of a small number of core elements, where each subregion within the hyperspace may correspond to different responses (Figure 3A). Therefore, various inputs may be clustered in the hyperspace, which may be called ‘classifier hyperspace', and relayed to outputs. In other words, how the signaling network responds to a specific set of stimuli depends on the activation levels and temporal dynamics of molecules in this theoretical hyperspace. However, in the TLR signaling network, there is only one element in the core of the bow-tie network that precludes the capability to generate differential outputs alone. Differential outputs are attained by modulation of subnetworks that are MyD88-independent pathways, by the small GTPase subnetwork, and by the PIPs subnetwork. The MyD88-dependent pathway may only function to trigger the activation of the downstream signaling system (Figure 3B). In this case, differences of responses for each stimuli are greatly influenced by the activity of the classifier hyperspace composed of TICAM1 for the MyD88-independent pathway, small GTPases including cell division cycle 42 (Cdc42), ras-related C3 botulinum toxin substrate 1 (Rac1), rat sarcoma viral oncogene homolog (Ras), ras homolog gene family A (RhoA), and PIPs. The essential idea behind the classifier hyperspace is that it implies that a certain abstract representation exists in the signal-transduction process, similar to a learning layer of certain types of neural networks. In other words, a signal-transduction network is an evolved network that can classify various stimuli into a limited number of categories where each category triggers a specific sequence of responses. This classification depends on the activity level and temporal dynamics, often called attractor dynamics (Strogatz, 1994), of the components involved. Figure 3 indicates a simple view in which the activity levels of each component appear to be used for classification, but classification can generally be made by attractor dynamics where each attractor can be interpreted as a symbol corresponding to our subjectively labeled interpretation of cellular responses (Hao, 1991). If this insight is correct, it suggests the existence of a common principle on how the signal-transduction network generates various responses to a broad range of stimuli in a consistent manner. This is an important hypothesis that needs to be experimentally verified.
Figure 3.
The core of bow-tie networks may have ‘classifier hyperspace' where reactions to various inputs can be classified within subregions within the hyperspace that consists of activation levels of core elements of the bow-tie structure. (A) EGFR signaling and GPCR signaling network has a small numbers of core elements such as small GTPases, PIPs, and non-RTKs for EGFR signaling, and calcium, cAMP, and inositol phosphate for GPCR signaling. For example, a group of stimuli may all activate core elements in the way that can be classified into region C1, for example, and these stimuli triggers transcription of genes and physiological responses as denoted as C1. (B) TLR signaling network has a salient feature where possible bow-tie core is composed of a single element, MyD88, thus there is no classifier hyperspace created within the bow-tie network. Differences of responses against various stimuli are modulated by the activation of elements in collateral pathways, such as small GTPases, PIPs, and TICAM1 involved in MyD88-independent pathway, and MyD88 pathway essentially calibrates the whole network. Thus, for TLR signaling network, the classifier hyperspace has four dimensions.
Conclusion
A comprehensive TLR signaling network that provides an overall network architecture of molecular interaction was created based on papers published so far. Although this map is far from complete in covering all interactions of the TLR signaling network, it represents a comprehensive body of knowledge available today. The map reveals the existence of a possible bow-tie network accompanied with collateral subnetworks that involve MyD88-independent pathways, small GTPase, and PIPs. The central bow-tie network relies on MyD88, which is a nonredundant core element of the network. This makes the whole system susceptible to the removal of MyD88 as seen in the phenotype of MyD88−/−-deficient mouse (Akira, 2000). This is a weakness of the system. Comparison with other signaling networks such as the EGFR signaling network and GPCR signaling network illustrates several characteristic features of the TLR signaling network as well as common features, which we proposed as a ‘classifier hyperspace'. This is interesting because similar operational principles on how to generate different responses to various input stimuli have emerged from investigating the structure of networks alone. Further elaboration of the concept and experimental verification of this hypothesis will be important in signal-transduction research in the future. While extensive feedback loops exist, we have noticed that only a few negative feedback loops have been reported so far. We consider that there may be a number of undiscovered negative feedback loops in this signaling network. We hope this map will contribute to system-wide studies of TLR signaling as well as immunology in general. However, the map is not complete and a number of undiscovered interactions are predicted; the map will be updated in collaboration with experts in the field.
Supplementary Material
Supplementary Information
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure Legends
Acknowledgments
This research was supported in part by the Exploratory Research for Advanced Technology (ERATO) and the Solution-Oriented Research for Science and Technology (SORST) programs (Japan Science and Technology Organization), an NEDO Grant (New Energy and Industrial Technology Development Organization) of the Japanese Ministry of Economy, Trade, and Industry (METI), the Special Coordination Funds for Promoting Science and Technology and the Center of Excellence Program for Keio University (Ministry of Education, Culture, Sports, Science, and Technology), and The Genome Network Project by Ministry of Education, Culture, Sports, Science, and Technology.
The authors declare that there is no financial conflict.
References
- Akira S (2000) Toll-like receptors: lessons from knockout mice. Biochem Soc Trans 28: 551–556 [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K (2004) Toll-like receptor signalling. Nat Rev Immunol 4: 499–511 [DOI] [PubMed] [Google Scholar]
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA (2001) Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413: 732–738 [DOI] [PubMed] [Google Scholar]
- Arbibe L, Mira JP, Teusch N, Kline L, Guha M, Mackman N, Godowski PJ, Ulevitch RJ, Knaus UG (2000) Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat Immunol 1: 533–540 [DOI] [PubMed] [Google Scholar]
- Beutler B (2004) Inferences, questions and possibilities in Toll-like receptor signalling. Nature 430: 257–263 [DOI] [PubMed] [Google Scholar]
- Chu WM, Ostertag D, Li ZW, Chang L, Chen Y, Hu Y, Williams B, Perrault J, Karin M (1999) JNK2 and IKKbeta are required for activating the innate response to viral infection. Immunity 11: 721–731 [DOI] [PubMed] [Google Scholar]
- Csete M, Doyle J (2004) Bow ties, metabolism and disease. Trends Biotechnol 22: 446–450 [DOI] [PubMed] [Google Scholar]
- Funahashi A, Kitano H (2003) CellDesigner: a process diagram editor for gene-regulatory and biochemical networks. Biosilico 1: 159–162 [Google Scholar]
- Goh KC, deVeer MJ, Williams BR (2000) The protein kinase PKR is required for p38 MAPK activation and the innate immune response to bacterial endotoxin. EMBO J 19: 4292–4297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao B (1991) Symbolic dynamics and characterization of complexity. Physica D 51: 161–176 [Google Scholar]
- Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A (2001) The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410: 1099–1103 [DOI] [PubMed] [Google Scholar]
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S (2000) A Toll-like receptor recognizes bacterial DNA. Nature 408: 740–745 [DOI] [PubMed] [Google Scholar]
- Hoebe K, Janssen E, Beutler B (2004) The interface between innate and adaptive immunity. Nat Immunol 5: 971–974 [DOI] [PubMed] [Google Scholar]
- Hoffmann JA, Reichhart JM (2002) Drosophila innate immunity: an evolutionary perspective. Nat Immunol 3: 121–126 [DOI] [PubMed] [Google Scholar]
- Hucka M, Finney A, Sauro HM, Bolouri H, Doyle JC, Kitano H, Arkin AP, Bornstein BJ, Bray D, Cornish-Bowden A, Cuellar AA, Dronov S, Gilles ED, Ginkel M, Gor V, Goryanin II, Hedley WJ, Hodgman TC, Hofmeyr JH, Hunter PJ, Juty NS, Kasberger JL, Kremling A, Kummer U, Le Novere N, Loew LM, Lucio D, Mendes P, Minch E, Mjolsness ED, Nakayama Y, Nelson MR, Nielsen PF, Sakurada T, Schaff JC, Shapiro BE, Shimizu TS, Spence HD, Stelling J, Takahashi K, Tomita M, Wagner J, Wang J (2003) The Systems Biology Markup Language (SBML): a medium for representation and exchange of biochemical network models. Bioinformatics 19: 524–531 [DOI] [PubMed] [Google Scholar]
- Iwami KI, Matsuguchi T, Masuda A, Kikuchi T, Musikacharoen T, Yoshikai Y (2000) Cutting edge: naturally occurring soluble form of mouse Toll-like receptor 4 inhibits lipopolysaccharide signaling. J Immunol 165: 6682–6686 [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R (2004) Toll-like receptor control of the adaptive immune responses. Nat Immunol 5: 987–995 [DOI] [PubMed] [Google Scholar]
- Janes KA, Albeck JG, Gaudet S, Sorger PK, Lauffenburger DA, Yaffe MB (2005) A systems model of signaling identifies a molecular basis set for cytokine-induced apoptosis. Science 310: 1646–1653 [DOI] [PubMed] [Google Scholar]
- Kim SO, Ono K, Tobias PS, Han J (2003) Orphan nuclear receptor Nur77 is involved in caspase-independent macrophage cell death. J Exp Med 197: 1441–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinjyo I, Hanada T, Inagaki-Ohara K, Mori H, Aki D, Ohishi M, Yoshida H, Kubo M, Yoshimura A (2002) SOCS1/JAB is a negative regulator of LPS-induced macrophage activation. Immunity 17: 583–591 [DOI] [PubMed] [Google Scholar]
- Kitano H (2004) Biological robustness. Nat Rev Genet 5: 826–837 [DOI] [PubMed] [Google Scholar]
- Kitano H, Funahashi A, Matsuoka Y, Oda K (2005) Using process diagrams for the graphical representation of biological networks. Nat Biotechnol 23: 961–966 [DOI] [PubMed] [Google Scholar]
- Liew FY, Xu D, Brint EK, O'Neill LA (2005) Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol 5: 446–458 [DOI] [PubMed] [Google Scholar]
- Matsuki T, Nakae S, Sudo K, Horai R, Iwakura Y (2006) Abnormal T cell activation caused by the imbalance of the IL-1/IL-1R antagonist system is responsible for the development of experimental autoimmune encephalomyelitis. Int Immunol 18: 399–407 [DOI] [PubMed] [Google Scholar]
- Nakagawa R, Naka T, Tsutsui H, Fujimoto M, Kimura A, Abe T, Seki E, Sato S, Takeuchi O, Takeda K, Akira S, Yamanishi K, Kawase I, Nakanishi K, Kishimoto T (2002) SOCS-1 participates in negative regulation of LPS responses. Immunity 17: 677–687 [DOI] [PubMed] [Google Scholar]
- Oda K, Matsuoka Y, Funahashi A, Kitano H (2005) A comprehensive pathway map of epidermal growth factor receptor signaling. Mol Syst Biol 1: 2005.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B (1998) Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282: 2085–2088 [DOI] [PubMed] [Google Scholar]
- Roach JC, Glusman G, Rowen L, Kaur A, Purcell MK, Smith KD, Hood LE, Aderem A (2005) The evolution of vertebrate Toll-like receptors. Proc Natl Acad Sci USA 102: 9577–9582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryo A, Suizu F, Yoshida Y, Perrem K, Liou YC, Wulf G, Rottapel R, Yamaoka S, Lu KP (2003) Regulation of NF-kappaB signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA. Mol Cell 12: 1413–1426 [DOI] [PubMed] [Google Scholar]
- Sarkar SN, Peters KL, Elco CP, Sakamoto S, Pal S, Sen GC (2004) Novel roles of TLR3 tyrosine phosphorylation and PI3 kinase in double-stranded RNA signaling. Nat Struct Mol Biol 11: 1060–1067 [DOI] [PubMed] [Google Scholar]
- Slack JL, Schooley K, Bonnert TP, Mitcham JL, Qwarnstrom EE, Sims JE, Dower SK (2000) Identification of two major sites in the type I interleukin-1 receptor cytoplasmic region responsible for coupling to pro-inflammatory signaling pathways. J Biol Chem 275: 4670–4678 [DOI] [PubMed] [Google Scholar]
- Strogatz S (1994) Nonlinear Dynamics and Chaos: With Applications in Physics, Biology, Chemistry, and Engineering. New York: Perseus Books [Google Scholar]
- Xu H, An H, Yu Y, Zhang M, Qi R, Cao X (2003) Ras participates in CpG oligodeoxynucleotide signaling through association with toll-like receptor 9 and promotion of interleukin-1 receptor-associated kinase/tumor necrosis factor receptor-associated factor 6 complex formation in macrophages. J Biol Chem 278: 36334–36340 [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S (2003) Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301: 640–643 [DOI] [PubMed] [Google Scholar]
References for TLR Pathway Map
- Abo A, Pick E, Hall A, Totty N, Teahan CG, Segal AW (1991) Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature 353: 668–670 [DOI] [PubMed] [Google Scholar]
- Adachi M, Fukuda M, Nishida E (1999) Two co-existing mechanisms for nuclear import of MAP kinase: passive diffusion of a monomer and active transport of a dimer. EMBO J 18: 5347–5358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JM, Houston H, Allen J, Lints T, Harvey R (1992) The hematopoietically expressed vav proto-oncogene shares homology with the dbl GDP-GTP exchange factor, the bcr gene and a yeast gene (CDC24) involved in cytoskeletal organization. Oncogene 7: 611–618 [PubMed] [Google Scholar]
- Ahn NG, Seger R, Bratlien RL, Diltz CD, Tonks NK, Krebs EG (1991) Multiple components in an epidermal growth factor-stimulated protein kinase cascade. In vitro activation of a myelin basic protein/microtubule-associated protein 2 kinase. J Biol Chem 266: 4220–4227 [PubMed] [Google Scholar]
- Akira S (2003) Toll-like receptor signaling. J Biol Chem 278: 38105–38108 [DOI] [PubMed] [Google Scholar]
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA (2001) Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413: 732–738 [DOI] [PubMed] [Google Scholar]
- Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A (1999) Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285: 736–739 [DOI] [PubMed] [Google Scholar]
- Aliprantis AO, Yang RB, Weiss DS, Godowski P, Zychlinsky A (2000) The apoptotic signaling pathway activated by Toll-like receptor-2. EMBO J 19: 3325–3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders HJ, Banas B, Schlondorff D (2004) Signaling danger: toll-like receptors and their potential roles in kidney disease. J Am Soc Nephrol 15: 854–867 [DOI] [PubMed] [Google Scholar]
- Anderson KE, Coadwell J, Stephens LR, Hawkins PT (1998) Translocation of PDK-1 to the plasma membrane is important in allowing PDK-1 to activate protein kinase B. Curr Biol 8: 684–691 [DOI] [PubMed] [Google Scholar]
- Anrather J, Csizmadia V, Soares MP, Winkler H (1999) Regulation of NF-kappaB RelA phosphorylation and transcriptional activity by p21(ras) and protein kinase Czeta in primary endothelial cells. J Biol Chem 274: 13594–13603 [DOI] [PubMed] [Google Scholar]
- Arbibe L, Mira JP, Teusch N, Kline L, Guha M, Mackman N, Godowski PJ, Ulevitch RJ, Knaus UG (2000) Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat Immunol 1: 533–540 [DOI] [PubMed] [Google Scholar]
- Arenzana-Seisdedos F, Turpin P, Rodriguez M, Thomas D, Hay RT, Virelizier JL, Dargemont C (1997) Nuclear localization of I kappa B alpha promotes active transport of NF-kappa B from the nucleus to the cytoplasm. J Cell Sci 110 (Part 3): 369–378 [DOI] [PubMed] [Google Scholar]
- Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK (2002) Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem 277: 15028–15034 [DOI] [PubMed] [Google Scholar]
- Backer JM (2000) Phosphoinositide 3-kinases and the regulation of vesicular trafficking. Mol Cell Biol Res Commun 3: 193–204 [DOI] [PubMed] [Google Scholar]
- Balendran A, Hare GR, Kieloch A, Williams MR, Alessi DR (2000) Further evidence that 3-phosphoinositide-dependent protein kinase-1 (PDK1) is required for the stability and phosphorylation of protein kinase C (PKC) isoforms. FEBS Lett 484: 217–223 [DOI] [PubMed] [Google Scholar]
- Bannerman DD, Erwert RD, Winn RK, Harlan JM (2002) TIRAP mediates endotoxin-induced NF-kappaB activation and apoptosis in endothelial cells. Biochem Biophys Res Commun 295: 157–162 [DOI] [PubMed] [Google Scholar]
- Bard F, Patel U, Levy JB, Jurdic P, Horne WC, Baron R (2002) Molecular complexes that contain both c-Cbl and c-Src associate with Golgi membranes. Eur J Cell Biol 81: 26–35 [DOI] [PubMed] [Google Scholar]
- Baud V, Liu ZG, Bennett B, Suzuki N, Xia Y, Karin M (1999) Signaling by proinflammatory cytokines: oligomerization of TRAF2 and TRAF6 is sufficient for JNK and IKK activation and target gene induction via an amino-terminal effector domain. Genes Dev 13: 1297–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann B, Weber CK, Troppmair J, Whiteside S, Israel A, Rapp UR, Wirth T (2000) Raf induces NF-kappaB by membrane shuttle kinase MEKK1, a signaling pathway critical for transformation. Proc Natl Acad Sci USA 97: 4615–4620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelloni A, Santi S, Sirri A, Riccio M, Faenza I, Zini N, Cecchi S, Ferri A, Auron P, Maraldi NM, Marmiroli S (1999) Phosphatidylinositol 3-kinase translocation to the nucleus is induced by interleukin 1 and prevented by mutation of interleukin 1 receptor in human osteosarcoma Saos-2 cells. J Cell Sci 112 (Part 5): 631–640 [DOI] [PubMed] [Google Scholar]
- Beaty CD, Franklin TL, Uehara Y, Wilson CB (1994) Lipopolysaccharide-induced cytokine production in human monocytes: role of tyrosine phosphorylation in transmembrane signal transduction. Eur J Immunol 24: 1278–1284 [DOI] [PubMed] [Google Scholar]
- Belham C, Wu S, Avruch J (1999) Intracellular signalling: PDK1—a kinase at the hub of things. Curr Biol 9: R93–96 [DOI] [PubMed] [Google Scholar]
- Ben-Levy R, Hooper S, Wilson R, Paterson HF, Marshall CJ (1998) Nuclear export of the stress-activated protein kinase p38 mediated by its substrate MAPKAP kinase-2. Curr Biol 8: 1049–1057 [DOI] [PubMed] [Google Scholar]
- Beutler B (2004) Inferences, questions and possibilities in Toll-like receptor signalling. Nature 430: 257–263 [DOI] [PubMed] [Google Scholar]
- Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, Shirakawa AK, Farber JM, Segal DM, Oppenheim JJ, Kwak LW (2002) Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science 298: 1025–1029 [DOI] [PubMed] [Google Scholar]
- Bird TA, Schooley K, Dower SK, Hagen H, Virca GD (1997) Activation of nuclear transcription factor NF-kappaB by interleukin-1 is accompanied by casein kinase II-mediated phosphorylation of the p65 subunit. J Biol Chem 272: 32606–32612 [DOI] [PubMed] [Google Scholar]
- Bishop AL, Hall A (2000) Rho GTPases and their effector proteins. Biochem J 348 (Part 2): 241–255 [PMC free article] [PubMed] [Google Scholar]
- Bokoch GM, Vlahos CJ, Wang Y, Knaus UG, Traynor-Kaplan AE (1996) Rac GTPase interacts specifically with phosphatidylinositol 3-kinase. Biochem J 315 (Part 3): 775–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bol GF, Jurrmann N, Brigelius-Flohe R (2005) Cellular trafficking of the IL-1RI-associated kinase-1 requires intact kinase activity. Biochem Biophys Res Commun 332: 279–287 [DOI] [PubMed] [Google Scholar]
- Bonnard M, Mirtsos C, Suzuki S, Graham K, Huang J, Ng M, Itie A, Wakeham A, Shahinian A, Henzel WJ, Elia AJ, Shillinglaw W, Mak TW, Cao Z, Yeh WC (2000) Deficiency of T2K leads to apoptotic liver degeneration and impaired NF-kappaB-dependent gene transcription. EMBO J 19: 4976–4985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet MC, Weil R, Dam E, Hovanessian AG, Meurs EF (2000) PKR stimulates NF-kappaB irrespective of its kinase function by interacting with the IkappaB kinase complex. Mol Cell Biol 20: 4532–4542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, Hurley P, Chien M, Chai S, Hitotsumatsu O, McNally E, Pickart C, Ma A (2004) The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol 5: 1052–1060 [DOI] [PubMed] [Google Scholar]
- Borgatti P, Martelli AM, Bellacosa A, Casto R, Massari L, Capitani S, Neri LM (2000) Translocation of Akt/PKB to the nucleus of osteoblast-like MC3T3-E1 cells exposed to proliferative growth factors. FEBS Lett 477: 27–32 [DOI] [PubMed] [Google Scholar]
- Bornancin F, Parker PJ (1996) Phosphorylation of threonine 638 critically controls the dephosphorylation and inactivation of protein kinase Calpha. Curr Biol 6: 1114–1123 [DOI] [PubMed] [Google Scholar]
- Bornancin F, Parker PJ (1997) Phosphorylation of protein kinase C-alpha on serine 657 controls the accumulation of active enzyme and contributes to its phosphatase-resistant state. J Biol Chem 272: 3544–3549 [DOI] [PubMed] [Google Scholar]
- Boulet I, Ralph S, Stanley E, Lock P, Dunn AR, Green SP, Phillips WA (1992) Lipopolysaccharide- and interferon-gamma-induced expression of hck and lyn tyrosine kinases in murine bone marrow-derived macrophages. Oncogene 7: 703–710 [PubMed] [Google Scholar]
- Bowman EP, Uhlinger DJ, Lambeth JD (1993) Neutrophil phospholipase D is activated by a membrane-associated Rho family small molecular weight GTP-binding protein. J Biol Chem 268: 21509–21512 [PubMed] [Google Scholar]
- Brint EK, Xu D, Liu H, Dunne A, McKenzie AN, O'Neill LA, Liew FY (2004) ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat Immunol 5: 373–379 [DOI] [PubMed] [Google Scholar]
- Bulut Y, Faure E, Thomas L, Equils O, Arditi M (2001) Cooperation of Toll-like receptor 2 and 6 for cellular activation by soluble tuberculosis factor and Borrelia burgdorferi outer surface protein A lipoprotein: role of Toll-interacting protein and IL-1 receptor signaling molecules in Toll-like receptor 2 signaling. J Immunol 167: 987–994 [DOI] [PubMed] [Google Scholar]
- Bulut Y, Faure E, Thomas L, Karahashi H, Michelsen KS, Equils O, Morrison SG, Morrison RP, Arditi M (2002) Chlamydial heat shock protein 60 activates macrophages and endothelial cells through Toll-like receptor 4 and MD2 in a MyD88-dependent pathway. J Immunol 168: 1435–1440 [DOI] [PubMed] [Google Scholar]
- Burns K, Clatworthy J, Martin L, Martinon F, Plumpton C, Maschera B, Lewis A, Ray K, Tschopp J, Volpe F (2000) Tollip, a new component of the IL-1RI pathway, links IRAK to the IL-1 receptor. Nat Cell Biol 2: 346–351 [DOI] [PubMed] [Google Scholar]
- Burns K, Janssens S, Brissoni B, Olivos N, Beyaert R, Tschopp J (2003) Inhibition of interleukin 1 receptor/Toll-like receptor signaling through the alternatively spliced, short form of MyD88 is due to its failure to recruit IRAK-4. J Exp Med 197: 263–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MP, Hanly JA, Moynagh PN (2005) Pellino3 is a novel upstream regulator of p38 MAPK and activates CREB in a p38-dependent manner. J Biol Chem 280: 27759–27768 [DOI] [PubMed] [Google Scholar]
- Callaghan J, Simonsen A, Gaullier JM, Toh BH, Stenmark H (1999) The endosome fusion regulator early-endosomal autoantigen 1 (EEA1) is a dimer. Biochem J 338 (Part 2): 539–543 [PMC free article] [PubMed] [Google Scholar]
- Cantrell D (1998) Lymphocyte signalling: a coordinating role for Vav? Curr Biol 8: R535–R538 [DOI] [PubMed] [Google Scholar]
- Cardone MH, Salvesen GS, Widmann C, Johnson G, Frisch SM (1997) The regulation of anoikis: MEKK-1 activation requires cleavage by caspases. Cell 90: 315–323 [DOI] [PubMed] [Google Scholar]
- Carruth LM, Demczuk S, Mizel SB (1991) Involvement of a calpain-like protease in the processing of the murine interleukin 1 alpha precursor. J Biol Chem 266: 12162–12167 [PubMed] [Google Scholar]
- Casamayor A, Morrice NA, Alessi DR (1999) Phosphorylation of Ser-241 is essential for the activity of 3-phosphoinositide-dependent protein kinase-1: identification of five sites of phosphorylation in vivo. Biochem J 342 (Part 2): 287–292 [PMC free article] [PubMed] [Google Scholar]
- Cataldi A, Centurione L, Di Pietro R, Rapino M, Bosco D, Grifone G, Garaci F, Rana R (2003) Protein kinase C zeta nuclear translocation mediates the occurrence of radioresistance in friend erythroleukemia cells. J Cell Biochem 88: 144–151 [DOI] [PubMed] [Google Scholar]
- Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, Van Ness K, Greenstreet TA, March CJ, Kronheim SR, Druck T, Cannizzaro LA, Huebner K, Black RA (1992) Molecular cloning of the interleukin-1 beta converting enzyme. Science 256: 97–100 [DOI] [PubMed] [Google Scholar]
- Chamaillard M, Hashimoto M, Horie Y, Masumoto J, Qiu S, Saab L, Ogura Y, Kawasaki A, Fukase K, Kusumoto S, Valvano MA, Foster SJ, Mak TW, Nunez G, Inohara N (2003) An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol 4: 702–707 [DOI] [PubMed] [Google Scholar]
- Channavajhala PL, Wu L, Cuozzo JW, Hall JP, Liu W, Lin LL, Zhang Y (2003) Identification of a novel human kinase supporter of Ras (hKSR-2) that functions as a negative regulator of Cot (Tpl2) signaling. J Biol Chem 278: 47089–47097 [DOI] [PubMed] [Google Scholar]
- Chen L, Fischle W, Verdin E, Greene WC (2001) Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science 293: 1653–1657 [DOI] [PubMed] [Google Scholar]
- Chen LF, Greene WC (2004) Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol 5: 392–401 [DOI] [PubMed] [Google Scholar]
- Chen LF, Mu Y, Greene WC (2002a) Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. EMBO J 21: 6539–6548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LY, Zuraw BL, Liu FT, Huang S, Pan ZK (2002b) IL-1 receptor-associated kinase and low molecular weight GTPase RhoA signal molecules are required for bacterial lipopolysaccharide-induced cytokine gene transcription. J Immunol 169: 3934–3939 [DOI] [PubMed] [Google Scholar]
- Chen RH, Sarnecki C, Blenis J (1992) Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol Cell Biol 12: 915–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung PC, Campbell DG, Nebreda AR, Cohen P (2003) Feedback control of the protein kinase TAK1 by SAPK2a/p38alpha. EMBO J 22: 5793–5805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung PC, Nebreda AR, Cohen P (2004) TAB3, a new binding partner of the protein kinase TAK1. Biochem J 378: 27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J, Melnick M, Solidakis GP, Tsichlis PN (2005) Tpl2 (tumor progression locus 2) phosphorylation at Thr290 is induced by lipopolysaccharide via an Ikappa-B kinase-beta-dependent pathway and is required for Tpl2 activation by external signals. J Biol Chem 280: 20442–20448 [DOI] [PubMed] [Google Scholar]
- Cho J, Tsichlis PN (2005) Phosphorylation at Thr-290 regulates Tpl2 binding to NF-kappaB1/p105 and Tpl2 activation and degradation by lipopolysaccharide. Proc Natl Acad Sci USA 102: 2350–2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KB, Wong F, Harlan JM, Chaudhary PM, Hood L, Karsan A (1998) Lipopolysaccharide mediates endothelial apoptosis by a FADD-dependent pathway. J Biol Chem 273: 20185–20188 [DOI] [PubMed] [Google Scholar]
- Chrivia JC, Kwok RP, Lamb N, Hagiwara M, Montminy MR, Goodman RH (1993) Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature 365: 855–859 [DOI] [PubMed] [Google Scholar]
- Chu ZL, McKinsey TA, Liu L, Qi X, Ballard DW (1996) Basal phosphorylation of the PEST domain in the I(kappa)B(beta) regulates its functional interaction with the c-rel proto-oncogene product. Mol Cell Biol 16: 5974–5984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang TH, Ulevitch RJ (2004) Triad3A, an E3 ubiquitin–protein ligase regulating Toll-like receptors. Nat Immunol 5: 495–502 [DOI] [PubMed] [Google Scholar]
- Coelho PS, Klein A, Talvani A, Coutinho SF, Takeuchi O, Akira S, Silva JS, Canizzaro H, Gazzinelli RT, Teixeira MM (2002) Glycosylphosphatidylinositol-anchored mucin-like glycoproteins isolated from Trypanosoma cruzi trypomastigotes induce in vivo leukocyte recruitment dependent on MCP-1 production by IFN-gamma-primed-macrophages. J Leukocyte Biol 71: 837–844 [PubMed] [Google Scholar]
- Cohen S, Achbert-Weiner H, Ciechanover A (2004) Dual effects of IkappaB kinase beta-mediated phosphorylation on p105 Fate: SCF(beta-TrCP)-dependent degradation and SCF(beta-TrCP)-independent processing. Mol Cell Biol 24: 475–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Orian A, Ciechanover A (2001) Processing of p105 is inhibited by docking of p50 active subunits to the ankyrin repeat domain, and inhibition is alleviated by signaling via the carboxyl-terminal phosphorylation/ubiquitin-ligase binding domain. J Biol Chem 276: 26769–26776 [DOI] [PubMed] [Google Scholar]
- Colbran RJ, Schworer CM, Hashimoto Y, Fong YL, Rich DP, Smith MK, Soderling TR (1989) Calcium/calmodulin-dependent protein kinase II. Biochem J 258: 313–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colicelli J (2004) Human RAS superfamily proteins and related GTPases. Sci STKE 2004: RE13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronella-Wood J, Terrand J, Sun H, Chen QM (2004) c-Fos phosphorylation induced by H2O2 prevents proteasomal degradation of c-Fos in cardiomyocytes. J Biol Chem 279: 33567–33574 [DOI] [PubMed] [Google Scholar]
- Cory GO, Lovering RC, Hinshelwood S, MacCarthy-Morrogh L, Levinsky RJ, Kinnon C (1995) The protein product of the c-cbl protooncogene is phosphorylated after B cell receptor stimulation and binds the SH3 domain of Bruton's tyrosine kinase. J Exp Med 182: 611–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JA (1988) Interactive properties of calmodulin. Biochem J 249: 621–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo P, Schuebel KE, Ostrom AA, Gutkind JS, Bustelo XR (1997) Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature 385: 169–172 [DOI] [PubMed] [Google Scholar]
- Crews CM, Alessandrini A, Erikson RL (1992) The primary structure of MEK, a protein kinase that phosphorylates the ERK gene product. Science 258: 478–480 [DOI] [PubMed] [Google Scholar]
- Cuenda A, Alonso G, Morrice N, Jones M, Meier R, Cohen P, Nebreda AR (1996) Purification and cDNA cloning of SAPKK3, the major activator of RK/p38 in stress- and cytokine-stimulated monocytes and epithelial cells. EMBO J 15: 4156–4164 [PMC free article] [PubMed] [Google Scholar]
- Dalton S, Treisman R (1992) Characterization of SAP-1, a protein recruited by serum response factor to the c-fos serum response element. Cell 68: 597–612 [DOI] [PubMed] [Google Scholar]
- Dang PM, Fontayne A, Hakim J, El Benna J, Perianin A (2001) Protein kinase C zeta phosphorylates a subset of selective sites of the NADPH oxidase component p47phox and participates in formyl peptide-mediated neutrophil respiratory burst. J Immunol 166: 1206–1213 [DOI] [PubMed] [Google Scholar]
- Davis M, Hatzubai A, Andersen JS, Ben-Shushan E, Fisher GZ, Yaron A, Bauskin A, Mercurio F, Mann M, Ben-Neriah Y (2002) Pseudosubstrate regulation of the SCF(beta-TrCP) ubiquitin ligase by hnRNP-U. Genes Dev 16: 439–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon K, Blank JL (1999) MEK kinase 3 directly activates MKK6 and MKK7, specific activators of the p38 and c-Jun NH2-terminal kinases. J Biol Chem 274: 16604–16610 [DOI] [PubMed] [Google Scholar]
- Deak JC, Cross JV, Lewis M, Qian Y, Parrott LA, Distelhorst CW, Templeton DJ (1998a) Fas-induced proteolytic activation and intracellular redistribution of the stress-signaling kinase MEKK1. Proc Natl Acad Sci USA 95: 5595–5600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak M, Clifton AD, Lucocq LM, Alessi DR (1998b) Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J 17: 4426–4441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhase M, Hayakawa M, Chen Y, Karin M (1999) Positive and negative regulation of IkappaB kinase activity through IKKbeta subunit phosphorylation. Science 284: 309–313 [DOI] [PubMed] [Google Scholar]
- Deng L, Wang C, Spencer E, Yang L, Braun A, You J, Slaughter C, Pickart C, Chen ZJ (2000) Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103: 351–361 [DOI] [PubMed] [Google Scholar]
- Derijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, Karin M, Davis RJ (1994) JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell 76: 1025–1037 [DOI] [PubMed] [Google Scholar]
- Dhillon AS, Kolch W (2002) Untying the regulation of the Raf-1 kinase. Arch Biochem Biophys 404: 3–9 [DOI] [PubMed] [Google Scholar]
- Diaz-Meco MT, Berra E, Municio MM, Sanz L, Lozano J, Dominguez I, Diaz-Golpe V, Lain de Lera MT, Alcamí J, Payá CV, Arenzana-Seisdedos F, Virelizier JL, Moscat J (1993) A dominant negative protein kinase C zeta subspecies blocks NF-kappa B activation. Mol Cell Biol 13: 4770–4775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiDonato J, Mercurio F, Rosette C, Wu-Li J, Suyang H, Ghosh S, Karin M (1996) Mapping of the inducible IkappaB phosphorylation sites that signal its ubiquitination and degradation. Mol Cell Biol 16: 1295–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C (2004) Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303: 1529–1531 [DOI] [PubMed] [Google Scholar]
- Diehl GE, Yue HH, Hsieh K, Kuang AA, Ho M, Morici LA, Lenz LL, Cado D, Riley LW, Winoto A (2004) TRAIL-R as a negative regulator of innate immune cell responses. Immunity 21: 877–889 [DOI] [PubMed] [Google Scholar]
- Diekmann D, Abo A, Johnston C, Segal AW, Hall A (1994) Interaction of Rac with p67phox and regulation of phagocytic NADPH oxidase activity. Science 265: 531–533 [DOI] [PubMed] [Google Scholar]
- Dumitru CD, Ceci JD, Tsatsanis C, Kontoyiannis D, Stamatakis K, Lin JH, Patriotis C, Jenkins NA, Copeland NG, Kollias G, Tsichlis PN (2000) TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell 103: 1071–1083 [DOI] [PubMed] [Google Scholar]
- Dunne A, Ejdeback M, Ludidi PL, O'Neill LA, Gay NJ (2003) Structural complementarity of Toll/interleukin-1 receptor domains in Toll-like receptors and the adaptors Mal and MyD88. J Biol Chem 278: 41443–41451 [DOI] [PubMed] [Google Scholar]
- Edwards AS, Newton AC (1997) Phosphorylation at conserved carboxyl-terminal hydrophobic motif regulates the catalytic and regulatory domains of protein kinase C. J Biol Chem 272: 18382–18390 [DOI] [PubMed] [Google Scholar]
- El Benna J, Faust RP, Johnson JL, Babior BM (1996) Phosphorylation of the respiratory burst oxidase subunit p47phox as determined by two-dimensional phosphopeptide mapping. Phosphorylation by protein kinase C, protein kinase A, and a mitogen-activated protein kinase. J Biol Chem 271: 6374–6378 [DOI] [PubMed] [Google Scholar]
- El-Benna J, Dang PM, Gougerot-Pocidalo MA, Elbim C (2005) Phagocyte NADPH oxidase: a multicomponent enzyme essential for host defenses. Arch Immunol Ther Exp (Warsz) 53: 199–206 [PubMed] [Google Scholar]
- Fagerlund R, Kinnunen L, Kohler M, Julkunen I, Melen K (2005) NF-kappaB is transported into the nucleus by importin alpha3 and importin alpha4. J Biol Chem 280: 15942–15951 [DOI] [PubMed] [Google Scholar]
- Fan CM, Maniatis T (1991) Generation of p50 subunit of NF-kappa B by processing of p105 through an ATP-dependent pathway. Nature 354: 395–398 [DOI] [PubMed] [Google Scholar]
- Farnell MB, He H, Genovese K, Kogut MH (2003) Pharmacological analysis of signal transduction pathways required for oxidative burst in chicken heterophils stimulated by a Toll-like receptor 2 agonist. Int Immunopharmacol 3: 1677–1684 [DOI] [PubMed] [Google Scholar]
- Fass DM, Butler JE, Goodman RH (2003) Deacetylase activity is required for cAMP activation of a subset of CREB target genes. J Biol Chem 278: 43014–43019 [DOI] [PubMed] [Google Scholar]
- Faust LR, el Benna J, Babior BM, Chanock SJ (1995) The phosphorylation targets of p47phox, a subunit of the respiratory burst oxidase. Functions of the individual target serines as evaluated by site-directed mutagenesis. J Clin Invest 96: 1499–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Longmore GD (2005) The LIM protein Ajuba influences interleukin-1-induced NF-kappaB activation by affecting the assembly and activity of the protein kinase Czeta/p62/TRAF6 signaling complex. Mol Cell Biol 25: 4010–4022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, Coyle AJ, Liao SM, Maniatis T (2003a) IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol 4: 491–496 [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, Palsson-McDermott EM, Bowie AG, Jefferies CA, Mansell AS, Brady G, Brint E, Dunne A, Gray P, Harte MT, McMurray D, Smith DE, Sims JE, Bird TA, O'Neill LA (2001) Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature 413: 78–83 [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, Rowe DC, Barnes BJ, Caffrey DR, Visintin A, Latz E, Monks B, Pitha PM, Golenbock DT (2003b) LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF. J Exp Med 198: 1043–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KA, Rowe DC, Golenbock DT (2004) Endotoxin recognition and signal transduction by the TLR4/MD2-complex. Microbes Infect 6: 1361–1367 [DOI] [PubMed] [Google Scholar]
- Fontayne A, Dang PM, Gougerot-Pocidalo MA, El-Benna J (2002) Phosphorylation of p47phox sites by PKC alpha, beta II, delta, and zeta: effect on binding to p22phox and on NADPH oxidase activation. Biochemistry 41: 7743–7750 [DOI] [PubMed] [Google Scholar]
- Frost JA, Steen H, Shapiro P, Lewis T, Ahn N, Shaw PE, Cobb MH (1997) Cross-cascade activation of ERKs and ternary complex factors by Rho family proteins. EMBO J 16: 6426–6438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs SY, Chen A, Xiong Y, Pan ZQ, Ronai Z (1999) HOS, a human homolog of Slimb, forms an SCF complex with Skp1 and Cullin1 and targets the phosphorylation-dependent degradation of IkappaB and beta-catenin. Oncogene 18: 2039–2046 [DOI] [PubMed] [Google Scholar]
- Fujioka S, Niu J, Schmidt C, Sclabas GM, Peng B, Uwagawa T, Li Z, Evans DB, Abbruzzese JL, Chiao PJ (2004) NF-kappaB and AP-1 connection: mechanism of NF-kappaB-dependent regulation of AP-1 activity. Mol Cell Biol 24: 7806–7819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Koyasu S (2003) PI3K and negative regulation of TLR signaling. Trends Immunol 24: 358–363 [DOI] [PubMed] [Google Scholar]
- Fukunaga R, Hunter T (1997) MNK1, a new MAP kinase-activated protein kinase, isolated by a novel expression screening method for identifying protein kinase substrates. EMBO J 16: 1921–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi-Tago M, Tago K, Andoh K, Sonoda Y, Tominaga S, Kasahara T (2005) Functional role of c-Src in IL-1-induced NF-kappa B activation: c-Src is a component of the IKK complex. J Biochem (Tokyo) 137: 189–197 [DOI] [PubMed] [Google Scholar]
- Ge B, Gram H, Di Padova F, Huang B, New L, Ulevitch RJ, Luo Y, Han J (2002) MAPKK-independent activation of p38alpha mediated by TAB1-dependent autophosphorylation of p38alpha. Science 295: 1291–1294 [DOI] [PubMed] [Google Scholar]
- Gerritsen ME, Williams AJ, Neish AS, Moore S, Shi Y, Collins T (1997) CREB-binding protein/p300 are transcriptional coactivators of p65. Proc Natl Acad Sci USA 94: 2927–2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Karin M (2002) Missing pieces in the NF-kappaB puzzle. Cell 109 (Suppl): S81–S96 [DOI] [PubMed] [Google Scholar]
- Ghosh S, Strum JC, Sciorra VA, Daniel L, Bell RM (1996) Raf-1 kinase possesses distinct binding domains for phosphatidylserine and phosphatidic acid. Phosphatidic acid regulates the translocation of Raf-1 in 12-O-tetradecanoylphorbol-13-acetate-stimulated Madin–Darby canine kidney cells. J Biol Chem 271: 8472–8480 [DOI] [PubMed] [Google Scholar]
- Giese KP, Fedorov NB, Filipkowski RK, Silva AJ (1998) Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science 279: 870–873 [DOI] [PubMed] [Google Scholar]
- Gil J, Garcia MA, Gomez-Puertas P, Guerra S, Rullas J, Nakano H, Alcami J, Esteban M (2004) TRAF family proteins link PKR with NF-kappa B activation. Mol Cell Biol 24: 4502–4512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardin SE, Boneca IG, Carneiro LA, Antignac A, Jehanno M, Viala J, Tedin K, Taha MK, Labigne A, Zahringer U, Coyle AJ, DiStefano PS, Bertin J, Sansonetti PJ, Philpott DJ (2003a) Nod1 detects a unique muropeptide from Gram-negative bacterial peptidoglycan. Science 300: 1584–1587 [DOI] [PubMed] [Google Scholar]
- Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ (2003b) Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem 278: 8869–8872 [DOI] [PubMed] [Google Scholar]
- Girardin SE, Tournebize R, Mavris M, Page AL, Li X, Stark GR, Bertin J, DiStefano PS, Yaniv M, Sansonetti PJ, Philpott DJ (2001) CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri. EMBO Rep 2: 736–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gon Y, Asai Y, Hashimoto S, Mizumura K, Jibiki I, Machino T, Ra C, Horie T (2004) A20 inhibits toll-like receptor 2- and 4-mediated interleukin-8 synthesis in airway epithelial cells. Am J Respir Cell Mol Biol 31: 330–336 [DOI] [PubMed] [Google Scholar]
- Gonzalez FA, Seth A, Raden DL, Bowman DS, Fay FS, Davis RJ (1993) Serum-induced translocation of mitogen-activated protein kinase to the cell surface ruffling membrane and the nucleus. J Cell Biol 122: 1089–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez GA, Montminy MR (1989) Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell 59: 675–680 [DOI] [PubMed] [Google Scholar]
- Gratton JP, Morales-Ruiz M, Kureishi Y, Fulton D, Walsh K, Sessa WC (2001) Akt down-regulation of p38 signaling provides a novel mechanism of vascular endothelial growth factor-mediated cytoprotection in endothelial cells. J Biol Chem 276: 30359–30365 [DOI] [PubMed] [Google Scholar]
- Grimes CA, Jope RS (2001) CREB DNA binding activity is inhibited by glycogen synthase kinase-3 beta and facilitated by lithium. J Neurochem 78: 1219–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan H, Hou S, Ricciardi RP (2005) DNA binding of repressor nuclear factor-kappaB p50/p50 depends on phosphorylation of Ser337 by the protein kinase A catalytic subunit. J Biol Chem 280: 9957–9962 [DOI] [PubMed] [Google Scholar]
- Guinamard R, Fougereau M, Seckinger P (1997) The SH3 domain of Bruton's tyrosine kinase interacts with Vav, Sam68 and EWS. Scand J Immunol 45: 587–595 [DOI] [PubMed] [Google Scholar]
- Gupta S, Seth A, Davis RJ (1993) Transactivation of gene expression by Myc is inhibited by mutation at the phosphorylation sites Thr-58 and Ser-62. Proc Natl Acad Sci USA 90: 3216–3220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar AM, O'Mahony DS, Ozinsky A, Underhill DM, Aderem A, Klebanoff SJ, Wilson CB (2001) Cutting edge: functional interactions between toll-like receptor (TLR) 2 and TLR1 or TLR6 in response to phenol-soluble modulin. J Immunol 166: 15–19 [DOI] [PubMed] [Google Scholar]
- Han L, Colicelli J (1995) A human protein selected for interference with Ras function interacts directly with Ras and competes with Raf1. Mol Cell Biol 15: 1318–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy MP, O'Neill LA (2004) The murine IRAK2 gene encodes four alternatively spliced isoforms, two of which are inhibitory. J Biol Chem 279: 27699–27708 [DOI] [PubMed] [Google Scholar]
- Harhaj EW, Maggirwar SB, Sun SC (1996) Inhibition of p105 processing by NF-kappaB proteins in transiently transfected cells. Oncogene 12: 2385–2392 [PubMed] [Google Scholar]
- Hasan U, Chaffois C, Gaillard C, Saulnier V, Merck E, Tancredi S, Guiet C, Briere F, Vlach J, Lebecque S, Trinchieri G, Bates EE (2005) Human TLR10 is a functional receptor, expressed by B cells and plasmacytoid dendritic cells, which activates gene transcription through MyD88. J Immunol 174: 2942–2950 [DOI] [PubMed] [Google Scholar]
- Hatakeyama S, Kitagawa M, Nakayama K, Shirane M, Matsumoto M, Hattori K, Higashi H, Nakano H, Okumura K, Onoe K, Good RA (1999) Ubiquitin-dependent degradation of IkappaBalpha is mediated by a ubiquitin ligase Skp1/Cul 1/F-box protein FWD1. Proc Natl Acad Sci USA 96: 3859–3863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa J, Depatie C, Ohmichi M, Mercola D (2003) The activation of c-Jun NH2-terminal kinase (JNK) by DNA-damaging agents serves to promote drug resistance via activating transcription factor 2 (ATF2)-dependent enhanced DNA repair. J Biol Chem 278: 20582–20592 [DOI] [PubMed] [Google Scholar]
- Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A (2001) The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410: 1099–1103 [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S (2004) Signaling to NF-kappaB. Genes Dev 18: 2195–2224 [DOI] [PubMed] [Google Scholar]
- Heil F, Ahmad-Nejad P, Hemmi H, Hochrein H, Ampenberger F, Gellert T, Dietrich H, Lipford G, Takeda K, Akira S, Wagner H, Bauer S (2003) The Toll-like receptor 7 (TLR7)-specific stimulus loxoribine uncovers a strong relationship within the TLR7, 8 and 9 subfamily. Eur J Immunol 33: 2987–2997 [DOI] [PubMed] [Google Scholar]
- Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S (2004) Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303: 1526–1529 [DOI] [PubMed] [Google Scholar]
- Heissmeyer V, Krappmann D, Hatada EN, Scheidereit C (2001) Shared pathways of IkappaB kinase-induced SCF(betaTrCP)-mediated ubiquitination and degradation for the NF-kappaB precursor p105 and IkappaBalpha. Mol Cell Biol 21: 1024–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heissmeyer V, Krappmann D, Wulczyn FG, Scheidereit C (1999) NF-kappaB p105 is a target of IkappaB kinases and controls signal induction of Bcl-3–p50 complexes. EMBO J 18: 4766–4778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S (2002) Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol 3: 196–200 [DOI] [PubMed] [Google Scholar]
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S (2000) A Toll-like receptor recognizes bacterial DNA. Nature 408: 740–745 [DOI] [PubMed] [Google Scholar]
- Heyninck K, De Valck D, Vanden Berghe W, Van Criekinge W, Contreras R, Fiers W, Haegeman G, Beyaert R (1999) The zinc finger protein A20 inhibits TNF-induced NF-kappaB-dependent gene expression by interfering with an RIP- or TRAF2-mediated transactivation signal and directly binds to a novel NF-kappaB-inhibiting protein ABIN. J Cell Biol 145: 1471–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyninck K, Kreike MM, Beyaert R (2003) Structure-function analysis of the A20-binding inhibitor of NF-kappa B activation, ABIN-1. FEBS Lett 536: 135–140 [DOI] [PubMed] [Google Scholar]
- Hibi M, Lin A, Smeal T, Minden A, Karin M (1993) Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev 7: 2135–2148 [DOI] [PubMed] [Google Scholar]
- Hipskind RA, Rao VN, Mueller CG, Reddy ES, Nordheim A (1991) Ets-related protein Elk-1 is homologous to the c-fos regulatory factor p62TCF. Nature 354: 531–534 [DOI] [PubMed] [Google Scholar]
- Hirschfeld M, Weis JJ, Toshchakov V, Salkowski CA, Cody MJ, Ward DC, Qureshi N, Michalek SM, Vogel SN (2001) Signaling by toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect Immun 69: 1477–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebe K, Du X, Georgel P, Janssen E, Tabeta K, Kim SO, Goode J, Lin P, Mann N, Mudd S, Crozat K, Sovath S, Han J, Beutler B (2003) Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature 424: 743–748 [DOI] [PubMed] [Google Scholar]
- Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR (2000) Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature 406: 86–90 [DOI] [PubMed] [Google Scholar]
- Horng T, Barton GM, Flavell RA, Medzhitov R (2002) The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature 420: 329–333 [DOI] [PubMed] [Google Scholar]
- Huang J, Gao X, Li S, Cao Z (1997) Recruitment of IRAK to the interleukin 1 receptor complex requires interleukin 1 receptor accessory protein. Proc Natl Acad Sci USA 94: 12829–12832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Yang J, Lin Y, Walker C, Cheng J, Liu ZG, Su B (2004) Differential regulation of interleukin 1 receptor and Toll-like receptor signaling by MEKK3. Nat Immunol 5: 98–103 [DOI] [PubMed] [Google Scholar]
- Hughes K, Edin S, Antonsson A, Grundstrom T (2001) Calmodulin-dependent kinase II mediates T cell receptor/CD3- and phorbol ester-induced activation of IkappaB kinase. J Biol Chem 276: 36008–36013 [DOI] [PubMed] [Google Scholar]
- Hyakushima N, Mitsuzawa H, Nishitani C, Sano H, Kuronuma K, Konishi M, Himi T, Miyake K, Kuroki Y (2004) Interaction of soluble form of recombinant extracellular TLR4 domain with MD-2 enables lipopolysaccharide binding and attenuates TLR4-mediated signaling. J Immunol 173: 6949–6954 [DOI] [PubMed] [Google Scholar]
- Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y (1997) Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 275: 90–94 [DOI] [PubMed] [Google Scholar]
- Idriss H, Kumar A, Casas-Finet JR, Guo H, Damuni Z, Wilson SH (1994) Regulation of in vitro nucleic acid strand annealing activity of heterogeneous nuclear ribonucleoprotein protein A1 by reversible phosphorylation. Biochemistry 33: 11382–11390 [DOI] [PubMed] [Google Scholar]
- Illenberger D, Schwald F, Pimmer D, Binder W, Maier G, Dietrich A, Gierschik P (1998) Stimulation of phospholipase C-beta2 by the Rho GTPases Cdc42Hs and Rac1. EMBO J 17: 6241–6249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inohara N, Koseki T, del Peso L, Hu Y, Yee C, Chen S, Carrio R, Merino J, Liu D, Ni J, Nunez G (1999) Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-kappaB. J Biol Chem 274: 14560–14567 [DOI] [PubMed] [Google Scholar]
- Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, Fukase K, Inamura S, Kusumoto S, Hashimoto M, Foster SJ, Moran AP, Fernandez-Luna JL, Nunez G (2003) Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J Biol Chem 278: 5509–5512 [DOI] [PubMed] [Google Scholar]
- Irie T, Muta T, Takeshige K (2000) TAK1 mediates an activation signal from toll-like receptor(s) to nuclear factor-kappaB in lipopolysaccharide-stimulated macrophages. FEBS Lett 467: 160–164 [DOI] [PubMed] [Google Scholar]
- Ishida A, Shigeri Y, Taniguchi T, Kameshita I (2003) Protein phosphatases that regulate multifunctional Ca2+/calmodulin-dependent protein kinases: from biochemistry to pharmacology. Pharmacol Ther 100: 291–305 [DOI] [PubMed] [Google Scholar]
- Ishii KJ, Takeshita F, Gursel I, Gursel M, Conover J, Nussenzweig A, Klinman DM (2002) Potential role of phosphatidylinositol 3 kinase, rather than DNA-dependent protein kinase, in CpG DNA-induced immune activation. J Exp Med 196: 269–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani T, Takaesu G, Ninomiya-Tsuji J, Shibuya H, Gaynor RB, Matsumoto K (2003) Role of the TAB2-related protein TAB3 in IL-1 and TNF signaling. EMBO J 22: 6277–6288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam S, Hassan F, Mu MM, Ito H, Koide N, Mori I, Yoshida T, Yokochi T (2004) Piceatannol prevents lipopolysaccharide (LPS)-induced nitric oxide (NO) production and nuclear factor (NF)-kappaB activation by inhibiting IkappaB kinase (IKK). Microbiol Immunol 48: 729–736 [DOI] [PubMed] [Google Scholar]
- Iwami KI, Matsuguchi T, Masuda A, Kikuchi T, Musikacharoen T, Yoshikai Y (2000) Cutting edge: naturally occurring soluble form of mouse Toll-like receptor 4 inhibits lipopolysaccharide signaling. J Immunol 165: 6682–6686 [DOI] [PubMed] [Google Scholar]
- Jaffer ZM, Chernoff J (2002) p21-activated kinases: three more join the Pak. Int J Biochem Cell Biol 34: 713–717 [DOI] [PubMed] [Google Scholar]
- Jahr TG, Ryan L, Sundan A, Lichenstein HS, Skjak-Braek G, Espevik T (1997) Induction of tumor necrosis factor production from monocytes stimulated with mannuronic acid polymers and involvement of lipopolysaccharide-binding protein, CD14, and bactericidal/permeability-increasing factor. Infect Immun 65: 89–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens S, Burns K, Tschopp J, Beyaert R (2002) Regulation of interleukin-1- and lipopolysaccharide-induced NF-kappaB activation by alternative splicing of MyD88. Curr Biol 12: 467–471 [DOI] [PubMed] [Google Scholar]
- Jeannin P, Renno T, Goetsch L, Miconnet I, Aubry JP, Delneste Y, Herbault N, Baussant T, Magistrelli G, Soulas C, Romero P, Cerottini JC, Bonnefoy JY (2000) OmpA targets dendritic cells, induces their maturation and delivers antigen into the MHC class I presentation pathway. Nat Immunol 1: 502–509 [DOI] [PubMed] [Google Scholar]
- Jefferies CA, O'Neill LA (2004) Bruton's tyrosine kinase (Btk)-the critical tyrosine kinase in LPS signalling? Immunol Lett 92: 15–22 [DOI] [PubMed] [Google Scholar]
- Jiang Z, Johnson HJ, Nie H, Qin J, Bird TA, Li X (2003) Pellino 1 is required for interleukin-1 (IL-1)-mediated signaling through its interaction with the IL-1 receptor-associated kinase 4 (IRAK4)-IRAK-tumor necrosis factor receptor-associated factor 6 (TRAF6) complex. J Biol Chem 278: 10952–10956 [DOI] [PubMed] [Google Scholar]
- Jiang Z, Ninomiya-Tsuji J, Qian Y, Matsumoto K, Li X (2002) Interleukin-1 (IL-1) receptor-associated kinase-dependent IL-1-induced signaling complexes phosphorylate TAK1 and TAB2 at the plasma membrane and activate TAK1 in the cytosol. Mol Cell Biol 22: 7158–7167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GB, Brunn GJ, Kodaira Y, Platt JL (2002) Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol 168: 5233–5239 [DOI] [PubMed] [Google Scholar]
- Jurk M, Heil F, Vollmer J, Schetter C, Krieg AM, Wagner H, Lipford G, Bauer S (2002) Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat Immunol 3: 499. [DOI] [PubMed] [Google Scholar]
- Kanayama A, Seth RB, Sun L, Ea CK, Hong M, Shaito A, Chiu YH, Deng L, Chen ZJ (2004) TAB2 and TAB3 activate the NF-kappaB pathway through binding to polyubiquitin chains. Mol Cell 15: 535–548 [DOI] [PubMed] [Google Scholar]
- Kanoh H, Sakane F, Imai S, Wada I (1993) Diacylglycerol kinase and phosphatidic acid phosphatase—enzymes metabolizing lipid second messengers. Cell Signal 5: 495–503 [DOI] [PubMed] [Google Scholar]
- Karandikar M, Xu S, Cobb MH (2000) MEKK1 binds raf-1 and the ERK2 cascade components. J Biol Chem 275: 40120–40127 [DOI] [PubMed] [Google Scholar]
- Kariko K, Ni H, Capodici J, Lamphier M, Weissman D (2004) mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem 279: 12542–12550 [DOI] [PubMed] [Google Scholar]
- Karin M, Liu Z, Zandi E (1997) AP-1 function and regulation. Curr Opin Cell Biol 9: 240–246 [DOI] [PubMed] [Google Scholar]
- Kassenbrock CK, Hunter S, Garl P, Johnson GL, Anderson SM (2002) Inhibition of Src family kinases blocks epidermal growth factor (EGF)-induced activation of Akt, phosphorylation of c-Cbl, and ubiquitination of the EGF receptor. J Biol Chem 277: 24967–24975 [DOI] [PubMed] [Google Scholar]
- Kavita U, Mizel SB (1995) Differential sensitivity of interleukin-1 alpha and -beta precursor proteins to cleavage by calpain, a calcium-dependent protease. J Biol Chem 270: 27758–27765 [DOI] [PubMed] [Google Scholar]
- Kawai T, Sato S, Ishii KJ, Coban C, Hemmi H, Yamamoto M, Terai K, Matsuda M, Inoue J, Uematsu S, Takeuchi O, Akira S (2004) Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat Immunol 5: 1061–1068 [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Kawashima S (1996) Regulation of the calpain-calpastatin system by membranes [review]. Mol Membr Biol 13: 217–224 [DOI] [PubMed] [Google Scholar]
- Kawasaki K, Akashi S, Shimazu R, Yoshida T, Miyake K, Nishijima M (2000) Mouse toll-like receptor 4.MD-2 complex mediates lipopolysaccharide-mimetic signal transduction by Taxol. J Biol Chem 275: 2251–2254 [DOI] [PubMed] [Google Scholar]
- Keranen LM, Dutil EM, Newton AC (1995) Protein kinase C is regulated in vivo by three functionally distinct phosphorylations. Curr Biol 5: 1394–1403 [DOI] [PubMed] [Google Scholar]
- Khosravi-Far R, Solski PA, Clark GJ, Kinch MS, Der CJ (1995) Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol Cell Biol 15: 6443–6453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan R, Bres V, Ng RW, Coudart MP, El Messaoudi S, Sardet C, Jin DY, Emiliani S, Benkirane M (2003) Post-activation turn-off of NF-kappa B-dependent transcription is regulated by acetylation of p65. J Biol Chem 278: 2758–2766 [DOI] [PubMed] [Google Scholar]
- Kim AH, Khursigara G, Sun X, Franke TF, Chao MV (2001) Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol Cell Biol 21: 893–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Diebold BA, Kim JI, Kim J, Lee JY, Park JB (2004) Rho is involved in superoxide formation during phagocytosis of opsonized zymosans. J Biol Chem 279: 21589–21597 [DOI] [PubMed] [Google Scholar]
- Kim SO, Ono K, Tobias PS, Han J (2003) Orphan nuclear receptor Nur77 is involved in caspase-independent macrophage cell death. J Exp Med 197: 1441–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Hernandez LD, Galan JE, Janeway CA, Jr, Medzhitov R, Flavell RA (2002) IRAK-M is a negative regulator of Toll-like receptor signaling. Cell 110: 191–202 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Yamamoto K, Saido T, Kawasaki H, Oppenheim JJ, Matsushima K (1990) Identification of calcium-activated neutral protease as a processing enzyme of human interleukin 1 alpha. Proc Natl Acad Sci USA 87: 5548–5552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollewe C, Mackensen AC, Neumann D, Knop J, Cao P, Li S, Wesche H, Martin MU (2004) Sequential autophosphorylation steps in the interleukin-1 receptor-associated kinase-1 regulate its availability as an adapter in interleukin-1 signaling. J Biol Chem 279: 5227–5236 [DOI] [PubMed] [Google Scholar]
- Kopp E, Medzhitov R, Carothers J, Xiao C, Douglas I, Janeway CA, Ghosh S (1999) ECSIT is an evolutionarily conserved intermediate in the Toll/IL-1 signal transduction pathway. Genes Dev 13: 2059–2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostura MJ, Tocci MJ, Limjuco G, Chin J, Cameron P, Hillman AG, Chartrain NA, Schmidt JA (1989) Identification of a monocyte specific pre-interleukin 1 beta convertase activity. Proc Natl Acad Sci USA 86: 5227–5231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll M, Margottin F, Kohl A, Renard P, Durand H, Concordet JP, Bachelerie F, Arenzana-Seisdedos F, Benarous R (1999) Inducible degradation of IkappaBalpha by the proteasome requires interaction with the F-box protein h-betaTrCP. J Biol Chem 274: 7941–7945 [DOI] [PubMed] [Google Scholar]
- Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA (1995) Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science 267: 2000–2003 [DOI] [PubMed] [Google Scholar]
- Kumar S, McDonnell PC, Gum RJ, Hand AT, Lee JC, Young PR (1997) Novel homologues of CSBP/p38 MAP kinase: activation, substrate specificity and sensitivity to inhibition by pyridinyl imidazoles. Biochem Biophys Res Commun 235: 533–538 [DOI] [PubMed] [Google Scholar]
- Kurosaki T, Kurosaki M (1997) Transphosphorylation of Bruton's tyrosine kinase on tyrosine 551 is critical for B cell antigen receptor function. J Biol Chem 272: 15595–15598 [DOI] [PubMed] [Google Scholar]
- Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, Finberg RW (2000) Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol 1: 398–401 [DOI] [PubMed] [Google Scholar]
- Laherty CD, Hu HM, Opipari AW, Wang F, Dixit VM (1992) The Epstein–Barr virus LMP1 gene product induces A20 zinc finger protein expression by activating nuclear factor kappa B. J Biol Chem 267: 24157–24160 [PubMed] [Google Scholar]
- Laherty CD, Perkins ND, Dixit VM (1993) Human T cell leukemia virus type I Tax and phorbol 12-myristate 13-acetate induce expression of the A20 zinc finger protein by distinct mechanisms involving nuclear factor kappa B. J Biol Chem 268: 5032–5039 [PubMed] [Google Scholar]
- Lallena MJ, Diaz-Meco MT, Bren G, Paya CV, Moscat J (1999) Activation of IkappaB kinase beta by protein kinase C isoforms. Mol Cell Biol 19: 2180–2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang V, Janzen J, Fischer GZ, Soneji Y, Beinke S, Salmeron A, Allen H, Hay RT, Ben-Neriah Y, Ley SC (2003) betaTrCP-mediated proteolysis of NF-kappaB1 p105 requires phosphorylation of p105 serines 927 and 932. Mol Cell Biol 23: 402–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T, Golenbock DT (2004) TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol 5: 190–198 [DOI] [PubMed] [Google Scholar]
- Lauw FN, Caffrey DR, Golenbock DT (2005) Of mice and man: TLR11 (finally) finds profilin. Trends Immunol 26: 509–511 [DOI] [PubMed] [Google Scholar]
- Lawrence T, Bebien M, Liu GY, Nizet V, Karin M (2005) IKKalpha limits macrophage NF-kappaB activation and contributes to the resolution of inflammation. Nature 434: 1138–1143 [DOI] [PubMed] [Google Scholar]
- Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ (1998) Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science 281: 2042–2045 [DOI] [PubMed] [Google Scholar]
- Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A (2002) Chromatin–IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416: 603–607 [DOI] [PubMed] [Google Scholar]
- LeBouder E, Rey-Nores JE, Rushmere NK, Grigorov M, Lawn SD, Affolter M, Griffin GE, Ferrara P, Schiffrin EJ, Morgan BP, Labeta MO (2003) Soluble forms of Toll-like receptor (TLR)2 capable of modulating TLR2 signaling are present in human plasma and breast milk. J Immunol 171: 6680–6689 [DOI] [PubMed] [Google Scholar]
- Lee CW, Chien CS, Yang CM (2004) Lipoteichoic acid-stimulated p42/p44 MAPK activation via Toll-like receptor 2 in tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 286: L921–L930 [DOI] [PubMed] [Google Scholar]
- Lee J, Mira-Arbibe L, Ulevitch RJ (2000) TAK1 regulates multiple protein kinase cascades activated by bacterial lipopolysaccharide. J Leukocyte Biol 68: 909–915 [PubMed] [Google Scholar]
- Lee JY, Lowell CA, Lemay DG, Youn HS, Rhee SH, Sohn KH, Jang B, Ye J, Chung JH, Hwang DH (2005) The regulation of the expression of inducible nitric oxide synthase by Src-family tyrosine kinases mediated through MyD88-independent signaling pathways of Toll-like receptor 4. Biochem Pharmacol 70: 1231–1240 [DOI] [PubMed] [Google Scholar]
- Lee KK, Murakawa M, Takahashi S, Tsubuki S, Kawashima S, Sakamaki K, Yonehara S (1998) Purification, molecular cloning, and characterization of TRP32, a novel thioredoxin-related mammalian protein of 32 kDa. J Biol Chem 273: 19160–19166 [DOI] [PubMed] [Google Scholar]
- Leitges M, Sanz L, Martin P, Duran A, Braun U, Garcia JF, Camacho F, Diaz-Meco MT, Rennert PD, Moscat J (2001) Targeted disruption of the zetaPKC gene results in the impairment of the NF-kappaB pathway. Mol Cell 8: 771–780 [DOI] [PubMed] [Google Scholar]
- Lenormand P, Sardet C, Pages G, L'Allemain G, Brunet A, Pouyssegur J (1993) Growth factors induce nuclear translocation of MAP kinases (p42mapk and p44mapk) but not of their activator MAP kinase kinase (p45mapkk) in fibroblasts. J Cell Biol 122: 1079–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Bin LH, Li F, Liu Y, Chen D, Zhai Z, Shu HB (2005) TRIP6 is a RIP2-associated common signaling component of multiple NF-kappaB activation pathways. J Cell Sci 118: 555–563 [DOI] [PubMed] [Google Scholar]
- Li S, Strelow A, Fontana EJ, Wesche H (2002) IRAK-4: a novel member of the IRAK family with the properties of an IRAK-kinase. Proc Natl Acad Sci USA 99: 5567–5572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Hu J, Li L (2004) Characterization of Tollip protein upon lipopolysaccharide challenge. Mol Immunol 41: 85–92 [DOI] [PubMed] [Google Scholar]
- Liew FY, Liu H, Xu D (2005a) A novel negative regulator for IL-1 receptor and Toll-like receptor 4. Immunol Lett 96: 27–31 [DOI] [PubMed] [Google Scholar]
- Liew FY, Xu D, Brint EK, O'Neill LA (2005b) Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol 5: 446–458 [DOI] [PubMed] [Google Scholar]
- Limatola C, Schaap D, Moolenaar WH, van Blitterswijk WJ (1994) Phosphatidic acid activation of protein kinase C-zeta overexpressed in COS cells: comparison with other protein kinase C isotypes and other acidic lipids. Biochem J 304 (Part 3): 1001–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, DeMartino GN, Greene WC (1998) Cotranslational biogenesis of NF-kappaB p50 by the 26S proteasome. Cell 92: 819–828 [DOI] [PubMed] [Google Scholar]
- Lin R, Mamane Y, Hiscott J (1999a) Structural and functional analysis of interferon regulatory factor 3: localization of the transactivation and autoinhibitory domains. Mol Cell Biol 19: 2465–2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Cunningham ET Jr, Mu Y, Geleziunas R, Greene WC (1999b) The proto-oncogene Cot kinase participates in CD3/CD28 induction of NF-kappaB acting through the NF-kappaB-inducing kinase and IkappaB kinases. Immunity 10: 271–280 [DOI] [PubMed] [Google Scholar]
- Lippe R, Miaczynska M, Rybin V, Runge A, Zerial M (2001) Functional synergy between Rab5 effector Rabaptin-5 and exchange factor Rabex-5 when physically associated in a complex. Mol Biol Cell 12: 2219–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscovitch M, Ben-Av P, Danin M, Faiman G, Eldar H, Livneh E (1993) Phospholipase D-mediated hydrolysis of phosphatidylcholine: role in cell signalling. J Lipid Mediat 8: 177–182 [PubMed] [Google Scholar]
- Liu W, Quinto I, Chen X, Palmieri C, Rabin RL, Schwartz OM, Nelson DL, Scala G (2001) Direct inhibition of Bruton's tyrosine kinase by IBtk, a Btk-binding protein. Nat Immunol 2: 939–946 [DOI] [PubMed] [Google Scholar]
- Lu Y, Wahl LM (2005) Production of matrix metalloproteinase-9 by activated human monocytes involves a phosphatidylinositol-3 kinase/Akt/IKKalpha/NF-kappaB pathway. J Leukocyte Biol 78: 259–265 [DOI] [PubMed] [Google Scholar]
- Luciano BS, Hsu S, Channavajhala PL, Lin LL, Cuozzo JW (2004) Phosphorylation of threonine 290 in the activation loop of Tpl2/Cot is necessary but not sufficient for kinase activity. J Biol Chem 279: 52117–52123 [DOI] [PubMed] [Google Scholar]
- MacKichan ML, Logeat F, Israel A (1996) Phosphorylation of p105 PEST sequence via a redox-insensitive pathway up-regulates processing of p50 NF-kappaB. J Biol Chem 271: 6084–6091 [DOI] [PubMed] [Google Scholar]
- Malcolm KC, Ross AH, Qiu RG, Symons M, Exton JH (1994) Activation of rat liver phospholipase D by the small GTP-binding protein RhoA. J Biol Chem 269: 25951–25954 [PubMed] [Google Scholar]
- Malek S, Chen Y, Huxford T, Ghosh G (2001) IkappaBbeta, but not IkappaBalpha, functions as a classical cytoplasmic inhibitor of NF-kappaB dimers by masking both NF-kappaB nuclear localization sequences in resting cells. J Biol Chem 276: 45225–45235 [DOI] [PubMed] [Google Scholar]
- Marais R, Wynne J, Treisman R (1993) The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell 73: 381–393 [DOI] [PubMed] [Google Scholar]
- Marie I, Durbin JE, Levy DE (1998) Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J 17: 6660–6669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmiroli S, Bavelloni A, Faenza I, Sirri A, Ognibene A, Cenni V, Tsukada J, Koyama Y, Ruzzene M, Ferri A, Auron PE, Toker A, Maraldi NM (1998) Phosphatidylinositol 3-kinase is recruited to a specific site in the activated IL-1 receptor I. FEBS Lett 438: 49–54 [DOI] [PubMed] [Google Scholar]
- Martin M, Rehani K, Jope RS, Michalek SM (2005) Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol 6: 777–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massari P, Henneke P, Ho Y, Latz E, Golenbock DT, Wetzler LM (2002) Cutting edge: immune stimulation by neisserial porins is toll-like receptor 2 and MyD88 dependent. J Immunol 168: 1533–1537 [DOI] [PubMed] [Google Scholar]
- Matsuzawa A, Saegusa K, Noguchi T, Sadamitsu C, Nishitoh H, Nagai S, Koyasu S, Matsumoto K, Takeda K, Ichijo H (2005) ROS-dependent activation of the TRAF6–ASK1–p38 pathway is selectively required for TLR4-mediated innate immunity. Nat Immunol 6: 587–592 [DOI] [PubMed] [Google Scholar]
- McBride HM, Rybin V, Murphy C, Giner A, Teasdale R, Zerial M (1999) Oligomeric complexes link Rab5 effectors with NSF and drive membrane fusion via interactions between EEA1 and syntaxin 13. Cell 98: 377–386 [DOI] [PubMed] [Google Scholar]
- McCoy CE, Campbell DG, Deak M, Bloomberg GB, Arthur JS (2005) MSK1 activity is controlled by multiple phosphorylation sites. Biochem J 387: 507–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay LI, Cidlowski JA (1999) Molecular control of immune/inflammatory responses: interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr Rev 20: 435–459 [DOI] [PubMed] [Google Scholar]
- McWhirter SM, Fitzgerald KA, Rosains J, Rowe DC, Golenbock DT, Maniatis T (2004) IFN-regulatory factor 3-dependent gene expression is defective in Tbk1-deficient mouse embryonic fibroblasts. Proc Natl Acad Sci USA 101: 233–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means TK, Wang S, Lien E, Yoshimura A, Golenbock DT, Fenton MJ (1999) Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol 163: 3920–3927 [PubMed] [Google Scholar]
- Meng F, Lowell CA (1997) Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of Src-family kinases Hck, Fgr, and Lyn. J Exp Med 185: 1661–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng W, Swenson LL, Fitzgibbon MJ, Hayakawa K, Ter Haar E, Behrens AE, Fulghum JR, Lippke JA (2002) Structure of mitogen-activated protein kinase-activated protein (MAPKAP) kinase 2 suggests a bifunctional switch that couples kinase activation with nuclear export. J Biol Chem 277: 37401–37405 [DOI] [PubMed] [Google Scholar]
- Meyer TE, Habener JF (1993) Cyclic adenosine 3′,5′-monophosphate response element binding protein (CREB) and related transcription-activating deoxyribonucleic acid-binding proteins. Endocr Rev 14: 269–290 [DOI] [PubMed] [Google Scholar]
- Meylan E, Burns K, Hofmann K, Blancheteau V, Martinon F, Kelliher M, Tschopp J (2004) RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol 5: 503–507 [DOI] [PubMed] [Google Scholar]
- Miranda-Vizuete A, Gustafsson JA, Spyrou G (1998) Molecular cloning and expression of a cDNA encoding a human thioredoxin-like protein. Biochem Biophys Res Commun 243: 284–288 [DOI] [PubMed] [Google Scholar]
- Mizukami Y, Yoshioka K, Morimoto S, Yoshida K (1997) A novel mechanism of JNK1 activation. Nuclear translocation and activation of JNK1 during ischemia and reperfusion. J Biol Chem 272: 16657–16662 [DOI] [PubMed] [Google Scholar]
- Mori M, Yoneyama M, Ito T, Takahashi K, Inagaki F, Fujita T (2004) Identification of Ser-386 of interferon regulatory factor 3 as critical target for inducible phosphorylation that determines activation. J Biol Chem 279: 9698–9702 [DOI] [PubMed] [Google Scholar]
- Moritz A, De Graan PN, Gispen WH, Wirtz KW (1992) Phosphatidic acid is a specific activator of phosphatidylinositol-4-phosphate kinase. J Biol Chem 267: 7207–7210 [PubMed] [Google Scholar]
- Mosley B, Urdal DL, Prickett KS, Larsen A, Cosman D, Conlon PJ, Gillis S, Dower SK (1987) The interleukin-1 receptor binds the human interleukin-1 alpha precursor but not the interleukin-1 beta precursor. J Biol Chem 262: 2941–2944 [PubMed] [Google Scholar]
- Mullen GE, Kennedy MN, Visintin A, Mazzoni A, Leifer CA, Davies DR, Segal DM (2003) The role of disulfide bonds in the assembly and function of MD-2. Proc Natl Acad Sci USA 100: 3919–3924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller CW, Harrison SC (1995) The structure of the NF-kappa B p50:DNA-complex: a starting point for analyzing the Rel family. FEBS Lett 369: 113–117 [DOI] [PubMed] [Google Scholar]
- Muller J, Ory S, Copeland T, Piwnica-Worms H, Morrison DK (2001) C-TAK1 regulates Ras signaling by phosphorylating the MAPK scaffold, KSR1. Mol Cell 8: 983–993 [DOI] [PubMed] [Google Scholar]
- Murray JT, Panaretou C, Stenmark H, Miaczynska M, Backer JM (2002) Role of Rab5 in the recruitment of hVps34/p150 to the early endosome. Traffic 3: 416–427 [DOI] [PubMed] [Google Scholar]
- Muta T, Takeshige K (2001) Essential roles of CD14 and lipopolysaccharide-binding protein for activation of toll-like receptor (TLR)2 as well as TLR4 reconstitution of TLR2- and TLR4-activation by distinguishable ligands in LPS preparations. Eur J Biochem 268: 4580–4589 [DOI] [PubMed] [Google Scholar]
- Muzio M, Stockwell BR, Stennicke HR, Salvesen GS, Dixit VM (1998) An induced proximity model for caspase-8 activation. J Biol Chem 273: 2926–2930 [DOI] [PubMed] [Google Scholar]
- Nakano H, Shindo M, Sakon S, Nishinaka S, Mihara M, Yagita H, Okumura K (1998) Differential regulation of IkappaB kinase alpha and beta by two upstream kinases, NF-kappaB-inducing kinase and mitogen-activated protein kinase/ERK kinase kinase-1. Proc Natl Acad Sci USA 95: 3537–3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K, Hatakeyama S, Maruyama S, Kikuchi A, Onoe K, Good RA, Nakayama KI (2003) Impaired degradation of inhibitory subunit of NF-kappa B (I kappa B) and beta-catenin as a result of targeted disruption of the beta-TrCP1 gene. Proc Natl Acad Sci USA 100: 8752–8757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitani G, Bortoletto N, Racioppi L, Lanzavecchia A, D'Oro U (2003) Activation of src-family tyrosine kinases by LPS regulates cytokine production in dendritic cells by controlling AP-1 formation. Eur J Immunol 33: 2832–2841 [DOI] [PubMed] [Google Scholar]
- Naumann M, Wulczyn FG, Scheidereit C (1993) The NF-kappa B precursor p105 and the proto-oncogene product Bcl-3 are I kappa B molecules and control nuclear translocation of NF-kappa B. EMBO J 12: 213–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisimoto Y, Freeman JL, Motalebi SA, Hirshberg M, Lambeth JD (1997) Rac binding to p67(phox). Structural basis for interactions of the Rac1 effector region and insert region with components of the respiratory burst oxidase. J Biol Chem 272: 18834–18841 [DOI] [PubMed] [Google Scholar]
- Nordberg J, Arner ES (2001) Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med 31: 1287–1312 [DOI] [PubMed] [Google Scholar]
- Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G (2001) Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem 276: 4812–4818 [DOI] [PubMed] [Google Scholar]
- Ohashi K, Burkart V, Flohe S, Kolb H (2000) Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol 164: 558–561 [DOI] [PubMed] [Google Scholar]
- Ojaniemi M, Glumoff V, Harju K, Liljeroos M, Vuori K, Hallman M (2003) Phosphatidylinositol 3-kinase is involved in Toll-like receptor 4-mediated cytokine expression in mouse macrophages. Eur J Immunol 33: 597–605 [DOI] [PubMed] [Google Scholar]
- Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, Chow JC, Strauss JF III (2001) The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem 276: 10229–10233 [DOI] [PubMed] [Google Scholar]
- Opitz B, Schroder NW, Spreitzer I, Michelsen KS, Kirschning CJ, Hallatschek W, Zahringer U, Hartung T, Gobel UB, Schumann RR (2001) Toll-like receptor-2 mediates Treponema glycolipid and lipoteichoic acid-induced NF-kappaB translocation. J Biol Chem 276: 22041–22047 [DOI] [PubMed] [Google Scholar]
- Orian A, Gonen H, Bercovich B, Fajerman I, Eytan E, Israel A, Mercurio F, Iwai K, Schwartz AL, Ciechanover A (2000) SCF(beta) (−TrCP) ubiquitin ligase-mediated processing of NF-kappaB p105 requires phosphorylation of its C-terminus by IkappaB kinase. EMBO J 19: 2580–2591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlicek SL, Hanke JH, English BK (1999) The src family-selective tyrosine kinase inhibitor PP1 blocks LPS and IFN-gamma-mediated TNF and iNOS production in murine macrophages. Shock 12: 350–354 [DOI] [PubMed] [Google Scholar]
- Ory S, Zhou M, Conrads TP, Veenstra TD, Morrison DK (2003) Protein phosphatase 2A positively regulates Ras signaling by dephosphorylating KSR1 and Raf-1 on critical 14-3-3 binding sites. Curr Biol 13: 1356–1364 [DOI] [PubMed] [Google Scholar]
- Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T (2003a) TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat Immunol 4: 161–167 [DOI] [PubMed] [Google Scholar]
- Oshiumi H, Sasai M, Shida K, Fujita T, Matsumoto M, Seya T (2003b) TIR-containing adapter molecule (TICAM)-2, a bridging adapter recruiting to toll-like receptor 4 TICAM-1 that induces interferon-beta. J Biol Chem 278: 49751–49762 [DOI] [PubMed] [Google Scholar]
- Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB (1999) NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature 401: 82–85 [DOI] [PubMed] [Google Scholar]
- Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A (2000) The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA 97: 13766–13771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombella VJ, Rando OJ, Goldberg AL, Maniatis T (1994) The ubiquitin–proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell 78: 773–785 [DOI] [PubMed] [Google Scholar]
- Pan ZK (2004) Toll-like receptors and TLR-mediated signaling: more questions than answers. Am J Physiol Lung Cell Mol Physiol 286: L918–L920 [DOI] [PubMed] [Google Scholar]
- Pathak SK, Bhattacharyya A, Pathak S, Basak C, Mandal D, Kundu M, Basu J (2004) Toll-like receptor 2 and mitogen- and stress-activated kinase 1 are effectors of Mycobacterium avium-induced cyclooxygenase-2 expression in macrophages. J Biol Chem 279: 55127–55136 [DOI] [PubMed] [Google Scholar]
- Patterson RL, Boehning D, Snyder SH (2004) Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu Rev Biochem 73: 437–465 [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B (1998) Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282: 2085–2088 [DOI] [PubMed] [Google Scholar]
- Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ (1995) Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem 270: 7420–7426 [DOI] [PubMed] [Google Scholar]
- Raingeaud J, Whitmarsh AJ, Barrett T, Derijard B, Davis RJ (1996) MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol 16: 1247–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao N, Nguyen S, Ngo K, Fung-Leung WP (2005) A novel splice variant of interleukin-1 receptor (IL-1R)-associated kinase 1 plays a negative regulatory role in toll/IL-1R-induced inflammatory signaling. Mol Cell Biol 25: 6521–6532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassa JC, Meyers JL, Zhang Y, Kudaravalli R, Ross SR (2002) Murine retroviruses activate B cells via interaction with toll-like receptor 4. Proc Natl Acad Sci USA 99: 2281–2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re F, Strominger JL (2002) Monomeric recombinant MD-2 binds toll-like receptor 4 tightly and confers lipopolysaccharide responsiveness. J Biol Chem 277: 23427–23432 [DOI] [PubMed] [Google Scholar]
- Reddy SA, Lin YF, Huang HJ, Samanta AK, Liao WS (2004) The IL-1 receptor accessory protein is essential for PI 3-kinase recruitment and activation. Biochem Biophys Res Commun 316: 1022–1028 [DOI] [PubMed] [Google Scholar]
- Ren XD, Bokoch GM, Traynor-Kaplan A, Jenkins GH, Anderson RA, Schwartz MA (1996) Physical association of the small GTPase Rho with a 68-kDa phosphatidylinositol 4-phosphate 5-kinase in Swiss 3T3 cells. Mol Biol Cell 7: 435–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusch HP, Zimmermann S, Schaefer M, Paul M, Moelling K (2001) Regulation of Raf by Akt controls growth and differentiation in vascular smooth muscle cells. J Biol Chem 276: 33630–33637 [DOI] [PubMed] [Google Scholar]
- Rhee SG, Bae YS (1997) Regulation of phosphoinositide-specific phospholipase C isozymes. J Biol Chem 272: 15045–15048 [DOI] [PubMed] [Google Scholar]
- Romano PR, Garcia-Barrio MT, Zhang X, Wang Q, Taylor DR, Zhang F, Herring C, Mathews MB, Qin J, Hinnebusch AG (1998) Autophosphorylation in the activation loop is required for full kinase activity in vivo of human and yeast eukaryotic initiation factor 2alpha kinases PKR and GCN2. Mol Cell Biol 18: 2282–2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckdeschel K, Mannel O, Schrottner P (2002) Divergence of apoptosis-inducing and preventing signals in bacteria-faced macrophages through myeloid differentiation factor 88 and IL-1 receptor-associated kinase members. J Immunol 168: 4601–4611 [DOI] [PubMed] [Google Scholar]
- Ryo A, Suizu F, Yoshida Y, Perrem K, Liou YC, Wulf G, Rottapel R, Yamaoka S, Lu KP (2003) Regulation of NF-kappaB signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA. Mol Cell 12: 1413–1426 [DOI] [PubMed] [Google Scholar]
- Saccani S, Marazzi I, Beg AA, Natoli G (2004) Degradation of promoter-bound p65/RelA is essential for the prompt termination of the nuclear factor kappaB response. J Exp Med 200: 107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H (1998) Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J 17: 2596–2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh T, Yamamoto M, Miyagishi M, Taira K, Nakanishi M, Fujita T, Akira S, Yamamoto N, Yamaoka S (2005) A20 is a negative regulator of IFN regulatory factor 3 signaling. J Immunol 174: 1507–1512 [DOI] [PubMed] [Google Scholar]
- Sakurai H, Miyoshi H, Toriumi W, Sugita T (1999) Functional interactions of transforming growth factor beta-activated kinase 1 with IkappaB kinases to stimulate NF-kappaB activation. J Biol Chem 274: 10641–10648 [DOI] [PubMed] [Google Scholar]
- Salmeron A, Janzen J, Soneji Y, Bump N, Kamens J, Allen H, Ley SC (2001) Direct phosphorylation of NF-kappaB1 p105 by the IkappaB kinase complex on serine 927 is essential for signal-induced p105 proteolysis. J Biol Chem 276: 22215–22222 [DOI] [PubMed] [Google Scholar]
- Sanchez P, De Carcer G, Sandoval IV, Moscat J, Diaz-Meco MT (1998) Localization of atypical protein kinase C isoforms into lysosome-targeted endosomes through interaction with p62. Mol Cell Biol 18: 3069–3080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz L, Diaz-Meco MT, Nakano H, Moscat J (2000) The atypical PKC-interacting protein p62 channels NF-kappaB activation by the IL-1-TRAF6 pathway. EMBO J 19: 1576–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar SN, Peters KL, Elco CP, Sakamoto S, Pal S, Sen GC (2004) Novel roles of TLR3 tyrosine phosphorylation and PI3 kinase in double-stranded RNA signaling. Nat Struct Mol Biol 11: 1060–1067 [DOI] [PubMed] [Google Scholar]
- Sato S, Sugiyama M, Yamamoto M, Watanabe Y, Kawai T, Takeda K, Akira S (2003) Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-kappa B and IFN-regulatory factor-3, in the Toll-like receptor signaling. J Immunol 171: 4304–4310 [DOI] [PubMed] [Google Scholar]
- Satoh S, Hijikata M, Handa H, Shimotohno K (1999) Caspase-mediated cleavage of eukaryotic translation initiation factor subunit 2alpha. Biochem J 342 (Part 1): 65–70 [PMC free article] [PubMed] [Google Scholar]
- Scheid MP, Woodgett JR (2003) Unravelling the activation mechanisms of protein kinase B/Akt. FEBS Lett 546: 108–112 [DOI] [PubMed] [Google Scholar]
- Scherer DC, Brockman JA, Chen Z, Maniatis T, Ballard DW (1995) Signal-induced degradation of I kappa B alpha requires site-specific ubiquitination. Proc Natl Acad Sci USA 92: 11259–11263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe RF, Brenner DA (2002) Role of glycogen synthase kinase-3 in TNF-alpha-induced NF-kappaB activation and apoptosis in hepatocytes. Am J Physiol Gastrointest Liver Physiol 283: G204–G211 [DOI] [PubMed] [Google Scholar]
- Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ (1999) Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem 274: 17406–17409 [DOI] [PubMed] [Google Scholar]
- Seth A, Alvarez E, Gupta S, Davis RJ (1991) A phosphorylation site located in the NH2-terminal domain of c-Myc increases transactivation of gene expression. J Biol Chem 266: 23521–23524 [PubMed] [Google Scholar]
- Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J (2003) Triggering the interferon antiviral response through an IKK-related pathway. Science 300: 1148–1151 [DOI] [PubMed] [Google Scholar]
- Sheng M, Thompson MA, Greenberg ME (1991) CREB: a Ca(2+)-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science 252: 1427–1430 [DOI] [PubMed] [Google Scholar]
- Shibuya H, Yamaguchi K, Shirakabe K, Tonegawa A, Gotoh Y, Ueno N, Irie K, Nishida E, Matsumoto K (1996) TAB1: an activator of the TAK1 MAPKKK in TGF-beta signal transduction. Science 272: 1179–1182 [DOI] [PubMed] [Google Scholar]
- Shimokawa N, Qiu CH, Seki T, Dikic I, Koibuchi N (2004) Phosphorylation of JNK is involved in regulation of H(+)-induced c-Jun expression. Cell Signal 16: 723–729 [DOI] [PubMed] [Google Scholar]
- Shuto T, Xu H, Wang B, Han J, Kai H, Gu XX, Murphy TF, Lim DJ, Li JD (2001) Activation of NF-kappa B by nontypeable Hemophilus influenzae is mediated by toll-like receptor 2-TAK1-dependent NIK-IKK alpha/beta-I kappa B alpha and MKK3/6-p38 MAP kinase signaling pathways in epithelial cells. Proc Natl Acad Sci USA 98: 8774–8779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer WD, Brown HA, Jiang X, Sternweis PC (1996) Regulation of phospholipase D by protein kinase C is synergistic with ADP-ribosylation factor and independent of protein kinase activity. J Biol Chem 271: 4504–4510 [DOI] [PubMed] [Google Scholar]
- Singh R, Wang B, Shirvaikar A, Khan S, Kamat S, Schelling JR, Konieczkowski M, Sedor JR (1999) The IL-1 receptor and Rho directly associate to drive cell activation in inflammation. J Clin Invest 103: 1561–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhirunnusorn P, Suzuki S, Kawasaki N, Saiki I, Sakurai H (2005) Critical roles of threonine 187 phosphorylation in cellular stress-induced rapid and transient activation of transforming growth factor-beta-activated kinase 1 (TAK1) in a signaling complex containing TAK1-binding protein TAB1 and TAB2. J Biol Chem 280: 7359–7368 [DOI] [PubMed] [Google Scholar]
- Smiley ST, King JA, Hancock WW (2001) Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol 167: 2887–2894 [DOI] [PubMed] [Google Scholar]
- Smith MF Jr, Mitchell A, Li G, Ding S, Fitzmaurice AM, Ryan K, Crowe S, Goldberg JB (2003) Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for Helicobacter pylori-induced NF-kappa B activation and chemokine expression by epithelial cells. J Biol Chem 278: 32552–32560 [DOI] [PubMed] [Google Scholar]
- Sohn HW, Gu H, Pierce SK (2003) Cbl-b negatively regulates B cell antigen receptor signaling in mature B cells through ubiquitination of the tyrosine kinase Syk. J Exp Med 197: 1511–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer E, Jiang J, Chen ZJ (1999) Signal-induced ubiquitination of IkappaBalpha by the F-box protein Slimb/beta-TrCP. Genes Dev 13: 284–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standaert ML, Bandyopadhyay G, Kanoh Y, Sajan MP, Farese RV (2001) Insulin and PIP3 activate PKC-zeta by mechanisms that are both dependent and independent of phosphorylation of activation loop (T410) and autophosphorylation (T560) sites. Biochemistry 40: 249–255 [DOI] [PubMed] [Google Scholar]
- Stefanova I, Corcoran ML, Horak EM, Wahl LM, Bolen JB, Horak ID (1993) Lipopolysaccharide induces activation of CD14-associated protein tyrosine kinase p53/56lyn. J Biol Chem 268: 20725–20728 [PubMed] [Google Scholar]
- Stephens LR, Jackson TR, Hawkins PT (1993) Agonist-stimulated synthesis of phosphatidylinositol(3,4,5)-trisphosphate: a new intracellular signalling system? Biochim Biophys Acta 1179: 27–75 [DOI] [PubMed] [Google Scholar]
- Stokoe D, Engel K, Campbell DG, Cohen P, Gaestel M (1992) Identification of MAPKAP kinase 2 as a major enzyme responsible for the phosphorylation of the small mammalian heat shock proteins. FEBS Lett 313: 307–313 [DOI] [PubMed] [Google Scholar]
- Stovall SH, Yi AK, Meals EA, Talati AJ, Godambe SA, English BK (2004) Role of vav1- and src-related tyrosine kinases in macrophage activation by CpG DNA. J Biol Chem 279: 13809–13816 [DOI] [PubMed] [Google Scholar]
- Stylianou E, Saklatvala J (1998) Interleukin-1. Int J Biochem Cell Biol 30: 1075–1079 [DOI] [PubMed] [Google Scholar]
- Su W, Chardin P, Yamazaki M, Kanaho Y, Du G (2006) RhoA-mediated phospholipase D1 signaling is not required for the formation of stress fibers and focal adhesions. Cell Signal 18: 469–478 [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Fujita M, Koide N, Mori I, Yoshida T, Mori H, Yokochi T (2004) 2-aminopurine inhibits lipopolysaccharide-induced nitric oxide production by preventing IFN-beta production. Microbiol Immunol 48: 957–963 [DOI] [PubMed] [Google Scholar]
- Sun H, Charles CH, Lau LF, Tonks NK (1993) MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell 75: 487–493 [DOI] [PubMed] [Google Scholar]
- Suyang H, Phillips R, Douglas I, Ghosh S (1996) Role of unphosphorylated, newly synthesized I kappa B beta in persistent activation of NF-kappa B. Mol Cell Biol 16: 5444–5449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Chiba T, Kobayashi M, Takeuchi M, Suzuki T, Ichiyama A, Ikenoue T, Omata M, Furuichi K, Tanaka K (1999) IkappaBalpha ubiquitination is catalyzed by an SCF-like complex containing Skp1, cullin-1, and two F-box/WD40-repeat proteins, betaTrCP1 and betaTrCP2. Biochem Biophys Res Commun 256: 127–132 [DOI] [PubMed] [Google Scholar]
- Sweet MJ, Leung BP, Kang D, Sogaard M, Schulz K, Trajkovic V, Campbell CC, Xu D, Liew FY (2001) A novel pathway regulating lipopolysaccharide-induced shock by ST2/T1 via inhibition of Toll-like receptor 4 expression. J Immunol 166: 6633–6639 [DOI] [PubMed] [Google Scholar]
- Takaesu G, Kishida S, Hiyama A, Yamaguchi K, Shibuya H, Irie K, Ninomiya-Tsuji J, Matsumoto K (2000) TAB2, a novel adaptor protein, mediates activation of TAK1 MAPKKK by linking TAK1 to TRAF6 in the IL-1 signal transduction pathway. Mol Cell 5: 649–658 [DOI] [PubMed] [Google Scholar]
- Takatsuna H, Kato H, Gohda J, Akiyama T, Moriya A, Okamoto Y, Yamagata Y, Otsuka M, Umezawa K, Semba K, Inoue J (2003) Identification of TIFA as an adapter protein that links tumor necrosis factor receptor-associated factor 6 (TRAF6) to interleukin-1 (IL-1) receptor-associated kinase-1 (IRAK-1) in IL-1 receptor signaling. J Biol Chem 278: 12144–12150 [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S (2005) Toll-like receptors in innate immunity. Int Immunol 17: 1–14 [DOI] [PubMed] [Google Scholar]
- Takeshita F, Gursel I, Ishii KJ, Suzuki K, Gursel M, Klinman DM (2004) Signal transduction pathways mediated by the interaction of CpG DNA with Toll-like receptor 9. Semin Immunol 16: 17–22 [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S (1999) Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity 11: 443–451 [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Kawai T, Muhlradt PF, Morr M, Radolf JD, Zychlinsky A, Takeda K, Akira S (2001) Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int Immunol 13: 933–940 [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, Modlin RL, Akira S (2002) Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol 169: 10–14 [DOI] [PubMed] [Google Scholar]
- Tan Y, Rouse J, Zhang A, Cariati S, Cohen P, Comb MJ (1996) FGF and stress regulate CREB and ATF-1 via a pathway involving p38 MAP kinase and MAPKAP kinase-2. EMBO J 15: 4629–4642 [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T, Ogasawara K, Takaoka A, Tanaka N (2001) IRF family of transcription factors as regulators of host defense. Annu Rev Immunol 19: 623–655 [DOI] [PubMed] [Google Scholar]
- Tegethoff S, Behlke J, Scheidereit C (2003) Tetrameric oligomerization of IkappaB kinase gamma (IKKgamma) is obligatory for IKK complex activity and NF-kappaB activation. Mol Cell Biol 23: 2029–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC (2002) Oligosaccharides of hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med 195: 99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teusch N, Lombardo E, Eddleston J, Knaus UG (2004) The low molecular weight GTPase RhoA and atypical protein kinase Czeta are required for TLR2-mediated gene transcription. J Immunol 173: 507–514 [DOI] [PubMed] [Google Scholar]
- Thomson S, Clayton AL, Hazzalin CA, Rose S, Barratt MJ, Mahadevan LC (1999) The nucleosomal response associated with immediate-early gene induction is mediated via alternative MAP kinase cascades: MSK1 as a potential histone H3/HMG-14 kinase. EMBO J 18: 4779–4793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J, Elliston KO, Ayala JM, Casano FJ, Chin J, Ding GJF, Egger LA, Gaffney EP, Limjuco G, Palyha OC, Raju SM, Rolando AM, Salley JP, Yamin TT, Lee TD, Shively JE, MacCross M, Mumford RA, Schmidt JA, Tocci MJ (1992) A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 356: 768–774 [DOI] [PubMed] [Google Scholar]
- Tian J, Karin M (1999) Stimulation of Elk1 transcriptional activity by mitogen-activated protein kinases is negatively regulated by protein phosphatase 2B (calcineurin). J Biol Chem 274: 15173–15180 [DOI] [PubMed] [Google Scholar]
- Ting JP, Davis BK (2005) CATERPILLER: a novel gene family important in immunity, cell death, and diseases. Annu Rev Immunol 23: 387–414 [DOI] [PubMed] [Google Scholar]
- Tobiume K, Saitoh M, Ichijo H (2002) Activation of apoptosis signal-regulating kinase 1 by the stress-induced activating phosphorylation of pre-formed oligomer. J Cell Physiol 191: 95–104 [DOI] [PubMed] [Google Scholar]
- Tolias KF, Cantley LC (1999) Pathways for phosphoinositide synthesis. Chem Phys Lipids 98: 69–77 [DOI] [PubMed] [Google Scholar]
- Tolias KF, Cantley LC, Carpenter CL (1995) Rho family GTPases bind to phosphoinositide kinases. J Biol Chem 270: 17656–17659 [DOI] [PubMed] [Google Scholar]
- Tolias KF, Couvillon AD, Cantley LC, Carpenter CL (1998) Characterization of a Rac1- and RhoGDI-associated lipid kinase signaling complex. Mol Cell Biol 18: 762–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topham MK, Prescott SM (1999) Mammalian diacylglycerol kinases, a family of lipid kinases with signaling functions. J Biol Chem 274: 11447–11450 [DOI] [PubMed] [Google Scholar]
- Torok AM, Bouton AH, Goldberg JB (2005) Helicobacter pylori induces interleukin-8 secretion by Toll-like receptor 2- and Toll-like receptor 5-dependent and -independent pathways. Infect Immun 73: 1523–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku T, Curotto Kurzydlowski K, Narayanan N, MacLennan DH (1994) Identification of Ser38 as the site in cardiac sarcoplasmic reticulum Ca(2+)-ATPase that is phosphorylated by Ca2+/calmodulin-dependent protein kinase. J Biol Chem 269: 26492–26496 [PubMed] [Google Scholar]
- Tran K, Merika M, Thanos D (1997) Distinct functional properties of IkappaB alpha and IkappaB beta. Mol Cell Biol 17: 5386–5399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukel C, Raffatellu M, Humphries AD, Wilson RP, Andrews-Polymenis HL, Gull T, Figueiredo JF, Wong MH, Michelsen KS, Akcelik M, Adams LG, Baumler AJ (2005) CsgA is a pathogen-associated molecular pattern of Salmonella enterica serotype Typhimurium that is recognized by Toll-like receptor 2. Mol Microbiol 58: 289–304 [DOI] [PubMed] [Google Scholar]
- Ueno Y, Kume N, Miyamoto S, Morimoto M, Kataoka H, Ochi H, Nishi E, Moriwaki H, Minami M, Hashimoto N, Kita T (1999) Lysophosphatidylcholine phosphorylates CREB and activates the jun2TRE site of c-jun promoter in vascular endothelial cells. FEBS Lett 457: 241–245 [DOI] [PubMed] [Google Scholar]
- Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A (1999) The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401: 811–815 [DOI] [PubMed] [Google Scholar]
- Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H (2002) HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem 277: 15107–15112 [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD (2001) Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem 70: 535–602 [DOI] [PubMed] [Google Scholar]
- Verma IM, Sassone-Corsi P (1987) Proto-oncogene fos: complex but versatile regulation. Cell 51: 513–514 [DOI] [PubMed] [Google Scholar]
- Vermeulen L, De Wilde G, Van Damme P, Vanden Berghe W, Haegeman G (2003) Transcriptional activation of the NF-kappaB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1). EMBO J 22: 1313–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vihinen M, Mattsson PT, Smith CI (1997) BTK, the tyrosine kinase affected in X-linked agammaglobulinemia. Front Biosci 2: d27–d42 [DOI] [PubMed] [Google Scholar]
- Visintin A, Latz E, Monks BG, Espevik T, Golenbock DT (2003) Lysines 128 and 132 enable lipopolysaccharide binding to MD-2, leading to Toll-like receptor-4 aggregation and signal transduction. J Biol Chem 278: 48313–48320 [DOI] [PubMed] [Google Scholar]
- Vora P, Youdim A, Thomas LS, Fukata M, Tesfay SY, Lukasek K, Michelsen KS, Wada A, Hirayama T, Arditi M, Abreu MT (2004) Beta-defensin-2 expression is regulated by TLR signaling in intestinal epithelial cells. J Immunol 173: 5398–5405 [DOI] [PubMed] [Google Scholar]
- Wald D, Qin J, Zhao Z, Qian Y, Naramura M, Tian L, Towne J, Sims JE, Stark GR, Li X (2003) SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol 4: 920–927 [DOI] [PubMed] [Google Scholar]
- Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ (2001) TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412: 346–351 [DOI] [PubMed] [Google Scholar]
- Wang D, Westerheide SD, Hanson JL, Baldwin AS Jr (2000) Tumor necrosis factor alpha-induced phosphorylation of RelA/p65 on Ser529 is controlled by casein kinase II. J Biol Chem 275: 32592–32597 [DOI] [PubMed] [Google Scholar]
- Waskiewicz AJ, Flynn A, Proud CG, Cooper JA (1997) Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J 16: 1909–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Iwamura T, Shinoda T, Fujita T (1997) Regulation of NFKB1 proteins by the candidate oncoprotein BCL-3: generation of NF-kappaB homodimers from the cytoplasmic pool of p50-p105 and nuclear translocation. EMBO J 16: 3609–3620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterfield M, Jin W, Reiley W, Zhang M, Sun SC (2004) IkappaB kinase is an essential component of the Tpl2 signaling pathway. Mol Cell Biol 24: 6040–6048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werts C, Tapping RI, Mathison JC, Chuang TH, Kravchenko V, Saint Girons I, Haake DA, Godowski PJ, Hayashi F, Ozinsky A, Underhill DM, Kirschning CJ, Wagner H, Aderem A, Tobias PS, Ulevitch RJ (2001) Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat Immunol 2: 346–352 [DOI] [PubMed] [Google Scholar]
- Wesche H, Gao X, Li X, Kirschning CJ, Stark GR, Cao Z (1999) IRAK-M is a novel member of the Pelle/interleukin-1 receptor-associated kinase (IRAK) family. J Biol Chem 274: 19403–19410 [DOI] [PubMed] [Google Scholar]
- Wesche H, Henzel WJ, Shillinglaw W, Li S, Cao Z (1997) MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity 7: 837–847 [DOI] [PubMed] [Google Scholar]
- Wick MJ, Ramos FJ, Chen H, Quon MJ, Dong LQ, Liu F (2003) Mouse 3-phosphoinositide-dependent protein kinase-1 undergoes dimerization and trans-phosphorylation in the activation loop. J Biol Chem 278: 42913–42919 [DOI] [PubMed] [Google Scholar]
- Winston JT, Strack P, Beer-Romero P, Chu CY, Elledge SJ, Harper JW (1999) The SCFbeta–TRCP–ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IkappaBalpha and beta-catenin and stimulates IkappaBalpha ubiquitination in vitro. Genes Dev 13: 270–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witowsky JA, Johnson GL (2003) Ubiquitylation of MEKK1 inhibits its phosphorylation of MKK1 and MKK4 and activation of the ERK1/2 and JNK pathways. J Biol Chem 278: 1403–1406 [DOI] [PubMed] [Google Scholar]
- Wu C, Ghosh S (1999) Beta-TrCP mediates the signal-induced ubiquitination of IkappaBbeta. J Biol Chem 274: 29591–29594 [DOI] [PubMed] [Google Scholar]
- Wu J, Harrison JK, Dent P, Lynch KR, Weber MJ, Sturgill TW (1993) Identification and characterization of a new mammalian mitogen-activated protein kinase kinase, MKK2. Mol Cell Biol 13: 4539–4548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, McMurray CT (2001) Calmodulin kinase II attenuation of gene transcription by preventing cAMP response element-binding protein (CREB) dimerization and binding of the CREB-binding protein. J Biol Chem 276: 1735–1741 [DOI] [PubMed] [Google Scholar]
- Wyllie DH, Kiss-Toth E, Visintin A, Smith SC, Boussouf S, Segal DM, Duff GW, Dower SK (2000) Evidence for an accessory protein function for Toll-like receptor 1 in anti-bacterial responses. J Immunol 165: 7125–7132 [DOI] [PubMed] [Google Scholar]
- Xu H, An H, Yu Y, Zhang M, Qi R, Cao X (2003) Ras participates in CpG oligodeoxynucleotide signaling through association with toll-like receptor 9 and promotion of interleukin-1 receptor-associated kinase/tumor necrosis factor receptor-associated factor 6 complex formation in macrophages. J Biol Chem 278: 36334–36340 [DOI] [PubMed] [Google Scholar]
- Xu S, Robbins D, Frost J, Dang A, Lange-Carter C, Cobb MH (1995) MEKK1 phosphorylates MEK1 and MEK2 but does not cause activation of mitogen-activated protein kinase. Proc Natl Acad Sci USA 92: 6808–6812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S (2003a) Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301: 640–643 [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Sanjo H, Uematsu S, Kaisho T, Hoshino K, Takeuchi O, Kobayashi M, Fujita T, Takeda K, Akira S (2002) Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature 420: 324–329 [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Uematsu S, Hoshino K, Kaisho T, Takeuchi O, Takeda K, Akira S (2003b) TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol 4: 1144–1150 [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Yamazaki S, Uematsu S, Sato S, Hemmi H, Hoshino K, Kaisho T, Kuwata H, Takeuchi O, Takeshige K, Saitoh T, Yamaoka S, Yamamoto N, Yamamoto S, Muta T, Takeda K, Akira S (2004) Regulation of Toll/IL-1-receptor-mediated gene expression by the inducible nuclear protein IkappaBzeta. Nature 430: 218–222 [DOI] [PubMed] [Google Scholar]
- Yang F, Tang E, Guan K, Wang CY (2003) IKK beta plays an essential role in the phosphorylation of RelA/p65 on serine 536 induced by lipopolysaccharide. J Immunol 170: 5630–5635 [DOI] [PubMed] [Google Scholar]
- Yang X, Chang HY, Baltimore D (1998) Autoproteolytic activation of pro-caspases by oligomerization. Mol Cell 1: 319–325 [DOI] [PubMed] [Google Scholar]
- Yaron A, Gonen H, Alkalay I, Hatzubai A, Jung S, Beyth S, Mercurio F, Manning AM, Ciechanover A, Ben-Neriah Y (1997) Inhibition of NF-kappa-B cellular function via specific targeting of the I-kappa-B–ubiquitin ligase. EMBO J 16: 6486–6494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaron A, Hatzubai A, Davis M, Lavon I, Amit S, Manning AM, Andersen JS, Mann M, Mercurio F, Ben-Neriah Y (1998) Identification of the receptor component of the IkappaBalpha–ubiquitin ligase. Nature 396: 590–594 [DOI] [PubMed] [Google Scholar]
- Yeung KC, Rose DW, Dhillon AS, Yaros D, Gustafsson M, Chatterjee D, McFerran B, Wyche J, Kolch W, Sedivy JM (2001) Raf kinase inhibitor protein interacts with NF-kappaB-inducing kinase and TAK1 and inhibits NF-kappaB activation. Mol Cell Biol 21: 7207–7217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenke FT, King CC, Bohl BP, Bokoch GM (1999) Identification of a central phosphorylation site in p21-activated kinase regulating autoinhibition and kinase activity. J Biol Chem 274: 32565–32573 [DOI] [PubMed] [Google Scholar]
- Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S (2004) A toll-like receptor that prevents infection by uropathogenic bacteria. Science 303: 1522–1526 [DOI] [PubMed] [Google Scholar]
- Zhang F, Romano PR, Nagamura-Inoue T, Tian B, Dever TE, Mathews MB, Ozato K, Hinnebusch AG (2001) Binding of double-stranded RNA to protein kinase PKR is required for dimerization and promotes critical autophosphorylation events in the activation loop. J Biol Chem 276: 24946–24958 [DOI] [PubMed] [Google Scholar]
- Zhang G, Ghosh S (2002) Negative regulation of toll-like receptor-mediated signaling by Tollip. J Biol Chem 277: 7059–7065 [DOI] [PubMed] [Google Scholar]
- Zheng Y, Bagrodia S, Cerione RA (1994) Activation of phosphoinositide 3-kinase activity by Cdc42Hs binding to p85. J Biol Chem 269: 18727–18730 [PubMed] [Google Scholar]
- Zhong H, May MJ, Jimi E, Ghosh S (2002) The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Mol Cell 9: 625–636 [DOI] [PubMed] [Google Scholar]
- Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, Ghosh S (1997) The transcriptional activity of NF-kappaB is regulated by the IkappaB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell 89: 413–424 [DOI] [PubMed] [Google Scholar]
- Ziegler SF, Wilson CB, Perlmutter RM (1988) Augmented expression of a myeloid-specific protein tyrosine kinase gene (hck) after macrophage activation. J Exp Med 168: 1801–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann S, Moelling K (1999) Phosphorylation and regulation of Raf by Akt (protein kinase B). Science 286: 1741–1744 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure Legends