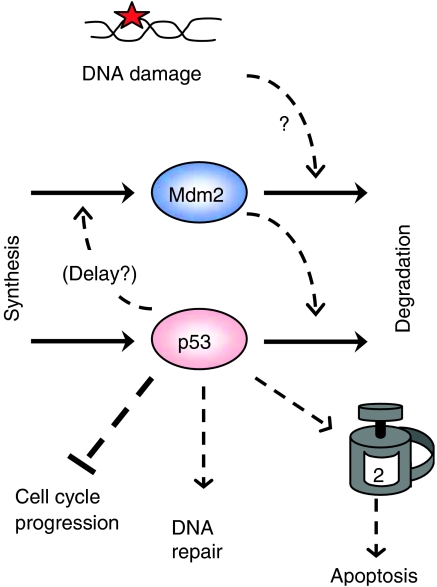
The tumor suppressor protein, p53, is a major guardian of genomic integrity in human cells (Hanahan and Weinberg, 2000). When DNA is damaged by ionizing radiation (as a result, for example, of unprotected sun worship), p53 is one of the first proteins to know about it (Figure 1 ). In response, p53's job is, first, to put the brakes on DNA synthesis and mitosis to prevent the transmission of damaged DNA to cell progeny. Secondly, p53 induces production of DNA repair proteins to fix the problem, if possible. If this is not possible, p53's third job is to trigger the cell-death program (apoptosis) to destroy irreparably damaged cells before they can cause any mischief. Cell lines that lack functional p53 rapidly accumulate genomic abnormalities that are often correlated with metastatic tumors. For these reasons, p53 has been subjected to intense scrutiny by an army of molecular cell biologists.
Above the clamor and confusion of this battleground stands the work of Uri Alon, Arnold Levine and their colleagues. In 2000, they reported that protein levels of p53 and its negative regulator (Mdm2) show dampened oscillations in populations of human breast cancer cells that have been damaged by gamma radiation (Lev Bar-Or et al, 2000). A few years later, Lahav et al (2004) showed beautifully that single cells can show repeated pulses of p53 and Mdm2—pulses of undampened amplitude at regular 5–6 h intervals—in response to DNA damage. Given the limited time frame of observations (16 h), the team could only observe 0, 1 or 2 pulses in any single cell. Noting a distinct correlation between radiation dose and average number of p53 pulses, they guessed that the extent of DNA damage might be encoded in the number of p53 pulses generated. In a study published in Molecular Systems Biology (Geva-Zatorsky et al, 2006), the team now reports surprising results of long observations (30–70 h) on hundreds of cells at four different radiation doses (measured in ‘Grays'):
Some cells, damaged by either 5 or 10 Gy, show sustained oscillations for the entire time of observation (up to 10 peaks in 60 h). Radiation dose determines not the number of pulses but rather the probability that an irradiated cell oscillates permanently or not.
Whether the damage is large, small or nonexistent, some cells show irregular fluctuations of p53 and Mdm2 with a broad distribution of interpulse intervals from 8–12 h.
Oscillations in the 4–7 h window are distinctive in their regular periodicity and shape and in their highly variable amplitude.
These unexpected results raise a number of interesting questions.
How can cells show such robust oscillations for a seemingly indefinite period of time? Long trains of undampened oscillations suggest that the p53 control system is capable of autonomous ‘limit cycle' oscillations, as in the case of circadian rhythms. (Limit cycles are recurrent solutions—of constant amplitude and period—of systems of nonlinear ordinary differential equations.) In the case of p53 and Mdm2, a potential source of oscillations has been evident for years: p53 upregulates transcription of the mdm2 gene, and Mdm2 protein promotes proteolysis of p53. ‘Negative feedback' like this is often used by cells to achieve homeostasis: an unanticipated upswing of p53 generates more Mdm2, which pushes p53 back down to a stable steady-state level. However, under suitable conditions, a negative feedback loop can undergo sustained oscillations as p53 and Mdm2 repeatedly overshoot and undershoot the intended steady state. It appears that mammalian cells use the p53–Mdm2 feedback loop for both purposes—to maintain a stable, steady, low level of p53 in the unperturbed state, and to generate indefinitely repeated pulses of p53 when DNA is damaged. The damage signal must push the control system across a bifurcation point separating the stable steady state from the stable limit cycle.
Theoretically inclined molecular biologists have known for years that sustained oscillations in a pure negative feedback loop are rather difficult to achieve. Oscillations are more robust when negative feedback is combined with long time delays, extremely nonlinear response functions and/or concurrent positive feedback loops. Of these, the feature that might be crucial for p53 regulation remains a question for the future.
Figure 1.
p53 protein level rises in response to DNA damage and triggers three responses.
Why do individual cells under identical conditions show great variability in response to radiation damage? The fact that individual breast cancer cells respond to DNA damage in highly variable ways may be due to genetic variation among cells in the culture (cancer cells are notoriously heterogeneous, because of genomic instability). In addition, stochastic events in the DNA repair process may determine whether a damaged cell shows characteristic 6-h oscillations or not. The initial dose of radiation produces much DNA damage (approximately 30 double-strand breaks per cell per Gy), most of which is probably repaired rapidly. However, some cells may incur lesions that cannot be repaired and continue generating a signal that puts the cell into a permanent p53 oscillation. Considering that the experimental cell line is derived from breast cancer tissue, the DNA repair system may already be compromised and unable to cope with certain kinds of rare defects. Presumably, the higher the dose, the more likely such lesions, because the probability that an irradiated cell oscillates increases with dose.
The long observation times of Geva-Zatorsky et al show, convincingly, that the oscillator's amplitude is much more variable than its period. Exploring a hybrid deterministic-stochastic model of molecular events within single cells, Geva-Zatorsky et al conclude that their observed fluctuations in Mdm2 amplitude (but not in period) could be attributed to slow variations in the basal synthesis rate of p53, provided these variations have a fundamental period of about twice the natural rhythm of p53 peaks. (The p53–Mdm2 oscillator effectively ignores fluctuations of much higher or lower frequency.)
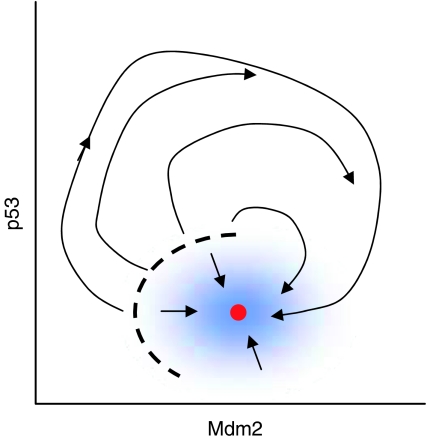
Why do undamaged cells exhibit irregular fluctuations? Noise might also be the key to this behavior. In undamaged cells, p53 is presumably attracted to a stable (homeostatic) steady state. However, this steady state is likely to be ‘excitable'. meaning that sufficiently large fluctuations of p53 or Mdm2 levels can carry the control system across a threshold, inducing a transient pulse of p53, which disappears as the control system comes back to the stable steady state. Depending on the size of p53 and Mdm2 fluctuations compared to the distance from steady state to threshold, this sort of excitable system can show nearly periodic excitations by a phenomenon called stochastic resonance (Figure 2 ) (Muratov et al, 2005).
Figure 2.
Small perturbations (blue zone) away from the stable steady state (red dot) are immediately damped out (short arrows), but larger perturbations, beyond the threshold (dashed line), generate transient activation of p53. What do you expect will happen if this p53–Mdm2 control system is continually buffeted by stochastic fluctuations?
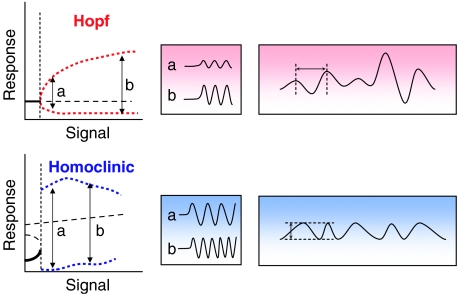
How do p53 oscillations arise? That the oscillator's amplitude is much more variable than its period is relevant to the type of bifurcation that separates the homeostatic response from the oscillatory state. Theoreticians distinguish two types of bifurcation to limit cycles: ‘Hopf' bifurcation (fixed period, increasing amplitude of response) and ‘homoclinic' bifurcation (fixed amplitude, increasing frequency of response) (Figure 3 ). Both types of bifurcation have been used by theoreticians to model the p53 oscillator (Ciliberto et al, 2005; Ma et al, 2005). Hopf bifurcations arise naturally in negative feedback loops, but the amplitude of oscillation may be too small to drive reliable responses. Homoclinic bifurcations require more complex networks with both positive and negative feedback. Their constant, large amplitude output pulses are easy to count, but their initial response to stimulation may be quite sluggish. Based on short observations times, Lahav et al concluded that p53 pulses are ‘quantal' (i.e., fixed amplitude), which suggests a homoclinic bifurcation, but the more extensive observations of Geva-Zatorsky et al suggest that a Hopf bifurcation is more likely (Figure 3).
Figure 3.
Two types of bifurcations to limit cycle oscillations: Hopf (red) and homoclinic (blue). ‘Signal' is some ‘input parameter' to the control system. ‘Response' is some ‘output variable' governed by the control system. At signal=0, the control system is sitting at a stable steady state (bold solid line) with low level of response. As signal increases, the steady state loses stability at a bifurcation point (vertical dashed line). Beyond the bifurcation point, the steady state is unstable (bold dashed line) and the system oscillates between maximal and minimal response values (red and blue dotted curves). Typical oscillations at two different signal strengths (a and b) are illustrated in the middle panel. If the signal strength is fluctuating between a and b, the Hopf bifurcation is expected to give an oscillating response of roughly constant period and highly variable amplitude (far right). The homoclinic bifurcation, by contrast, is expected to give an oscillating response of roughly constant amplitude and highly variable period.
What is the function, if any, of these p53 pulses? It is tempting to speculate that p53 pulse trains are the standard response of normal human cells to lingering DNA damage. Cells may count p53 pulses and trigger apoptosis beyond a critical number. However, it would be premature to draw such conclusions. The cancer cells under study seem to be deficient in downstream apoptotic responses, because they happily generate p53 pulses for days without committing suicide. They may also be deficient in some crucial aspect of p53 regulation—who knows?—and the whole phenomenon of periodic pulses may be irrelevant to normal (untransformed) cells. That would be very disappointing indeed!
So there is much work yet to be done. Are periodic p53 pulses observed in normal cells in response to DNA damage? Do the pulses stop when the damage is repaired? If repair is blocked, do repeated pulses of p53 trigger apoptosis? If all these speculations are confirmed, what are the crucial molecular mechanisms underlying the responses? How do cells count p53 pulses? All the technology needed to address these questions is available and answers will undoubtedly be appearing soon.
References
- Ciliberto A, Novak B, Tyson JJ (2005) Steady states and oscillations in the p53/Mdm2 network. Cell Cycle 4: 488–493 [DOI] [PubMed] [Google Scholar]
- Geva-Zatorsky N, Rosenfeld N, Itzkovitz S, Milo R, Sigal A, Dekel E, Yarnitzky T, Polak P, Liron Y, Kam Z, Lahav G, Alon U (2006) Oscillations and variability in the p53 system. Mol Syst Biol 2006.0033. doi:10.1038/msb4100068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100: 57–70 [DOI] [PubMed] [Google Scholar]
- Lahav G, Rosenfeld N, Sigal A, Geva-Zatorsky N, Levine AJ, Elowitz MB, Alon U (2004) Dynamics of the p53–Mdm2 feedback loop in individual cells. Nat Genet 36: 147–150 [DOI] [PubMed] [Google Scholar]
- Lev Bar-Or R, Maya R, Segel LA, Alon U, Levine AJ, Oren M (2000) Generation of oscillations by the p53–Mdm2 feedback loop: a theoretical and experimental study. Proc Natl Acad Sci USA 97: 11250–11255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Wagner J, Rice JJ, Hu W, Levine AJ, Stolovitzky GA (2005) A plausible model for the digital response of p53 to DNA damage. Proc Natl Acad Sci USA 102: 14266–14271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muratov CB, Vanden Eijnden E, Weinan E (2005) Self-induced stochastic resonance in excitable systems. Physica D 210: 227–240 [Google Scholar]