Introduction
Crohn's disease is a chronic inflammatory disorder that can affect any part of the gastrointestinal tract from the mouth to the anus. The disease is characterized by transmural inflammation that can be complicated by the development of fibrotic strictures, perforation, abscess formation, and fistulization. Perianal fistulas may arise from inflamed or infected anal glands (fistula-in-ano) and/or penetration of fissures or ulcers of the rectum or anal canal.[1] Classification of fistulas in Crohn's disease is based on the origin and terminus of the fistulous tract. Fistulas may develop between 2 segments of bowel (enteroenteric fistula), a segment of bowel and an adjacent organ (eg, enterovesicular), or between a segment of bowel and the skin (enterocutaneous).[2,3] Fistulas that communicate with the skin are known as external fistulas; those that communicate with adjacent structures within the abdomen and pelvis are known as internal fistulas.[2] Population-based estimates of the lifetime risk of fistula development in Crohn's disease range from 14% to 38%.[4–6] The development of fistulas may precede or coincide with the diagnosis of Crohn's disease. One cohort study estimated the rate of fistula formation preceding the diagnosis of Crohn's disease to be 45%.[4,5,7] Rates of spontaneous fistula closure are low, with estimates ranging from as low as 6% to 13% in placebo-treated patients in randomized controlled trials of 6-mercaptopurine (6-MP) and infliximab.[8,9] Spontaneous remission rates of simple fistulas-in-ano have been reported to be as high as 50%.[7,10]
Identifying Perianal Disease
Perianal fistulas cause significant morbidity and diminish patients' quality of life. Consequences of perianal fistulas include perianal drainage, pain, abscess formation, dyschezia, dyspareunia, and fecal incontinence. The identification and classification of perianal fistulas in Crohn's disease is essential to providing safe and effective treatment for this patient population.
Clinicians should have a high index of suspicion for perianal fistulas in any patient suffering from Crohn's disease. Consequently, all patients should be asked about symptoms that might suggest perianal disease. Symptoms include perianal drainage (purulent or enteric material), discomfort (including dyspareunia and dyschezia), and swelling. Perianal fistulas can occur with or without active disease involving the rectosigmoid region. It is important to inquire about symptoms that might suggest active inflammation, including diarrhea with or without blood, rectal urgency, tenesmus, incontinence, and abdominal discomfort, as well as systemic symptoms of fever, night sweats, and weight loss. In addition, extraintestinal manifestations, including asymmetric large joint arthritis and skin lesions such as pyoderma gangrenosum, uveitis, and oral ulcers, may be a clue to active Crohn's disease, even in the patient with few or no bowel symptoms.
A perianal and rectal examination should be performed routinely in patients with Crohn's disease. The clinician should first inspect the perianal region for evidence of induration and erythema, fistulous tract openings, multiple or eccentric anal fissures, ulcerations, and/or large, broad-based skin tags. In the presence of fistulous openings, gentle compression adjacent to the orifice may result in the expulsion of purulent or enteric material suggesting active inflammation of the tract. Pain and induration on digital ano-rectal examination should raise suspicion for perianal infection, with or without an organized abscess. The presence of perianal fluctuance suggests the presence of an abscess cavity. Digital rectal examination is also important for identification of strictures and internal openings of fistulas that sometimes can be as large as a centimeter or more in diameter.
When perianal disease is found, the clinician should keep the differential diagnosis in mind, including hidradenitis suppurativa, sexually transmitted diseases, squamous cell carcinoma, tuberculosis, and surgery or trauma to the perianal area.[11] In particular, hidradenitis suppurativa may be confused with complex fistulizing perianal Crohn's disease. The correct diagnosis is made on examination under anesthesia by probing of the cutaneous tracts and demonstrating that they only traverse to the subdermal space and do not communicate with the rectum or other parts of the digestive tract.[12]
Perianal Anatomy and Classification of Fistulas
Unfortunately, there are no validated clinical measures of fistulous disease activity. The most comprehensive measure of morbidity caused by perianal Crohn's disease is provided by The Perianal Disease Activity Index.[13] This index evaluates 5 categories affected by fistulas, including discharge, pain, restriction of sexual activity, type of perianal disease, and degree of induration – but it has yet to be validated.
Understanding perianal anatomy is imperative for classifying fistulas accurately. The anal canal comprises 2 muscular cylinders: the inner cylinder (internal anal sphincter) and external cylinder (external anal sphincter). The internal anal sphincter is a 3-cm-long thickened continuation of the circular smooth muscle of the rectum. The external sphincter is a 4-cm-long downward extension of the puborectalis muscle, which is skeletal muscle. Skeletal muscle above the puborectalis fans out to create the levator ani muscles that divide the perineum from the abdominal cavity (Figure 1).[14]
Figure 1.
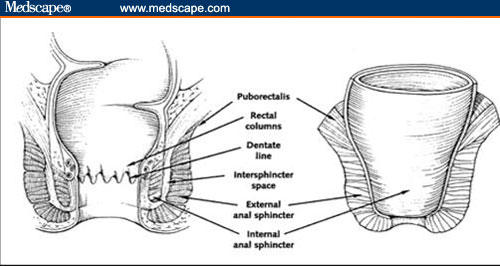
Anatomic relationships in the perianal region.
Republished from Schwartz DA, Pemberton JH, Sandborn WJ. Diagnosis and treatment of perianal fistulas in Crohn disease. Ann Intern Med. 2001;135(10):906–918. By permission from Mayo Foundation for Medical Education and Research. All rights reserved.
A number of classification systems for perianal fistulae exist. The system most commonly used is that of Parks and colleagues[15] that involves the external anal sphincter as the anatomic point of reference for description of the fistulous tract (Figure 2). Simple fistulas in-ano are low-lying tracts with a single external opening. These fistulas typically occur in patients without rectal disease involvement and tend to have an excellent response to fistulotomy, with healing rates ranging from 70% to 100% (Figure 3).[2]
Figure 2.
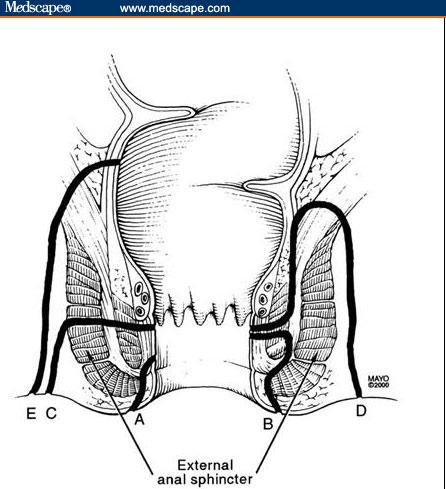
Park's Classification of perianal fistulas. (A) A superficial fistula tracks below both the internal anal sphincter and external anal sphincter complexes. (B) An intersphincteric fistula tracks between the internal anal sphincter and the external anal sphincter in the intersphincteric space. (C) A transsphincteric fistula tracks from the intersphincteric space through the external anal sphincter. (D) A suprasphincteric fistula leaves the intersphincteric space over the top of the puborectalis and penetrates the levator muscle before tracking down to the skin. (E) An extrasphincteric fistula tracks outside of the external anal sphincter and penetrates the levator muscle into the rectum.
Republished from Schwartz DA, Pemberton JH, Sandborn WJ. Diagnosis and treatment of perianal fistulas in Crohn disease. Ann Intern Med. 2001;135(10):906–918. By permission from Mayo Foundation for Medical Education and Research. All rights reserved.
Figure 3.
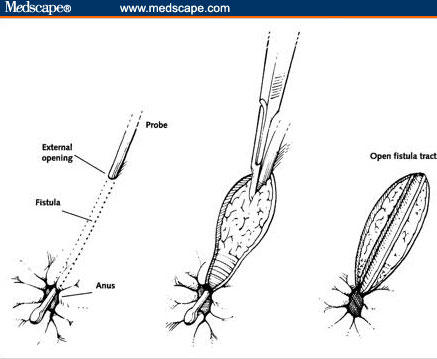
Fistulotomy.
Republished from Schwartz DA, Pemberton JH, Sandborn WJ. Diagnosis and treatment of perianal fistulas in Crohn disease. Ann Intern Med. 2001;135(10):906–918. By permission from Mayo Foundation for Medical Education and Research. All rights reserved.
Some controversy exists as to the appropriateness of traditional fistulotomy in patients with rectal disease. Some studies have demonstrated impaired wound healing post fistulotomy in patients with active inflammatory disease of the rectum.[16–18] Some experts recommend a more conservative management approach, with the placement of noncutting setons rather than traditional fistulotomy.[3] A complex perianal fistula is defined as any fistula with multiple openings; internal orifices above the dentate line; horseshoe tracts; high, blind extensions; and those that lie high above the anal sphincter, are supra- or extrasphincteric, or recur after fistulotomy.[1] Traditional fistulotomies should not be performed in patients with complex perianal fistulas because of the significant risk of rendering the patient incontinent as a result of transection of both the internal and external anal sphincter.[1,2,14]
Evaluation of Fistula Anatomy
Adequate evaluation of fistula anatomy requires a combined radiologic and surgical approach.[19–21] Examination under anesthesia (EUA) consists of visual inspection, palpation, and the passage of metal probes into fistula tracks under general anesthesia.
EUA has long been regarded as the gold standard for the evaluation and classification of perianal fistulas in Crohn's disease, and is the gold standard used in most studies.[14] However, recent studies comparing EUA with consensus diagnosis, pelvic magnetic resonance imaging (MRI), or anorectal endoscopic ultrasound (EUS) suggest that EUA will misclassify perianal fistulas in approximately 10% of patients, with the potential to adversely affect therapeutic outcomes.[22–24]
Imaging modalities that have been studied for the evaluation of perianal fistulas in Crohn's disease include computed tomography (CT), fistulography, MRI, and EUS (Figure 4). Fistulography involves the insertion of a small catheter into the external opening of a fistula followed by injection of radiocontrast material directly into the track. Radiographs are then taken from different angles.[25] Fistulography does not allow direct visualization of the anal sphincter anatomy and can be very uncomfortable for patients. A single retrospective study comparing the accuracy of fistulography to operative findings showed that fistulograms were correct in only 4 out of 25 patients (16%) with a false-positive rate of 12%.[26] As a result, fistulograms are not particularly useful in the evaluation of perianal fistulas.
Figure 4.
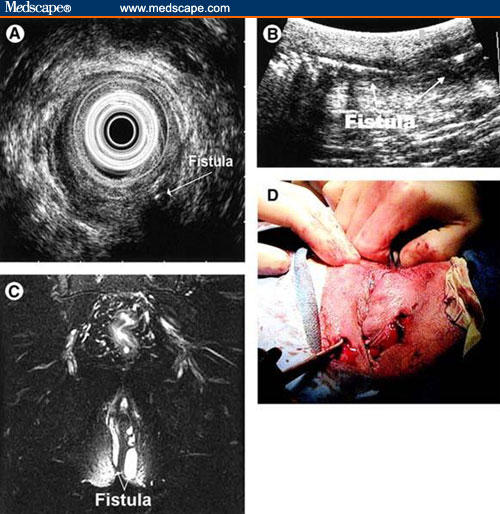
Imaging modalities in the evaluation of perianal fistulas.
Example of a patient illustrating the use of all 3 diagnostic modalities. The patient presented with recurrent perianal pain and drainage. (A) The radial EUS image shows a transsphincteric fistula with horseshoeing. (B) The linear ultrasound image shows a long fistula tract with air in it. (C) The MRI image illustrates a complex horseshoe fistula. (D) The patient then had an EUA that confirmed a horseshoe fistula.
Republished from Schwartz DA, Wiersema MJ, Dudiak KM, et al. A comparison of endoscopic ultrasound, magnetic resonance imaging, and exam under anesthesia for evaluation of Crohn's perianal fistulas. Gastroenterology. 2001;121(5):1064–1072. Copyright 2001, with permission form American Gastroenterological Association.
Although CT allows better visualization of structures outside the bowel lumen, its use in evaluating perianal fistulas is less clear. Fistulas are identified as linear tracks extending from the bowel that contain air or contrast material on CT scan. CT has limited resolution, making it difficult to distinguish between soft tissue and inflammatory streaking.[27] Additionally, CT scan does not reliably identify the levator ani muscle, making fistula classification difficult.[28] In 1993, Schratter-Sehn and colleagues[29] performed a study comparing CT and EUS to operative findings and/or clinical course for the evaluation of perianal fistulas and found EUS to be much more sensitive than CT scan (82% vs 24%). Unlike EUS, CT exposes patients to significant quantities of ionizing radiation. For these reasons, the role of CT scan in the evaluation of perianal fistulas in Crohn's disease is limited.
The limitations of fistulography and CT scan have led to the evaluation of newer technologies, such as MRI and EUS. Initial prospective studies involving MRI in the evaluation of fistula in-ano unrelated to inflammatory bowel disease using EUA as the gold standard have shown conflicting results, with concordance rates of 85% and 42%, respectively, in different studies.[30–32] More recently, inter- and intraobserver agreement among expert radiologists was excellent (k = 0.92).[21,32] A recent study from St. Mark's Hospital in London, United Kingdom, demonstrated that the therapeutic impact of MRI in the evaluation of primary cryptoglandular fistula in-ano was 10%, meaning that the surgical approach was changed based on the MRI findings in 10% of patients.[21] In 2004, Schaefer and colleagues[33] performed a study assessing a new MR-imaging protocol with high-resolution subtraction MR-fistulography for evaluation of fistulas in Crohn's disease and cryptoglandular sepsis vs results of surgical exploration. They found complete agreement between subtraction MR-fistulography and surgical findings in 89% of cases, results that were comparable to standard MR-imaging protocols. A group from the Mayo clinic in Rochester, Minnesota, performed a blinded, prospective comparison between EUS, MRI, and EUA for the evaluation of perianal fistulas in 34 patients with Crohn's disease.[20] Fistulas were classified according to the Park's criteria, and consensus gold standards were determined for each patient. Accuracy (defined as > 85% agreement with the consensus gold standard) for EUS, MRI, and EUA was 91%, 87%, and 91%, respectively. When any 2 modalities were combined, the accuracy was 100%, suggesting that EUA in combination with either EUS or pelvic MRI was the best approach for evaluating and classifying perianal fistulas.
Some very interesting data have emerged regarding the utility of MRI in following the radiologic course of fistulous tracts in response to infliximab therapy.[34,35] Van Assche and colleagues[35] examined clinical and radiologic response in 18 patients before and after treatment with infliximab. They performed a double-blind, placebo-controlled trial with infliximab given at 5 or 10 mg/kg vs placebo at weeks 0, 2, and 6. At baseline all patients underwent an EUA and colonoscopic evaluation. MRI was performed at inclusion and at week 6. Seven other patients received infliximab infusions as part of an expanded-access program. These patients received induction dosing followed by maintenance infusions at 8-week intervals. This study confirmed that MRI was reliable in the assessment of fistula tracts, with good interobserver agreement (P < .001). All 18 patients had signs of active inflammation at baseline. Seven of the 18 patients had perianal fluid collections. After short-term infliximab treatment, 11 patients had responded clinically (defined as cessation of fistula drainage). However, 8 of these 11 had persistent tracts with varying degrees of residual inflammation on MRI. After 46 weeks of therapy, MRI signs of inflammation had resolved in 3 of 6 patients. These data would suggest a potential role for pelvic MRI in the assessment of underlying fistula anatomy and inflammation in response to anti-tumor necrosis factor-alpha therapy.
Most studies involving the use of endosonography (blind transrectal and EUS) in the evaluation of perianal fistulas have focused on patients without Crohn's disease.[25] A prospective, blinded study comparing EUS with CT scan in 25 patients with Crohn's disease using surgery or fistulography as the gold standard showed EUS to be superior to CT scan, with sensitivities of 82% vs 24%, respectively.[29] Several studies have compared MRI with endosonography. Studies focusing on noninflammatory bowel disease-related fistulas showed MRI to be superior to EUS in imaging fistulas.[31,36] However, both of these studies used 7-MHz rigid radial scanning ultrasound probes which have been shown to be less sensitive when compared with more flexible probes. In contrast, Orsoni and colleagues[37] found EUS to be more sensitive than MRI in the evaluation of Crohn's disease-related fistulas in a prospective pilot study involving 22 patients, using surgical evaluation as the gold standard. Agreement of EUS and MRI with surgical findings was 82% and 50%, respectively. The poor sensitivity of MRI demonstrated in this study may be accounted for by the use of a body coil rather than a pelvic-phased array coil, which provides better resolution.
A study published in The American Journal of Gastroenterology in 2003 examined the role of transperineal ultrasound in the evaluation of fistula response to infliximab therapy in patients with Crohn's disease.[34] (There are no other therapeutic trials using these various imaging modalities to evaluate fistula closure as a primary endpoint.) Thirty-five patients with Crohn's-related perianal fistulas were treated with infliximab for up to 48 weeks. Primary outcome measures of complete fistula closure and radiologic healing were assessed using an imaging score of perianal fistula severity based on the Parks criteria.[15] At 8 weeks, after the first 2 induction doses of infliximab given at weeks 0 and 2, clinical fistula closure occurred in 49% of patients. The radiologic score was significantly better in those patients with clinical fistula closure than in those with no clinical improvement (P = .023). At 56 weeks, clinical fistula closure occurred in 46% of patients. Clinical scores correlated with radiologic scores (R = 0.52; P < .001). The proportion of patients with significant radiologic improvement increased from 14% at 8 weeks to 43% at 56 weeks (P = .015). These data would suggest that in responders to induction dosing, prolonged therapy with infliximab would result in significant healing of fistula tracts, which may be assessed by transperineal ultrasound. However, there are no data to suggest which ultrasound modality (transperineal ultrasound or transrectal EUS) is more accurate. Also, it remains to be determined whether improved or complete radiographic healing would translate to a prolonged duration of response after discontinuation of infliximab.
Concluding Remarks
The diagnosis of perianal fistulas in patients with Crohn's disease is often challenging and crucial to the determination of the appropriate therapy. Clinicians should have a high index of suspicion for perianal disease in any patient with Crohn's disease, particularly those with rectosigmoid involvement. A thorough history and physical examination with attention to symptoms and signs suggestive of perianal fistulizing disease followed by preoperative pelvic imaging is important in elucidating the anatomy of the fistulous tract. Consultation and collaboration with a colon and rectal surgeon experienced in the management of perianal disease in the patient with inflammatory bowel disease is essential. Preoperative imaging with EUS or MRI in combination with EUA approaches 100% accuracy in the classification of perianal fistulas and will help guide decisions regarding surgical and medical intervention.
Contributor Information
Jennifer Jones, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota.
William Tremaine, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota.
References
- 1.Judge TA, Lichenstein GR. Kirsner's Inflammatory Bowel Diseases. 6th ed. New York: W.B. Saunders; 2004. Fistulizing Crohn's Disease; pp. 700–716. [Google Scholar]
- 2.Schwartz DA, Pemberton JH, Sandborn WJ. Diagnosis and treatment of perianal fistulas in Crohn disease. Ann Intern Med. 2001;135:906–918. doi: 10.7326/0003-4819-135-10-200111200-00011. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz DA, Herdman CR. Review article: The medical treatment of Crohn's perianal fistulas. Aliment Pharmacol Ther. 2004;19:953–967. doi: 10.1111/j.1365-2036.2004.01917.x. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz DA, Loftus EV, Jr, Tremaine WJ, et al. The natural history of fistulizing Crohn's disease in Olmsted County, Minnesota. Gastroenterology. 2002;122:875–880. doi: 10.1053/gast.2002.32362. [DOI] [PubMed] [Google Scholar]
- 5.Hellers G, Bergstrand O, Ewerth S, Holmstrom B. Occurrence and outcome after primary treatment of anal fistulae in Crohn's disease. Gut. 1980;21:525–527. doi: 10.1136/gut.21.6.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farmer RG, Hawk WA, Turnbull RB., Jr Clinical patterns in Crohn's disease: a statistical study of 615 cases. Gastroenterology. 1975;68(4 Pt 1):627–635. [PubMed] [Google Scholar]
- 7.Gray BK, Lockhartmummery HE, Morson BC. Crohn's disease of the anal region. Gut. 1965;6:515–524. doi: 10.1136/gut.6.6.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340:1398–1405. doi: 10.1056/NEJM199905063401804. [DOI] [PubMed] [Google Scholar]
- 9.Present DH, Korelitz BI, Wisch N, Glass JL, Sachar DB, Pasternack BS. Treatment of Crohn's disease with 6-mercaptopurine. A long-term, randomized, double-blind study. N Engl J Med. 1980;302:981–987. doi: 10.1056/NEJM198005013021801. [DOI] [PubMed] [Google Scholar]
- 10.Halme L, Sainio AP. Factors related to frequency, type, and outcome of anal fistulas in Crohn's disease. Dis Colon Rectum. 1995;38:55–59. doi: 10.1007/BF02053858. [DOI] [PubMed] [Google Scholar]
- 11.McLeod RS. Management of fistula-in-ano: 1990 Roussel Lecture. Can J Surg. 1991;34:581–585. [PubMed] [Google Scholar]
- 12.Wiseman M. Hidradenitis suppurativa: a review. Dermatol Ther. 2004;17:50–54. doi: 10.1111/j.1396-0296.2004.04007.x. [DOI] [PubMed] [Google Scholar]
- 13.Irvine EJ. Usual therapy improves perianal Crohn's disease as measured by a new disease activity index. McMaster IBD Study Group. J Clin Gastroenterol. 1995;20:27–32. [PubMed] [Google Scholar]
- 14.Sandborn WJ, Fazio VW, Feagan BG, Hanauer SB American Gastroenterological Association Clinical Practice C. AGA technical review on perianal Crohn's disease. Gastroenterology. 2003;125:1508–1530. doi: 10.1016/j.gastro.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 15.Parks AG, Gordon PH, Hardcastle JD. A classification of fistula-in-ano. Br J of Surg. 1976;63:1–2. doi: 10.1002/bjs.1800630102. [DOI] [PubMed] [Google Scholar]
- 16.Levien DH, Surrell J, Mazier WP. Surgical treatment of anorectal fistula in patients with Crohn's disease. Surg Gynecol Obstet. 1989;169:133–136. [PubMed] [Google Scholar]
- 17.Radcliffe AG, Ritchie JK, Hawley PR, Lennard-Jones JE, Northover JM. Anovaginal and rectovaginal fistulas in Crohn's disease. Dis Colon Rectum. 1988;31:94–99. doi: 10.1007/BF02562636. [DOI] [PubMed] [Google Scholar]
- 18.Nordgren S, Fasth S, Hulten L. Anal fistulas in Crohn's disease: incidence and outcome of surgical treatment. Int J Colorectal Dis. 1992;7:214–218. doi: 10.1007/BF00341224. [DOI] [PubMed] [Google Scholar]
- 19.Beets-Tan RG, Beets GL, van der Hoop AG, et al. Preoperative MR imaging of anal fistulas: Does it really help the surgeon? Radiology. 2001;218:75–84. doi: 10.1148/radiology.218.1.r01dc0575. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz DA, Wiersema MJ, Dudiak KM, et al. A comparison of endoscopic ultrasound, magnetic resonance imaging, and exam under anesthesia for evaluation of Crohn's perianal fistulas. Gastroenterology. 2001;121:1064–1072. doi: 10.1053/gast.2001.28676. [DOI] [PubMed] [Google Scholar]
- 21.Buchanan GN, Halligan S, Williams AB, et al. Magnetic resonance imaging for primary fistula in ano. Br J Surg. 2003;90:877–881. doi: 10.1002/bjs.4125. [DOI] [PubMed] [Google Scholar]
- 22.Spencer JA, Chapple K, Wilson D, Ward J, Windsor AC, Ambrose NS. Outcome after surgery for perianal fistula: predictive value of MR imaging. AJR Am J Roentgen. 1998;171:403–406. doi: 10.2214/ajr.171.2.9694464. [DOI] [PubMed] [Google Scholar]
- 23.Chapple KS, Spencer JA, Windsor AC, Wilson D, Ward J, Ambrose NS. Prognostic value of magnetic resonance imaging in the management of fistula-in-ano. Dis Colon Rectum. 2000;43:511–516. doi: 10.1007/BF02237196. [DOI] [PubMed] [Google Scholar]
- 24.Beckingham IJ, Spencer JA, Ward J, Dyke GW, Adams C, Ambrose NS. Prospective evaluation of dynamic contrast enhanced magnetic resonance imaging in the evaluation of fistula in ano. Br J Surg. 1996;83:1396–1398. doi: 10.1002/bjs.1800831022. [DOI] [PubMed] [Google Scholar]
- 25.UpToDate. The role of imaging tests in the evaluation of anal abscesses and fistulas. UpToDate, Inc.; 2005. Available at: http://patients.uptodate.com/topic.asp?file=gi_dis/24663 Accessed April 27, 2005
- 26.Kuijpers HC, Schulpen T. Fistulography for fistula-in-ano. Is it useful? Dis Colon Rectum. 1985;28:103–104. doi: 10.1007/BF02552656. [DOI] [PubMed] [Google Scholar]
- 27.Yousem DM, Fishman EK, Jones B. Crohn disease: perirectal and perianal findings at CT. Radiology. 1988;167:331–334. doi: 10.1148/radiology.167.2.3357940. [DOI] [PubMed] [Google Scholar]
- 28.Guillaumin E, Jeffrey RB, Jr, Shea WJ, Asling CW, Goldberg HI. Perirectal inflammatory disease: CT findings. Radiology. 1986;161:153–157. doi: 10.1148/radiology.161.1.3763858. [DOI] [PubMed] [Google Scholar]
- 29.Schratter-Sehn AU, Lochs H, Vogelsang H, Schurawitzki H, Herold C, Schratter M. Endoscopic ultrasonography versus computed tomography in the differential diagnosis of perianorectal complications in Crohn's disease. Endoscopy. 1993;25:582–586. doi: 10.1055/s-2007-1010409. [DOI] [PubMed] [Google Scholar]
- 30.Scholefield JH, Berry DP, Armitage NC, Wastie ML. Magnetic resonance imaging in the management of fistula in ano. Int J Colorectal Dis. 1997;12:276–279. doi: 10.1007/s003840050105. [DOI] [PubMed] [Google Scholar]
- 31.Lunniss PJ, Barker PG, Sultan AH, et al. Magnetic resonance imaging of fistula-in-ano. Dis Colon Rectum. 1994;37:708–718. doi: 10.1007/BF02054416. [DOI] [PubMed] [Google Scholar]
- 32.Buchanan GN, Halligan S, Taylor S, Williams A, Cohen R, Bartram C. MRI of fistula in ano: inter- and intraobserver agreement and effects of directed education. AJR Am J Roentgen. 2004;183:135–140. doi: 10.2214/ajr.183.1.1830135. [DOI] [PubMed] [Google Scholar]
- 33.Schaefer O, Lohrmann C, Langer M. Assessment of anal fistulas with high-resolution subtraction MR-fistulography: comparison with surgical findings. J Magn Reson Imaging. 2004;19:91–98. doi: 10.1002/jmri.10436. [DOI] [PubMed] [Google Scholar]
- 34.Rasul I, Wilson SR, MacRae H, Irwin S, Greenberg GR. Clinical and radiological responses after infliximab treatment for perianal fistulizing Crohn's disease. Am J Gastroenterol. 2004;99:82–88. doi: 10.1046/j.1572-0241.2003.04009.x. [DOI] [PubMed] [Google Scholar]
- 35.Van Assche G, Vanbeckevoort D, Bielen D, et al. Magnetic resonance imaging of the effects of infliximab on perianal fistulizing Crohn's disease. Am J Gastroenterol. 2003;98:332–339. doi: 10.1111/j.1572-0241.2003.07241.x. [DOI] [PubMed] [Google Scholar]
- 36.Hussain SM, Stoker J, Schouten WR, Hop WC, Lameris JS. Fistula in ano: endoanal sonography versus endoanal MR imaging in classification. Radiology. 1996;200:475–481. doi: 10.1148/radiology.200.2.8685344. [DOI] [PubMed] [Google Scholar]
- 37.Orsoni P, Barthet M, Portier F, Panuel M, Desjeux A, Grimaud JC. Prospective comparison of endosonography, magnetic resonance imaging and surgical findings in anorectal fistula and abscess complicating Crohn's disease. Br J Surg. 1999;86:360–364. doi: 10.1046/j.1365-2168.1999.01020.x. [DOI] [PubMed] [Google Scholar]


