Abstract
The activity of CTP:phosphocholine cytidylyltransferase, a rate-limiting enzyme in phosphatidylcholine biosynthesis, is modulated by its interaction with lipid bilayers [Kent, C. (1997) Biochim. Biophys. Acta 1348, 79–90]. Its regulation is of central importance in the maintenance of membrane lipid homeostasis. Here we show evidence that the stored curvature elastic stress in the lipid membrane's monolayer modulates the activity of CTP:phosphocholine cytidylyltransferase. Our results show how a purely physical feedback signal could play a key role in the control of membrane lipid synthesis.
Membrane phospholipid synthesis and turnover are tightly regulated, and homeostatic control of membrane phospholipid content and composition is essential for cell growth and survival (1). Phosphatidylcholine (PtdCho) is a major membrane phospholipid and a precursor to other membrane lipids. Hence, regulation of CTP:phosphocholine cytidylyltransferase (CCT) is critical for membrane biogenesis and for the production of new membrane during the S phase of the cell cycle (1, 2). Furthermore, inhibition of CCT by using either biochemical (3–6) or genetic (7) methods triggers programmed cell death. CCT activity is modulated by the association of its amphipathic helical domain with phospholipid bilayers (1, 8–10). The enzyme binds tightly to PtdCho bilayers containing fatty acids and diacylglycerol (11–16). Model studies with synthetic peptides corresponding to the 11-residue repeat motif in the amphipathic helical domain of CCT show that membrane binding induces a conformation change from a random coil in the aqueous solvent to a helical structure when associated with bilayers (9). This conformational change accelerates CCT activity by dramatically increasing the affinity of the enzyme for cytidine triphosphate (17). In contrast, CCT activation is blocked by lipids such as hexadecylphosphocholine (HexPC), 1-O-octadecyl-2-O-methyl-rac-glycerophosphocholine, and sphingosine (3–5, 18, 19).
Although the sensitivity of CCT to the lipid environment is established, the specific biophysical property of the bilayer that regulates the association of the enzyme with membranes containing a diverse mixture of phospholipids has not been elucidated. The binding of CCT to PtdCho vesicles that incorporate negatively charged lipids, such as phosphatidylserine or fatty acid, has been attributed to an electrostatic interaction between the anionic lipids and the positively charged amino acid residues in the amphipathic helix (20). However, electrostatic interactions cannot account for the stimulation of CCT activity by uncharged lipids, such as diacylglycerol. Such activating lipids share a common feature, namely the ability to form aggregates in which the polar/apolar interface bends toward the polar environment, and are known as type II amphiphiles. By contrast, lipid inhibitors of CCT, such as HexPC, are characterized by their ability to form aggregates in which the polar/apolar interface curves away from the polar region. These are known as type I amphiphiles. The observation that type II lipids activate CCT has been pointed out previously (21), but the physical origins of the activation have remained obscure. In this report, we present a model for the interaction between CCT and an amphiphilic membrane and test both its qualitative and quantitative predictions.
To build our model of CCT/membrane interactions, we first need to understand something about the local intermolecular forces between amphiphiles in a membrane. When incorporated into a bilayer membrane, both type I and type II lipids impart a desire for interfacial curvature, because of the nonuniform distribution of lateral pressure, π(z), between the amphiphiles. At mechanical equilibrium, the summed lateral pressure distribution must equal zero, but the first moment of the lateral pressure is in general nonzero
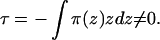 |
1 |
We will call τ the torque tension, because it is the torque stored in a monolayer, which is forced to remain flat. Physically, it is the fact that a bilayer consists of two monolayers back to back that constrains each monolayer to remain flat. For our purposes, the most useful way of quantifying the torque tension is in terms of a stored curvature elastic energy. We use the Helfrich Hamiltonian (22) for the curvature elastic energy per amphiphile in a monolayer
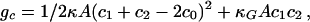 |
2 |
where A is the cross-sectional area per molecule, c1 and c2 are the principal curvatures at the interface (defined to be negative for curvature toward the water), c0 is the spontaneous curvature of the monolayer, κ is the bending rigidity of the monolayer, and κG is the Gaussian curvature modulus. The geometry of the monolayer with no torque tension is spherical, and the curvature elastic energy per molecule stored in a flat monolayer membrane relative to this state is equal to 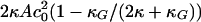 (23). The torque tension of the monolayer is related to the curvature elastic parameters via
(23). The torque tension of the monolayer is related to the curvature elastic parameters via 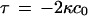 (24). Thus in a homogeneous multicomponent membrane, the stored curvature elastic energy and torque tension may be found from a knowledge of the effective values of c0 and the bending rigidities of the monolayer, parameters that have been measured for a number of biologically relevant amphiphiles.
(24). Thus in a homogeneous multicomponent membrane, the stored curvature elastic energy and torque tension may be found from a knowledge of the effective values of c0 and the bending rigidities of the monolayer, parameters that have been measured for a number of biologically relevant amphiphiles.
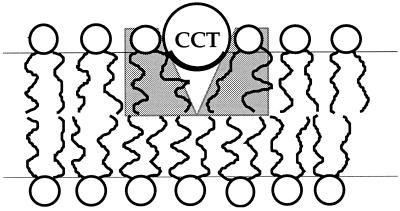
For the type II lipid amphiphile, dioleoylphosphatidylethanolamine (DOPE), we calculate that the total stored curvature elastic energy for each DOPE molecule in a flat monolayer is approximately 1 kBT (23, 25, 26). Qualitatively, we can see that the partial submersion of the amphipathic α-helical binding domain of CCT into an amphiphilic monolayer allows the release of at least some of this stored curvature elastic energy for type II amphiphiles (Fig. 1). Given the extent of the CCT-binding domain of approximately 78 Å (9), 19 or more lipids may become partially curvature relaxed during binding, which represents a potentially significant contribution to the membrane association energy. We therefore anticipate that the stored curvature elasticity may play a significant role in modulating the partitioning of CCT into the membrane. Because the geometry of the bound CCT α-helix cannot release the stored curvature elastic energy for a type I amphiphile, we postulate that it is both the sign and magnitude of τ, which can potentially modulate the membrane association of CCT and hence its activity.
Figure 1.
The CCT-binding helix is seen in cross section bound to the membrane. The geometry of the hydrophobic part of the binding domain (dark line) allows the nearby amphiphiles (gray background) to splay. The molecules are approximately to scale.
The suggestion that type II amphiphiles might play a key role in the functioning of biological membranes was discussed some 20 years ago (27, 28), and the possibility that κ and c0 might affect the properties of membrane-spanning proteins was clearly recognized by Gruner (29, 30). This paper pursues that idea for a protein that does not span the membrane but associates with the monolayer surface.
Materials and Methods
To test our hypothesis of CCT activity regulation by stored membrane curvature elastic stresses, we isothermally varied τ by systematically altering the lipid composition of large unilamellar vesicles (LUVs) and then measured the activity of purified CCT in their presence. We used LUVs to ensure that 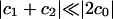 and that the binding of CCT would cause an insignificant percentage change in the monolayer area. This means that we can discount any effects of imposed membrane curvature or membrane compression on the activity of CCT. The CCT activity was measured in the presence of LUVs made from mixtures of six different amphiphiles, dioleoylphosphatidylcholine (DOPC), DOPE, dimyristoylphosphatidylcholine (DMPC), octaethyleneglycol monohexadecyl ether (C16EO8), all purchased from Fluka, monomyristoylphosphatidylcholine (lyso-MPC), purchased from Sigma-Aldrich, and HexPC, purchased from Calbiochem–Novabiochem. All amphiphiles were stated to be better than 99% pure and were checked by TLC.
and that the binding of CCT would cause an insignificant percentage change in the monolayer area. This means that we can discount any effects of imposed membrane curvature or membrane compression on the activity of CCT. The CCT activity was measured in the presence of LUVs made from mixtures of six different amphiphiles, dioleoylphosphatidylcholine (DOPC), DOPE, dimyristoylphosphatidylcholine (DMPC), octaethyleneglycol monohexadecyl ether (C16EO8), all purchased from Fluka, monomyristoylphosphatidylcholine (lyso-MPC), purchased from Sigma-Aldrich, and HexPC, purchased from Calbiochem–Novabiochem. All amphiphiles were stated to be better than 99% pure and were checked by TLC.
The LUVs were prepared by a freeze–thaw technique. The lipid in chloroform solutions was dried down as a thin film by using a rotary evaporator followed by 1.5 h of lyophilization at 0.1 torr (1 torr = 133 Pa). The dried lipids were suspended in deionized water by gentle shaking and then lyophilized again. Buffer solution (100 mM Tris⋅HCl/100 mM NaCl/2.5 mM EDTA/5 mM DTT/20 mM MgCl2, pH 7.4) was added, and the mixture was vortexed for 15 min (until all solids were suspended). The mixture was sonicated in an ultrasonic bath for 20 min. The resulting suspension was flash frozen as a thin film by using liquid nitrogen and then allowed to thaw by standing at room temperature for 30 min. The freeze–thaw process was repeated three times. The suspension of vesicles that resulted was sonicated a second time for 30 seconds and subjected to five freeze–thaw cycles. Mean vesicle diameters were determined by using a Coulter N4 Plus Particle Sizer. Measurements of scattered light were made at 30°, 50°, and 90° to the incident beam. All mixtures used in the assays had vesicles with a mean diameter 1.95 μm. Preparations containing vesicles with diameters <0.8 μm in diameter, which amounted to >4% of the total vesicle population, were discarded.
CCT was obtained from extracts of Spodoptera frugiperda (Sf9) cells that had been infected with a recombinant baculovirus construct containing rat CCT cDNA under the control of the polyhedrin promoter (31). The enzyme was isolated in a delipidated form as reported previously and used within 24 h of its isolation (17). Cytidine triphosphate (CTP) (Sigma–Aldrich) at a concentration of 15 mM was dissolved in the same buffer solution as was used to produce the vesicle solution. Twenty microliters of the CTP solution was added to 50-μl aliquots of purified CCT. A suspension of the appropriate LUVs was then added to the CCT/CTP mixture at a lipid concentration of 1 mM (in the final assay volume of 100 μl). The CCT concentration was in the range 1.8–2.0 μg in final assay volume. The reaction was started by the addition of 10 μl (14C) choline phosphate (1,000–1,500 dpm/nmol) (Nycomed Amersham) followed by a few seconds of rapid mixing before incubation in capped tubes at 37°C for 20 min. The reaction was terminated on submersing the tubes in boiling water for 2–3 min. Charcoal slurry was added to the mixture, which was centrifuged at 12,000 × g for 1 min. The pellet recovered was washed with distilled water followed by centrifugation. CDP-choline was eluted from the charcoal by sequential washing and centrifugation with elution solvent consisting of 60% ethanol, 37% water, and 13% 0.88 NH4OH. Supernatants were decanted into scintillation vials and the solvent evaporated before the addition of scintillation fluid and determination of radioactivity. Control experiments were conducted in which either the vesicles or the CCT were omitted from the reaction mixtures. CDP-choline recovery standards were used to correct for nonspecific CCT binding and recovery. The decays per minute for each sample were corrected for background, and the data were divided by the mean value obtained for the standard lipid system (normally pure DOPC LUVs) to obtain the relative activity, S.
Results
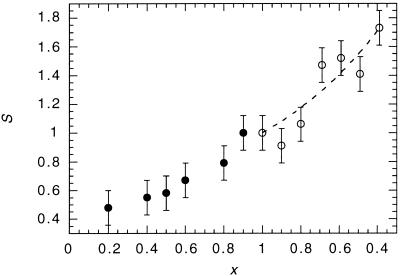
In Fig. 2, we show the variation in the activity of CCT in binary lipid vesicles consisting of DMPC with DOPC, and of DOPC with DOPE, relative to the activity of CCT in DOPC vesicles. The data shown are the average of at least three separate sets of measurements. Adding DOPC to DMPC or adding DOPE to DOPC increases activity monotonically. Both the DMPC/DOPC and DOPC/DOPE binary systems have been studied in extensive detail, and neither shows evidence of phase separation over the entire composition range, nor are there any changes in the distribution of vesicle diameters. Therefore, gross inhomogeneities are unlikely to be the cause of the observed changes in CCT activity. A physical property of the bilayer that we know is changing monotonically in this experiment is the spontaneous curvature and, by inference, the torque tension and the associated stored curvature elastic energy.
Figure 2.
Composite plot showing the relative activity, S, of CCT as a function of the mol fraction of DOPC, x, in DMPC/DOPC (solid circles) and DOPC/DOPE (open circles) vesicles. The curve through the data points in the DOPC/DOPE part of the plot is obtained from Eq. 5.
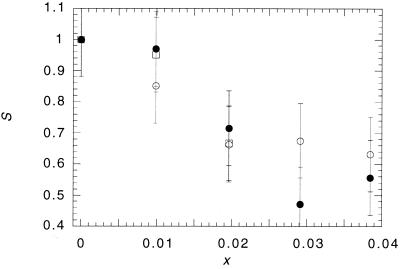
We have already pointed out that we would predict that adding type I amphiphiles to the membrane should lower the activity of CCT. This is because the addition of type I amphiphiles to a membrane composed of type II amphiphiles reduces the desire to bend toward the water. In Fig. 3, we show the effects on CCT activity of different concentrations of three type I amphiphiles, HexPC, C16EO8, and lyso-MPC, added to DOPE/DOPC vesicles (1:9 mol ratio). All three amphiphiles cause a rapid decrease in CCT activity, with gradients that are greater than those reported in Fig. 2.
Figure 3.
Plot showing the effect of lyso-MPC (open circles), HexPC (open squares) and C16EO8 (solid circles) as a function of their fractional molar composition in DOPC/DOPE vesicles (9:1 mol ratio) on the relative activity of CCT.
Discussion and Analysis
Increasing the amount of DOPC in DMPC/DOPC vesicles increases τ, because DOPC contains cis-unsaturated acyl chains, and chain unsaturation increases the propensity for acyl chain splay and hence the desire for monolayer curvature toward the polar region. Because this desire cannot be realized, the effect of increasing the proportion of cis-unsaturated chains in the monolayer is to increase the average value of A from 67 Å2 for 100 mol% DMPC to 76 Å2 for 100 mol% DOPC (32). Adding DOPE to DOPE/DOPC vesicles also increases the desire for curvature toward the water, but now it is because of the lower hydrophilicity of the phosphatidylethanolamine headgroup (33). However, the consequent increase in τ is therefore accompanied by a reduction in A to approximately 69 Å2 at 60 mol% DOPE (25). This means that as we travel from left to right in Fig. 2 the area per molecule initially increases and then falls as we pass through the 100 mol% DOPC datum. The data are therefore inconsistent with the activity of CCT depending on molecular area. Furthermore, because we observe variations in CCT activity with DMPC/DOPC vesicles where the amphiphilic headgroup is unchanged and in DOPE/DOPC vesicles where the chains are unchanged, we can also exclude specific headgroup or chain effects on CCT activity. The data are, however, qualitatively consistent with variations in the stored curvature elastic stress.
We now proceed to be somewhat more quantitative by creating a very simplified model of the interaction of CCT with the membrane and the effect of this on the activity. We will use this model to determine whether the hypothesis that the variations in S are because of changes in stored curvature elastic stress is plausible.
The partitioning of CCT into the cytosolic leaflet of a membrane will make a negative contribution to the average value of τ. Thus the partitioning of the helix into the bilayer is energetically more favorable for membranes with more positive values of τ and concomitantly greater amounts of stored curvature elastic energy. Of course, there are other free energy contributions to CCT binding, such as the sequestration of the hydrophobic residues on the binding domain, electrostatic interactions between peptide and lipids, and the energy of folding. However, for the purposes of this simplistic model, we will assume that all of these contributions to the binding energy are independent of the membrane composition and that only the stored curvature elastic energy will vary with the lipid composition. Because biochemical studies indicate that CCT is largely inactive in the cytosol, we will assume that its activity depends only on its partitioning into the monolayer. Next we make the simplifying assumptions that it is only the annulus of lipids surrounding the long sides of the bound α-helix that are allowed to splay. They will splay to some value c1 allowed by the geometry of the binding domain and to simplify our calculations, we assume that the membrane remains flat and there is no deformation of the membrane thickness (Fig. 1). Because the amphiphilic chains may splay only in one direction (perpendicular to the axis of the α-helix), the binding of CCT allows for only locally cylindrical interfacial curvature. This means that the curvature elastic energy per lipid released on CCT binding is equal to 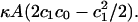 This is less than the total stored curvature elastic energy, which can be released only if locally spherical curvature is allowed. The membrane/water partition coefficient, K, for CCT is then
This is less than the total stored curvature elastic energy, which can be released only if locally spherical curvature is allowed. The membrane/water partition coefficient, K, for CCT is then
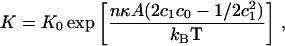 |
3 |
where the factor K0 represents the free energy contributions to partitioning that we are assuming remain invariant with respect to membrane composition, and n is equal to the number of amphiphiles surrounding the binding domain (length ≈ 78 Å). The relative activity of the enzyme, S, in the presence of binary lipid LUVs of composition (x) is then given by
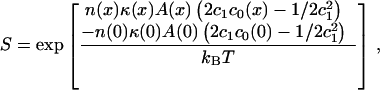 |
4 |
in which c0(x), κ(x), and A(x) will be determined by adding the values for the two lipid components in proportion to their mol fractions. Using average molecular properties in our determination of S implies that there is no preferential partitioning of more stressed lipids to the vicinity of the CCT. Because it is quite likely that in fact the more stressed lipids will aggregate around the CCT, we must accept the approximate nature of the model.
Reliable experimental values of c0, κ, and A are known for DOPC and DOPE (25, 26, 32, 34) (Table 1), and we are therefore able to fit S (x) for mixtures of these lipids (Fig. 2). The model fits the data well with a value of c1 of (−2.1 ± 0.2) × 10−3 Å−1. This degree of molecular splay for lipids surrounding the CCT is not unreasonable given the simplifications of the model. We now use this value of c1 to estimate the change in S when we add type I amphiphiles to 9:1 mol ratio DOPC/DOPE vesicles. The radius of the normal micelles formed by type I amphiphiles gives us an estimate of c0 in the region of 1/20 to 1/10 Å−1 (note that the curvature is positive). In the case of very small mol fractions of type I amphiphile, as in Fig. 3, the precise values of the molecular area and bending modulus of the type I amphiphile are not significant. By using A = 68 Å2 and κ = 0.5 kBT, we estimate that S = 0.74 to 0.8 at x = 0.04. The model predicts a rapid decrease in CCT activity. This is also seen in the data, where the measurements indicate S = 0.6 ± 0.1 at x = 0.04. Given the simplifications of the model, the agreement is reasonable.
Table 1.
Curvature elastic and geometric lipid parameters
| κ/kBT | c0/Å−1 | A/Å2 | |
|---|---|---|---|
| DOPC | 9 | −1/160 | 76 |
| DOPE | 13 | −1/53 | 65 |
The model indicates that the rapid deactivation of CCT by type I amphiphiles is because of the high degree of positive spontaneous curvature of these molecules. Because lyso-MPC and HexPC and C16EO8 deactivate CCT to a similar degree, it is clear that the effect does not depend directly on chemical interactions between CCT and amphiphilic headgroups. Rather it appears that the fact that all of the deactivators form type I lyotropic phases affects CCT activity.
Conclusions
Our analysis provides a plausible explanation for the modulation of CCT activity by nonionic and zwitterionic lipids. We have not presented comparative measurements on the effect of anionic lipids on CCT binding and activity, because electrostatic and curvature elastic effects are extremely difficult to disentangle. For example, fatty acids are activators of CCT activity, and it has been suggested that this is an electrostatic effect. However, it is known that fatty acids in combination with PCs give rise to type II lyotropic liquid crystalline phases, such as the inverse bicontinuous cubic phases (35) and the inverse hexagonal phase (36). Phosphatidylserines are capable of forming inverse hexagonal phases in the presence of screening, monovalent cations (37). One might then expect that positively charged amino acid residues on CCT would bind electrostatically to phosphatidylserine headgroups, but once bound the lipid chains are also able to splay around the embedded amphipathic helix. This means that these anionic lipids are expected to be activators of CCT because of both electrostatic and curvature elastic effects.
Although we are not yet at the stage of being able to disentangle curvature elastic and charge effects, we can make comparisons of the effects of charge and curvature elasticity from previously published data. Johnson et al. (38) have measured the partition coefficient of the CCT-binding domain in egg-PC and 1:1 molar ratio egg-PC/oleic acid vesicles. They find that the partition coefficient is 1.8 ± 0.6 greater in egg-PC/oleic acid vesicles. On the assumption that the partition coefficient and activity are directly correlated, we can see that this is comparable to the 1.6-fold increase we see between DOPC vesicles and 1:1 molar ratio DOPC/DOPE vesicles (Fig. 2). We can also obtain an estimate of the curvature elastic energy tied up in the binding energy of CCT and compare this to measurements of the binding energy of anionic lipids. For a 1:1 molar ratio of DOPC to DOPE, our fit to the data tells us that the binding energy is ≈0.65 kBT, equivalent to an additional drop in the free energy of binding, compared with DOPC, of −0.4 kcal⋅mol−1. This is the same drop in binding free energy that has been measured between the egg-PC and 1:1 egg-PC/oleic acid vesicles (38). It therefore seems reasonably clear that the effects of curvature elasticity are significant and are comparable to electrostatic effects, and compositionally driven changes in the membrane torque tension would be capable of causing significant alterations in CCT activity.
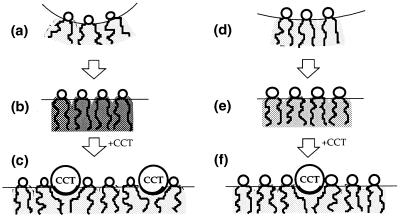
Eukaryotic cells might therefore use membrane torque tension as a feedback signal to regulate PtdCho synthesis (Fig. 4). It seems appropriate that the cell should use the torque tension to accomplish this, because it is the maintenance of the torque tension within a critical range of values that ensures that the membrane bilayer does not undergo a phase transition into a porous state. It also seems reasonable that the cell should use a nonspecific physical signal for this purpose, because this is the simplest and most robust means of ensuring the membrane's integrity. Gruner (29) pointed to the pivotal importance of the spontaneous curvature in altering the stored curvature elastic stress, and this is clearly the major effect in the data that we have measured. However, it is also clear that, in principle, the bending rigidity of the monolayer may also be a determinant used in controlling cell lipid homeostasis. It remains to be seen whether in practice cells make use of the rigidity.
Figure 4.
How the cell might regulate membrane composition can be seen from the effect of CCT binding on the torque tension of a DOPE and DOPC monolayer. (a) A curvature relaxed DOPE monolayer is flattened, b, with a resultant increase in torque tension, indicated by the dark shading in the chain region. (c) The partitioning of the helical CCT-binding domain into the monolayer allows DOPE molecules close by to splay and hence reduce their torque tension. (d) DOPC has a lower propensity for interfacial curvature and hence when it is flattened, e, the torque tension is less than a third of that of DOPE, as indicated by the lighter shading. (f) Hence the energy released on binding CCT is less than for DOPE, and fewer CCT molecules partition into the monolayer.
The monolayer torque tension may also be an important regulator of a variety of key enzymes and biochemical processes that are reversibly associated with biological membranes. Although it is controversial whether there is a correlation between PKC enzyme activity and the magnitude of the stored elastic stress (39), there are other examples of extrinsic membrane proteins that are modulated by membrane lipid composition (e.g., phospholipases C and A2, phosphatidate phosphohydrolase, and diacylglycerol kinase) (40, 41). It will be important to determine the extent to which these proteins are controlled by the stored elastic energy in a manner similar to CCT.
Acknowledgments
This work was supported by the Royal Society, the Engineering and Physical Sciences Research Council, the National Institutes of Health Grant GM45737, Cancer Center (CORE), Support Grant CA21765 and the American and Lebanese Syrian Associated Charities.
Abbreviations
- CCT
CTP:phosphocholine cytidylyltransferase
- C16EO8
octaethyleneglycol monohexadecyl ether
- DMPC
dimyristoylphosphatidylcholine
- DOPC
dioleoylphosphatidylcholine
- DOPE
dioleoylphosphatidylethanolamine
- HexPC
hexadecylphosphocholine
- lyso-MPC
monomyristoylphosphatidylcholine
- LUV
large unilamellar vesicle
- PtdCho
phosphatidylcholine
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.160260697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.160260697
References
- 1.Kent C. Biochim Biophys Acta. 1997;1348:79–90. doi: 10.1016/s0005-2760(97)00112-4. [DOI] [PubMed] [Google Scholar]
- 2.Jackowski S. J Biol Chem. 1994;269:3858–3867. [PubMed] [Google Scholar]
- 3.Boggs K P, Rock C O, Jackowski S. J Biol Chem. 1995;270:7757–7764. doi: 10.1074/jbc.270.13.7757. [DOI] [PubMed] [Google Scholar]
- 4.Boggs K P, Rock C O, Jackowski S. J Biol Chem. 1995;270:11612–11618. doi: 10.1074/jbc.270.19.11612. [DOI] [PubMed] [Google Scholar]
- 5.Boggs K P, Rock C O, Jackowski S. Biochim Biophys Acta. 1998;1389:1–12. doi: 10.1016/s0005-2760(97)00145-8. [DOI] [PubMed] [Google Scholar]
- 6.Baburina I, Jackowski S. J Biol Chem. 1998;273:2169–2173. doi: 10.1074/jbc.273.4.2169. [DOI] [PubMed] [Google Scholar]
- 7.Cui Z, Houweling M, Chen M H, Record M, Chap H, Vance D E, Terce F. J Biol Chem. 1996;271:14668–14671. doi: 10.1074/jbc.271.25.14668. [DOI] [PubMed] [Google Scholar]
- 8.Johnson J E, Cornell R B. Biochem J. 1994;33:4327–4335. doi: 10.1021/bi00180a029. [DOI] [PubMed] [Google Scholar]
- 9.Dunne S J, Cornell R B, Johnson J E, Glover N R, Tracey A S. Biochemistry. 1996;35:11975–11984. doi: 10.1021/bi960821+. [DOI] [PubMed] [Google Scholar]
- 10.Johnson J E, Aebersold R, Cornell R B. Biochim Biophys Acta. 1997;1324:273–284. doi: 10.1016/s0005-2736(96)00233-7. [DOI] [PubMed] [Google Scholar]
- 11.Choy P C, Farren S B, Vance D E. Can J Biochem. 1979;57:605–612. doi: 10.1139/o79-076. [DOI] [PubMed] [Google Scholar]
- 12.Cornell R, Vance D E. Biochim Biophys Acta. 1987;919:37–48. doi: 10.1016/0005-2760(87)90215-3. [DOI] [PubMed] [Google Scholar]
- 13.Cornell R B. Biochemistry. 1991;30:5881–5888. doi: 10.1021/bi00238a011. [DOI] [PubMed] [Google Scholar]
- 14.Cornell R B. Biochemistry. 1991;30:9810. doi: 10.1021/bi00238a011. [DOI] [PubMed] [Google Scholar]
- 15.Cornell R B. Biochemistry. 1991;30:5873–5880. doi: 10.1021/bi00238a010. [DOI] [PubMed] [Google Scholar]
- 16.Jamil H, Yao Z, Vance D E. J Biol Chem. 1990;265:4332–4339. [PubMed] [Google Scholar]
- 17.Yang W, Boggs K P, Jackowski S. J Biol Chem. 1995;270:23951–23957. doi: 10.1074/jbc.270.41.23951. [DOI] [PubMed] [Google Scholar]
- 18.Geilen C C, Haase A, Wieder T, Arndt D, Zeisig R, Reutter W. J Lipid Res. 1994;35:625–632. [PubMed] [Google Scholar]
- 19.Sohal P S, Cornell R B. J Biol Chem. 1990;265:11746–11750. [PubMed] [Google Scholar]
- 20.Arnold R S, Cornell R B. Biochemistry. 1996;35:9917–9924. doi: 10.1021/bi960397c. [DOI] [PubMed] [Google Scholar]
- 21.Jamil H, Hatch G M, Vance D E. Biochem J. 1993;291:419–427. doi: 10.1042/bj2910419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helfrich W. Z Naturforsch. 1973;28c:693–703. doi: 10.1515/znc-1973-11-1209. [DOI] [PubMed] [Google Scholar]
- 23.Templer R H, Khoo B J, Seddon J M. Langmuir. 1998;14:7427–7434. [Google Scholar]
- 24.Helfrich W. In: Physics of Defects. Balian R, Kléman M, Poirier J P, editors. Amsterdam: North–Holland; 1981. pp. 715–755. [Google Scholar]
- 25.Kozlov M M, Leikin S, Rand R P. Biophys J. 1994;67:1603–1611. doi: 10.1016/S0006-3495(94)80633-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Z, Rand R P. Biophys J. 1997;73:267–276. doi: 10.1016/S0006-3495(97)78067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan A, Rilfors L, Wieslander Å, Lindblom G. Eur J Biochem. 1981;116:215–220. doi: 10.1111/j.1432-1033.1981.tb05321.x. [DOI] [PubMed] [Google Scholar]
- 28.Lindblom G, Brentel I, Sjölund M, Wikander G, Wieslander Å. Biochemistry. 1986;25:7502–7510. doi: 10.1021/bi00371a037. [DOI] [PubMed] [Google Scholar]
- 29.Gruner S M. Proc Natl Acad Sci USA. 1985;82:3665–3669. doi: 10.1073/pnas.82.11.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gruner S M. Adv Chem Ser. 1994;235:129–149. [Google Scholar]
- 31.Luche M M, Rock C O, Jackowski S. Arch Biochem Biophys. 1993;301:114–118. doi: 10.1006/abbi.1993.1122. [DOI] [PubMed] [Google Scholar]
- 32.Costigan, S. C., Booth, P. J. & Templer, R. H. (2000) Biophys. Biochim. Acta-Biomembranes, in press. [DOI] [PubMed]
- 33.Templer R H, Castle S J, Curran A R, Klug D R. Faraday Disc. 1999;111:41–53. doi: 10.1039/a806472e. [DOI] [PubMed] [Google Scholar]
- 34.Keller S L, Bezrukov S M, Gruner S M, Tate M W, Vodyanoy I, Parsegian V A. Biophys J. 1993;65:23–27. doi: 10.1016/S0006-3495(93)81040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Templer R H, Seddon J M, Duesing P, Winter R, Erbes J. J Phys Chem B. 1998;102:7262–7271. [Google Scholar]
- 36.Seddon J M, Templer R H, Warrender N A, Huang Z, Cevc G, Marsh D. Biochim Biophys Acta-Biomembranes. 1997;1327:131–147. doi: 10.1016/s0005-2736(97)00047-3. [DOI] [PubMed] [Google Scholar]
- 37.Cevc G, Seddon J M, Marsh D. Biochim Biophys Acta. 1985;814:141–150. [Google Scholar]
- 38.Johnson J E, Rao N M, Hui S-W, Cornell R B. Biochemistry. 1998;37:9509–9519. doi: 10.1021/bi980340l. [DOI] [PubMed] [Google Scholar]
- 39.Giorgione J R, Kraayenhof R, Epand R M. Biochemistry. 1996;37:10956–10960. doi: 10.1021/bi980185a. [DOI] [PubMed] [Google Scholar]
- 40.Cornell R B, Arnold R S. Chem Phys Lipids. 1996;81:215–227. [Google Scholar]
- 41.Goñi F M, Basáñez G, Ruiz-Argüello M B, Alonso A. Faraday Disc. 1999;111:55–68. doi: 10.1039/a806352d. [DOI] [PubMed] [Google Scholar]